Abstract
Influenza virus infection is a significant public health problem; however factors affecting the incidence and severity of disease have not been fully elucidated. The present study sought to examine the role of sex and stress in mediating susceptibility to an influenza viral infection in mice. Male and female mice underwent repeated cycles of restraint (RST) stress, followed by an influenza A/PR8 virus infection. Following these manipulations, levels of circulating corticosterone, lung proinflammatory cytokine gene expression and sickness behavior were examined. The data indicate sex differences in several aspects of the response to the A/PR8 virus infection. The kinetics of lung interleukin-1β mRNA expression were faster in infected males compared to females, while circulating corticosterone levels were elevated in infected females, but not in males. Anorexia and reduced saccharin consumption began earlier and symptoms were more pronounced in infected males than in females. In addition, RST modulated the response to the A/PR8 virus infection. Proinflammatory cytokine gene expression in response to infection was enhanced and sickness behavior was modulated by RST in both males and females. These data suggest that males mount more vigorous immune and behavioral responses to influenza viral infection compared to females, and stress exacerbates the response in both males and females. In conclusion, complex interactions between biological and behavioral factors are involved in mediating individual differences in health and disease. Additional studies may help uncover some of the factors contributing to the individual differences in susceptibility to influenza infection.
Keywords: Restraint stress, proinflammatory cytokine, glucocorticoid, influenza virus, sickness behavior, mice
Introduction
Influenza viral infection is one of the major causes of morbidity and mortality in humans. Although substantial research efforts have focused on understanding the pathophysiology of influenza viral infection, it remains a major public health hazard as new and highly virulent strains continue to emerge. This was dramatically illustrated with the world-wide outbreak of the 2009 H1N1swine-origin influenza pandemic. Those who tend to suffer the most are persons with weakened immune systems including children, the elderly or those with a compromised immune system (Bender and Small, 1993; Ludwig et al., 2003; Webby and Webster, 2003). In the case of the 2009 influenza A pandemic strain, pregnant women, young people without pre-existing immunity to swine-type influenza and those persons with underlying medical conditions were most severely affected (CDC, 2010). The mechanisms involved in the response to influenza viral infection have been studied extensively using a murine model of influenza virus infection. In this model, intranasal administration of influenza virus resulted in viral replication in the epithelium of both the upper and lower respiratory tract. Viral replication in the respiratory epithelium stimulated the secretion of proinflammatory cytokines and host factors designed to enhance cellular resistance to infection (Konstantinos and Sheridan, 2001). In addition, murine influenza viral infection activated the hypothalamus-pituitary-adrenal axis (Hermann et al., 1994) and induced sickness behavior (Avitsur et al., 2009).
Studies have revealed significant differences in disease resistance between males and females. Accumulating evidence indicated that males are more susceptible to infectious diseases, whereas females are more vulnerable to autoimmune disorders (Han 2001; Bucher 2002). Behavioral and environmental factors such as mating competition and nutrition were implicated in the unequal susceptibility of males and females to infectious disease (Zuk and McKean 1996). Several reports further attributed these sex differences to variations in the organism’s response to an immune challenge since females mount more vigorous humoral immune responses, while males usually show enhanced cellular immune activity (Alexander and Stimson, 1988; Barna et al., 1996; Knoblich et al., 1983). Along these lines, sex differences were reported in morbidity and mortality from influenza viral infection. During the 1918 influenza pandemic, more men in the United States died from viral infection than did women; however, underlying pathologies, such as the tuberculosis infection most prevalent in the male population at that time, may have played a significant role in driving influenza-related death during that pandemic (Noymer, 2010). More recently, two studies have reported a higher proportion of severely infected women versus men during the 2009 H1N1 swine-origin pandemic (Chitnis, et al. 2010; Koegelenberg, et. al. 2010). These studies showed that females were more likely to be hospitalized with severe respiratory infection than were infected males. Conversely, a report from Argentina details higher mortality in older men during the 2009 H1N1 pandemic versus women (Roriz-Cruz, et. al 2010). As with any epidemiological study, factors in addition to sex that were not considered in the analysis may play a critical role in determining susceptibility to severe influenza infection.
Sex differences were also documented in the behavioral symptoms of diseases. Illness is typically accompanied by behavioral symptoms such as reduced locomotion and exploratory activity, anorexia and anhedonia (Avitsur and Yirmiya, 1999; Dantzer et al., 2007). Sickness behavior may be a strategy important for the survival of the individual, in that it promotes energy conservation and reduces the risk of encountering predators (Hart, 1988; Kent, et al., 1992). Proinflammatory cytokines secreted as a part of the inflammatory process have been implicated in mediating sickness behavior (Dantzer et al., 2007). Interestingly, studies have shown that the kinetics and severity of several aspects of sickness behavior were enhanced in female compared to male rats (Avitsur et al., 1995; Avitsur and Yirmiya, 1999; Yee and Prendergast, 2010, Yirmiya et al., 1995).
