Abstract
Signal Transducer and Activator of Transcription (STAT) family members direct the differentiation of T helper cells, with specific STAT proteins promoting distinct effector subsets. STAT6 is required for the development of T helper 2 (Th2) cells, whereas STAT3 promotes differentiation of Th17 and follicular helper T cell subsets. We demonstrated that STAT3 was also activated during Th2 cell development and was required for the expression of Th2-cell associated cytokines and transcription factors. STAT3 bound directly to Th2-cell associated gene loci and was required for the ability of STAT6 to bind target genes. In vivo, STAT3-deficiency in T cells eliminated the allergic inflammation in mice sensitized and challenged with ovalbumin, or transgenic for constitutively active STAT6. Thus, STAT3 cooperates with STAT6 in promoting Th2 cell development. These results demonstrate that differentiating T helper cells integrate multiple STAT protein signals during Th2 cell development.
INTRODUCTION
CD4+ T helper cells differentiate into several distinct subsets to provide host protection against a variety of pathogens. Each T helper cell lineage expresses specific transcription factors and cytokines that confer specific effector functions. T helper 2 (Th2) cells secrete interleukin (IL)-4, IL-5, and IL-13, which are important for immunity against extracellular parasites and for providing B cell help leading to antibody production. However, non-protective Th2 cell responses contribute to asthma, allergy and other allergic diseases (Zhu and Paul, 2008).
The differentiation of Th2 cells occurs when a naïve T cell is activated by antigen in the presence of IL-4. The IL-4 receptor is composed of the common cytokine receptor gamma subunit and the IL-4Rα chain. IL-4R is expressed at low levels on the surface of naïve CD4+ T cells, but upon antigen stimulation IL-4R surface expression is increased. IL-4 binding to the IL-4R results in recruitment of Signal Transducer and Activator of Transcription 6 (STAT6). STAT6 then becomes phosphorylated, dimerizes, and translocates to the nucleus where it can activate transcription of Th2 cell specific genes (Nelms et al., 1999).
There are several transcription factors associated with establishing the Th2 cell phenotype. STAT6 induces expression of GATA3 which is considered the Th2 cell master regulator (Ouyang et al., 2000). Other transcription factors important for Th2 cell development are induced upon TCR stimulation including NFAT family members, IRF4, Jun family members, and c-MAF (Ansel et al., 2006; Lee et al., 2006; Murphy and Reiner, 2002). More recently BATF was suggested to play a role in promoting the Th2 cell phenotype (Betz et al., 2010). The expression of each transcription factor is necessary for optimal Th2 cell development.
Though particular cytokines are thought to be promoters of specific Th lineages, a differentiating Th cell receives multiple cytokine signals. IL-4 and STAT6 clearly play a dominant role in Th2 cell differentiation. Transgenic mice expressing IL-4 or constitutively active STAT6 are characterized by the development of spontaneous allergic inflammation (Sehra et al., 2008; Tepper et al., 1990). Development of allergic disease is completely dependent on IL-4 since allergic inflammation is diminished in mice deficient in IL-4 or STAT6 (Akimoto et al., 1998; Brusselle et al., 1995; Kuperman et al., 1998; Sehra et al., 2008). However, IL-2 and STAT5 are also necessary for optimal Th2 cell differentiation through direct effects on Th2 cell cytokines and Il4ra expression (Ben-Sasson et al., 1990; Cote-Sierra et al., 2004; Kagami et al., 2001; Liao et al., 2008; Zhu et al., 2003). Moreover, although STAT3 is required for the differentiation and effector function of both Th17 and Tfh cells, IL-6 activated STAT3 promotes Maf expression, a factor required for IL-4 expression in Th2 cells (Mathur et al., 2007; Nurieva et al., 2008; Yang et al., 2007; Yang et al., 2005). STAT3 directly binds the Maf and Batf loci (Durant et al., 2010). IL-6 also induces SOCS1 and NFATc2 that respectively decrease Th1 cell differentiation and increase IL-4 production during Th2 cell development (Diehl et al., 2000; Diehl et al., 2002). However, the requirement for STAT3 in Th2 cell development has not been defined.
In this report we demonstrate that STAT3 is required for Th2 cytokine production and transcription factor expression. STAT3 was activated throughout Th2 cell differentiation and when ectopically expressed with STAT6 could augment Th2 cell cytokine production. STAT3 was also required for Th2 cell-mediated allergic inflammation. Thus, in the presence of activated STAT6, STAT3 promotes optimal Th2 cell differentiation and cytokine production.
RESULTS
STAT3 is activated during Th2 cell differentiation
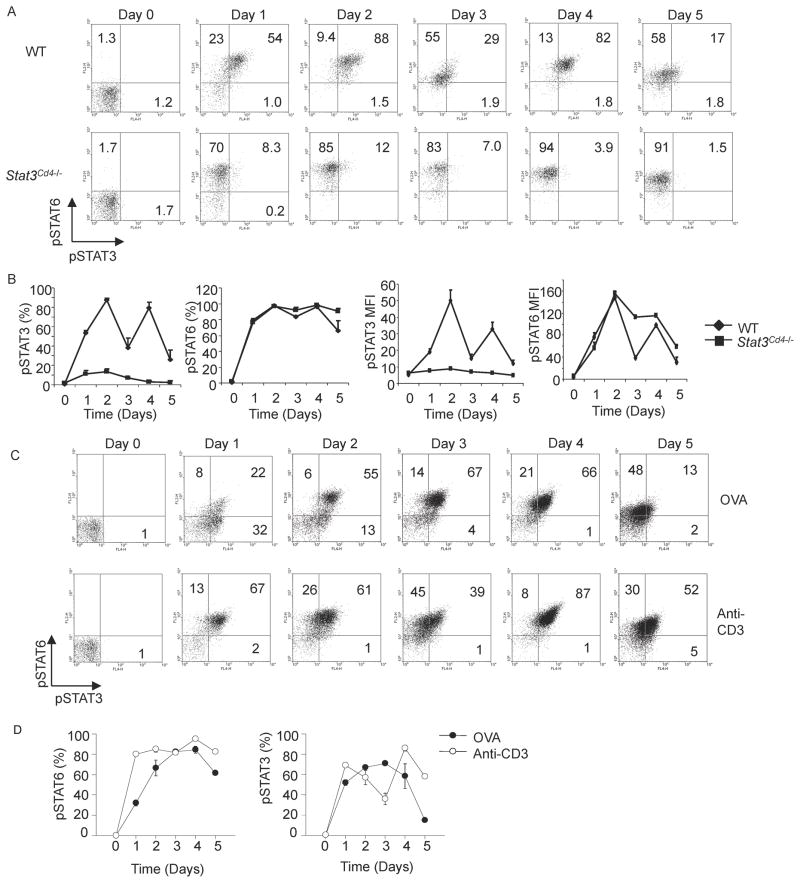
STAT6 activation is critical in Th2 cell differentiation. Though several cytokines important in Th2 differentiation and cytokine production signal through STAT3, the activation of STAT3 during Th2 development has not been carefully examined. To define STAT3 activation throughout Th2 differentiation, wild type and STAT3-deficient Th2 cells were assessed for intracellular phospho-STAT3 and phospho-STAT6 each day during Th2 differentiation. Wild type Th2 cells were nearly all phospho-STAT6 positive early on in differentiation and remained phospho-STAT6 positive throughout differentiation (Figure 1A and B). Wild type Th2 cells also demonstrated a high percentage of phospho-STAT3 positive cells throughout Th2 differentiation. STAT3 phosphorylation occurred early in differentiation, peaked at 48 hours and decreased by 72 hours (Figure 1A and B). There was a second peak of STAT3 phosphorylation after addition of further cytokines at 72 hours (Figure 1A and B). The initial induction of STAT3 phosphorylation was independent of IL-4 signaling because it was identical between wild type and STAT6-deficient cells during the first three days of Th2 cultures (Figure S1B). However, STAT6-deficient Th2 cells displayed reduced phospho-STAT3 during the last 2 days of differentiation, suggesting that genes downstream of STAT6 were at least partially responsible for maintaining STAT3 phosphorylation (Figure S1B). Similar patterns of pSTAT6 and pSTAT3 are observed in Th2 cultures of DO11.10 TCR transgenic T cells stimulated with anti-CD3 or ovalbumin (Figure 1C and D), although with antigen stimulation, pSTAT6 rose more slowly at culture initiation, and pSTAT3 decreased more dramatically at the end of culture. Taken together these data show that STAT3 becomes phosphorylated during Th2 differentiation.
