Rheumatoid arthritis (RA) is a disease of the synovial membrane and the resultant inflammatory synovitis is directly or indirectly responsible for the deformities seen in the digits.1 An untreated rheumatoid digital deformity moves from a passively correctable flexible deformity to a deformity with limited motion, and finally a fixed deformity. Although the initial inciting factor is synovitis, the progression of the digital deformity results from a combination of synovitis, adhesions, shortening and/or rupture of the ligaments, intrinsic muscles, and extrinsic tendons leading to poor joint position and joint ankylosis. When evaluating digital deformities for surgical treatment, the surgeon needs to classify them into flexible or a fixed deformities. Although many options are available for the treatment of flexible deformities, these options become progressively limited with increasing joint stiffness, and joint fusion is the only reliable option for a fixed interphalangeal joint deformity in RA. For a deformed digit with limited motion, the aim is to recover flexibility first.
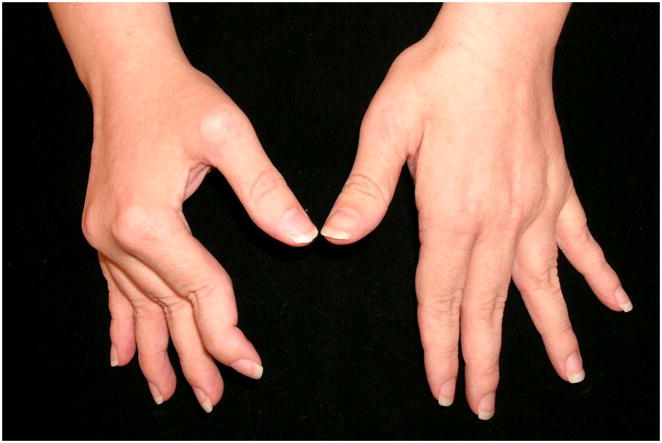
The two classic digital deformities seen in RA are swan-neck deformity (SND) and boutonniere deformity (BND) (Fig. 1).2 The incidence of uncorrectable SND and BND is estimated to be between 8% and 16% during the first 2 years after the onset of systemic disease, and the prevalence of finger deformities in patients with established RA is approximately 14% for SND and 36% for BND.3 Although both occur at the proximal interphalangeal joint (PIPJ), a SND can result from synovitis at the wrist, metacarpophalangeal joint (MCPJ), PIPJ, or the distal interphalangeal joint (DIPJ), whereas a BND always results from PIPJ synovitis. It is not uncommon for the same hand to develop both deformities simultaneously (Fig. 1A).4 A patient with a SND is unable to make a full fist due to the hyperextended posture of the PIPJ, whereas in a BND the patient is able to make a fist, but is unhappy with the flexed-posture of the PIPJ when the digit is extended (Fig. 1B). A SND therefore causes a functional problem whereas a BND is more of an aesthetic concern. The surgical treatment of SND places the digit in a functional posture, whereas the treatment of BND risks changing the flexed posture of the PIPJ into a functionally limiting extended posture.
Figure 1.
40-year-old lady with bilateral rheumatoid hand deformities
A. : Boutonniere deformities of left small, left ring, and right small fingers with simultaneous swan neck deformities of left long, right long, and right ring fingers.
B. : Note inability to make a fist on the right hand (predominantly swan neck deformity) compared to the left hand (predominantly boutonniere deformity)
C. : Radiograph
Although most attention in rheumatoid digits is directed towards the evaluation of SND and BND, one must not forget to examine the digit for the presence of other rheumatoid pathology. The concomitant conditions can include flexor tenosynovitis that can lead to trigger digits and rupture of the flexor tendons, and synovitis of the interphalangeal joints. The treatment of these conditions when detected early is rewarding. It is important to realize that the correction of digital deformities is last in the priority of surgical procedures for the rheumatoid hand and wrist. The surgical treatment of the rheumatoid wrist, MCPJ and thumb deformities takes priority because the results are good and these procedures contribute significantly to improving the quality of life. The results of surgery in rheumatoid SND and BND are unpredictable, and the patient may regain adequate function once the proximal deformities have been addressed or adapt quite well to the disease, despite obvious digital deformities. The number of RA patients in whom a surgeon can predictably improve the patient’s function by performing surgery for digital deformities are few and the surgeon must consider the outcome carefully before offering surgical correction.
ANATOMY AND KINEMATICS
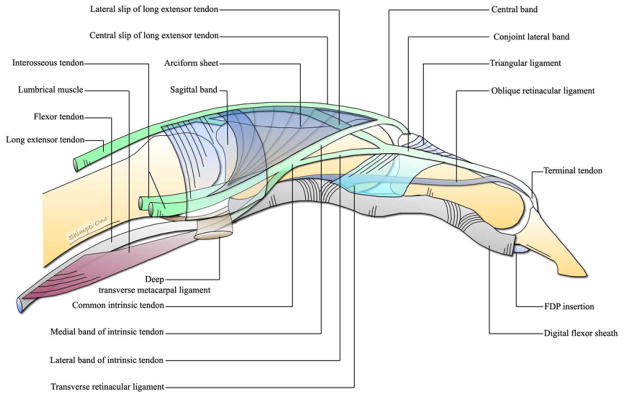
An appreciation of the anatomy and kinematics of the extensor apparatus is essential for understanding the pathogenesis and treatment of the rheumatoid digital deformities.5–7 The extensor apparatus is composed of a musculotendinous and a ligamentous system. The musculotendinous system comprises the radial nerve-innervated extrinsic extensors [extensor digitorum communis (EDC), extensor indicis proprius (EIP), and extensor digiti minimi quinti (EDQM)], and the intrinsic muscles [palmar interossei (PI), dorsal interossei (DI), and lumbricals] supplied by the ulnar and median nerves. The ligamentous system comprises of four ligaments, namely the sagittal band, triangular ligament, transverse retinacular ligament (TRL), and oblique retinacular ligament (ORL). These ligaments maintain equilibrium between the intrinsic and extrinsic musculotendinous systems.
Musculotendinous system
Extensor tendon
As the extrinsic extensor tendon passes over the MCPJ, it becomes flattened and band like. At the junction of the proximal and middle third of the proximal phalanx, it divides into three slips: a central slip and two lateral slips. The central slip continues in the midline and at the level of the neck of the proximal phalanx it receives the medial band of the intrinsic tendon on both sides. It then continues as the central band, which inserts into the base of the dorsal aspect of the middle phalanx. Each lateral slip merges with the respective lateral bands of the intrinsic tendon to form a conjoint lateral band. The conjoint lateral bands on both sides join with each other on the dorsum of the finger at the level of middle and distal third of the middle phalanx, forming the terminal tendon, which inserts onto the dorsal aspect of the base of the distal phalanx. The extensor tendon is considered to have two more insertions in addition to two described above. The first is onto the lateral aspect of the volar plate of the MCPJ via the sagittal band. The second is by an inconstant slip from the deep surface of the tendon to the base of the dorsal aspect of the proximal phalanx.7 (Fig. 2 & 3)
Figure 2.

Anatomy of the extensor apparatus (dorsal view)
Figure 3.