A variety of psychosocial and physiological factors have been shown to affect susceptibility to viral infection (Avitsur et al., 2006; Meagher et al., 2010). One of the key factors implicated in modulating disease severity is the exposure to chronic stressors. Studies have shown that chronic stress increased susceptibility and frequency of disease, prolonged healing times, and increased incidence of secondary health complications associated with viral infection (Bailey et al., 2003). Specifically, studies have demonstrated that chronic stress significantly altered the pathophysiology of influenza viral infection resulting in enhanced viral replication in the lungs of infected mice (Sheridan et al., 1998). Interestingly, reports indicated that males and females also differ in their physiological response to stress (reviewed by Kajantie and Phillips 2006). This raised the possibility that sex differences in the prevalence of disease could at least in part be explained by sex differences in the nature of the physiological response to stress. Indeed, earlier studies from our lab suggested sex differences in the effects of neonatal stress on the response to an influenza viral infection (Avitsur et al., 2006; 2009). These studies indicated that males and females differ in the kinetics and degree of the hormonal, immune and behavioral response to the virus and its modulation by neonatal stress.
The present study sought to examine more thoroughly the differences in the responses of males and females to an influenza viral infection. In addition, since stress modulated various aspects of the response to infection, and males and females differ in their response to stress, the interaction between sex and stress in modulating the response to an influenza viral infection was examined. Three aspects of the response to viral infection were assessed. First, following infection or injury proinflammatory cytokines (interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α) were secreted from activated immune cells and played an important role in modulating the acute phase response (Julkunen et al., 2001). Thus, in the present study lung proinflammatory cytokines gene expression was determined. Second, studies have shown that the hypothalamic pituitary (HPA) axis played an important role in mediating the response to infection and stress. Thus, levels of the HPA hormone corticosterone were measured. Third, the secretion of proinflammatory cytokines during an immune response was associated with the induction of sickness behavior (Dantzer et al., 2007). Thus, in the present study aspects of the behavioral response to infection were assessed.
Materials And Methods
Animals
Subjects were male and female C57BL/6 mice (Charles River Inc., Wilmington, MA), aged 6-8 weeks. Animals were housed in an American Association for the Accreditation of Laboratory Animal Care (AAALAC) accredited facility. Males and females were housed in separate rooms. Mice were given free access to food and water and were maintained on a 12-hour light/dark cycle (lights on at 6 AM). Animal care procedures were approved by the Ohio State University Institutional Laboratory Animal Care And Use Committee (ILACUC).
Restraint stress (RST)
Individual mice were placed in well-ventilated 50-ml centrifuge tubes at 5:00 PM and removed at 9:00 AM (Hermann et al., 1994). To control for the lack of food and water during the time of RST, control mice were food and water-deprived (FWD) at the time of RST.
Measurement of Plasma Corticosterone
Plasma was frozen at −70°C until analysis. Corticosterone was quantified using the DA rat corticosterone kit (ICN Biomedicals, Costa Mesa, CA) according to the manufacturer’s instructions. Intra-assay and inter-assay variability for the kit are 7%. The detection limit of the assay was 25 ng/ml.
RNA Isolation and Real Time PCR
RNA was harvested from the lungs using the Trizol extraction method (GibcoBRL, Rockville, MD). Reverse transcription was carried out with a commercially available Promega kit (Madison, WI). Real Time PCR was carried out using a 7000 Sequence Detecting System as previously described (Ghoshal et al., 2001).
Primer Design and Concentration
Primers and probes specific for the indicated cytokines were developed using Primer Express Software from PE Biosystems (Foster City, CA.). The final concentration for the PCR reaction is 900 nM for the primers and 100 nM for the probe. Cytokine probes are labeled at the 5′ end with the reporter dye 6-carboxy-fluorescein (FAM) and the quencher dye 6-carboxy-tetramethyl-rhodamine (TAMRA) at the 3′ end. The 18S probe is labeled at the 5′ end with VIC and TAMRA at the 3′ end. Labeled probes are synthesized by PE Biosystems.
Virus and Infection
Influenza A/PR8 virus was grown in the allantoic fluid of embryonic eggs and the viral titer was determined by hemagglutination assay. Mice were infected intranasally with 0.05 ml of 0.064 HAU influenza A/PR8 virus diluted in saline. Prior to infection, mice were anesthetized by an intramuscular injection of a Ketamine/Xylazine solution (62.4/3.52 mg/kg) diluted in saline. Sham infected mice were anesthetized as described and administered with 0.05 ml of sterile saline intranasally.
Procedure
Experiment 1
Male and female mice underwent four overnight FWD or RST cycles. On the morning after the third cycle mice of each group (male/female; FWD/RST) were randomly assigned into non-infected (NI) and infected (INF) sub groups. NI mice underwent sham infection and INF mice were infected with influenza virus as described. Mice from all groups were sacrificed on days 5 or 9 post infection (pi) (n=5-8/group/day). Mice were decapitated and trunk blood was collected into heparinized tubes to assess plasma corticosterone levels. Lungs were collected from all mice to assess cytokine gene expression. Since there were no significant differences between NI controls of the same sex and stress treatment sacrificed on different days, data from these groups were collapsed.
Experiment 2
Illness and the secretion of proinflammatory cytokines are typically accompanied by behavioral symptoms such as reduced locomotion and exploratory activity, anorexia and anhedonia (Avitsur and Yirmiya, 1999; Dantzer et al., 2007). In the following experiment, sex and stress effects on sickness behavior were examined. Specifically, anorexia (body weight and food consumption) and anhedonia (preference for sweetened solution) were assessed in male and female mice following influenza viral infection and RST stress.
