Introduction
Parkinson’s disease (PD) is a neurodegenerative movement disorder characterized by the loss of dopaminergic nigrostriatal neurons leading to bradykinesia, tremor and muscle rigidity. These symptoms have been attributed to the loss of dopamine, which subsequently leads to pathophysiological alterations in the basal ganglia and its intricate connections to the rest of the brain.
The classic rate model of PD basal ganglia dysfunction proposed that the foundational pathophysiological symptom was the asymmetry between the direct and indirect striatal output pathways caused by dopamine depletion (Albin et al. 1989; DeLong 1990). The original rate model was subsequently modified to include additional details of neuronal firing pattern changes and oscillatory activity in the basal ganglia (Hammond et al. 2007; Arbuthnott and Garcia-Munoz 2009). Additional refinements including the notion of a hyperdirect pathway and reciprocal connectivity between various basal ganglia structures have been recently added. For current reviews, see (Hammond et al. 2007; Galvan and Wichmann 2008; Weinberger et al. 2009). Even after the refinements, the original model of the direct and indirect basal ganglia pathways and their alterations in PD remains a major working model for PD pathophysiology.
The models of normal and parkinsonian basal ganglia function have been supported by many electrophysiological studies. In the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) lesioned primate model, Bergman and colleagues demonstrated an increase in STN firing rate from normal to parkinsonian state (Bergman et al. 1994), and a similar increase in SNR neuronal firing rate (Wichmann et al. 1999). Bergman and colleagues also showed that the primate STN and SNR became more bursty after MPTP treatment (Bergman et al. 1994; Wichmann et al. 1999). The same increases of rate and burstiness in STN and SNR have been confirmed in the lesioned rat model of PD (Breit et al. 2001; Breit et al. 2006; Breit et al. 2007). The synchronized oscillations represented by the local field potentials (LFPs) have also been shown to have characteristic patterns in the normal and parkinsonian state. Brown and colleagues showed that normal rats have a LFP peak in the 70 Hz region, similar to human PD patients medicated with levodopa (LD) and undergoing intraoperative recordings (Brown et al. 2001; Brown et al. 2002). Mallet and colleagues showed that anesthetized hemiparkinsonian rats showed a prominent beta LFP peak in the activated state which normal rats did not have (Mallet et al. 2008). Together, these and other animal studies demonstrate that the neurophysiological properties of STN and SNR are altered in response to nigrostriatal denervation and dopamine depletion.
While no studies to date have examined the effects of chronic LD therapy on STN or SNR neuronal firing rates or patterns, the effects of acute dopamine agonist therapy have been examined in the human STN. Recordings in PD patients undergoing functional neurosurgery for deep brain stimulation show that apomorphine increases burst discharges in the STN but does not alter firing rate at optimal doses. However, apomorphine decreases STN firing rates in the dyskinetic state (Lozano et al. 2000). Also, the prominent beta peak in STN LFPs has been shown to be greatly reduced when the patient is acutely given apomorphine or LD (Brown et al. 2001; Giannicola et al. 2010).
Although these studies in PD patients have contributed to our understanding of the neurophysiological properties of these structures, in clinical studies it is not practical for patients to be off anti-PD medications for an extended period. Clinical studies have shown that greater than 2 weeks of washout from LD is necessary to remove beneficial effects of LD therapy in PD patients (Fahn 2005). Such long washouts are not well tolerated by patients and have potential risk of significant morbidity and mortality (Newman et al. 2009). Therefore, conducting electrophysiological studies in PD patients in the complete “off” state devoid of all influences of LD or other dopaminergic medications is virtually impossible.
To overcome these difficulties, we designed a study to evaluate the effects of chronic intermittent LD treatments at optimal doses in the MPTP treated stable hemiparkinsonian monkey model of PD. We focused on the STN because of its important role in the indirect pathway of the corticostriatal-thalamocortical loop, and on the SNR because of its importance in mediating both motor and nonmotor symptoms of PD.
Materials and methods
Two adult female Macaca mulatta were housed according to standards set forth in the NIH ‘Guide for the Care and Use of Laboratory Animals’. All procedures were carried out in strict compliance with the “Principles of Laboratory Animal Care” (NIH Publication No. 86-23, revised 1985) and were approved by the local institutional animal care and use committee.
Assessing parkinsonism and chronic LD therapy
Detailed information on our model and behavioral paradigm can be found elsewhere (Lieu et al. 2010). Briefly, monkeys were assessed behaviorally using a primate parkinsonism rating scale (from 0, normal, to 100, severely parkinsonian) which was modeled after Part III of the Unified Parkinson's Disease Rating Scale (mUPDRS) (Subramanian et al. 2010). Ratings were performed by a blinded investigator and were spaced at least three days apart.
Intra-carotid injections of MPTP were administered to render the monkeys hemiparkinsonian (HP) on the right side of their body. Both monkeys received an initial dose of 0.5mg/kg MPTP, followed by two weeks of observation. If the original dose did not result in a stable unilateral HP state, repeated doses were given. The final result was a HP state which was stable for more than six months.
Once behavioral stability was documented, recording chambers were surgically implanted to permit chronic single cell extracellular neuronal recording from the left STN and SN in the stable HP state and on chronic LD treatment. LD/carbidopa (CD) therapy was initiated at an oral dose of 100mg/25mg twice a day and gradually escalated by 100mg/25mg every 72 hours until no further improvement in mUPDRS scores was observed. This was defined to be the optimal dose and was subsequently held constant throughout the dosing period. Serum dopamine levels were measured in a single monkey to check that dopamine was being successfully delivered, showing dopamine concentrations of 1.022 ng/mL before starting LD dosing and 17.04 ng/mL while on optimal LD dosing. Occasionally, the monkeys would refuse oral intake (defined by missing three successive doses). When this occurred, injectable benserazide and methyl ester of LD (25/100) were injected SQ/IM. At the point of stable mUPDRS score improvement and steady twice daily LD/CD dosing for at least one week, extracellular neuronal recording resumed. Dyskinetic activity was not seen on the stable dosing regimen (Lieu et al. 2010).