Anatomy of the extensor apparatus (view from radial aspect)
Interossei
There are three palmar and four dorsal interosseous muscles. The interossei originate between the metacarpal shafts and insert into the base of the proximal phalanx and the lateral band. The interosseous tendon passes volar to the axis of the MCPJ, but remains dorsal to the deep transverse metacarpal ligament, contributes fibers to the sagittal band, and forms the intrinsic tendon (Fig. 3). On the ulnar side of the digit, the intrinsic tendon is formed by the tendons of the palmar and dorsal interossei, whereas on the radial side it is formed by the interossei (palmar and dorsal) and the lumbrical tendon (Fig. 2). At the junction of the proximal and middle third of the proximal phalanx, each intrinsic tendon divides into the medial and lateral bands. The medial band joins with the central slip of the extensor tendon to form the central band, and the lateral band joins with the lateral slips of the extensor tendon to form the conjoint lateral band. (Fig. 2 & 3)
The PI do not have an insertion onto the long finger, whereas the DI do not have insertion onto the small finger. The PI causes adduction and flexion at the index, ring and small finger MCPJ, whereas the DI causes abduction and flexion of the index, long and ring fingers MCPJ. Their action on the interphalangeal joints depends to some extent on the position of the MCPJ. When the MCPJ is in extension, the interossei are under tension and their pull is transmitted to the central band and terminal tendon causing extension of the interphalangeal joints. As the MCPJ flexes, the interossei become progressively lax and their action of extension of the interphalangeal joints decreases, and when the MCPJ is in full flexion, they no longer contribute to interphalangeal joints extension.7, 8
Lumbrical
The lumbrical muscle originates from the FDP muscle in the palm, passes volar to the axis of the MCPJ, and combines with interosseous tendon to form the intrinsic tendon (Fig. 2 & 3). Unlike the interossei tendons which contribute towards formation of the intrinsic tendons on both sides of the digit (except small finger), the lumbrical tendon is present only on the radial aspect of the digit and contributes to formation of only the radial intrinsic tendon. Although both the lumbrical and interosseous tendons pass volar to the axis of the MCPJ, the lumbrical tendon passes volar to the deep transverse metacarpal ligament whereas the interosseous tendons pass dorsal to this ligament (Fig. 2 & 3). The lumbrical muscle extends the interphalangeal joints and flexes the MCPJ. However unlike the interossei, it can extend the interphalangeal joint irrespective of MCPJ position. This is because it has a mobile origin on the FDP. As the MCPJ is flexed the FDP moves proximally, maintaining tension on the lumbrical and allowing it to extend the interphalangeal joints.6
Ligamentous system
Sagittal band
It is a fibrous sheet that invests the extensor tendon on the dorsum of the MCPJ and stretches to the volar plate and the deep transverse metacarpal ligament on the palmar aspect. It receives fibers from the extensor tendon on the dorsal aspect and from the interosseous tendons on the palmar aspect. The principle function of the sagittal band is to extend the proximal phalanx. In addition, it acts as a static tether by stabilizing and maintaining the extensor tendon in the midline and as a dynamic tether by allowing proximal and distal gliding of the extensor tendons during finger flexion and extension. The sagittal band continues distally as a thin aponeurotic sheet (arciform sheet). This triangular shaped sheet extends between the extensor tendon and the intrinsic tendon and is composed of a layered criss-cross fiber pattern, which changes its geometric arrangement as the finger flexes and extends. This arrangement allows the intrinsic tendons to be displaced volarly in flexion and to return to the dorsum of the finger in extension.9 (Fig. 2 & 3)
Triangular ligament
It is situated dorsally over the proximal half of the middle phalanx. It is composed of transverse fibers that extend between the medial sides of the conjoint lateral bands. They maintain the dorsal position of the conjoint lateral bands and limit palmar and lateral shift during flexion of the PIPJ. (Fig. 2)
Transverse retinacular ligament (TRL)
It is situated on the lateral aspect of the proximal half of the middle phalanx. It consists of transverse fibers that extend from the lateral side of the conjoint lateral band to the volar plate of the PIPJ and the fibrous flexor sheath. These transverse fibers continue onto the dorsum merging with the fibers of the triangular ligament. The TRL overlies the collateral ligaments of the PIPJ and their main function is to prevent dorsomedial shift of the conjoint lateral band when the finger is extended. (Fig. 3)
Oblique retinacular ligament (ORL)
It is situated on the lateral aspect of the digit and has an oblique volar to dorsal course. It begins on the lateral aspect of the proximal phalanx and the fibrous flexor sheath at the level of the neck of the proximal phalanx and courses distally and dorsally. It passes volar to the axis of the PIPJ, superficial to the collateral ligament, deep to the TRL, along the side of the middle phalanx and inserts into the conjoint lateral band at the level of the neck of middle phalanx. It is consistently palmar to the PIP and dorsal to the DIPJ axis of rotation. (Fig. 3) The ORL is said to coordinate movement of the interphalangeal joints and act as a dynamic tenodesis to aid the conjoint lateral band in extension of the DIP joint.10 Extension of the PIPJ joint places the ORL under tension and it prevents easy active or passive flexion of the DIP joint.11
PATHOLOGY AND TREATMENT
Swan Neck Deformity(SND)
It is characterized by hyperextension of the PIPJ with reciprocal flexion of the MCPJ and DIPJ (Fig. 4).12
Figure 4.
Classic swan neck deformity of the right hand in a 42-year-old lady
Pathogenesis
A SND can occur as a result of pathology at the wrist, MCPJ, PIPJ or the DIPJ. The key to treatment of a flexible SND is to determine the joint that is primarily responsible for development of the SND. It is therefore essential to understand how synovitis at each of these joints can lead to the development of a SND. (Fig. 5)
Figure 5.
Pathogenesis of a swan neck deformity
Primary PIPJ pathology
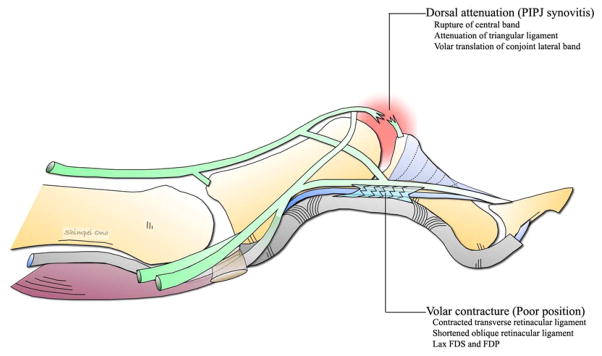
The synovial lining of the PIPJ especially the synovium within the palmar synovial pouch becomes inflamed and hyperplastic to form a pannus. This distends the joint and stretches the dorsal surface of the volar plate, the check rein ligaments, and along the dorsal surface of the FDS tendon. Later the FDP may also become involved and the tendon sheath filled with hyperplastic tissue and free fluid. This synovitis leads to stretching, weakening and eventually destruction of the volar plate and collateral ligaments, and the insertion of the FDS resulting in loss of palmar restraint at the PIPJ. This allows the normal extensor forces to cause abnormal hyperextension of the PIPJ that in turn relaxes the normal tension on the conjoint lateral bands leading to dorsal migration.13, 14 A relaxed conjoint lateral band loses the ability to extend the DIPJ. In addition, the hyperextension of the PIPJ stretches the FDP increasing its flexor action of the DIPJ, and causes the loss of the mechanical advantage of the ORL in extending the DIPJ. This loss of conjoint lateral band and ORL tension and increased tension of the FDP results in a DIPJ flexion deformity.12 Over time, adhesions develop between the central slip and the dorsally translated conjoint lateral bands converting the flexible deformity to a fixed deformity.