Male and female mice were individually housed at least 24 hours before the beginning of measurements during which they received a short exposure to saccharine solution (3M, Sigma, St. Louis, MO). Baseline measurements began four days prior to influenza infection. Mice were weighed, returned to their home cages and provided with weighed food, water and saccharine bottles. Mice, food pellets and drinking bottles were weighed again 24 hr later. Following baseline measurements, mice underwent three overnight FWD or RST cycles. On the morning after the third cycle mice were assigned into NI and INF sub groups. NI mice underwent sham infection and INF mice were infected with influenza virus as described (n=8-12/group). Following infection animals were weighed and returned to their home cage. Twentyfour hours after infection measurements resumed. Mice were weighed, and provided with weighed food, water and saccharine bottles. Similar to the baseline measurements, food and drinking bottles were weighed again every 24 hr for 9 days after infection. Occasionally food pellets were dropped by the animals or a bottle leaked; when this occurred, data for that measurement that day were discarded.
Statistical analysis
Experiment 1
Cytokine mRNA levels are expressed as the fold increase in expression of non-infected controls of the same sex and stress group. Cytokine mRNA, and plasma corticosterone levels were analyzed using a three-factor ANOVA with stress (FWD/RST), sex (male/female) and infection (NI/INF day 5/INF day 9) as between subject factors. In cases were significant interaction between at least two factors were revealed unpaired means comparisons were used to assess differences between experimental groups. Differences were considered significant at p<0.05.
Experiment 2
To control for individual differences in baseline measurements, data are presented as “difference from baseline” measurements. The effect of RST stress on body weight change prior to infection was examined by subtracting body weights measured after three cycles of FWD/RST from weights measured before the stress. The effect of influenza virus infection on body weight change was examined by subtracting weights measured on each day post infection from weights measured on the day of infection. Average food and fluid consumption of the three baseline days was calculated. Daily food and fluid consumption were subtracted from these average baseline measures. Data describing water consumption are not presented since mice consumed small amounts of water in the presence of the sweet solutions and no differences between experimental groups in water consumption was detected.
The effect of RST on body weight change prior to infection was analyzed using a two-way ANOVA with stress (FWD/RST) and sex (male/female) as between subject factors. Infection-induced changes in body weight, food and saccharin consumption data were analyzed using a repeated-measures three factor ANOVA with stress (FWD/RST), sex (male/female) and infection (NI/INF) as between subject factors. In cases were significant interactions between at least two factors were revealed unpaired means comparisons were used to assess differences between experimental groups. Differences were considered significant at p<0.05.
Results
Experiment 1
Corticosterone
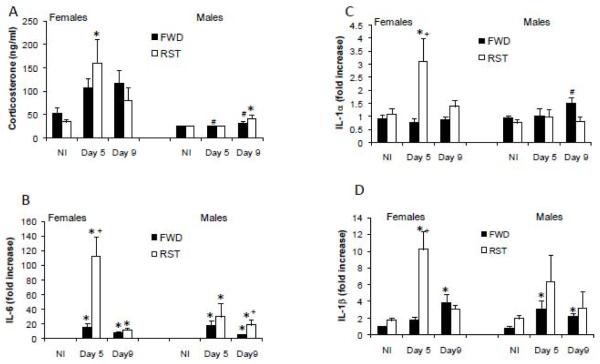
ANOVA revealed a significant main effect for infection (F(2,87)=3.7, p<0.05) and sex (F(1,87)=19.8, p<0.0001), and an interaction between the effects of infection and sex (F(2,87)=3.4, p<0.05) on circulating corticosterone levels. Corticosterone levels were elevated in infected RST females on day 5 pi, and in infected RST males on day 9 pi, compared to the corresponding NI group. Levels of corticosterone in infected females were significantly higher compared to infected males (Fig. 1A).
Fig. 1.
Effect of food and water deprivation (FWD) or restraint stress (RST) on influenza virus-induced corticosterone secretion (A), lung gene expression of IL-6 (B) IL-1α (C) and IL-1β (D) in non-infected (NI) and influenza A/PR8 virus infected (INF) male and female mice. Circulating corticosterone levels were determined using RIA on days 5 (Day 5) and 9 (Day 9) post infection. Changes in gene expression were assessed using real-time PCR. The fold-increase in gene expression levels were determined by comparing the levels of cytokine expression to lungs harvested from sham-infected controls of the same group. Data are presented as mean ± SEM.
* Significantly different from corresponding NI group (same sex and stress manipulation) (p<0.05).
+ Significantly different from corresponding FWD group (same sex and day of infection) (p<0.05).
# Significantly different from corresponding female group (same stress manipulation and day of infection) (p<0.05).
Interleukin-6 gene expression
ANOVA performed on levels of interleukin (IL)-6 gene expression revealed a significant main effect for stress, sex (F(1,87)=11.2, 4, respectively, p<0.005) and infection (F(2,87)=17.3, p<0.0001), an interaction between the effects of infection and stress, between the effects of infection and sex and between sex and stress, and an interaction between the effects of sex, stress and infection (F(2,87)=7.5, 4.6, 4, 5.5, respectively, p<0.05).