Figure 1. STAT3 is Activated During Th2 cell Differentiation.
(A) Wild type (WT) and Stat3Cd4−/ − naïve CD4+ T cells were activated with anti-CD3 and anti-CD28 and cultured in IL-4 and anti-IFN-γ (Th2 conditions). Each day during differentiation, cells were stained for intracellular phospho-STAT3 and phospho-STAT6. Numbers in flow cytometry dot plots indicate the percentages of cells in each quadrant.
(B) Graphical representation of percent phospo-STAT-positive cells and mean fluorescent intensity (mean ± s.d.). Data are an average of two mice and are representative of 2–3 experiments.
(C) Naïve DO11.10 TCR transgenic T cells were stimulated with anti-CD3 or ovalbumin (OVA) in the presence of antigen-presenting cells and cultured under Th2 conditions. Each day during differentiation, cells were stained for intracellular phospho-STAT3 and phospho-STAT6. Numbers in flow cytometry dot plots indicate the percentages of cells in each quadrant.
(D) Graphical representation of average percent phospho-STAT-positive cells for DO11.10 TCR transgenic T cells stimulated with OVA or anti-CD3 (mean ± s.d.). Data are an average of two mice and are representative of 2 experiments.
STAT3-deficient cells are defective in Th2 cell differentiation
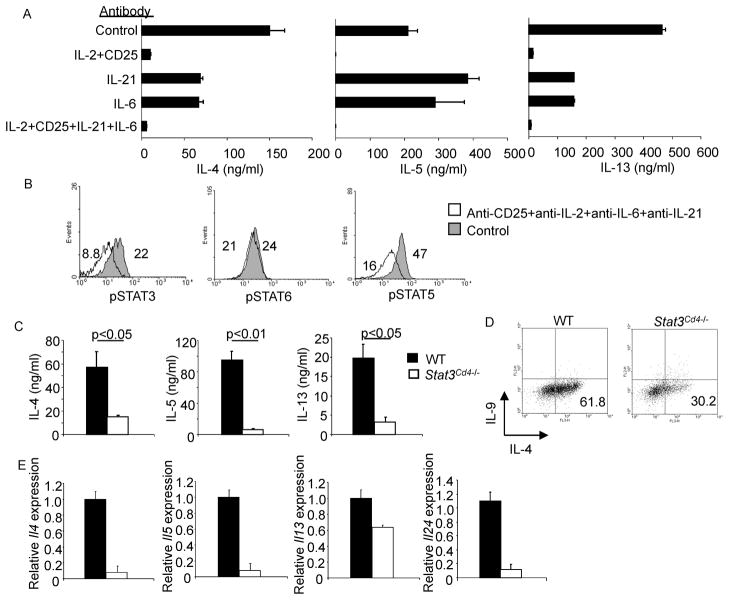
To determine which cytokines were activating STAT3 during Th2 differentiation we cultured Th2 cells in the presence of antibodies to cytokines known to activate STAT3. A combination of anti-IL-2 and anti-CD25 decreased Th2 cytokine production coincident with a decrease in pSTAT5 (Figure 2A and data not shown), similar to previous results (Cote-Sierra et al., 2004). Antibodies to IL-6 or IL-21 decreased IL-4 and IL-13 production, although they had no effect on IL-5 production (Figure 2A). Although the individual antibodies did not have a substantial effect on pSTAT3, a combination of antibodies to IL-2, CD25, IL-6 and IL-21 decreased pSTAT3, as well as pSTAT5, without affecting pSTAT6 (Figure 2B and data not shown). Thus several cytokines play redundant roles in the activation of STAT3 during Th2 differentiation.
Figure 2. Reduced Th2 Cytokine Production in the Absence of STAT3.
(A) WT naïve CD4+ T cells were activated with anti-CD3 and anti-CD28 and cultured under Th2 conditions, in the presence or absence of antibodies to the indicated cytokines and receptors (20 μg/ml per antibody) for five days before stimulation with anti-CD3. Twenty-four hours after stimulation supernatants were tested for cytokine concentration using ELISA (mean ± s.d.). Results are representative of experiments with four mice.
(B) Cells cultured as in (A) with control antibody or the combination of antibodies indicated were tested for amount of pSTAT by intracellular staining on day 4 of Th2 culture. Numbers represent mean fluorescence intensity. Results are representative of experiments with four mice.
(C) WT and Stat3Cd4−/ − naïve CD4+ T cells were activated with anti-CD3 and anti-CD28 and cultured with IL-4 + anti-IFN-γ (Th2 conditions). After 5 days of differentiation cells were collected and counted. Differentiated cells (1x106) were then re-stimulated with anti-CD3 (4 μg/ml) for 24 hours. Cell-free supernatant was collected and tested for various cytokines using ELISA. Data are the mean ± s.d. of results from two mice and representative of more than 5 experiments. Students t test was performed to calculate p values.
(D) Cells activated and cultured as in A were re-stimulated with anti-CD3 for 5 hours. Following stimulation cells were stained with anti-IL-4 and anti-IL-9. Numbers in flow cytometry dot plots indicate the percentages of cells in the quadrant.
(E) After re-stimulation of differentiated Th2 cells with anti-CD3 and recovery of supernatants as described in (A), cell pellets were collected, RNA was isolated, and quantitative PCR was performed for the indicated cytokines (mean ± s.d.). Data are representative of two experiments.