Primary MCPJ pathology
Synovitis of the MCPJ can lead to weakening of the insertion of the long extensors on the dorsal base of the proximal phalanx. This transmits the extensor force to the base of the middle phalanx resulting in hyperextension of the PIPJ. On the volar side, the synovitis causes attenuation of the volar plate resulting in volar subluxation of the MCPJ. Over time, volar subluxation results in shortening of the intrinsic muscles leading to PIPJ hyperextension and ultimately SND. This is compounded by synovitis in the flexor tendon sheath which restricts flexor tendon excursion concentrating the flexor force on the proximal phalanx resulting in MCPJ flexion. Adhesions of intrinsic muscle tendons around the MCPJ from rheumatoid synovitis can also cause intrinsic contracture. A few authors have proposed spasm of the intrinsic muscles as a result of irritation from MCPJ synovitis as a cause, whereas others have suggested that the afferent pain stimuli from the inflamed MCPJ capsule triggers a ‘protective pain reflex’ which produces spasm in the intrinsic muscle supplied by the same spinal cord segment.15 Secondary fibrosis in the persistently contracting muscle leads to contracture.
Primary DIPJ pathology
DIPJ synovitis can cause weakening and rupture of the terminal extensor tendon insertion leading to development of a mallet deformity. The proximal migration of the terminal extensor insertion will cause the lateral bands to become lax. All the power of the common extrinsic extensor will now be directed towards the central slip that inserts into the middle phalanx. Over time, the volar supporting structures of the PIPJ are weakened and the PIPJ is forced into hyperextension resulting in a SND.
Primary wrist pathology
Synovitis at the wrist joint can lead to carpal collapse, carpal supination, and ulnar translation. Carpal collapse leads to a relative lengthening (relaxation) of the long flexor and extensor tendons. The interosseous muscle can then overpower the action of extrinsics and lead to the MCPJ flexion and PIPJ extension. This is aided by carpal supination and ulnar translation that lead to a flexion deformity at the wrist and the MCPJ. This results in a posture of MCPJ flexion, which over a prolonged period causes a physiological shortening of the intrinsic muscles.
Management
Although the pathogenesis appears quite complicated, the evaluation of a SND is relatively simple and logical. The first step is to determine the passive range of motion at the PIPJ. This will classify the deformity in one of three types.
A. Full passive range of PIPJ motion
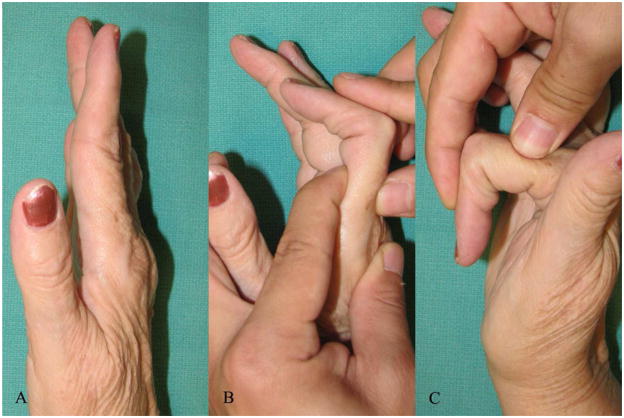
In patients with a normal range of passive PIPJ motion, the next step is to evaluate the MCPJ. The MCPJ is examined to determine the condition of the articular surfaces and the presence of intrinsic muscle shortening. Passive testing of joint stability will help in assessing attenuation or complete rupture of ligaments and a grinding test may be done to determine degree of joint cartilage damage. Once again this is better confirmed on a radiograph. The assessment of intrinsic tightness is done using the Finochietto-Bunnell test.16 This test examines the effect of MCPJ position on PIPJ flexion. Under normal circumstances, PIPJ flexion can be accomplished with the MCPJ fully extended. If the intrinsic muscles are shortened, PIPJ flexion will be limited as MCPJ extension increases. The test is considered positive when there is less flexion of the PIPJ when the MCPJ is held extended than when the MCPJ joint is flexed (Fig. 6). One can test for shortening of ulnar and radial intrinsics by performing the test with the MCPJ alternately radially and ulnarly deviated. In cases with an associated ulnar drift deformity, the ulnar intrinsic muscles are shorter than the radial intrinsic muscles. On the other hand, the radial intrinsic muscles are weaker and atrophic from lack of use.17 In cases with long standing intrinsic muscle shortening, extension of the MCPJ will not be possible. This is due to the secondary shortening of the capsular structures of the MP joint, tightness of the palmar skin, and flexor tendon adhesions.
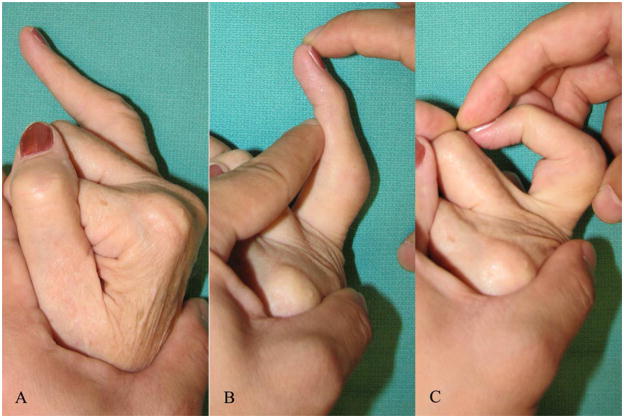
Figure 6.
Finochietto-Bunnell test for assessing intrinsic tightness
A. : 67-year-old lady with a flexible swan neck deformity of the right index finger
B. : PIPJ flexion is restricted when the MCPJ is hyperextended
C. : Improved range of PIPJ flexion when the MCPJ is flexed
The DIPJ is evaluated next to determine the condition of the articular surfaces and if the insertion of the terminal tendon into the base of the dorsum of the distal phalanx is preserved. Stiffness at the DIPJ with grating on motion suggests articular damage. This can be confirmed by a radiograph. The integrity of the terminal tendon can be determined by asking the patient to actively extend the DIPJ, with the examiner correcting the PIPJ hyperextension.
Treatment
The treatment of the MCPJ should take precedence in patients with an abnormal MCPJ. An intrinsic release for intrinsic contracture and/or MCPJ implant arthroplasty for MCPJ articular damage or volar subluxation should be done. If the articular surface of the MCPJ is preserved and there is no volar subluxation, but an ulnar drift deformity exists, the released radial intrinsic can be used for a crossed intrinsic transfer to correct the ulnar drift deformity of the adjacent long, ring, and small fingers. The treatment of an abnormal DIPJ (loss of terminal tendon or articular damage) is DIPJ fusion.
The surgical treatment of a flexible hyperextension deformity at the PIPJ aims to create a palmar restraint that will allow flexion and extension but prevent hyperextension. A tenodesis is most often used for this purpose. This tenodesis can be done using either the FDS (superficialis tenodesis)18–20 or the conjoint lateral band.10, 21, 22 Many variations of both have been described over time and the choice between them is mostly a matter of personal preference. The most important aspect of tenodesis is to fix the tendon to bone, instead of soft tissue (collateral ligament, pulley, flexor sheath etc) as a soft tissue attachment invariably attenuates over time. We prefer the superficialis tenodesis as it is simple, provides a sturdier checkrein against PIPJ hyperextension, and can be done in the palm thus avoiding dissection in zone 2.23 The superficialis tenodesis does not address the mallet deformity in subjects with an intact terminal tendon insertion. The conjoint lateral band tenodesis corrects the PIPJ hyperextension and DIPJ flexion simultaneously. However the quality of the conjoint lateral band can vary from patient to patient and dissecting it in a rheumatoid digit can be challenging.