Unpaired comparisons revealed that levels of IL-6 mRNA were significantly elevated in infected males and females (day 5 and 9 pi) compared to NI. In addition, in RST females, levels of IL-6 mRNA on day 5 pi were significantly higher that that on day 9 pi. Furthermore, RST significantly increased IL-6 mRNA levels compared to FWD. This effect was evident on day 5 pi in females, and on day 9 pi in males (Fig. 1B).
Interleukin-1α gene expression
ANOVA performed on levels of IL-1α gene expression revealed an interaction between sex and stress, and between infection and stress (F(2,87)=8.9 and 3.3 respectively, p<0.05). Unpaired comparisons revealed that in females RST elevated IL-1α expression compared to FWD-infected as well as NI on day 5 pi. RST had no effect on IL-1α gene expression in males (Fig. 1C).
Interleukin-1β gene expression
ANOVA on levels of IL-1β gene expression revealed a significant main effect for stress (F(1,82)=11.5, p<0.005) and infection (F(2,82)=11.9, p<0.0001), as well as an interaction between stress and infection (F(2,82)=7, p<0.005). Unpaired comparisons revealed that infection significantly elevated IL-1β gene expression in FWD males on days 5 and 9 pi, and in FWD females on day 9 pi compared to the corresponding NI group. In addition, in RST females infection elevated IL-1β gene expression on day 5 pi compared to NI, and RST significantly elevated IL-1β mRNA in females on day 5 pi compared to FWD (Fig. 1D).
Experiment 2
Body weight
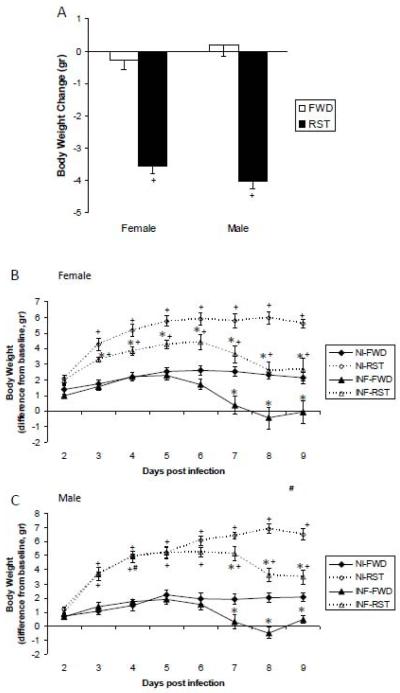
ANOVA performed on body weight changes following three cycles of FWD/RST (prior to infection) revealed a significant main effect for stress (F(1,76) = 172.146, p<.0001), indicating that RST mice lost more weight compared to FWD mice (Fig. 2A). ANOVA performed on body weight change following infection revealed a significant main effect for stress and infection (F(1,504)= 221.8, 35.1, respectively, p<0.0001), an interaction between the effects of stress and time of measurement, infection and time of measurement, sex and time of measurement (F(7,504)= 33.4, 34.1, 4.7, respectively, p<0.0001), and between sex, stress and time of measurement (F(7,504)= 2.4, p<0.05) (Fig 2B and 2C).
Fig. 2.
(A) Body weight change in response to three overnight food and water deprivation (FWD) or restraint stress (RST) cycles in male and female mice. Animals were weighed prior to the beginning of the experiment, and again after three nights of FWD or RST (prior to infection). “Body weight change” was calculated by subtracting weights measured after three cycles of FWD/RST from weights measured before the stress. Data are presented as mean ± SEM body weight change. (B-C) Body weight change following influenza A/PR8 viral infection in animals recovering from FWD/RST. Following RST stress mice typically show a gradual increase in body weight. Thus, Animals were weighed on the day of infection (after termination of FWD/RST), and again every 24 hr for 9 days. The effect of influenza virus infection on body weight change was examined by subtracting weights measured on each day post infection from weights measured on the day of infection. Data representing body weight change in non-infected (NI) and influenza A/PR8 virus infected (INF) female (B) and male (C) mice are presented as mean ± SEM difference from body weight measured on the day of infection
* Significantly different from corresponding non-infected (NI) group (same sex, stress manipulation and day of infection) (p<0.05).
+ Significantly different from corresponding FWD group (same sex, infection manipulation and day of infection) (p<0.05).
# Significantly different from corresponding female group (same stress and infection manipulations and day of infection) (p<0.05).
Unpaired comparisons revealed a gradual weight gain on days 3-9 pi in RST males and females compared to FWD, probably compensating for body weight loss during RST. A/PR8 viral infection induced a significant body weight loss in all groups. In FWD males and females and in RST males infection-induced body weight loss was evident on days 7-9 pi. In RST females, the kinetics of the effect was faster as infection-induced body weight loss was evident beginning on day 3 pi and continued throughout all days of measurement.