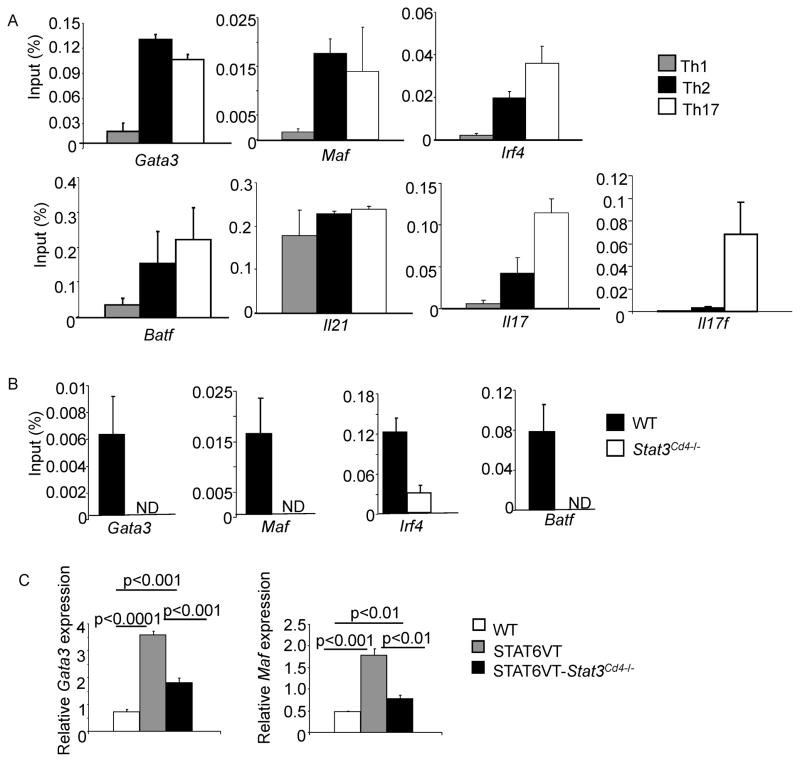
To specifically define if the activation of STAT3 during Th2 development reflected a requirement for STAT3 in this process, we used mice that have a floxed Stat3 allele, mated to mice expressing Cre from a Cd4 transgene (referred to as Stat3Cd4−/− mice). As previously described, T cell development in mice with STAT3-deficient T cells is undistinguishable from wild type mice (Mathur et al., 2007). Moreover, growth, proliferation and apoptosis of STAT3-deficient Th2 cells were not obviously different from wild type cultures (Figure S2A–C). Importantly, STAT6 phosphorylation was not dependent on STAT3 as a similar pattern was observed in STAT3-deficient cultures (Figure 1A and B). To examine differentiation, naïve CD4+ T cells were isolated from spleens of wild type and Stat3Cd4−/− mice and cultured under Th1, Th2, or Th17 conditions. STAT3-deficient Th1 cells produced similar amounts of the cytokines IFN-γ and GM-CSF as wild type Th1 cells, although STAT3 was required for the generation of cells secreting IL-17A and IL-17F (Figure S2D). Although wild type Th2 cells secreted high amounts of IL-4, IL-5 and IL-13, STAT3-deficient CD4+ T cells cultured under Th2 skewing conditions had diminished IL-4, IL-5, and IL-13 production and gene expression, and expression of the gene encoding the Th2 cell-associated cytokine IL-24 (Figure 2C–E). STAT3-deficient Th2 cells had only a modest increase in IFNγ production, suggesting that they were not differentiating into Th1 cells, and did not acquire expression of Foxp3 mRNA (Figure S2E and data not shown).
STAT3 is required for Th2 cell transcription factor expression
Previous studies have demonstrated STAT3-deficient CD4+ cells have reduced CD25 expression (Akaishi et al., 1998), and IL-2 signaling is required for Th2 differentiation at multiple levels including the expression of Il4ra (Liao et al., 2008). To determine if CD25 or IL-4Rα expression was decreased on STAT3-deficient cells during Th2 differentiation, we examined surface expression throughout differentiation. The percent of receptor positive cells was comparable between wild type and STAT3-deficient Th2 cells (Figure S3), and although the MFI of CD25 was decreased on the fourth day of differentiation, adding exogenous IL-2 during differentiation did not rescue Th2 cytokine production in STAT3-deficient cultures (data not shown). Normal IL-4Rα expression in STAT3-deficient Th2 cultures was consistent with normal STAT6 activation in the absence of STAT3 (Figure 1A and B). These data suggest that the reduction in Th2 cytokine production in STAT3-deficient Th2 cells is not due to reduced CD25 or IL-4Rα expression.
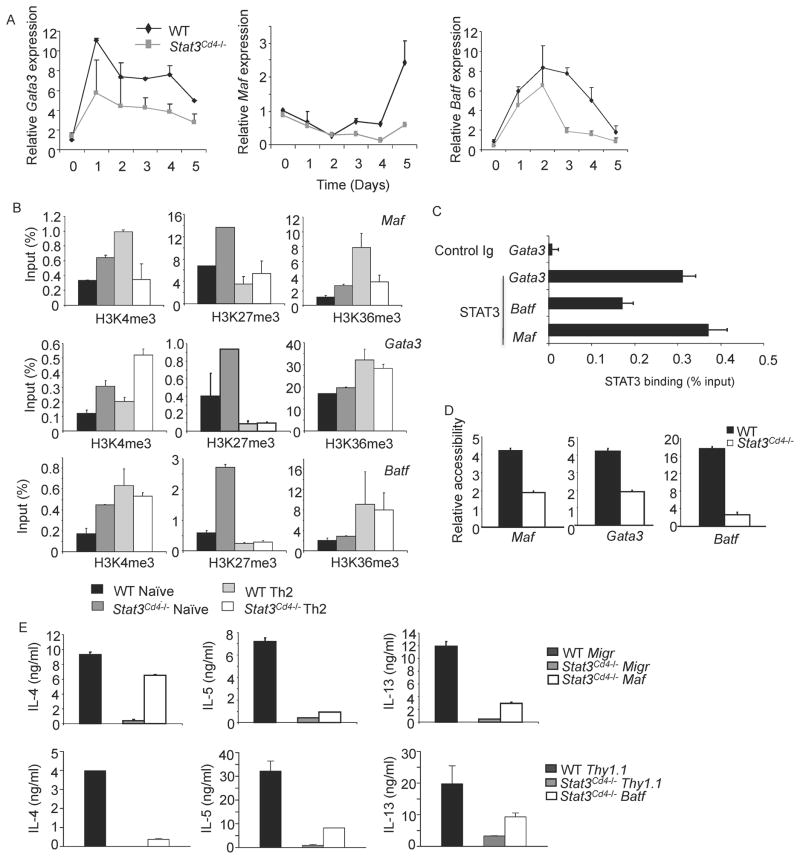
We then examined the expression of transcription factors associated with establishing the Th2 phenotype including Gata3, Batf, Maf, Gfi1, Irf4, and the IL-6 target Socs1, during the differentiation process. Expression of Gata3 was decreased throughout differentiation; Maf and Batf expression were more affected during the last three days of differentiation (Figure 3A). GATA3 protein was also decreased 24 hours after the initiation of culture, but endogenous IL-2 or IL-4 production was not affected by STAT3-deficiency at this time point (Figure S4A and B, and data not shown). Gfi1 and Irf4 expression were less affected by STAT3-deficiency, and Socs1 expression was only decreased during the last three days of differentiation (Figure S4C), agreeing with our data that IFNγ was not induced in STAT3-deficient Th2 cultures (Diehl et al., 2000). The expression of Gata3, Maf, Batf and Irf4 is also decreased at day 5 of differentiation (Figures 3A and S4D). The loss of Gata3 and Maf expression in STAT6-deficient Th2 cells was greater than in STAT3-deficient Th2 cells. However, STAT3 but not STAT6, was required for optimal Irf4 expression (Figure S4D and S1C).
Figure 3. Reduced Th2-Specific Transcription Factor Expression in the Absence of STAT3.
(A) WT and Stat3Cd4−/− naïve CD4+ T cells were differentiated under Th2 conditions and cells were isolated each day during differentiation. RNA isolated from cells was analyzed for gene expression using qPCR (mean ± s.d.). Data are representative of experiments with 4 mice.
(B) WT and Stat3Cd4−/ − naïve CD4+ T cells were used directly or differentiated as in (A) for five days. Chromatin immunoprecipitation was performed for the indicated histone modifications and qPCR was used to determine the amounts of each modification (mean ± s.d.).
(C) Chromatin immunoprecipitation from WT naïve CD4+ T cells with control or STAT3 antibodies followed by qPCR for detection of the indicated loci (mean ± s.d.).