Electrophysiology
All recordings were done in awake, behaving animals. Wakefulness was monitored by eye blink reflex and responsiveness of animal to investigators while in the restraint chair. Animals were trained to allow passive limb movements by experimenters in order to examine somatotopic responses during recordings (Starr et al. 2000). Extracellular single cell recordings were carried out using glass coated platinum-iridium microelectrodes (impedance 0.5–1.0 Mega-ohms, FHC) or tungsten microelectrodes (0.5–2.0 Mega-ohms, FHC). The STN and SNR nuclei were systematically sampled, recording each neuron encountered at the target depth range, with tracts typically separated laterally from each other by 1 mm. The electrical signal was amplified (MDA-4I BAK or ISO-80 WPI), filtered (200–10,000 Hz, Krohn-Hite), monitored on an audio loudspeaker, and displayed on a digital oscilloscope to ensure good signal isolation. The signals were digitally sampled at 25000 samples per second (Spike2, CED). Simultaneously, LFPs were filtered (3–500Hz) and digitized at 1000 samples per second.
Localization within the nuclei was confirmed in five ways. First, the depth of the electrode tip was correlated to a rhesus brain atlas. Second, firing characteristics of landmarks in the brain were monitored as reported previously (Starr et al. 2000). Third, on some tracts, after a neuron had been recorded for at least 60 seconds for later analysis, somatotopic responses were examined by flexing and extending the monkey’s arm or leg during the recording (for example, see supplement Figure S2). Fourth, recording tracts were histologically confirmed in the STN and SNR. Fifth, the root mean square (RMS) was calculated on the activity recorded along each track. RMS has been used clinically to determine the borders of STN, as the overall activity in STN is higher than that superior and inferior to STN (Moran et al. 2006; Snellings et al. 2009).
Data analysis
During offline analysis, interspike intervals (ISIs) were generated using Spike2’s template matching spike sorting algorithm. Neuron sorting and isolation was further refined using principal component analysis on the spike waveforms. In each case, records were comprised of at least 400 spikes and had duration between 60 and 120 seconds.
In addition to firing rates, seven measures of the firing patterns were employed. First, the coefficient of variation (CV) of the ISIs was computed for each recording. A low CV indicates a regularly firing cell. Second, the burst index was computed as the mean of the ISI distribution divided by the mode of the ISI distribution (Hutchison et al. 1997). A higher burst index indicates a cell that tends to fire in bursts. Third and fourth, the percent of spikes in bursts and percentage of time in bursts were calculated using the Poisson-surprise method (Legendy and Salcman 1985). Bursts were confirmed if their Poisson surprise value was greater than or equal to 3 (Aldridge and Gilman 1991; Wichmann and Soares 2006). This provided a sliding time-window view of the burstiness of each spike train, a different perspective than the other burstiness metrics that process the entire recording at once. Fifth, the density discharge histogram (DDH) (Kaneoke and Vitek 1996) of each recording was computed and compared to the DDH of a random Poisson spiketrain. If the DDH of the recording was not significantly different from the Poisson DDH by a chi-square test, the firing pattern was classified as ‘Poisson’. If the pattern was significantly different, the pattern was classified as ‘regular’ or ‘bursty’ depending on whether the variance of the DDH was less than one or greater than one, respectively (Levy et al. 2001). Sixth, the range of the DDH was computed. Bursty neurons had a larger DDH range because some bins contained large numbers of spikes. Seventh, the sample entropy was computed as a measure of spike randomness. The sample entropy is based on the approximate entropy measure introduced by Pincus, and is modified to reduce estimation bias (Pincus 1991; Richman and Moorman 2000). We used an embedding dimension of 2 and a tolerance of 0.2 times the standard deviation, based on empirical optimization and on previous literature. Darbin and colleagues have reported nonlinear features of monkey basal ganglia neuronal firing and Lafreniere-Roula and colleagues have recently reported an entropy reduction in STN recordings from human PD patients following apomorphine treatment (Lafreniere-Roula et al. 2010; Darbin et al. 2006). Thus we chose this additional metric to examine whether our chronic LD treatment might have a similar effect. The seven numeric firing pattern metrics were compared using the Wilcoxon-Mann-Whitney rank-sum test, and the categorical DDH classification was compared using Fisher’s 2×2 exact test (grouping “Poisson” and “bursty” categories together).
Spectra were generated from LFP signals using the Welch periodogram method, using 512-point discrete Fourier transforms with 128-point overlap windows. The periodograms were normalized using the power in the 65–80 Hz band to eliminate any bias due to 60Hz line noise. The spectral power was then summed in 5-Hz bins. Sums were compared in each frequency band between the On-LD and Off-LD conditions using an unpaired two-sided T-test. The centroids of power spectral density (PSD) segments cropped between 10–50Hz were computed to test for spectral power shifts in this region. The centroids were compared using an unpaired two-sided T-test. The spike-triggered average (STA) of the LFP signal was computed and compared to the STA of a randomly shuffled version of the same ISI sequence. A rank-sum test was used to compare the ratio of the peak-to-trough difference of the STA to the peak-to-trough difference of the randomized STA.