Food consumption
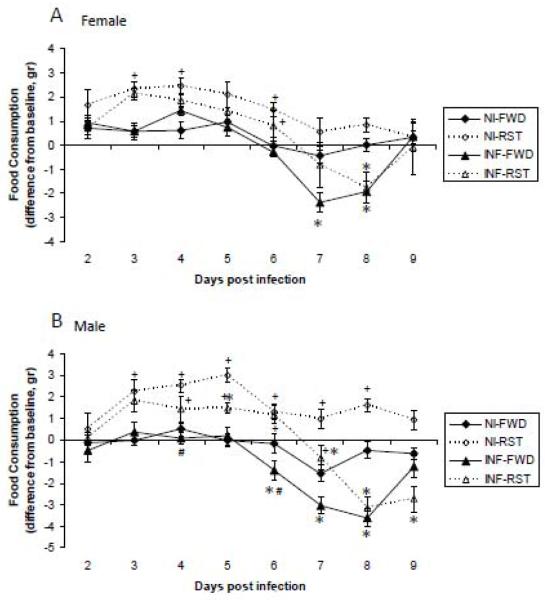
ANOVA revealed a significant main effect for stress, infection and sex (F(1,364)= 35.2, 24.2, 7.1, respectively, p<0.05) an interaction between the effects of stress and time of measurement, infection and time of measurement, sex and time of measurement (F(7,364)= 15, 57.9, 7.2, respectively, p<0.05), and between sex, infection and time of measurement (F(7,364)= 2.4, p<0.05) on food consumption.
Unpaired comparisons revealed that food consumption was increased by RST, and decreased by infection in a sex depended manner. In FWD animals, infection induced anorexia on days 7-8 pi in females and on days 6-9 in males. In RST mice, infection induced anorexia on day 8 pi in females and on days 5 and 7-9 in males. In addition, significant sex differences in food consumption were noted on days 4 and 6 pi. On these days, infected FWD females consumed more food compared the corresponding male group (Fig. 3).
Fig. 3.
Effect of food and water deprivation (FWD) or restraint stress (RST) on influenza A/PR8 virus-induced anorexia in non-infected (NI) and influenza virus infected (INF) female (A) and male (B) mice. Data are presented as mean ± SEM difference from baseline measurements.
* Significantly different from corresponding non-infected (NI) group (same sex, stress manipulation and day of infection) (p<0.05).
+ Significantly different from corresponding FWD group (same sex, infection manipulation and day of infection) (p<0.05).
# Significantly different from corresponding female group (same stress and infection manipulations and day of infection) (p<0.05).
Saccharin consumption
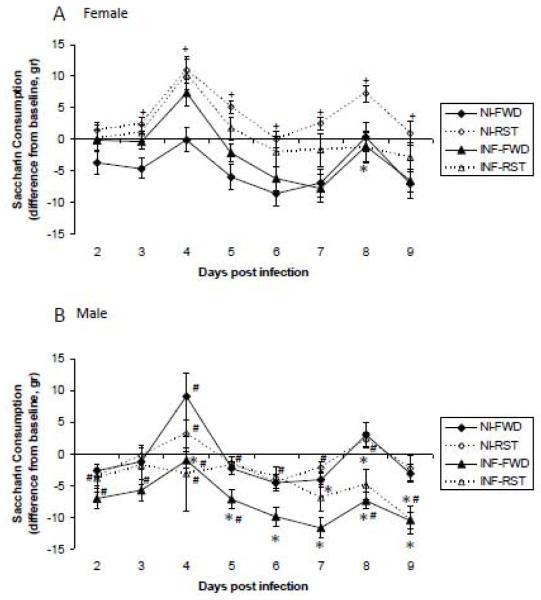
ANOVA revealed a significant interaction between the effects of stress and time of measurement and infection and time of measurement (F(7,322)=2.7, 7.7, respectively, p<0.05), and between sex, infection and time of measurement (F(7,322)= 2.3, p<0.05).
Unpaired comparisons revealed that saccharin consumption was increased by RST, and decreased by infection in a sex dependent manner. In FWD animals, infection reduced saccharin consumption on day 8 pi in females and on days 4-9 in males. In RST mice, infection reduced saccharin consumption on days 7-9 in males, while it had no effect in females. In addition, there were significant sex differences in saccharine consumption. In NI-RST mice, males consumed less saccharin compared to females on days 2 and 4-8 pi. In INF-FWD, males consumed less saccharin compared to females on days 2-5 and 8 pi. On day 9 pi, INF-RST males consumed less saccharin compared to the corresponding female group (Fig. 4).
Fig. 4.
Effect of food and water deprivation (FWD) or restraint stress (RST) on influenza A/PR8 virus-induced changes in saccharine consumption in non-infected (NI) and influenza virus infected (INF) female (A) and male (B) mice. Data are presented as mean ± SEM difference from baseline measurements.
* Significantly different from corresponding non-infected (NI) group (same sex, stress manipulation and day of infection) (p<0.05).
+ Significantly different from corresponding FWD group (same sex, infection manipulation and day of infection) (p<0.05).
# Significantly different from corresponding female group (same stress and infection manipulations and day of infection) (p<0.05).
Discussion
It is well recognized that susceptibility to infectious diseases is modulated by complex interactions between environmental and biological factors. The present study sought to examine the role of sex and stress in mediating the response to an influenza A/PR8 viral infection. Male and female mice underwent repeated cycles of RST stress, followed by A/PR8 viral infection. Following these manipulations, the hormonal, immune and behavioral responses to infection were examined.