(D) WT and Stat3Cd4−/ − naïve CD4+ T cells were differentiated as in (A) for five days. Nuclei were isolated and left untreated or treated with micrococcal nuclease for 10 minutes. Relative accessibility is defined as 2 to the power of the difference between the Ct value of qPCR of untreated and treated samples (mean ± s.d.).
(E) WT and Stat3Cd4−/ − naïve CD4+ T cells were differentiated as in (A). On Day 2 cells were transduced with control, Batf or Maf-expressing retrovirus. Sorted transduced cells were re-stimulated with anti-CD3 for 24 hours. Supernatant was tested for Th2 cytokines using ELISA (mean ± s.d.). Data are representative of 2 independent experiments.
To further define the effects of STAT3-deficiency on the loci most affected, we examined the presence of histone modifications trimethyl-H3K4 and − H3K36 that are associated with active genes, and trimethyl-H3K27, which is associated with repressed genes, at the Gata3, Batf and Maf loci. In naïve cells, there was either an increased or unchanged amount of trimethyl-H3K4 and H3K36, suggesting that STAT3 was not required for these modifications in unstimulated cells (Figure 3B). In contrast, there was a marked increase in H3K27 trimethylation in naïve STAT3-deficient T cells (Figure 3B), suggesting that STAT3 plays a role in preventing this modification, and correlating with STAT3 bound to these loci in naïve cells (Figure 3C). In contrast, differences in H3K27 methylation between wild type and STAT3-deficient Th2 cells were not observed. The Gata3 and Batf loci did not have decreased H3K4 and H3K36 methylation in STAT3-deficient Th2 cells compared to wild type Th2 cells. However, activating histone modifications were decreased at the Maf locus (Figure 3B). To determine if STAT3 was also affecting the accessibility of chromatin, we performed micrococcal nuclease assays with nuclei from wild type and STAT3-deficient Th2 cells. We observed decreased accessibility at all three loci, with the greatest difference at the Batf locus (Figure 3D).
We then tested whether transduction of any of these factors into STAT3-deficient Th2 cultures would result in a recovery of cytokine production. Although transduction of Gata3 into STAT3-deficient Th2 cells did not alter cytokine production (data not shown), transduction of either Maf or Batf resulted in a partial recovery of Th2 cytokine production, with Maf having the greatest effects on IL-4 production (Figure 3E). Thus, Maf and Batf likely represent STAT3 targets during Th2 development, and the defects in Th2 cytokine production in the absence of STAT3 are the result of effects on multiple downstream transcription factors.
STAT3 and STAT6 cooperate in promoting Th2 cell cytokine production
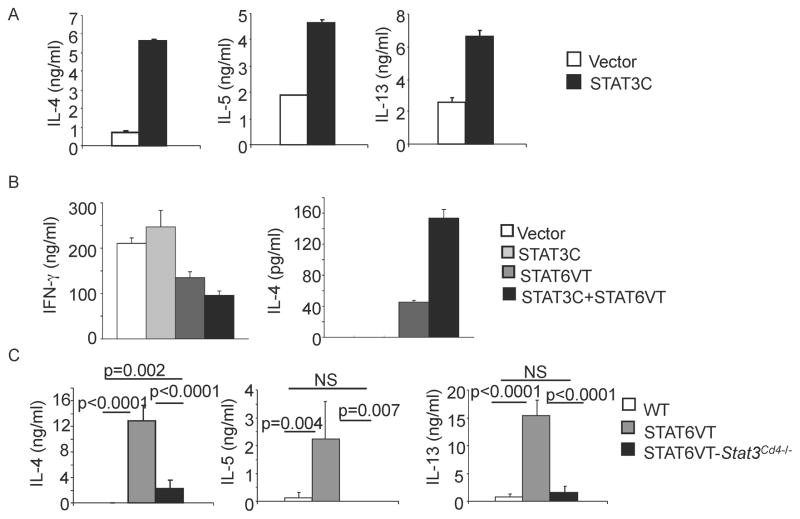
To determine if STAT6 and STAT3 are cooperating to promote Th2 cytokine production we used 2 previously described constitutively active STAT mutants. Both the STAT6VT and STAT3C have two amino acid mutations within the SH2 domain, which renders them constitutively active in the absence of a stimulus (Bromberg et al., 1999; Bruns et al., 2003). We first transduced STAT3C into naïve CD4+ T cells activated under non-skewing conditions (anti-IFN-γ ), which has previously been shown to increase IL-17 production (Mathur et al., 2007). Under non-skewing conditions transduction of STAT3C induced the production of Th2 cytokines (Figure 4A).
Figure 4. STAT3 and STAT6 Cooperate in Promoting Th2 Cytokine Production.
(A) Naïve CD4+ T cells were activated for 48 hours in the absence of skewing cytokines before being transduced with control or STAT3C-expressing retrovirus. After 5 days in culture, cells were sorted and re-stimulated with anti-CD3 (4 μg/ml) for 24 hours before cell-free supernatant was tested for cytokines using ELISA (mean ± s.d.).
(B) Naïve CD4+ T cells were activated for 48 hours under Th1 conditions (5 ng/ml IL–12 + 10 μg/ml anti-IL-4) before being transduced with control, STAT6VT-expressing, STAT3-expressing or both retroviruses. After 5 days in culture, cells were sorted and re-stimulated with anti-CD3 (4 μg/ml) for 24 hours before cell-free supernatant was tested for cytokines using ELISA (mean ± s.d.).
(C) CD4+ cells were isolated from WT, STAT6VT and STAT6VT-Stat3Cd4−/ − mice. Cells were then re-stimulated with anti-CD3 for 24 hours. Cell-free supernatant was collected and tested for various cytokines using ELISA. Data are representative of 2 experiments (average of 2–6 mice ± s.d.). Students t test was performed to calculate p values.
To directly test if the combination of constitutively active STAT6 and STAT3 could increase Th2 cytokine production compared to constitutively STAT6 alone, naïve T cells cultured under Th1 conditions were transduced with retroviruses expressing STAT6VT and STAT3C alone or in combination. Under these conditions, STAT3C alone did not increase IL-4 production, had modest effects on IFNγ production, and did not induce IL-17 production (Figure 4B and data not shown). Transduction of STAT6VT increased IL-4 production and decreased IFNγ, and co-transduction of STAT3C further increased the amount of IL-4 produced in these cultures (Figure 4B).
We then wanted to confirm the requirement for STAT3 for optimal Th2 differentiation in vivo in the presence of a constitutively active STAT6. Peripheral T cells in STAT6VT transgenic mice have an increased propensity towards a Th2 cytokine-secreting phenotype (Bruns et al., 2003; Sehra et al., 2008). To directly test if STAT3 is necessary for Th2 cytokine production in this in vivo system, we isolated CD4+ T cells from the spleens of wild type, STAT6VT transgenic, and STAT6VT transgenic crossed to Stat3Cd4−/− (STAT6VT- Stat3Cd4−/−) mice, and stimulated cells with anti-CD3 for 24 hours before cytokine production was assessed using ELISA. As shown previously, STAT6VT mice have increased production of IL-4, IL-5 and IL-13, whereas STAT6VT T cells lacking STAT3 produced Th2 cytokines in amounts similar to wild type cells (Figure 4C). These results demonstrate that STAT3 cooperates with STAT6 to promote Th2 cytokine production.