Results
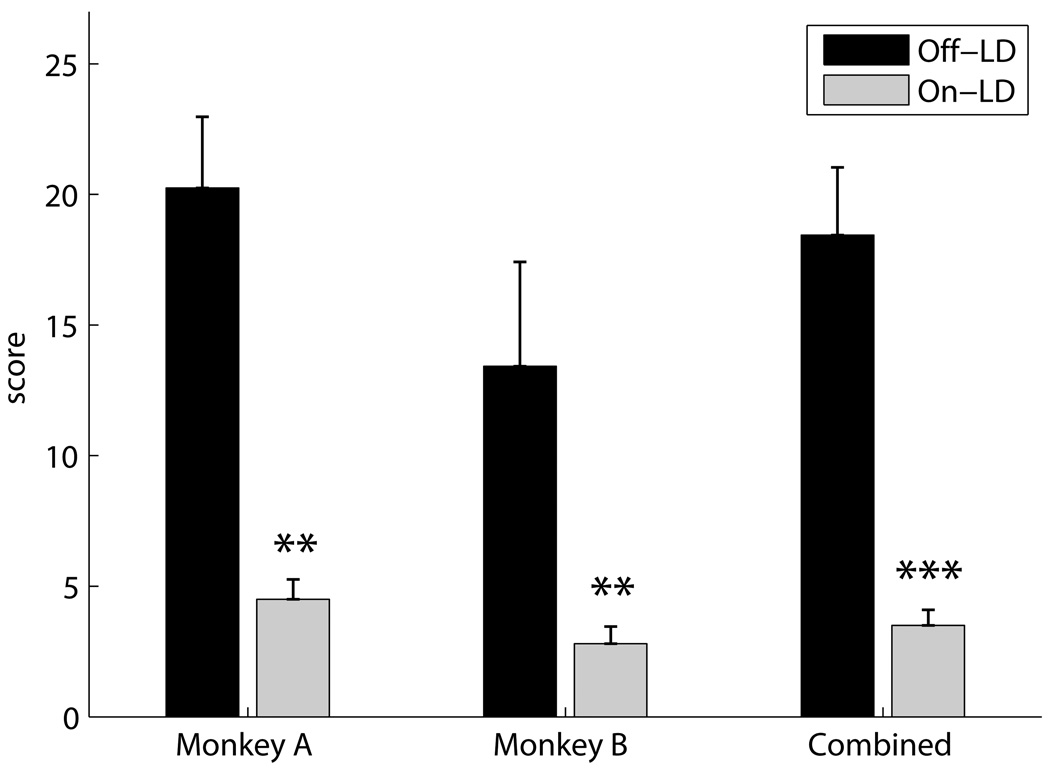
Chronic LD treatment substantially improved parkinsonism in both monkeys without causing drug induced dyskinesias. The mUPDRS score significantly decreased in the LD-treated state (77.7% for Monkey A and 79.1% for Monkey B, p < 0.001 unpaired two-sided T-test on pooled data, Figure 1).
Figure 1.
mUPDRS behavioral scores for each monkey separately and the pooled scores (** p < 0.01, *** p < 0.001, two-sided unpaired T-test). Black columns represent tests taken during off-LD periods (Monkey A: N=6, Monkey B: N=6, Combined: N=12), and gray columns represent tests taken during on-LD periods (Monkey A: N=3, Monkey B: N=5, Combined: N=8).
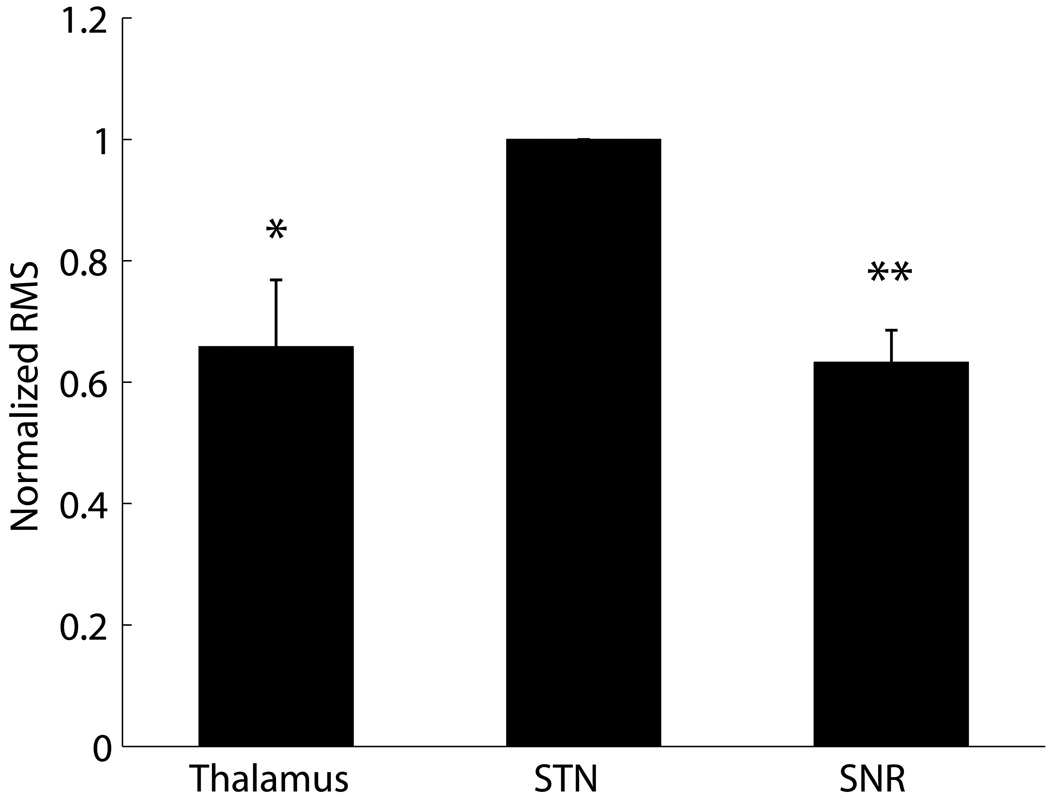
During microelectrode recordings, we calculated the RMS of the activity recorded along multiple tracks, one of many measures that allowed us to determine location within the various nuclei. The RMS increased as the electrode traveled out of the thalamus and into STN, and decreased upon exiting STN and entering SNR. Figure 2 shows normalized RMS values of recordings in thalamus, STN and SNR.