Technique of FDS tenodesis
A zigzag incision is made over the volar aspect of the MCPJ. The triangular skin flap is raised and both neurovascular bundles are identified and protected. The A1 is identified and the flexor sheath opened proximal to it. The FDS tendon is identified and the radial slip of the FDS is divided 1 cm proximal to A1 pulley and separated from the ulnar slip. A small bone anchor is inserted into the radial lateral aspect of the shaft of the metacarpal proximal to the neck. The divided slip of the FDS is sutured to the bone anchor in enough tension to maintain the PIPJ in 20° flexion. A synovectomy of the flexor tendons should be done simultaneously. This will improve the excursion of the FDP within the flexor sheath. Active mobilization in flexion should be started immediately and hyperextension blocked using a dorsal splint for 4–6 weeks.
B. Limited passive range of PIPJ motion
The active and passive ranges of motion at the PIPJ are limited in all positions of the MCPJ. This causes a significant disability as the patient is unable to flex the PIPJ to grasp objects. The primary cause of this limited range of motion is adhesion between the central band and dorsally translated conjoint lateral bands over the dorsum of the PIPJ. Over time, a contracture of the dorsal skin over the PIPJ further limits flexion. None of the previously described surgical techniques (tenodesis, joint fusion, intrinsic release etc.) can by themselves restore motion to the PIPJ. These techniques can only be used once the stiff PIPJ becomes flexible. This is done by releasing the adhesions and soft tissue contractures.
Treatment
One always attempts to release the adhesions and soft tissue contractures by manipulation under anesthesia. A surgical release of the joint is considered only in select cases if manipulation is not successful. Patient selection is very important because a surgical release will need compliance with a rigorous post-operative therapy regimen because surgery by itself can lead to increased adhesions and scarring. Before restoring motion at the PIPJ, it is essential to correct any contributing deformity at the MCPJ and the DIPJ and ensure that there are no advanced intra-articular changes at the PIPJ. One should also examine for the presence of digital flexor tenosynovitis, as this may restrict flexor tendon excursion. An RA patient with flexor tenosynovitis will have good passive range of motion but limited active range of motion, whereas a patient with joint damage will have limitation in both passive and active motion.24
Technique of manipulation of the PIPJ
This is preferably done under regional or local anesthesia as it allows an immediate assessment of the released PIPJ. One aims to manipulate the PIPJ into 80°–90° of flexion by stretching the soft tissues. This should be done gently to prevent rupture of the attenuated central band and/or fracture of the osteoporotic phalanges. Once adequate passive flexion of the PIPJ is obtained, the patient is asked to actively flex the joint. If the patient is under general anesthesia, traction is applied to the flexor tendons via an incision in the palm to determine if the flexor tendon excursion uses all of the passive PIPJ flexion obtained as a result of joint manipulation.25 If the patient is unable to do so, one should suspect tenosynovitis of the flexor tendons and perform a flexor tenosynovectomy from a volar approach. Once active and passive range of motion at the PIPJ is established the patient is started on a therapy program that gradually encourages active range of PIPJ flexion.
Technique of soft tissue release
The aim of the soft tissue release is to mobilize the dorsally displaced conjoint lateral bands. (Fig. 7) A curvilinear incision is made over the dorsum of the proximal phalanx and extends onto the middle phalanx in an oblique fashion. The skin flaps are raised and the central band is separated from the conjoint lateral bands by two longitudinal incisions on both sides of the central band extending from the mid-middle phalanx to mid-proximal phalanx. The conjoint lateral bands are mobilized on either side and the central band is separated from the dorsum of the proximal phalanx and the underlying joint capsule. One then attempts to gently bring the PIPJ into 80°–90° flexion. If there is difficult in getting flexion at the PIPJ one can do the following maneuvers’ in sequence till passive PIPJ flexion is achieved: 1) Resection of the dorsal PIPJ capsule; 2) Release of the dorsal portions of the radial and ulnar collateral ligaments from the proximal phalanx; 3) Z- lengthening of the central band.26 The patient is asked to actively flex his/her digit and shifting of the lateral bands volarly on flexion and relocation dorsally on extension should be observed. Only the proximal portion of the incision (till the PIPJ) is closed with the finger in flexion and the distal incision is left to heal spontaneously. The patient is started on a therapy program that gradually encourages range of PIPJ flexion.
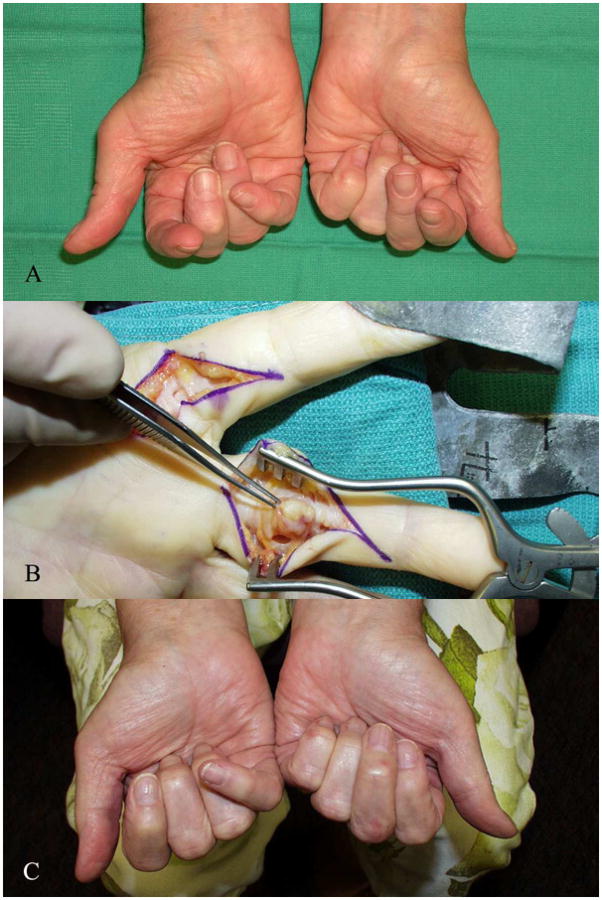
Figure 7.
Manipulation and soft tissue release for correction of stiff swan neck deformities
A. : 66-year-old female with stiff swan neck deformities of index, long, and ring fingers
B. : Longitudinal incision to separate conjoint lateral band from the central band with manipulation of the PIPJ
C. : One year follow-up showing improvement in range of flexion
C. No passive PIPJ motion
In RA patients with articular damage to the proximal interphalangeal joint surface and/or an unstable dislocated joint, the treatment choices are limited to joint fusion or implant arthroplasty. Although implant arthroplasty appears to be an attractive solution, in our opinion joint fusion is the more reliable option in RA patients. The ligamentous support (collateral ligaments, volar plate, and central band) of the PIPJ in RA is usually diseased and does not provide enough stability for implants. This is contrary to the situation seen in osteoarthritis, in which the supporting structures are intact and implant arthroplasty is predictable and reliable. Implant arthroplasty of the PIPJ in RA is limited to the ring and small fingers (index and long need good lateral stability and flexion is less important) in patients with good adjacent joints (no MCPJ disease), good dorsal skin, intact flexor tendons, and good soft tissue support.26 This combination of factors is rare in RA patients with no passive motion at the PIPJ and arthrodesis of the PIPJ in flexion is treatment of choice.
Boutonniere Deformity(BND)
It is characterized by a flexion deformity of the PIPJ with reciprocal extension of the MCPJ and DIPJ.27(Fig. 8)
Figure 8.