STAT3 binds Th2 cell-associated gene loci and defines the STAT6 binding pattern
To further examine the cooperation of STAT6 and STAT3 in enhancing Th2 cytokine production, binding of these proteins to gene targets was determined using chromatin immunoprecipitation. In Th2 cells, similar to binding in naïve T cells (Figure 3C), STAT3 directly binds a number of the same loci bound in Th17 cells, including Maf, Batf and Irf4, which also contribute to Th17 development (Bauquet et al., 2009; Betz et al., 2010; Brustle et al., 2007; Schraml et al., 2009). STAT3 was also bound to Gata3, and Il21, a cytokine produced by multiple Th cell subsets, but was more predominant at the Il17 and Il17f loci than in other Th subsets (Figure 5A).
Figure 5. STAT3 Binds Th2-Associated Gene Loci and Defines the STAT6 Binding Pattern.
(A) Naïve CD4+ T cells activated with anti-CD3 and anti-CD28 and cultured under Th1, Th2 or Th17 conditions for 3 days were used for ChIP performed with normal rabbit IgG or anti-STAT3 before qPCR was performed for the indicated genes. Data are expressed as percent input ± s.d. and control Ig background values are subtracted from the values indicated. Data are representative of 3–4 experiments with similar results.
(B) Naïve CD4+ T cells from WT and Stat3Cd4−/ − activated with anti-CD3 and anti-CD28 and cultured under Th2 conditions for 3 days were used for ChIP performed with normal rabbit IgG or anti-STAT6 and qPCR was performed for the indicated genes. Data are expressed as percent input ± s.d. and control Ig background values are subtracted from the values indicated. Data are representative of 3–4 experiments with similar results. ND, not detected.
(C) CD4+ cells were isolated from WT, STAT6VT and STAT6VT-Stat3Cd4−/ − mice. Cells were then re-stimulated with anti-CD3 for 6 hrs before RNA was isolated and quantitative PCR was performed. Data are representative of 2 experiments (average of 2–6 mice ± s.d.). Students t test was performed to calculate p values.
We then tested whether STAT3 has an effect on STAT6 binding to target genes. In Th2 cells, STAT6 binds to the Gata3, Maf, Batf and Irf4 genes (Figure 5B). However, in the absence of STAT3, STAT6 binding was either undetectable or greatly diminished (Figure 5B). This was concurrent with decreased locus accessibility in the absence of STAT3 (Figure 3D) and suggests that STAT3 is required to allow access for STAT6 to bind these loci and increase gene expression.
We then tested whether an active STAT6 was capable of inducing expression of Th2 transcription factors in the absence of STAT3. Expression of Gata3 and Maf were significantly increased in STAT6VT CD4+ T cells examined directly ex vivo, compared to cells from wild type mice. However, STAT3-deficient STAT6VT CD4+ T cells had reduced expression of both Gata3 and Maf, compared to T cells from STAT6VT transgenic mice on a wild type background (Figure 5C). Together, these data suggest that STAT3 facilitates the ability of STAT6 to bind target genes and promote the Th2 genetic program.
STAT3 in T cells is required for the development of allergic inflammation
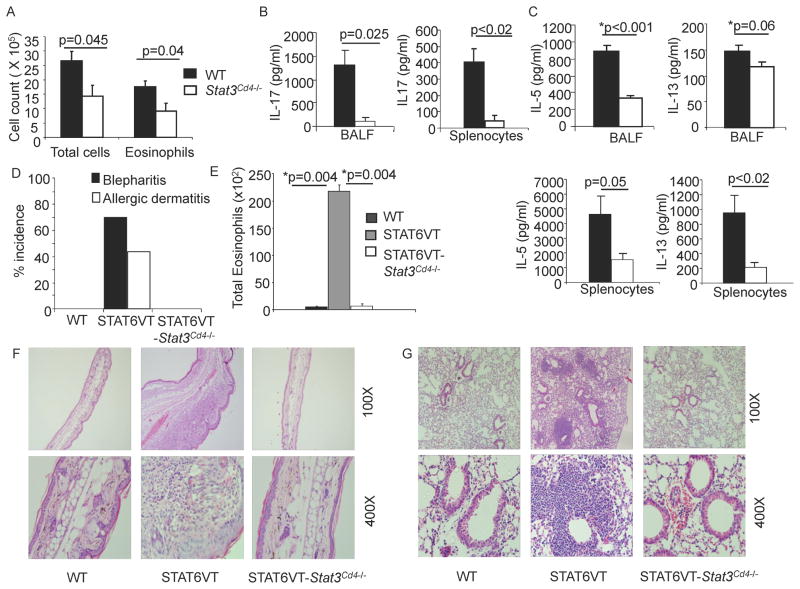
To test if STAT3 is also required for in vivo Th2 differentiation, wild type and Stat3Cd4−/− mice were sensitized with alum-adsorbed OVA. Two weeks after the second immunization, and following intranasal challenges, we observed that pulmonary inflammation, assessed by total cell number, and by number of eosinophils in the bronchoalveolar lavage (BAL), was decreased in Stat3Cd4−/− mice, compared to wild type mice (Figure 6A). Amounts of Th17 and Th2 cytokines were decreased in the BAL fluid, and in cultures of splenocytes stimulated in vitro with OVA analyzed using ELISA (Figure 6B and C). Although Th2 immunity is clearly decreased in vivo, the simultaneous requirement for Th17 cells in this model (Lewkowich et al., 2005; Schnyder-Candrian et al., 2006) complicates the interpretation of the requirement for STAT3-dependent Th2-mediated inflammation in vivo.
Figure 6. STAT3 Promotes the Development of Allergic Inflammation.
(A–C) WT and Stat3Cd4−/ − mice were immunized with OVA and Alum on days 0 and 7 and challenged as described in methods. After challenges, BAL cell numbers were determined by cell counting and flow cytometry (A), and cytokine levels were measured in BAL fluid and in culture supernatants from splenocytes stimulated with OVA for 72 h using ELISA. Data are average of 5–6 mice per group ± s.e.m
(D) Incidence of blepharitis and atopic dermatitis of WT, STAT6VT and STAT6VT-Stat3Cd4−/ − mice are shown. Incidence was determined by visual examination of mice (n=25 per group).
(E) Numbers of eosinophils (defined by flow cytometry) recovered in BAL. BAL data are representative of 2 independent experiments and shown as the average of 2 mice per group ± s.d. For A–C and E, Students t test was performed to calculate p values.
(F) Ear tissue from WT, STAT6VT and STAT6VT-Stat3Cd4−/ − mice were fixed and paraffin-embedded sections were stained with hematoxylin-eosin. Magnification is indicated in the panel and photomicrographs are representative of 10 mice per group
(G) Lungs from WT, STAT6VT and STAT6VT-Stat3Cd4−/ − mice were embedded in paraffin and stained with H & E. Magnification is indicated in the panel and photomicrographs are representative of 10 mice per group.
To investigate the requirement for STAT3 in allergic inflammation further, we used mice expressing STAT6VT in T cells that spontaneously develop multi-organ allergic inflammation, including pulmonary inflammation, blepharitis, and skin inflammation, all of which are completely dependent on IL-4 (Sehra et al., 2008; Sehra et al., 2010). The incidence of blepharitis in STAT6VT mice is nearly 75%, and is never observed in wild type mice. STAT6VT-Stat3Cd4−/− mice were protected from blepharitis and have 0% incidence even in older mice (Figure 6D). Approximately 40% of STAT6VT transgenic mice developed skin inflammation resembling atopic dermatitis, a condition not observed in wild type mice. As with blepharitis, STAT6VT transgenic mice lacking STAT3 in T cells were protected from skin inflammation, thickening of the skin and immune infiltration (Figure 6D and F). STAT6VT transgenic mice develop lung inflammation characterized by peri-bronchial and peri-arterial accumulation of eosinophils and lymphocytes. However STAT6VT-Stat3Cd4−/− mice, like wild type mice, had very few eosinophils infiltrating the lungs (Figure 6E and G). These results demonstrate that the loss of STAT3 in T cells protects mice from the development of Th2-mediated inflammatory diseases. Taken together, STAT3 and STAT6 proteins are both necessary for optimal Th2 development and in the context of the STAT6 signal, STAT3 enhances Th2 cell development.