Figure 2.
Normalized root-mean-square (RMS) power in neuronal recordings from nuclei at different depths. Each tract was normalized by the STN power. A rank-sum test was performed to test the difference between the Thalamus and STN recordings and between SNR and STN recordings (N=7 tracks, * p < 0.05, ** p < 0.01).
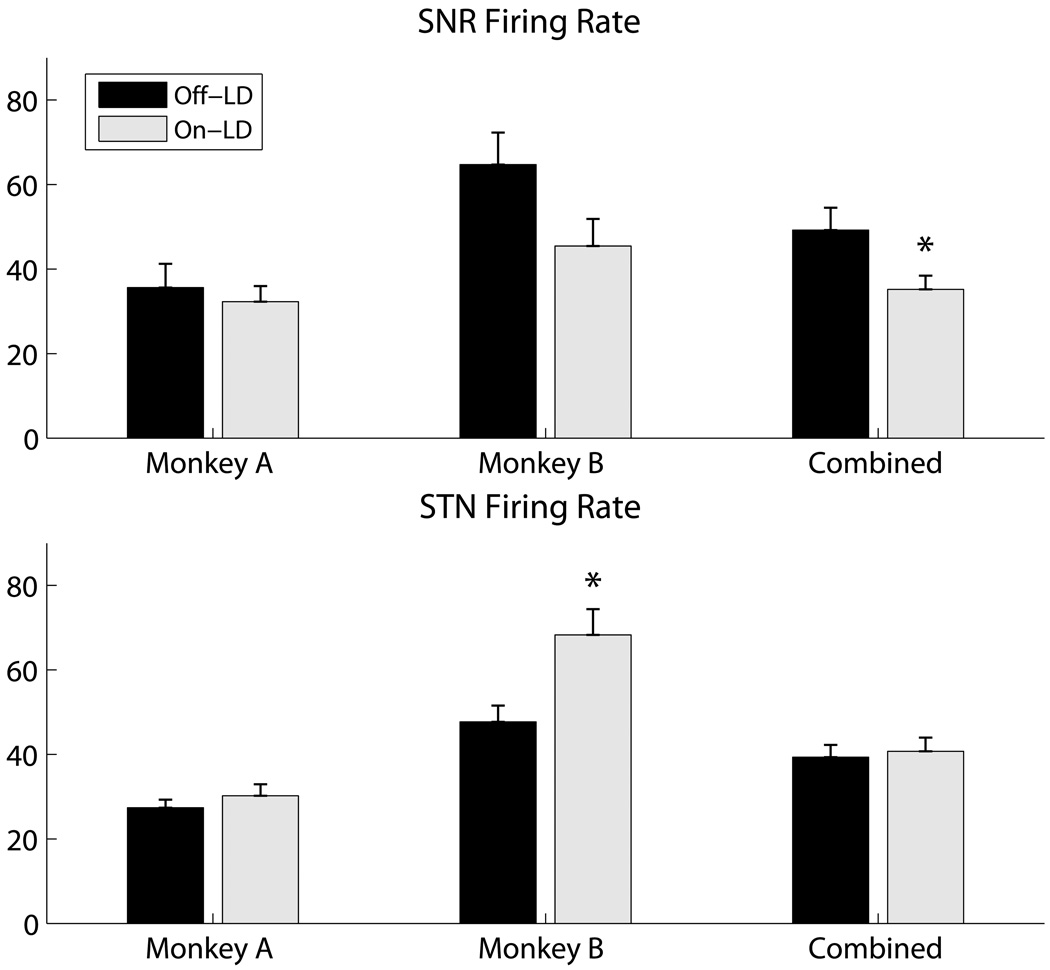
From the microelectrode recordings, we examined the firing rate and firing pattern of neurons in both nuclei before and after LD treatment. Although Monkey B showed an increase in STN firing rate, the pooled mean STN firing rate (± SEM) showed no significant change (42.6 ± 3.5 Hz to 41.3 ± 3.3 Hz, Figure 3). The SNR pooled mean firing rate on LD decreased (52.1 ± 5.7 Hz to 36.2 ± 3.3 Hz, p<0.013, Figure 3). In both the STN and the SNR Monkey B showed a larger effect than Monkey A, but both monkeys showed the same trends.
Figure 3.
Effects of LD on neuronal firing rates. Top and bottom rows: firing rates of SNR and STN neurons, respectively (mean ± SEM). Black columns represent data recorded before LD treatment (SNR: N=30; STN: N=50), and gray columns represent data recorded during LD treatment (SNR: N=39; STN: N=76) (* p < 0.05, t-test).
The quantification of the firing patterns did not show a significant difference in most measures in either nucleus (Table 1). The STN did not show any trend toward increasing or decreasing burstiness. The SNR showed a trend toward an increase in burstiness in all measures of burstiness, and this trend was statistically significant in the DDH range (p < 0.05).
Table 1.