Classic boutonniere deformity of the right hand in a 69-year-old lady
Pathogenesis
Unlike SND, there is only one etiological factor for the development of BND in RA, i.e. synovitis of the PIPJ.13, 14 The pathological process begins with intra-articular proliferation of the synovium in the dorsal synovial pouch, leading to distention of the capsulo-ligamentous apparatus and stretching of the central band. The synovium extends as a pannus across the joint and penetrates the bone beneath the collateral ligaments at their attachment to the head of the proximal phalanx. It creates erosions and detachment of the ligaments. The synovium stretches the conjoint lateral bands laterally, breaking through between the central band and the conjoint lateral bands. This combined with stretching of the central band leads to a volar displacement of the conjoint lateral bands over the convexity of the phalangeal condyles. The conjoint lateral bands are now volar to the axis of rotation of the PIPJ and become a flexor of the PIPJ instead of its normal role as an extensor. The stretched central band is no longer able to maintain full extension of the PIPJ and the volarly subluxed conjoint lateral bands maintain persistent PIPJ flexion. Eventually, the head of the proximal phalanx prolapses through the attenuated central slip like a button through a button hole (boutonniere deformity). (Fig. 9)
Figure 9.
Pathogenesis of a boutonniere deformity
The rupture of the central slip means that the interosseous and lumbrical muscle no longer have an insertion into the base of the middle phalanx and their entire force is now transmitted via the lateral bands to the distal phalanx with resultant hyperextension of the DIPJ. The proximal migration of the extensor apparatus as a result of the BND allows the pull of the extensor tendon to be concentrated on its insertion into the base of the proximal phalanx resulting in hyperextension of the MCPJ. Eventually, persistent PIPJ flexion leads to shortening and contracture of the volar plate, collateral ligaments, transverse retinacular ligament, and oblique retinacular ligaments. The shortening of the oblique retinacular ligaments maintains the hyperextension deformity at the DIPJ and limits active flexion of the DIPJ. The MCPJ hyperextension is exaggerated as a result of patient trying to compensate for increasing PIPJ flexion deformity. Passive correction of the BND is possible in the early stage, but later on becomes fixed due to fibrosis and contracture of the capsular structures.28, 29
Management
The soft tissue reconstruction of rheumatoid BND is unpredictable and often disappointing.30 Flatt stated that the correction of a BND caused by trauma was difficult, and that the correction of a BND caused by RA was virtually impossible.31 BND is not as functionally disabling as SND and rarely compromises PIPJ flexion and grip strength (Fig. 1B). One should not trade extension at the PIPJ for a stiff finger and a weak hand. Although this sounds like a pessimistic outlook, we feel that the surgical treatment of BND in RA should be tempered with a dose of realism and the surgeon’s ability to predictably improve the patient’s function. Like the SND, the first step is to determine the passive range of motion at the PIPJ. This will classify the deformity in one of three types.
A. Full passive range of PIPJ motion
In this group of patients, one is able to passively correct the PIPJ flexion deformity. They represent the early stage of the disease and the deformity is mild as soft tissue contractures have not yet occurred. On attempting active PIPJ extension they have a 10°–15° extension lag. One should then examine the PIPJ for evidence of active synovitis and ascertain if the posture of DIPJ hyperextension becomes better or worse with PIPJ extension. If DIPJ hyperextension becomes worse with PIPJ extension it indicates tightness of the ORL; this is tested using the Haines test.11 The PIPJ is held extended by the examiner who then assesses the resistance to passive flexion of the DIPJ (Fig. 10). Patients with tightness of the ORL have a much greater resistance to passive flexion of the DIPJ and DIPJ flexion is only possible when the PIPJ is allowed to flex.
Figure 10.
Haines test for assessing tightness of the oblique retinacular ligament
A. : 67-year-old lady with a flexible boutonniere deformity of the right small finger
B. : DIPJ flexion is restricted when the PIPJ is maintained in extension
C. : Improved range of DIPJ flexion when the PIPJ is allowed to flex
Treatment
A normal PIPJ in patients with early BND is best treated by splinting the PIPJ in extension. The splint should not extend to the DIPJ and care must be taken to avoid pressure necrosis of the skin around the PIPJ. The patients should be advised to continue to flex the DIPJ. This will maintain the lateral bands in a good position. In patients who are unable to flex the DIPJ while the PIPJ is maintained in complete extension due to a tight ORL, an extensor tenotomy will be required. If there is PIPJ synovitis that has not responded to oral medications a single intra-articular injection of corticosteroid can be effective. However if the synovitis is persistent synovectomy of the PIPJ is indicated.
Technique of extensor tenotomy
The aim of this tenotomy is to divide the terminal tendon insertion such that the contribution of the conjoint lateral bands to the terminal tendon is divided, but the contribution of the ORL to the terminal tendon is preserved.32, 33 A longitudinal incision is made over the dorsum of the middle phalanx. The attenuated fibers of the triangular ligament and the volarly displaced conjoint lateral bands are identified over the mid-middle phalanx. The triangular ligament and both conjoint lateral bands are divided transversely. This should be done proximal to the attachment of the ORL. A dynamic splint (reverse knuckle bender type) at the PIPJ helps restore proper balance between the joints in the post-operative period. A slight mallet deformity may occur after the tenotomy, but this is usually well tolerated. If the mallet deformity is significant the DIPJ is splinted for 4–6 weeks in extension. If this does not correct the mallet deformity a fusion of the DIPJ can be considered.34
B. Limited passive range of PIPJ motion
These patients have had the deformity for some period of time resulting in soft tissue changes, which prevents complete passive correction of the flexion deformity. On attempted active PIPJ extension, they have a pronounced extensor lag of 30°–40° and the MCPJ hyperextension is obvious. Before considering any surgical treatment the patient should undergo a trial of splinting to try and stretch out the collateral ligaments, volar plate, joint capsule and the volar skin. It is also important to establish that the flexor tendons are intact and functioning and that the joint surfaces are smooth on a radiograph. The aim of surgical treatment is to restore extensor force to the PIPJ using local tissues. This is done by excising the attenuated portion of the central band and repairing it, and bringing and maintaining the conjoint lateral bands dorsally. In theory, this should correct the BND, but in practice it is difficult to repair the central slip without limiting flexion – a high price to pay.34
Technique of extensor reconstruction
This procedure should be done under light sedation and digital block so that the surgeon can gauge the tension of the tendon reconstruction, with the patient participating in active finger extension and flexion. A dorsal curvilinear incision is made over the proximal interphalangeal joint extending from mid-middle phalanx to the mid proximal phalanx. The incision is curved over the PIPJ so that a skin flap will cover the repair of the central band.34 The central band is divided from the insertion onto the base of the middle phalanx leaving a cuff of tissue on the middle phalanx to allow repair later. The PIPJ is inspected and a synovectomy carried out, if needed. The central band is separated from the lateral bands proximally, the attenuated distal portion excised (approx 3–7mm), and central band advanced and reattached to the cuff of tissue at the base of the middle phalanx. Before excising the central band one must ensure that a repair is possible with the PIPJ flexed to 70°–80°. A correct judgment of tension is the most critical step in this procedure. This can be assessed with an alert patient participating in active finger movement. Next an incision is made volar to the conjoint lateral bands extending from the mid-proximal phalanx till the mid-middle phalanx on both sides. This incision divides the transverse retinacular ligament on either side, but care is taken to preserve the ORL, which runs below the TRL and inserts distally to the terminal tendon (Fig. 2B). The conjoint lateral bands can now be transposed from their displaced volar location to a more dorsal location. The lateral bands are sutured to each other over the mid proximal phalanx and to the central band over the PIPJ.35 At this point one examines if DIPJ flexion is restricted when the PIPJ is extended, and if needed an extensor tenotomy is carried out. The PIPJ is splinted in extension for 4–6 weeks to allow the repair of the central band to become strong. The DIPJ is left free and the patient is encouraged to move it. After 4–6 weeks the static splint is traded for a dynamic splint and a resting splint at night.