Male and female mice were infected with a low dose of A/PR8 virus. This infectious dose of A/PR8 virus was found to induce a mild increase in lung proinflammatory cytokine expression and circulating corticosterone levels. In addition, it induced moderate symptoms of sickness behavior peaking around day 7-8 post infection (Avitsur et al., 2006, 2009). Sex differences were revealed in several aspects of the response to the A/PR8 virus. First, the kinetics of lung IL-1β mRNA expression was faster in FWD infected males compared to females. Increased expression of IL-1β appeared in infected males on day 5 pi and remained elevated on day 9 pi while in infected females the increase in IL-1β appeared later - on day 9 pi. Second, male showed faster kinetics and augmented behavioral response to infection. The data revealed that anorexia and reduced saccharin consumption began earlier and symptoms were more pronounced in infected males than in females. Furthermore, circulating corticosterone levels were elevated in infected females, but not in males (Avitsur and Yirmiya, 1999; Yee and Prendergast, 2010). The low corticosterone response in males may have been due to the attenuated infection model used in this study. This mild infection may have been insufficient to induce a notable corticosterone response in males. Alternatively, corticosterone secretion may have peaked in males at different time point than those measured in this study (Avitsur et al., 2006). To sum up, males mount a faster IL-1β response to the viral infection, accompanied by more severe behavioral symptoms and blunted corticosterone response compared to females. To the best of our knowledge, most studies of the mechanism of the host response to influenza virus infection were conducted on males (Bonneau et al., 2007; Sheridan et al., 1998). Only recently, have we begun to examine the response of infected females and to compare selected aspects of the response to males (Avitsur et al., 2006; 2009). Accumulating data suggested that the kinetics and severity of the response of females differ significantly from males. These findings may have important implication for the understanding of health and disease in women.
Although the association between the behavioral, immune and endocrine responses to infection was not examined directly in this study, a possible link may be suggested. Studies have shown that proinflammatory cytokines secretion at early stages of the inflammatory process induced symptoms of sickness behavior (Dantzer 2006, Dantzer et al., 2007). Accordingly, it may be suggested that the enhanced IL-1β response observed in infected males was involved in the augmented behavioral response to infection. Furthermore, it was demonstrated that corticosterone down-regulated the effects of proinflammatory cytokines and suppressed sickness behavior (Dantzer 2006, Johnson et al., 1996). Thus, the increase in infection-induced corticosterone levels demonstrated in females may have suppressed the behavioral symptoms of infection.
In the present study, mice underwent repeated overnight RST stress prior to infection, allowing the examination of the effects of stress on the response to an influenza viral infection. The results indicated that the response to the infection was altered by RST in a sex-dependent manner. Expression of proinflammatory cytokines in the lungs was augmented by RST in both infected males and females, however this effect was more pronounced in females compared to males. Specifically, in infected males and females RST elevated lung IL-6 expression. RST-induced increase in IL-6 was evident in infected males later (day 9 pi) than in females (day 5 pi). In addition, expression of lung IL-1α and IL-1β was elevated by RST in infected females but not in males.
The current findings indicate that RST had no effect on circulating corticosterone levels of influenza infected animals. The lack of corticosterone response to RST was probably because several days had elapsed between the end of stress and hormone measurements, as stress-induced increase in corticosterone secretion was found to cease earlier (Sheridan et al., 1998).
The data further showed that the kinetics and severity of influenza virus-induced sickness behavior were modulated by sex and RST. Prior to infection, repeated cycles of RST induced a significant body weight loss. Following termination of stress a gradual weight gain accompanied by an increase in food and saccharin consumption was observed. The recovery process from stress altered some of the symptoms of sickness behavior in infected animals. Interestingly, whilst some of the symptoms appeared enhanced in RST mice, other symptoms were attenuated by RST. For instance, in infected males, anorexia was evident earlier in the RST group and lasted longer (day 5-9 pi) than in FWD group (day 6-8 pi). In females, on the other hand, anorexia appeared later in RST (day 8 pi) than in FWD mice (day 7-8 pi). Together these data demonstrated that RST enhanced the hormonal and cytokine response to influenza virus infection and modulated the behavioral symptoms of illness.
Studies have shown that variations in the timing of experimental stressors relative to the inflammatory response can influence the impact on immunity. Stress initiated prior to and continued after the immunological challenge will most likely be immunosuppressive. The onset of a stressor after the inflammatory response has begun was less likely to be immunomodulatory (Dobbs et al., 1993; Hermann et al., 1995). In the present study, stress began prior to immune challenge and terminated soon after infection. Thus, animals developed an inflammatory response to infection while recovering from stress. The data indicated that many aspects of the response to infection were altered in animals recovering from a stressor. The multifaceted mechanisms involved in mediating the interactions between inflammatory and neuroendocrine factors triggered by stress recovery and influenza infection are yet to be determined.
Health and well-being are influenced by a complex interaction between environmental demands and the individual’s capacity to cope with these demands. Understanding the origin and underlying mechanisms of individual differences in diseases vulnerability is one of the major challenges of current medical research. Accumulating studies, including the present report, indicated that whether one is male or female is an important factor in mediating the response to stress and infectious disease. Although sex differences in morbidity have been reported widely, health research has frequently overlooked sex differences and even fully omitted one sex, usually females (Lahelma et al., 1999). Studies on women’s health have generally focused on problems which concern exclusively or disproportionally women, such as reproductive health (Lahelma et al., 1999). In recent years, an increasing number of health studies has compared disease susceptibility of males and females, and begun to reveal the causes and mechanisms of these sex differences. It is now becoming apparent that sex differences in health are the result of complex interactions between biological, psychological, behavioral and social factors (Lahelma et al., 1999). Additional studies are needed in order to elucidate the characteristics of these interactions and their implications for the development of individual differences in health and disease.