Firing pattern metrics (mean ± SEM) comparing the SNR and STN in the off-LD and on-LD conditions.
| SNR | STN | |||
|---|---|---|---|---|
| Off-LD | On-LD | Off-LD | On-LD | |
| CV | 0.80 ± 0.06 | 0.86 ± 0.06 | 0.96 ± 0.06 | 0.95 ± 0.05 |
| Burst Index | 4.93 ± 1.42 | 9.17 ± 1.71 | 3.74 ± 0.68 | 3.23 ± 0.33 |
| Sample Entropy | 1.48 ± 0.07 | 1.58 ± 0.06 | 1.46 ± 0.63 | 1.48 ± 0.45 |
| DDH Range | 4.60 ± 0.21 | 5.44 ± 0.26* | 4.87 ± 0.19 | 4.95 ± 0.14 |
| Percent of time in bursts | 0.51 ± 0.16 | 1.08 ± 0.29 | 1.36 ± 0.34 | 1.26 ± 0.23 |
| Percent of spikes in bursts | 2.05 ± 0.64 | 5.4 ± 1.61 | 4.96 ± 1.28 | 4.84 ± 0.91 |
| Poisson DDH pattern classification (number of neurons) | Regular: 28 | Regular: 39 | Regular: 29 | Regular: 58 |
| Poisson: 1 | Poisson: 4 | Poisson: 5 | Poisson: 10 | |
| Bursty: 1 | Bursty: 7 | Bursty: 5 | Bursty: 8 | |
Only the DDH range showed a significant difference in SNR, with increased burstiness in the LD-treated condition (* p < 0.05, rank-sum test).
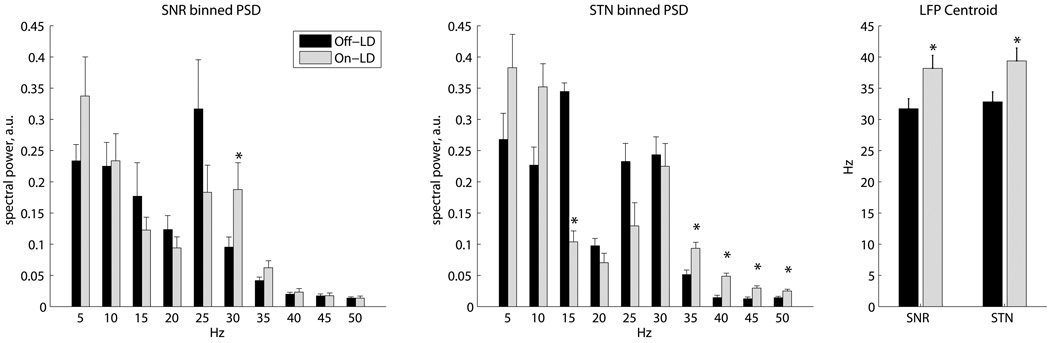
Local field potentials were also analyzed from the neural recordings. LFP spectra in SNR showed a decrease in the low beta frequencies (20–25 Hz) and an increase in higher beta frequencies (30–35 Hz) on LD (Figure 4). In the STN, there was a similar shift of spectral power into higher frequency bands, seen as a reduction in the 15–20 Hz band and an increase in the 35–55 Hz band on LD. The centroids of spectral power in the 10–50 Hz band were significantly higher in both the STN and SNR (p < 0.05). No significant differences in the spike-triggered averages were seen between the on-LD and off-LD groups (for more details, see online supplement, Figure S1).
Figure 4.
LD effects on spectral power of local field potentials (Off-LD SNR: N=28; On-LD SNR: N=16; Off-LD STN: N=36; On-LD STN: N=22). Left and center: power spectral densities (PSDs) were computed using Welch periodograms, normalized using the power in the 65–80Hz band, and summed into 5-Hz bins. These graphs display the normalized power in each bin, labeled at the lower edge of each bin, with SE errorbars (* p < 0.05, two-sided unpaired T-test). Right: centroids of PSD segments cropped between 10–50Hz to focus on the changes in this region (* p < 0.05, two-sided unpaired T-test).
Discussion
In this study we have focused on the firing rates and patterns in the STN and SNR in hemiparkinsonian rhesus nonhuman primates in the stable parkinsonian state and when exposed to chronic intermittent LD treatment, in order to mimic the clinical scenario of LD administration in PD patients.
Firing Rates
The firing rates we observed in the parkinsonian state are consistent with what has been reported in MPTP-treated primates and PD patients. Neuronal discharge rates in the parkinsonian state range from 26–50 Hz in the STN, and 45–86 Hz in the SNR (Bergman et al. 1994; Hutchison et al. 1998; Wichmann et al. 1999; Bejjani et al. 2000; Levy et al. 2000; Magnin et al. 2000; Theodosopoulos et al. 2003; Wichmann and Soares 2006).
The pooled mean STN firing rate did not show a significant change on LD. This fits with a previous report in human PD patients which showed that apomorphine did not change the STN firing rate in the human (Lozano et al. 2000; Levy et al. 2001). However, this result does not fit the rate model, which predicts that amelioration of PD symptoms would correlate with a reduced STN firing rate. Our result also contrasts with a previous study which showed a reduction in STN firing rate after apomorphine treatment (Kreiss et al. 1997). However, LD has important differences from apomorphine. apomorphine can act upon dopamine receptors anywhere, whereas LD mediates its effect only through surviving dopaminergic neural pathways. This may explain the difference.
In contrast to the STN, the firing rate of the SNR decreased significantly on LD. This result is similar to a previous report of acute apomorphine treatment in the normal primate demonstrating significant SNR decreases of firing rate but no significant changes in firing pattern after apomorphine treatment (Nevet et al. 2004). Our result is also similar to the report by Park and colleagues that dopamine D1 receptor agonist SKF38393 and D2 receptor agonist Quinpirole reduced the SNR firing rates (Park et al. 2007).