DISCUSSION
The paradigm that STAT family members promoted specific Th effector cell phenotypes was developed when the number of known effector subsets was more limited. We and other researchers initially defined that STAT4 is required for Th1 cell development, and STAT6 is required for Th2 cell development (Kaplan et al., 1996a; Kaplan et al., 1996b; Shimoda et al., 1996; Takeda et al., 1996; Thierfelder et al., 1996). However, this simple one STAT-one subset paradigm became more complicated when it was shown that STAT1 also contributed to Th1 differentiation (Afkarian et al., 2002; Lighvani et al., 2001), and STAT5 could function with STAT6 in the development of Th2 cells (Cote-Sierra et al., 2004; Takatori et al., 2005; Zhu et al., 2003). This was an important finding as STAT5, which is also critical for the development of T regulatory cells (Burchill et al., 2007; Yao et al., 2007), has different functions when activated in the presence of STAT6. Thus, the differentiating T helper cell is able to assimilate multiple signals and acquire the appropriate effector phenotype. In this report, we further our understanding of the integration of STAT signals by demonstrating that STAT3, which clearly promotes Th17 development in the absence of signals that promote other phenotypes, is required for the function of STAT6 during Th2 development.
Based on our data we propose the following model of Th2 development. STAT3 is bound to multiple Th2-associated transcription factor loci even in naïve cells, which limits repressive histone modifications. Upon activation in a pro-Th2 environment, STAT3 is activated by multiple cytokines promoting chromatin remodeling, and allowing STAT6 to bind and activate target genes. STAT3 also has direct effects on histone modifications at the Maf locus. Interestingly, we find that H3K4 methylation is STAT3-dependent at the Maf but not the Batf locus, whereas in Th17 cells the opposite pattern was observed (Durant et al., 2010). The similarity in STAT3 target genes in Th2 and Th17 cells suggests that STAT3 is able to initiate differentiation to both phenotypes, and it is the presence of the IL-4/STAT6 signal that promotes Th2 development at the expense of the Th17 program. IL-4 signaling has a similar effect on Treg development by decreasing STAT5 binding to the Foxp3 locus (O'Malley et al., 2009) and promoting an alternative T helper subset. Thus, STAT6 plays a definitive role in the outcome of Th differentiation in the presence of IL-4.
The exact targets of STAT3 required for Th2 development are not entirely clear and likely several targets are important. Although the Maf gene is a characterized target of STAT3 (Yang et al., 2005), and expression of Maf is deficient in the absence of STAT3, ectopic expression of Maf resulted in only a partial recovery of Th2 cytokine production. BATF, a transcription factor recently shown to promote Th2 development, also requires STAT3 for normal expression in Th2 cells, and transduction of Batf resulted in a partial recovery of Th2 cytokine production. We did not see recovery of Th2 cytokine production when Gata3 or Irf4 were ectopically expressed. This is distinct from STAT6-deficient cells where expression of GATA3 induces Th2 cytokine production (Chang et al., 2005; Ouyang et al., 2000). Together these data suggest that the defect in STAT3-deficient Th2 cultures is more complex than the absence of one factor, and recovery of Th2 cytokine expression may require the coordinated function of several factors.
The requirement for STAT3 in Th2 development is in contrast to the hyper-IgE syndrome that develops in patients with dominant negative STAT3 mutations (Ma et al., 2008; Milner et al., 2008). Although human STAT3 mutations are autosomal dominant, it is reasonable to expect that some STAT3 function is retained in these patients because, at least in mice, STAT3-deficiency is embryonic lethal (Takeda et al., 1997). Moreover, it is yet unclear how STAT3 mutants result in hyper-IgE syndrome. Like mice with STAT3-deficient T cells (Mathur et al., 2007; Yang et al., 2007), patients with hyper-IgE syndrome lack Th17 cells, though effects on Th2 cells in patients have not been clearly defined. However, mice with STAT3-deficient T cells do not have increased serum IgE (data not shown), suggesting that either human STAT3 mutants are not functionally equivalent to STAT3-deficiency, or that mutant STAT3 promotes hyper-IgE in cells other than T cells. Although mutant STAT3 in human B cells is required for IL-21-stimulated IgE production and generation of memory B and plasma cells, STAT3-deficiency in mouse B cells affects IgG responses but does not result in hyper-IgE syndrome (Avery et al., 2010; Avery et al., 2008; Fornek et al., 2006). The pathogenesis of hyper-IgE syndrome is clearly complex and additional mechanistic insight into STAT3-dependent functions likely requires introduction of STAT3 mutations into a mouse model.
Multiple signals contribute to the generation of differentiated T helper subsets. However, in this model there is a dominant signal, IL-4 in the case of Th2 cells, which defines the outcome of the differentiation process. It is clear that STAT3 is required for the development of Th17 cells, and that constitutively active STAT3 promotes the development of IL-17-secreting cells (Ma et al., 2008; Mathur et al., 2007; Milner et al., 2008; Nurieva et al., 2008; Yang et al., 2007; Yang et al., 2005). However, IL-4 provides a dominant signal that diminishes Th17 development (Harrington et al., 2005; Park et al., 2005) and decreases symptoms of autoimmunity in multiple models (Rocken et al., 1996). Thus, when both STAT3 and STAT6 signals are present in a cell, the pro-Th17 effects of STAT3 are reduced, while the pro-Th2 effects of STAT6 are amplified. Mechanistically this occurs through the binding of STAT3 to Th2 genes that facilitate the ability of STAT6 to activate genes necessary for Th2 development. Thus, multiple STAT proteins, activated by cytokines present in the milieu of a developing immune response, cooperate in defining the ultimate phenotype of the differentiating effector T cell.
EXPERIMENTAL PROCEDURES
Mice
Stat3fl/fl mice (Chiarle et al., 2005) with a Cd4-Cre (Stat3Cd4−/−) transgene and wild type mice (Harlan Laboratories, Indianapolis IN) were on a C57BL/6 background. WT mice in experiments using Stat3Cd4−/ − mice were Cre-negative littermates. STAT6VT (STAT6 with V547 and T548 mutated to alanine) transgenic mice were previously described (Bruns et al., 2003) were backcrossed to C57BL/6 mice (Harlan Laboratories). Stat3Cd4−/ − mice were mated to STAT6VT transgenic mice to generate STAT6VT- Stat3Cd4−/ −. All mice were maintained in specific pathogen-free conditions and experiments approved by the Indiana University Institutional Animal Care and Use Committee.
Quantitative RT-PCR
Total RNA was isolated from either un-stimulated or anti-CD3 (2μg/ml) re-stimulated cells using Trizol and reverse transcribed according to manufacturer’s instructions (Invitrogen Life Technologies, Carlsbad, CA). Quantitative PCR was performed with Taqman Fast Universal PCR Master Mix and commercially available primers for genes using the 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA). RNA was normalized to expression levels of β2-microglobulin and relative expression was calculated using the 2−ΔΔCt method.