C: No passive PIPJ motion
This can be either due to a very severe soft tissue contracture or a bony ankylosis resulting from erosion of the joint surfaces. A radiograph will easily differentiate between these two etiologies. In patients with a very severe soft tissue contracture it is possible to try stretching the soft tissue with series of plaster casts, then attempt a joint release followed by an extensor reconstruction. However such an extensive degree of surgery is seldom indicated.34 The surgical options in these patients and in patients with articular damage are limited to joint fusion or implant arthroplasty. The indications for implant arthroplasty in BND are even more limited when compared to SND. This is because a significant resection of the proximal phalanx is required to seat the implants in the flexed BND and this leads to instability as the collateral ligaments have to be sacrificed.34 Additionally an extensor reconstruction is needed, which adds to the period before mobilization can be started. Our treatment of choice of severe BND is fusion of the PIPJ.
Flexor Tenosynovitis
Synovitis is the hallmark of RA and in the hand it involves the synovial lining of the tendon sheath (tenosynovium) and the joint. Flexor tendons are covered by tenosynovium under the carpal tunnel and in the digital flexor sheath, whereas extensor tendons are covered by tenosynovium only under the extensor retinaculum. The changes in extensor tendon seen at the interphalangeal joints are due to underlying joint synovitis and not tenosynovitis.17
Pathogenesis
Synovitis involves the extensor tendons more frequently than the flexor tendons of the hand.1 As synovitis progresses the tendon is infiltrated and ultra structural changes take place in the tendinous tissue, which may include ischemic necrosis, eventually leading to loss of function and rupture without additional mechanical irritation. Three main groups of flexor tenosynovitis can be distinguished: isolated carpal tenosynovitis (20%), palmodigital tenosynovitis (50%), and diffuse tenosynovitis (30%). The index, long, and small fingers are most frequently involved. Rheumatoid nodules are three times more frequent in the flexor tendons than the extensor tendons and are usually confined to the profundus tendon.36 A flexor tendon rupture in the digital flexor sheath is almost always secondary to infiltrative synovitis and usually involves the FDS at the proximal interphalangeal joint (PIPJ).37 This is probably because the FDS splits into two slips diminishing the cross sectional area by half and increasing the surface in contact with the invading synovium in the distal third of the proximal phalanx near the vincula tendinum.17
Management
Tenosynovitis can be identified on the basis of three findings: swelling of the palmar aspect of the digit, discrepancy between active and passive range of motion, and palpable crepitus along the course of the flexor tendon on active and passive flexion of the digit.24 It can also cause triggering and pain when flexing the finger, loss of active interphalangeal flexion, and a SND in long standing cases.38 Tenosynovitis of the flexor tendons is diagnosed by asking the patient to flex the interphalangeal joints while applying some pressure over the tendon proximal to the A1 pulley of the flexor tendon sheath. The patient will experience discomfort, and the examiner will notice swelling, grinding and mild triggering from the hypertrophic synovium going in and out of the flexor tendon sheath. A definitive diagnosis is made when the patient cannot fully flex the IP joints actively, while full passive flexion is possible (Fig. 11A).17 A rheumatoid trigger finger presents as an intermittent somewhat painful triggering on active flexion and extension. Gentle palpation of the flexor sheath during active flexion will reveal a nodule moving with the tendon. A trigger finger must be distinguished from the snapping finger that is seen in early SND deformity, when the patient is able to correct the hyperextended position by sudden flexion of the PIPJ. This brings the lateral bands below the axis of rotation with a snap.
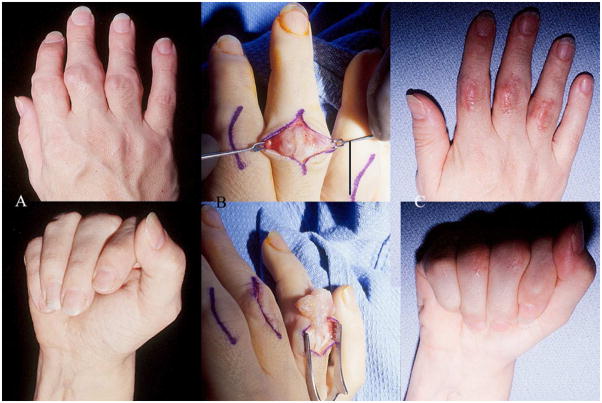
Figure 11.
Rheumatoid digital flexor tenosynovitis
A. : 69-year-old lady with restricted active flexion of the left index, left long and right index fingers due to digital flexor tenosynovitis
B. : Intra-operative picture showing the appearance of the left long finger prior to synovectomy and the left index finger following synovectomy.
C. : Post-operative result at 3 months showing improvement in range of active flexion
Treatment
Flexor tenosynovectomy is a safe and effective method for restoring function to rheumatoid patients with flexor tenosynovitis and to prevent further complications of flexor tenosynovitis like median nerve compression and flexor tendon rupture.39 The surgical technique for flexor synovectomy requires care to avoid injury to the median nerve, palmar arch, and digital neurovascular bundles. Flexor tenosynovitis may involve multiple sites in the same hand and it is important to do a complete flexor tenosynovectomy in a single stage. Residual synovitis in an unoperated finger will restrict post-operative mobility of the other fingers. The skin incisions must be planned carefully to allow complete access to the disease area without forceful retraction.
Technique of flexor tenosynovectomy
We use a Brunner type incision in the fingers and the palm, and an extended carpal tunnel incision if access is required more proximally. (Fig. 11) The skin flaps are raised carefully dissecting the neurovascular bundles till the sausage shaped mass of distended synovium is visualized. The flexor tendons are then delivered into the wound by alternatively pulling on the FDS and the FDP in the gaps between the annular pulleys. The area of tenosynovitis can then be excised from each tendon using a combination of sharp and blunt dissection. Occasionally the tenosynovitis has infiltrated into the tendon substance resulting in a frayed tendon surface. In such cases the frayed surface should be trimmed. A tendon defect resulting from excision of a nodule can be closed with a horizontal mattress suture. The slips of the FDS may be infiltrated with tenosynovitis and the surgeon tempted to excise the FDS tendon. This should be avoided as far as possible because of the progressive nature of the disease. The FDS is the prime flexor of the PIPJ and excising it may result in a SND. In the presence of intrinsic muscle shortening the lateral bands should be divided. They can be approached by blunt dissection on both sides at the base of the proximal phalanx using the same volar incision. While maintaining the MCPJ in extension, the extensor apparatus is divided from volar to dorsal until the PIPJ can be passively flexed.
The tourniquet is released and careful hemostasis achieved before loose skin closure with interrupted nylon 4.0/5.0 sutures. If the surgery was carried out under local anesthesia or a Biers block, the active range of motion can be visualized prior to skin closure and any residual diseased areas addressed. A generously padded volar plaster of Paris slab extending till the finger tips, and maintaining the fingers in an intrinsic plus posture and the wrist in moderate extension is applied. The hand is kept elevated and gentle mobilization started in 4–7 days.