One possible explanation for why the effects of chronic LD treatment and acute dopamine agonist treatment are similar in the SNR but different in the STN would be that based on the classic rate model of Albin and Delong, chronic LD treatment in the parkinsonian state affects the direct pathway more than the indirect pathway (Albin et al. 1989; DeLong 1990).
Another possible explanation may be related to better preservation of the SNpc-SNR connectivity than the SNpc-STN connectivity in these animals. Surviving dopaminergic connections from the SNpc would putatively enable exogenous LD mediated dopamine conversion and synaptic release into the SNR that would mimic the effects of apomorphine acting directly on dopamine receptors located in the SNR. Supporting this notion, Prescott and colleagues recently showed that in the SNR of human patients, LD increased stimulation-evoked plasticity (Prescott et al. 2009). They noted in passing that LD decreased SNR firing rates, but they did not systematically examine this decrease. Our results confirm their firing rate observation in a systematic way in the primate model, and further suggest that treatment-induced increases in available dopamine may have a larger effect on SNR firing rates than on STN firing rates in the parkinsonian condition. This may be due to a specific role of the small dopaminergic connection between the substantia nigra pars compacta (SNC) and the SNR in regulating neuronal firing (Kliem et al. 2007).
Firing Patterns
Previous studies have shown that cells in the primate STN and SNR become more bursty after MPTP-induced parkinsonism (Bergman et al. 1994; Wichmann et al. 1999; and Wichmann and Soares 2006). In our study we did not try to replicate this well-documented change between normal and parkinsonian monkeys, but instead focused on the difference between the parkinsonian state off-LD and on-LD.
In the STN, we did not observe any significant changes in the bursting spike patterns of cells from the LD treatment. This contrasts with previous studies that showed that apomorphine increased the burstiness (Levy et al. 2001) and reduced the entropy (Lafreniere-Roula et al. 2010) of STN neurons. This may again illustrate the difference between LD and dopamine agonists. Apomorphine directly acts upon the dopamine receptors, so it exerts its effect whether or not the normal dopamine pathways are operational. LD requires the operation of remaining dopaminergic neurons in physiological dopamine pathways to be converted to dopamine and exert its effect.
In the SNR there was a slight trend toward increasing burstiness on LD, which was significant in one out of the seven firing pattern measures we examined. This lack of reduction of the burstiness fits with the finding by Boraud and colleagues that the neuronal firing patterns in the related GPi nucleus do not become less bursty on LD (Boraud et al. 1998). Although our study design prevents conclusions about the “normalizing” effect of LD or lack thereof, we clearly demonstrate that in both the SNR and STN, LD did not reduce the bursting patterns that previous studies have associated with parkinsonism.
Local Field Potentials
Our LFP measurements show that LD decreased the STN LFP power in the 15–20 Hz band and increased power in the 35–55 Hz band. This fits with similar previous literature reports (Gatev et al. 2006; Hammond et al. 2007; Galvan and Wichmann 2008). Similarly, Sharott and colleagues showed that apomorphine delivery reduced the beta LFP peak in awake behaving rats (Sharott et al. 2005). In humans, several studies have shown that LD reduces the amplitude of the LFP beta peak in patients undergoing deep brain stimulator implants (Brown et al. 2001; Alonso-Frech et al. 2006; Kuhn et al. 2006; Giannicola et al. 2010; Lopez-Azcarate et al. 2010). Some human studies have reported seeing a peak in the high gamma band (60–90 Hz) on LD (Brown et al. 2001; Lopez-Azcarate et al. 2010), but we did not see any significant increase in the LFP power above 60 Hz (data not shown). It is possible that the increase we observed in low gamma band activity (35–55 Hz) in the STN may be a primate analogue to the human and rodent high gamma band increases (Hammond et al. 2007). Another explanation is suggested by the report by Alonso-Frech and colleagues that high gamma band activity was increased during dyskinesias in human patients (Alonso-Frech et al. 2006). The fact that our monkeys did not become dyskinetic may explain why we did not see an increase in STN high gamma activity.
To our knowledge, the current study is the first to examine the effects of LD on SNR LFPs in the primate. Our results show that LD treatment reduces SNR low beta LFP activity and increases the high beta LFP activity, similar to the changes in the STN. Like the STN, we did not see increases in SNR high gamma activity (above 60 Hz, data not shown). We did not see specific SNR increases in the 35–55 Hz power in 5-Hz bins on LD, but the centroid of the LFP spectral power in the 10–50 Hz band was shifted significantly higher in both the SNR and STN. Thus, overall, the synchronous synaptic input to the SNR changed along with the STN input in response to LD, although perhaps not quite as robustly. The differences in the STN LFP may be due to the direct cortical input pathway to the STN, which has recently been shown by optogenetic experiments to be highly important in the activation of basal ganglia pathways (Gradinaru et al. 2009).
Although the electrophysiological properties of the STN and SNR in parkinsonism have been studied, these properties are likely to be a combination of both the motor and nonmotor functions of these nuclei. Future studies are necessary to evaluate the electrophysiological properties of the STN and SNR in basal ganglia nonmotor circuits and subsequent physiological alterations as a result of dopamine depletion and dopamine replacement therapy.
Conclusion
In summary, our results show that chronic intermittent LD therapy in hemiparkinsonian rhesus nonhuman primates that mimics the clinical scenario of LD administration in PD patients reduces neuronal firing rates in the SNR but does not reduce the bursty firing patterns in the STN and SNR. We also corroborate previous studies showing that LD reduces the LFP spectral power in the low beta band and increases the power in the high beta band. LD is known to ameliorate motor symptoms of PD such as rigidity and akinesia, as well as some nonmotor symptoms. However, long term use of LD is known to be associated with disabling side effects such as dyskinesias and refractory nonmotor PD symptoms. Our results suggest that future research is warranted on the basal ganglia neuronal bursting activity and its relationship with the long term symptomatic effects of levodopa treatment.