Murine T Helper Cell Differentiation
Naïve CD4+CD62L+ T cells were purified from spleens using magnetic isolation (Miltenyi Biotec, Auburn, CA). Naïve CD4+ T cells (1x106 cells/ml of complete RPMI-1640 medium) were cultured with plate bound anti-CD3 (4 μg/ml 145–2C11) and soluble anti-CD28 (1 μg/ml; BD Pharmingen) under Th2 (IL-4 at 10 ng/ml (PeproTech, Rocky Hill, NJ) and anti-IFNγ at 10 μg/ml), Th1 with anti-IL-4 (10 μg/ml 11B11) and IL-12 (5 ng/ml; R&D Systems), or Th17 with anti-IFN-γ , anti-IL-4, TGF-β1 (2 ng/ml; R&D Systems) and IL-6 (100 ng/ml; PeproTech). Cells were expanded on day three after stimulation by adding half the dose of the original cytokines in fresh medium. After 5 days of culture, differentiated cells were re-stimulated with plate bound anti-CD3 at 4 μg/ml for 1 or 3 days, and the cell-free supernatant was collected after centrifugation and stored at −20°C until use. The levels of cytokines produced were determined using ELISA (reagents from BD Pharmingen or eBioscience). For cytokine blocking experiments Th2 cells were cultured as above in the presence of control antibody or blocking antibodies to IL-2, IL-6, IL-21, and/or CD25 (20 μg/ml, BD Pharmingen or R&D Systems) from the initiation of culture and additional antibodies added at day 3 during cell expansion. Naïve CD4+ T cells from DO11.10 mice were stimulated with plate bound anti-CD3 and anti-CD28, or 100 μg/ml OVA protein and anti-CD28, and cultured with anti-IFNγ and IL-4 and CD4-depleted irradiated APCs (1:5). On day 3 of culture cells were expanded and an additional dose of IL-4 was added.
Retroviral Transduction
Bicistronic retroviral vectors encoding mouse GATA3 or IRF4 and human CD4 (Ahyi et al., 2009; Chang et al., 2005), STAT6VT or c-MAF (kindly provided by Dr. I. C. Ho) and EGFP, or STAT3C (Mathur et al., 2007) or BATF (Williams et al., 2003) subcloned into the bicistronic retroviral vector containing the marker Thy1.1 and Thy1.1 empty vector (kindly provided by Dr. S. Goenka) were used to generate virus as described (Mathur et al., 2007). After 2 days of differentiation, T helper cells were transduced with retroviral supernatant containing polybrene. On day 5 transduced cells (EGFP, Thy1.1, or hCD4 positive cells) were sorted by flow cytometry before cytokine production and gene expression analyses.
Analysis of inflammation and Th2 responses in vivo
Immunization and challenge of mice was performed as described (Chang et al., 2010; O'Malley et al., 2009). Splenocytes from the mice were stimulated with OVA (100 μg/ml) for 72h and Th2 cytokines in cell-free supernatants were assessed using ELISA. Paraffin-embedded sections were stained with hematoxylin and eosin for evaluation of the infiltration of inflammatory cells by light microscopy. BAL was performed and eosinophils in the BAL were identified as described (Chang et al., 2010; O'Malley et al., 2009).
Intracellular Staining
After differentiation cells were re-stimulated with anti-CD3 (4 μg/ml) for 5 hours. Monensin was added to the cells for the final 2 hours of stimulation. Following stimulation cells were fixed with paraformaldehyde and permeablized with saponin. Cells were then stained with fluorochrome-conjugated anti-mouse IL-4 (BVD6-24G2; eBioscience). For intracellular phospho-STAT staining, T cells were collected and fixed with 1.5% formaldehyde for 10 minutes at room temperature. Following fixation cells were permeabilized for 15 minutes at 4°C with 100% methanol. Cells were then stained for phospho-STAT3 or phospho-STAT6 (BD Pharmingen) for 30 minutes at room temperature. Cells were analyzed by flow cytometry using FACSCalibur (Beckton Dickinson) and results were analyzed using WinMDI.
Chromatin Immunoprecipitation
ChIP assay was performed as previously described (Yu et al., 2007). Immunoprecipitations were perfromed with rabbit polyclonal antibodies (anti-STAT3 sc-482, anti-Stat6 sc-1698, rabbit IgG (Santa Cruz), H3K4me3 and M3K27me3 (Millipore), H3K36me3 (Abcam)). Quantification of DNA was performed using SYBR Green Fast PCR Master Mix (primer sequences provided in Supplementary Table I) using ABI 7500 Fast Real-time PCR System. To quantify immunoprecipitated DNA, a standard curve was generated from serial dilutions of Input DNA. To calculate ChIP results as a percentage of input, the amount of the immunoprecipitated DNA from the IgG control was subtracted from the amount of the immunoprecipitated DNA from the specific antibody ChIP followed by normalizing against the amount of the input DNA.
Nuclease accessibility assay
Nuclei were isolated from naïve CD4+ T cells or Th2 cells using preparation buffer (0.3 M sucrose, 60 mM KCl, 0.1 mM ethylene glycol tetraacetic acid [EGTA], 15 mM Tris-HCl (pH 7.5), 15 mM NaCl, and 5 mM MgCl2) containing 0.4% NP-40. Nuclei were pelleted by centrifugation at 200 rpm for 5 minutes and washed three times in preparation buffer without NP-40 containing protease inhibitors before samples were stored in preparation buffer at 80 °C. Aliquots of nuclei (1x106) were incubated with 0.2 U of micrococcal nuclease S7 (MNase) (Roche) in MNase digestion buffer (60 mM KCl, 15 mM Tris-HCl (pH 7.5), 15 mM NaCl, and 1 mM MgCl2, 0.5 mM Spermidine, 0.1 Mm PMSF) containing 5 Mm CaCl2 and protease inhibitor cocktail for 10min at 37 °C, and the reactions were terminated by the addition of stop buffer (20 mM EDTA, 1% SDS, and 100 μg/ml proteinase K). Genomic DNA was then purified using the high pure PCR product purification kit (Roche). Relative accessibility was quantified using SYBR Green Fast PCR Master using ABI 7500 Fast Real-time PCR System. Relative accessibility is defined as 2 to the power of the difference between the Ct value of qPCR of nuclease untreated and treated samples.
Supplementary Material
Acknowledgments
The authors thank Drs. IC Ho and EJ Taparowsky for respectively providing the c-Maf and Batf plasmids, Dr. B Zhou for DO11.10 mice, Drs J Blum, A Dent and S Goenka for review of this paper.