Treatment of trigger digits
Surgery for trigger fingers in rheumatoid patients consists of flexor tenosynovectomy as outlined earlier and A1 pulley release. These patients should be approached by Brunner incision, instead of the standard limited palmar incision used for release of triggers in non-rheumatoid patients. Some authors recommend resection of one slip of the FDS to provide greater space for passage of the FDP instead of A1 pulley release. They believe that release of the A1 pulley contributes towards the MCPJ ulnar drift deformity. This may be true for the index and middle fingers due to oblique line of the pull of the long flexors for these digits, although there are several factors responsible for the ulnar drift deformity.40
Treatment of ruptured flexor tendons
The management of a flexor tendon rupture within the digit is difficult, and a flexor tenosynovectomy performed early can prevent this.39 Rupture of the FDS is frequently overlooked as the patient can still fully flex the finger and integrity of the FDS tendons is not routinely examined by many surgeons. Palpating the volar aspect of the finger and finding an empty sheath, usually at the level of the proximal phalanx will establish the level of rupture.
Rupture of FDS: One should excise the FDS and carry out tenosynovectomy of the FDP
Rupture of FDP: If the rupture of the FDP has occurred distal to the FDS insertion, it may be possible to advance and repair it as the proximal end is usually caught at the FDS chiasm. However if repair is not possible or the rupture has occurred proximally, it is better to excise the FDP and do an arthrodesis or tenodesis of the distal interphalangeal joint. Irrespective of what is done for the ruptured FDP the most important consideration is to do a complete flexor tenosynovectomy of the FDS to protect it from rupture.
Rupture of FDS and FDP: This is a difficult situation and the chances of obtaining active flexion at the PIPJ are severely limited. Two options are available. One is to carry out flexor tenosynovectomy, insert a silicon rod, and perform a tendon graft procedure at a later date. The other is to fuse the interphalangeal joints and suture one of the long flexors to the base of the proximal phalanx (flexor sheath or bone) to increase the strength of flexion at the MCPJ.
Joint Synovitis
The development and progress of synovitis in the PIPJ has been described previously in the section on SND and BND.13, 14 The role of joint synovectomy (wrist, MCPJ, and IPJ) in rheumatoid arthritis has not been established. Whereas it has been shown that recurrence after flexor tenosynovectomy is rare and that tendon ruptures are prevented by tenosynovectomy, there has been no large series of cases that has shown that joint synovectomy can change the course of rheumatoid joint involvement. However most rheumatoid hand surgeons feel that joint synovectomy is useful in certain specific situations. It has a prophylactic value in patients that have a persistent smoldering synovitis, which is never fully controlled by medication and has not yet caused articular erosion. These patients respond to splinting and steroid injections for a few months and then flare again. If left untreated the synovitis will gradually lead to joint destruction. Joint synovectomy is unlikely to be beneficial in the presence of articular erosions, therefore early identification and excision of persistent synovitis is the basis of a prophylactic synovectomy. The results of soft tissue reconstructive procedures and implant arthroplasty at the PIPJ are unpredictable when compared to the wrist and MCPJ. The indications for PIPJ synovectomy are therefore broader and it can be done even in the presence of some joint destruction. It is a relatively minor procedure with little disability and a short hospitalization, and if it fails one could then turn to other reconstructive procedures. 39
Technique of PIPJ synovectomy
A curvilinear dorsal longitudinal skin incision on the dorsum of the PIPJ is used. (Fig. 12) If the synovial bulging is limited to one side a lateral incision can be used. If a dorsal incision is used the joint is entered either between the central slip and the lateral band or palmar to the lateral band depending on the location of the disease. If a lateral incision is used the joint is entered dorsal or palmar to the collateral ligament. Traction on the finger improves the visualization of the joint and a small rongeur is used to excise the synovium. A small curette is useful to clear the synovial recesses on the palmar and dorsal aspects of the head of the proximal phalanx. The joint is irrigated to wash out any free bits of synovium and the incision in the extensor mechanism is repaired with 4.0 vicryl sutures. An early BND may become more pronounced after a synovectomy and a prophylactic repair should be done immediately. The joint is splinted in extension till the patient is able to perform active extension and gentle mobilization started after 4–7 days.
Figure 12.
Technique of synovectomy of the PIPJ
A. : 56-year-old lady with synovitis of the PIPJ of the right index, long and ring fingers with limited active range of flexion
B. : Intra-operative picture showing the appearance of the left long finger prior to synovectomy and the left ring finger following synovectomy
C. : Post-operative result at 3 months showing improvement in range of active flexion
CONCLUSION
An experienced RA hand surgeon will strive to understand the patient’s needs and expectations for improvement, and attempt to match them with the available surgical options that can predictably improve the patient’s function. It is important to collaborate with the referring rheumatologist who has an intimate knowledge of the patient’s overall disease, psychosocial issues, and functional requirements before deciding whether an operation is needed and what type of procedures should be performed. The correction of digital deformities in RA is akin to putting the icing on the cake or scoring a point after touchdown in American football. The cake or the touchdown itself is more important and refers to correction of the more proximal deformities (thumb, MCPJ & wrist, extensor tendon reconstruction etc.) that have predictable outcomes and markedly improve the patients’ quality of life. This creates an environment of trust between the patient, the referring rheumatologist, and the hand surgeon. One can then proceed with the correction of digital deformities. It is also helpful to outline for the patient a sequence of treatment priorities in order to plan for a long-term relationship. Informing the rheumatologist of the plans will help in their management of the patients before and after surgery. Although the incidence of rheumatoid hand surgery appears to be decreasing there is still a need for hand surgeons to be involved in the care of the rheumatoid hands because a substantial number of patients may not respond to medications and will still develop hand deformities. It is also uncertain whether these new medications simply delay the inevitable progression of hand and wrist diseases. Hand surgery should remain a partner with rheumatology in the coordinated care of patients whose hand diseases may be treated by the continued innovations in arthroplasty procedures.