Research Highlights
Chronic intermittent levodopa therapy improves parkinsonism and reduces firing rates in the SNR but not in the STN in MPTP-treated macaques.
Chronic intermittent levodopa therapy does not reduce neuronal bursting in the SNR or STN in the non-human primate model of Parkinson's disease
Chronic intermittent levodopa therapy in optimal doses does not change neuronal firing entropy in SNR or STN in hemiparkinsonian monkeys.
Supplementary Material
Acknowledgements
This research was funded in part by the NIH NINDS RO1NS42402, HRSA DIBTH0632, PA Tobacco Settlement Funds Biomedical Research Grant, PSUHMC Movement Disorders Brain Repair Fund, NCCAMR21 AT001607 to Thyagarajan Subramanian, and GRSA to Tim Gilmour via PA Tobacco Settlement Funds (the Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions).
We thank Thomas Wichmann, MD (Emory University) and Michele Kliem, MS (Emory University) for their advice, technical help in the initial stages of the recording studies, and assistance with the burst detection algorithms. We thank Gurunathan Murugesan, PhD (Cleveland Clinic Foundation) and the Cleveland Clinic Laboratory staff for their assistance with the LD plasma measurements.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albin RL, et al. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12(10):366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Aldridge JW, Gilman S. The temporal structure of spike trains in the primate basal ganglia: afferent regulation of bursting demonstrated with precentral cerebral cortical ablation. Brain Res. 1991;543(1):123–138. doi: 10.1016/0006-8993(91)91055-6. [DOI] [PubMed] [Google Scholar]
- Alonso-Frech F, et al. Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson's disease. Brain. 2006;129(Pt 7):1748–1757. doi: 10.1093/brain/awl103. [DOI] [PubMed] [Google Scholar]
- Arbuthnott G, Garcia-Munoz M. Dealing with the devil in the detail - some thoughts about the next model of the basal ganglia. Parkinsonism Relat Disord. 2009;15 Suppl 3:S139–S142. doi: 10.1016/S1353-8020(09)70801-6. [DOI] [PubMed] [Google Scholar]
- Bejjani BP, et al. Bilateral subthalamic stimulation for Parkinson's disease by using three-dimensional stereotactic magnetic resonance imaging and electrophysiological guidance. J Neurosurg. 2000;92(4):615–625. doi: 10.3171/jns.2000.92.4.0615. [DOI] [PubMed] [Google Scholar]
- Bergman H, et al. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72(2):507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Boraud T, et al. Effects of L-DOPA on neuronal activity of the globus pallidus externalis (GPe) and globus pallidus internalis (GPi) in the MPTP-treated monkey. Brain Res. 1998;787(1):157–160. doi: 10.1016/s0006-8993(97)01563-1. [DOI] [PubMed] [Google Scholar]
- Breit S, et al. Unilateral lesion of the nigrostriatal pathway induces an increase of neuronal activity of the pedunculopontine nucleus, which is reversed by the lesion of the subthalamic nucleus in the rat. Eur J Neurosci. 2001;14(11):1833–1842. doi: 10.1046/j.0953-816x.2001.01800.x. [DOI] [PubMed] [Google Scholar]
- Breit S, et al. Effects of 6-hydroxydopamine-induced severe or partial lesion of the nigrostriatal pathway on the neuronal activity of pallido-subthalamic network in the rat. Exp Neurol. 2007;205(1):36–47. doi: 10.1016/j.expneurol.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Breit S, et al. Lesion of the pedunculopontine nucleus reverses hyperactivity of the subthalamic nucleus and substantia nigra pars reticulata in a 6-hydroxydopamine rat model. Eur J Neurosci. 2006;24(8):2275–2282. doi: 10.1111/j.1460-9568.2006.05106.x. [DOI] [PubMed] [Google Scholar]
- Brown P, et al. Oscillatory local field potentials recorded from the subthalamic nucleus of the alert rat. Exp Neurol. 2002;177(2):581–585. doi: 10.1006/exnr.2002.7984. [DOI] [PubMed] [Google Scholar]
- Brown P, et al. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J Neurosci. 2001;21(3):1033–1038. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbin O, et al. Nonlinear analysis of discharge patterns in monkey basal ganglia. Brain Res. 2006;1118(1):84–93. doi: 10.1016/j.brainres.2006.08.027. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13(7):281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Fahn S. Does levodopa slow or hasten the rate of progression of Parkinson's disease? J Neurol. 2005;252 Suppl 4:IV37–IV42. doi: 10.1007/s00415-005-4008-5. [DOI] [PubMed] [Google Scholar]
- Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119(7):1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatev P, et al. Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov Disord. 2006;21(10):1566–1577. doi: 10.1002/mds.21033. [DOI] [PubMed] [Google Scholar]
- Giannicola G, et al. The effects of levodopa and ongoing deep brain stimulation on subthalamic beta oscillations in Parkinson's disease. Exp Neurol. 2010;226(1):120–127. doi: 10.1016/j.expneurol.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, et al. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324(5925):354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, et al. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci. 2007;30(7):357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, et al. Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson's disease. Ann Neurol. 1998;44(4):622–628. doi: 10.1002/ana.410440407. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, et al. Effects of apomorphine on globus pallidus neurons in parkinsonian patients. Annals of Neurology. 1997;42(5):767–775. doi: 10.1002/ana.410420513. [DOI] [PubMed] [Google Scholar]