Research Highlights for Avitsur et al., “ Sex Differences In The Response To Influenza Virus Infection: Modulation By Stress”.
IL-1β mRNA was expressed in lungs of A/PR8 infected males earlier than in females
Circulating corticosterone was elevated in A/PR8 infected females, but not in males.
Sickness behavior was more severe in A/PR8 infected males than in females.
Stress enhanced proinflammatory cytokine expression in A/PR8 infected mice.
Stress altered the kinetics of sickness behavior in A/PR8 infected mice.
Acknowledgments
The authors would like to thank Nir Eyal, Hagit Furst and Jacqui Verity for excellent technical help. This study was supported by: NIH grants NIMH/RO1MH046801-18 and NIDCR/T32DE014320-8 to JFS, The Israel Science Foundation to RA (1053/04), The United States-Israel Binational Science Foundation to RA and JFS (2003105) and NIDCR/F30 DE017068 Individual Predoctoral Dental Scientist Fellowship to JWM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander J, Stimson WH. Sex hormones and the course of parasitic infection. Parasitol. Today. 1988;4:189–193. [Google Scholar]
- Avitsur R, Donchin O, Barak O, Cohen E, Yirmiya R. Behavioral effects of interleukin-1 beta: modulation by gender, estrus cycle, and progesterone. Brain Behav. Immun. 1995;9(3):234–41. doi: 10.1006/brbi.1995.1022. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Hunzeker J, Sheridan JF. Role of early stress in the individual differences in host response to viral infection. Brain, Behav. Immun. 2006;20(4):339–348. doi: 10.1016/j.bbi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Sheridan JF. Neonatal stress modulates sickness behavior. Brain, Behav. Immun. 2009;23(7):977–985. doi: 10.1016/j.bbi.2009.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitsur R, Yirmiya R. The immunobiology of sexual behavior: gender differences in the suppression of sexual activity during illness. Pharmacol. Biochem. Behav. 1999;64(4):787–96. doi: 10.1016/s0091-3057(99)00165-3. [DOI] [PubMed] [Google Scholar]
- Bailey M, Engler H, Hunzeker J, Sheridan JF. The hypothalamic-pituitary-adrenal axis and viral infection. Viral Immunol. 2003;16(2):141–157. doi: 10.1089/088282403322017884. [DOI] [PubMed] [Google Scholar]
- Barna M, Komatsu T, Bi Z, Reiss CS. Sex differences in susceptibility to viral infection of the central nervous system. J. Neuroimmunol. 1996;67:31–39. doi: 10.1016/0165-5728(96)00022-7. [DOI] [PubMed] [Google Scholar]
- Bender BS, Small PA., Jr. Heterotypic immune mice lose protection against influenza virus infection with senescence. J, Infect, Dis. 1993;168(4):873–880. doi: 10.1093/infdis/168.4.873. [DOI] [PubMed] [Google Scholar]
- Bonneau RH, Padgett DA, Sheridan JF. Twenty years of psychoneuroimmunology and viral infections in Brain, Behavior, and Immunity. Brain Behav. Immun. 2007;21(3):273–280. doi: 10.1016/j.bbi.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Butcher AR, Palethorpe HM, Grove DI. Effects of sex and age on the susceptibility of C57BL/6J mice to infection with Brachylaima cribbi and the course of infection in NOD SCID mice. Parasitol, Res. 2002;88(7):668–674. doi: 10.1007/s00436-002-0642-3. [DOI] [PubMed] [Google Scholar]
- CDC Novel H1N1 Flu The 2009 H1N1 Pandemic: Summary Highlights, April 2009-April 2010. http://www.cdc.gov/h1n1flu/cdcresponse.htm#. Updated: June 16, 2010.