This work was supported by U19 AI070448. GLS was supported by T32 AI060519. DP was supported by T32 HL07910.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- Ahyi AN, Chang HC, Dent AL, Nutt SL, Kaplan MH. IFN regulatory factor 4 regulates the expression of a subset of Th2 cytokines. J Immunol. 2009;183:1598–1606. doi: 10.4049/jimmunol.0803302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaishi H, Takeda K, Kaisho T, Shineha R, Satomi S, Takeda J, Akira S. Defective IL-2-mediated IL-2 receptor alpha chain expression in Stat3-deficient T lymphocytes. Int Immunol. 1998;10:1747–1751. doi: 10.1093/intimm/10.11.1747. [DOI] [PubMed] [Google Scholar]
- Akimoto T, Numata F, Tamura M, Takata Y, Higashida N, Takashi T, Takeda K, Akira S. Abrogation of bronchial eosinophilic inflammation and airway hyperreactivity in signal transducers and activators of transcription (STAT)6-deficient mice. J Exp Med. 1998;187:1537–1542. doi: 10.1084/jem.187.9.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- Avery DT, Deenick EK, Ma CS, Suryani S, Simpson N, Chew GY, Chan TD, Palendira U, Bustamante J, Boisson-Dupuis S, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med. 2010;207:155–171. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery DT, Ma CS, Bryant VL, Santner-Nanan B, Nanan R, Wong M, Fulcher DA, Cook MC, Tangye SG. STAT3 is required for IL-21-induced secretion of IgE from human naive B cells. Blood. 2008;112:1784–1793. doi: 10.1182/blood-2008-02-142745. [DOI] [PubMed] [Google Scholar]
- Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson SZ, Le Gros G, Conrad DH, Finkelman FD, Paul WE. IL-4 production by T cells from naive donors. IL-2 is required for IL-4 production. J Immunol. 1990;145:1127–1136. [PubMed] [Google Scholar]
- Betz BC, Jordan-Williams KL, Wang C, Kang SG, Liao J, Logan MR, Kim CH, Taparowsky EJ. Batf coordinates multiple aspects of B and T cell function required for normal antibody responses. J Exp Med. 2010;207:933–942. doi: 10.1084/jem.20091548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Bruns HA, Schindler U, Kaplan MH. Expression of a constitutively active Stat6 in vivo alters lymphocyte homeostasis with distinct effects in T and B cells. J Immunol. 2003;170:3478–3487. doi: 10.4049/jimmunol.170.7.3478. [DOI] [PubMed] [Google Scholar]
- Brusselle G, Kips J, Joos G, Bluethmann H, Pauwels R. Allergen-induced airway inflammation and bronchial responsiveness in wild-type and interleukin-4-deficient mice. Am J Respir Cell Mol Biol. 1995;12:254–259. doi: 10.1165/ajrcmb.12.3.7873190. [DOI] [PubMed] [Google Scholar]
- Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Zhang S, Thieu VT, Slee RB, Bruns HA, Laribee RN, Klemsz MJ, Kaplan MH. PU.1 expression delineates heterogeneity in primary Th2 cells. Immunity. 2005;22:693–703. doi: 10.1016/j.immuni.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Chiarle R, Simmons WJ, Cai H, Dhall G, Zamo A, Raz R, Karras JG, Levy DE, Inghirami G. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat Med. 2005;11:623–629. doi: 10.1038/nm1249. [DOI] [PubMed] [Google Scholar]
- Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, Zhu J, Paul WE. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl S, Anguita J, Hoffmeyer A, Zapton T, Ihle JN, Fikrig E, Rincon M. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity. 2000;13:805–815. doi: 10.1016/s1074-7613(00)00078-9. [DOI] [PubMed] [Google Scholar]
- Diehl S, Chow CW, Weiss L, Palmetshofer A, Twardzik T, Rounds L, Serfling E, Davis RJ, Anguita J, Rincon M. Induction of NFATc2 expression by interleukin 6 promotes T helper type 2 differentiation. J Exp Med. 2002;196:39–49. doi: 10.1084/jem.20020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant L, Watford WT, Ramos HL, Laurence A, Vahedi G, Wei L, Takahashi H, Sun HW, Kanno Y, Powrie F, O'Shea JJ. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornek JL, Tygrett LT, Waldschmidt TJ, Poli V, Rickert RC, Kansas GS. Critical role for Stat3 in T-dependent terminal differentiation of IgG B cells. Blood. 2006;107:1085–1091. doi: 10.1182/blood-2005-07-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Kagami S, Nakajima H, Suto A, Hirose K, Suzuki K, Morita S, Kato I, Saito Y, Kitamura T, Iwamoto I. Stat5a regulates T helper cell differentiation by several distinct mechanisms. Blood. 2001;97:2358–2365. doi: 10.1182/blood.v97.8.2358. [DOI] [PubMed] [Google Scholar]
- Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996a;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996b;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med. 1998;187:939–948. doi: 10.1084/jem.187.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GR, Kim ST, Spilianakis CG, Fields PE, Flavell RA. T helper cell differentiation: regulation by cis elements and epigenetics. Immunity. 2006;24:369–379. doi: 10.1016/j.immuni.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Kohl J, Belkaid Y, Wills-Karp M. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Schones DE, Oh J, Cui Y, Cui K, Roh TY, Zhao K, Leonard WJ. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor alpha-chain expression. Nat Immunol. 2008;9:1288–1296. doi: 10.1038/ni.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE, O'Shea JJ. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, Fulcher DA, Tangye SG, Cook MC. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O'Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH. Stat3 and Stat4 Direct Development of IL-17-Secreting Th Cells. J Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley JT, Sehra S, Thieu VT, Yu Q, Chang HC, Stritesky GL, Nguyen ET, Mathur AN, Levy DE, Kaplan MH. Signal transducer and activator of transcription 4 limits the development of adaptive regulatory T cells. Immunology. 2009;127:587–595. doi: 10.1111/j.1365-2567.2008.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Lohning M, Gao Z, Assenmacher M, Ranganath S, Radbruch A, Murphy KM. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity. 2000;12:27–37. doi: 10.1016/s1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocken M, Racke M, Shevach EM. IL-4-induced immune deviation as antigen-specific therapy for inflammatory autoimmune disease. Immunol Today. 1996;17:225–231. doi: 10.1016/0167-5699(96)80556-1. [DOI] [PubMed] [Google Scholar]
- Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehra S, Bruns HA, Ahyi AN, Nguyen ET, Schmidt NW, Michels EG, von Bulow GU, Kaplan MH. IL-4 is a critical determinant in the generation of allergic inflammation initiated by a constitutively active Stat6. J Immunol. 2008;180:3551–3559. doi: 10.4049/jimmunol.180.5.3551. [DOI] [PubMed] [Google Scholar]
- Sehra S, Yao Y, Howell MD, Nguyen ET, Kansas GS, Leung DY, Travers JB, Kaplan MH. IL–4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010;184:3186–3190. doi: 10.4049/jimmunol.0901860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- Takatori H, Nakajima H, Hirose K, Kagami S, Tamachi T, Suto A, Suzuki K, Saito Y, Iwamoto I. Indispensable role of Stat5a in Stat6-independent Th2 cell differentiation and allergic airway inflammation. J Immunol. 2005;174:3734–3740. doi: 10.4049/jimmunol.174.6.3734. [DOI] [PubMed] [Google Scholar]
- Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci U S A. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- Tepper RI, Levinson DA, Stanger BZ, Campos-Torres J, Abbas AK, Leder P. IL-4 induces allergic-like inflammatory disease and alters T cell development in transgenic mice. Cell. 1990;62:457–467. doi: 10.1016/0092-8674(90)90011-3. [DOI] [PubMed] [Google Scholar]
- Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, Ihle JN. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- Williams KL, Zullo AJ, Kaplan MH, Brutkiewicz RR, Deppmann CD, Vinson C, Taparowsky EJ. BATF transgenic mice reveal a role for activator protein-1 in NKT cell development. J Immunol. 2003;170:2417–2426. doi: 10.4049/jimmunol.170.5.2417. [DOI] [PubMed] [Google Scholar]
- Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ochando J, Yopp A, Bromberg JS, Ding Y. IL-6 plays a unique role in initiating c-Maf expression during early stage of CD4 T cell activation. J Immunol. 2005;174:2720–2729. doi: 10.4049/jimmunol.174.5.2720. [DOI] [PubMed] [Google Scholar]
- Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Thieu VT, Kaplan MH. Stat4 limits DNA methyltransferase recruitment and DNA methylation of the IL-18Ralpha gene during Th1 differentiation. Embo J. 2007;26:2052–2060. doi: 10.1038/sj.emboj.7601653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Cote-Sierra J, Guo L, Paul WE. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003;19:739–748. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
- Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.