Acknowledgments
Supported in part by a Midcareer Investigator Award in Patient-Oriented Research (K24 AR053120) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (to Dr. Kevin C. Chung).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schindele D, Kloss D, Herren D. Options in extensor tendon reconstruction in rheumatoid arthritis. In: Ian Trail MH, editor. Surgery of the Rheumatoid Hand and Wrist. Amsterdam: Elsevier; 2006. pp. 94–106. [Google Scholar]
- 2.Johnsson PM, Eberhardt K. Hand deformities are important signs of disease severity in patients with early rheumatoid arthritis. Rheumatology. 2009 Nov 1;48(11):1398–1401. doi: 10.1093/rheumatology/kep253. [DOI] [PubMed] [Google Scholar]
- 3.Eberhardt K, Johnson PM, Rydgren L. The occurrence and significance of hand deformities in early rheumatoid arthritis. Br J Rheumatol. 1991 Jun 1;30(3):211–213. doi: 10.1093/rheumatology/30.3.211. [DOI] [PubMed] [Google Scholar]
- 4.Tubiana R, Toth B. Rheumatoid arthritis: clinical types of deformities and management. In: CB WP, editor. Clinics in rheumatic diseases. Vol. 5. Philadelphia: WB Saunders; 1984. pp. 21–47. [PubMed] [Google Scholar]
- 5.Smith RJ. Balance and kinetics of the fingers under normal and pathological conditions. Clin Orthop Relat Res. 1974 Oct 1;(104):92–111. doi: 10.1097/00003086-197410000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Doyle J. Hand. In: Doyle J, Botte M, editors. Surgical anatomy of the hand and upper extremity. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 533–667. [Google Scholar]
- 7.Yu H, Chase R, Strauch B, editors. Atlas of hand anatomy and clinical implications. Mosby; 2003. [Google Scholar]
- 8.Tubiana R, Thomine JM, Mackin E. Movements of the hand and wrist. In: Tubiana R, Thomine JM, Mackin E, editors. Examination of the hand and wrist. St Louis: Mosby; 1996. [Google Scholar]
- 9.Schultz RJ, Furlong J, Storace A. Detailed anatomy of the extensor mechanism at the proximal aspect of the finger. J Hand Surg. 1981;6A:493–498. doi: 10.1016/s0363-5023(81)80110-4. [DOI] [PubMed] [Google Scholar]
- 10.Thompson J, Littler J, Upton J. The spiral oblique retinacular ligament (SORL) J Hand Surg. 1978;3A:482–487. doi: 10.1016/s0363-5023(78)80144-0. [DOI] [PubMed] [Google Scholar]
- 11.Haines RW. The extensor apparatus of the finger. J Anat. 1951;85:251–259. [PMC free article] [PubMed] [Google Scholar]
- 12.Heywood AW. The pathogenesis of the rheumatoid swan neck deformity. Hand. 1979 Jun 1;11(2):176–183. doi: 10.1016/s0072-968x(79)80031-5. [DOI] [PubMed] [Google Scholar]
- 13.Harrison SH. Rheumatoid deformities of the proximal interphalangeal joints of the hand. Ann Rheum Dis. 1969 Sep 1;28(5 Suppl):20–22. [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison SH. The proximal interphalangeal joint in rheumatoid arthritis. Hand. 1971 Sep 1;3(2):125–130. doi: 10.1016/0072-968x(71)90029-5. [DOI] [PubMed] [Google Scholar]
- 15.Dreyfus JN, Schnitzer TJ. Pathogenesis and differential diagnosis of the swan-neck deformity. Semin Arthritis Rheum. 1983 Nov 1;13(2):200–211. doi: 10.1016/0049-0172(83)90007-0. [DOI] [PubMed] [Google Scholar]
- 16.Bunnell S, Doherty E, Curtis R. Ischemic contracture, local, in the hand. Plast Reconstr Surg. 1946;3(4):424–433. doi: 10.1097/00006534-194807000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Lluch A. Examination of the rheumatoid hand and wrist. In: Trail I, Hayton M, editors. Surgery of the rheumatoid hand and wrist. Amsterdam: Elsevier; 2006. pp. 9–26. [Google Scholar]
- 18.Swanson A. Surgery of the hand in cerebral palsy and swan neck deformity. J Bone Joint Surg. 1960;42A:951–964. [Google Scholar]
- 19.Milford L. Sublimis tenodesis technique by Curtis. In: Edmonson A, Crenshaw A, editors. Campbell's Operative Orthopaedics. 6. St Louis: Mosby; 1980. p. 319. [Google Scholar]
- 20.Nalebuff E. The rheumatoid swan-neck deformity. Hand Clin. 1989;5(2):203–214. [PubMed] [Google Scholar]
- 21.Littler J. The finger extensor mechanism. Surg Clin North Am. 1967;47:415–432. doi: 10.1016/s0039-6109(16)38186-5. [DOI] [PubMed] [Google Scholar]
- 22.Zancolli E. In: The paralysed hand. Lamb D, editor. Edinburgh: Churchill Livingstone; 1987. pp. 163–167. [Google Scholar]
- 23.Nalebuff EA. The rheumatoid swan neck deformity. Hand Clin. 1989;5:407–420. [PubMed] [Google Scholar]
- 24.Boyer MI, Gelberman RH. Operative correction of swan-neck and boutonniere deformities in the rheumatoid hand. J Am Acad Orthop Surg. 1999 Jan 1;7(2):92–100. doi: 10.5435/00124635-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Nalebuff E. Surgical treatment of finger deformities in the rheumatoid hand. Surg Clin North Am. 1969;49:833–846. doi: 10.1016/s0039-6109(16)38892-2. [DOI] [PubMed] [Google Scholar]
- 26.Nalebuff EA, Millender LH. Surgical treatment of the swan-neck deformity in rheumatoid arthritis. Orthop Clin North Am. 1975 Jul 1;6(3):733–752. [PubMed] [Google Scholar]
- 27.Heywood AWB. Correction of the rheumatoid Boutonniere deformity. Journal of Bone and Joint Surgery. 1969;51A:1309–1314. doi: 10.2106/00004623-196951070-00009. [DOI] [PubMed] [Google Scholar]
- 28.Feldon P, Terrono AL, Nalebuff EA, Millender LH. Rheumatoid arthritis and other connective tissue disorders. In: Green DP, Hotchkiss RN, Pederson WC, Wolfe SW, editors. Greens Operative Hand Surgery. Vol. 1. Elsevier Health Sciences; 2005. [Google Scholar]
- 29.Zancolli E. Structural and dynamic bases of hand surgery. Philadelphia: JB Lippincott Company; 1968. [Google Scholar]
- 30.Kiefhaber TR, Strickland JW. Soft tissue reconstruction for rheumatoid swan-neck and boutonniere deformities: long-term results. J Hand Surg Am. 1993 Nov 1;18(6):984–989. doi: 10.1016/0363-5023(93)90387-I. [DOI] [PubMed] [Google Scholar]
- 31.Flatt A. Intra-articular thio-tepa in rheumatoid disease of the hands. Rheumatism. 1960;18:70–73. [PubMed] [Google Scholar]
- 32.Dolphin J. Extensor tenotomy for chronic boutonniere deformity of the finger: Report of two cases. J Bone Joint Surg. 1965;47A:161–164. [PubMed] [Google Scholar]
- 33.Fowler S. The hand in rheumatoid arthritis. Am Surg. 1963;29:403–404. [PubMed] [Google Scholar]
- 34.Nalebuff EA, Millender LH. Surgical treatment of the boutonniere deformity in rheumatoid arthritis. Orthop Clin North Am. 1975 Jul 1;6(3):753–763. [PubMed] [Google Scholar]
- 35.Urbaniak J, Hayes M. Chronic boutonniere deformity-an anatomic reconstruction. J Hand Surg. 1981;6A(4):379–383. doi: 10.1016/s0363-5023(81)80048-2. [DOI] [PubMed] [Google Scholar]
- 36.Saffar P. Flexor tendon synovectomy in rheumatoid arthritis. In: Trail I, Hayton M, editors. Surgery of the rheumatoid hand and wrist. Amsterdam: Elsevier; 2006. pp. 107–117. [Google Scholar]
- 37.Ertel AN, Millender LH, Nalebuff EA, McKay D, Leslie B. Flexor tendon ruptures in patients with rheumatoid arthritis. J Hand Surg Am. 1988;13A(6):860–866. doi: 10.1016/0363-5023(88)90260-2. [DOI] [PubMed] [Google Scholar]
- 38.Lluch A. The treatment of finger joint deformities in rheumatoid arthritis. In: Allieu Y, editor. The rheumatoid Hand and Wrist. Paris: Expansion Scientifique Publications; 1998. pp. 85–104. [Google Scholar]
- 39.Millender LH, Nalebuff EA. Preventive surgery - tenosynovectomy and synovectomy. Orthop Clin North Am. 1975 Jul 1;6(3):765–792. [PubMed] [Google Scholar]
- 40.Ferlic DC, Clayton ML. Flexor tenosynovectomy in the rheumatoid finger. J Hand Surg. 1978;3A:364–367. doi: 10.1016/s0363-5023(78)80039-2. [DOI] [PubMed] [Google Scholar]