- Chitnis AS, Truelove SA, Druckenmiller JK, Heffernan RT, Davis JP. Epidemiologic and clinical features among patients hospitalized in Wisconsin with 2009 H1N1 influenza A virus infections, April to August 2009. WMJ. 2010;109(4):201–208. [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Neurol. Clin. 2006;24(3):441–460. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Bluthe R, Castanon N, Kelley KW, Konsman JP, Laye S, Lestage J, Parnet P. Cytokines, sickness behavior and depression. In: Ader R, editor. Psychoneuroimmunology. Elsevier; 2007. pp. 281–318. [Google Scholar]
- Dobbs CM, Vasquez M, Glaser R, Sheridan JF. Mechanisms of stress-induced modulation of viral pathogenesis and immunity. J Neuroimmunol. 1993;48(2):151–160. doi: 10.1016/0165-5728(93)90187-4. [DOI] [PubMed] [Google Scholar]
- Ghoshal K, Majumder S, Zhu Q, Hunzeker J, Datta J, Shah M, Sheridan JF, Jacob ST. InXuenza virus infection induces metallothionein gene expression in the mouse liver and lung by overlapping but distinct molecular mechanisms. Mol Cell Biol. 2001;24:8301–8317. doi: 10.1128/MCB.21.24.8301-8317.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Lundberg P, Tanamachi B, Openshaw H, Longmate J, Cantin E. Gender influences herpes simplex virus type 1 infection in normal and gamma interferon-mutant mice. J. Virol. 2001;75(6):3048–3052. doi: 10.1128/JVI.75.6.3048-3052.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hermann G, Beck FM, Sheridan JF. Stress-induced glucocorticoid response modulates mononuclear cell trafficking during an experimental influenza viral infection. J, Neuroimmunol. 1995;56(2):179–186. doi: 10.1016/0165-5728(94)00145-e. [DOI] [PubMed] [Google Scholar]
- Hermann G, Tovar CA, Beck FM, Sheridan JF. Kinetics of glucocorticoid response to restraint stress and/or experimental influenza viral infection in two inbred strains of mice. J. Neuroimmunol. 1994;49(1-2):25–33. doi: 10.1016/0165-5728(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Johnson RW, Propes MJ, Shavit Y. Corticosterone modulates behavioral and metabolic effects of lipopolysaccharide. Am. J. Physiol. 1996;270:R192–R198. doi: 10.1152/ajpregu.1996.270.1.R192. [DOI] [PubMed] [Google Scholar]
- Julkunen I, Sareneva T, Pirhonen J, Ronni T, Melen K, Matikainen S. Molecular pathogenesis of influenza A virus infection and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 2001;12:171–80. doi: 10.1016/s1359-6101(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DIW. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe R, Kelley K, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol. Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Knoblich A, Gortz J, Harle-Grupp V, Falke D. Kinetics and genetics of herpes simplex virus-induced antibody formation in mice. Infect. Immun. 1983;39:15–23. doi: 10.1128/iai.39.1.15-23.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegelenberg CF, Irusen EM, Cooper R, Diacon AH, Taljaard JJ, Mowlana A, von Groote-Bidlingmaier F, Bolliger CT. High mortality from respiratory failure secondary to swine-origin influenza A (H1N1) in South Africa. QJM. 2010;103(5):319–325. doi: 10.1093/qjmed/hcq022. [DOI] [PubMed] [Google Scholar]
- Konstantinos AP, Sheridan JF. Stress and influenza viral infection: modulation of proinflammatory cytokine responses in the lung. Respir. Physiol. 2001;128(1):71–77. doi: 10.1016/s0034-5687(01)00266-3. [DOI] [PubMed] [Google Scholar]
- Lahelma E, Martikainen P, Rahkonen O, Silventoinen K. Gender differences in illhealth in Finland: Patterns, magnitude and change. Soc. Sci. Ned. 1999;48:7–19. doi: 10.1016/s0277-9536(98)00285-8. [DOI] [PubMed] [Google Scholar]
- Ludwig S, Planz O, Pleschka S, Wolff T. Influenza-virus-induced signaling cascades: targets for antiviral therapy? Trends Mol. Med. 2003;9(2):46–52. doi: 10.1016/s1471-4914(02)00010-2. [DOI] [PubMed] [Google Scholar]
- Meagher MW, Sieve AN, Johnson RR, Satterlee D, Belyavskyi M, Mi W, Prentice TW, Welsh TH, Jr., Welsh CJR. Neonatal maternal separation alters immune, endocrine, and behavioral responses to acute Theiler’s virus infection in adult mice. Behav. Genet. 2010;40:233–249. doi: 10.1007/s10519-010-9333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noymer A. The 1918 influenza pandemic affected sex differentials in mortality: Comment on Sawchuk. Am J Phys Anthropol. 2010 doi: 10.1002/ajpa.21405. DOI: 10.1002/ajpa.21405. [DOI] [PubMed] [Google Scholar]
- Roriz-Cruz M, Rosset I, Montero-Odasso M. Lower mortality from H1N1 influenza in older Argentineans: men more affected. J Am Geriatr Soc. 2010;58(9):1813–1815. doi: 10.1111/j.1532-5415.2010.03045.x. [DOI] [PubMed] [Google Scholar]
- Sheridan JF, Dobbs C, Jung J, Chu X, Konstantinos A, Padgett D, Glaser R. Stress-induced neuroendocrine modulation of viral pathogenesis and immunity. Ann N. Y. Acad. Sci. 1998;840:803–808. doi: 10.1111/j.1749-6632.1998.tb09618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webby RJ, Webster RG. Are we ready for pandemic influenza? Science. 2003;302(5650):1519–1522. doi: 10.1126/science.1090350. [DOI] [PubMed] [Google Scholar]
- Yee JR, Prendergast BJ. Sex-specific social regulation of inflammatory responses and sickness behaviors. Brain Behav, Immun. 2010;24(6):942–51. doi: 10.1016/j.bbi.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Avitsur R, Donchin O, Cohen E. Interleukin-1 inhibits sexual behavior in female but not in male rats. Brain Behav. Immun. 1995;9(3):220–33. doi: 10.1006/brbi.1995.1021. [DOI] [PubMed] [Google Scholar]
- Zuk M, McKean K. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 1996;26:1009–1024. [PubMed] [Google Scholar]