- Kaneoke Y, Vitek JL. Burst and oscillation as disparate neuronal properties. J Neurosci Methods. 1996;68(2):211–223. doi: 10.1016/0165-0270(96)00081-7. [DOI] [PubMed] [Google Scholar]
- Kliem MA, et al. Activation of nigral and pallidal dopamine D1-like receptors modulates basal ganglia outflow in monkeys. J Neurophysiol. 2007;98(3):1489–1500. doi: 10.1152/jn.00171.2007. [DOI] [PubMed] [Google Scholar]
- Kreiss DS, et al. The response of subthalamic nucleus neurons to dopamine receptor stimulation in a rodent model of Parkinson's disease. J Neurosci. 1997;17(17):6807–6819. doi: 10.1523/JNEUROSCI.17-17-06807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, et al. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson's disease. Eur J Neurosci. 2006;23(7):1956–1960. doi: 10.1111/j.1460-9568.2006.04717.x. [DOI] [PubMed] [Google Scholar]
- Lafreniere-Roula M, et al. Apomorphine reduces subthalamic neuronal entropy in parkinsonian patients. Exp Neurol. 2010;225(2):455–458. doi: 10.1016/j.expneurol.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol. 1985;53(4):926–939. doi: 10.1152/jn.1985.53.4.926. [DOI] [PubMed] [Google Scholar]
- Levy R, et al. Effects of apomorphine on subthalamic nucleus and globus pallidus internus neurons in patients with Parkinson's disease. J Neurophysiol. 2001;86(1):249–260. doi: 10.1152/jn.2001.86.1.249. [DOI] [PubMed] [Google Scholar]
- Levy R, et al. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci. 2000;20(20):7766–7775. doi: 10.1523/JNEUROSCI.20-20-07766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu CA, et al. Dyskinesias do not develop after chronic intermittent levodopa therapy in clinically hemiparkinsonian rhesus monkeys. Parkinsonism and Related Disorders. 2010 doi: 10.1016/j.parkreldis.2010.10.010. DOI: 10.1016/j.parkreldis.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Azcarate J, et al. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson's disease. J Neurosci. 2010;30(19):6667–6677. doi: 10.1523/JNEUROSCI.5459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano AM, et al. Neuronal recordings in Parkinson's disease patients with dyskinesias induced by apomorphine. Ann Neurol. 2000;47(4) Suppl 1:S141–S146. [PubMed] [Google Scholar]
- Magnin M, et al. Single-unit analysis of the pallidum, thalamus and subthalamic nucleus in parkinsonian patients. Neuroscience. 2000;96(3):549–564. doi: 10.1016/s0306-4522(99)00583-7. [DOI] [PubMed] [Google Scholar]
- Mallet N, et al. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci. 2008;28(18):4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A, et al. Real-time refinement of subthalamic nucleus targeting using Bayesian decision-making on the root mean square measure. Mov Disord. 2006;21(9):1425–1431. doi: 10.1002/mds.20995. [DOI] [PubMed] [Google Scholar]
- Nevet A, et al. Discharge rate of substantia nigra pars reticulata neurons is reduced in non-parkinsonian monkeys with apomorphine-induced orofacial dyskinesia. J Neurophysiol. 2004;92(4):1973–1981. doi: 10.1152/jn.01036.2003. [DOI] [PubMed] [Google Scholar]
- Newman EJ, et al. The parkinsonism-hyperpyrexia syndrome. Neurocrit Care. 2009;10(1):136–140. doi: 10.1007/s12028-008-9125-4. [DOI] [PubMed] [Google Scholar]
- Park YS, et al. Lesion of subthalamic nucleus in parkinsonian rats : effects of dopamine d(1) and d(2) receptor agonists on the neuronal activities of the substantia nigra pars reticulata. J Korean Neurosurg Soc. 2007;42(6):455–461. doi: 10.3340/jkns.2007.42.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci U S A. 1991;88(6):2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott IA, et al. Levodopa enhances synaptic plasticity in the substantia nigra pars reticulata of Parkinson's disease patients. Brain. 2009;132(Pt 2):309–318. doi: 10.1093/brain/awn322. [DOI] [PubMed] [Google Scholar]
- Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278(6):H2039–H2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Sharott A, et al. Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur J Neurosci. 2005;21(5):1413–1422. doi: 10.1111/j.1460-9568.2005.03973.x. [DOI] [PubMed] [Google Scholar]
- Snellings A, et al. Identification of the subthalamic nucleus in deep brain stimulation surgery with a novel wavelet-derived measure of neural background activity. J Neurosurg. 2009;111(4):767–774. doi: 10.3171/2008.11.JNS08392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr PA, et al. Electrophysiological localization of the substantia nigra in the parkinsonian nonhuman primate. J Neurosurg. 2000;93(4):704–710. doi: 10.3171/jns.2000.93.4.0704. [DOI] [PubMed] [Google Scholar]
- Subramanian T, et al. Detection of MPTP-induced substantia nigra hyperechogenicity in Rhesus monkeys by transcranial ultrasound. Ultrasound Med Biol. 2010;36(4):604–609. doi: 10.1016/j.ultrasmedbio.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosopoulos PV, et al. Locations of movement-related cells in the human subthalamic nucleus in Parkinson's disease. Mov Disord. 2003;18(7):791–798. doi: 10.1002/mds.10446. [DOI] [PubMed] [Google Scholar]
- Weinberger M, et al. Pathological subthalamic nucleus oscillations in PD: can they be the cause of bradykinesia and akinesia? Exp Neurol. 2009;219(1):58–61. doi: 10.1016/j.expneurol.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Wichmann T, et al. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp Brain Res. 1999;125(4):397–409. doi: 10.1007/s002210050696. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Soares J. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J Neurophysiol. 2006;95(4):2120–2133. doi: 10.1152/jn.01013.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.