Abstract
The role of hydrophobicity in the attachment of enteropathogens to gastrointestinal mucosa is controversial. In vitro binding of Escherichia coli RDEC-1 to rabbit intestine is dependent on the expression of pili. We examined in vitro adherence of piliated RDEC-1 after altering either the hydrophobicity of the organisms, the hydrophobicity of the substrate for attachment, or the surface tension of the suspending liquid. Hydrophobicity of RDEC-1 was determined using four complementary methods. In each assay piliated RDEC-1 demonstrated relatively more hydrophobic properties compared with both organisms grown to suppress pilus expression and a mutant that cannot express mannose-resistant pili. When piliated RDEC-1 were pretreated with tetramethyl urea to disrupt hydrophobic bonds surface hydrophobicity decreased. Concurrently, bacterial adherence to rabbit ileal microvillus membranes, mucus and mucin was reduced. Binding of piliated organisms to hydrophobic surfaces was significantly higher compared to both nonpiliated bacteria and the adherence of piliated RDEC-1 to relatively hydrophilic surfaces. Addition of propanol reduced the surface tension of the suspending liquid, and decreased adhesion of piliated RDEC-1 to polystyrene by 80%. Conversely, adherence of piliated organisms to a hydrophilic surface increased 12-fold after lowering the surface tension of the suspending liquid. We conclude that hydrophobic properties have a role in mediating in vitro adherence of this E. coli enteric pathogen.
Full text
PDF


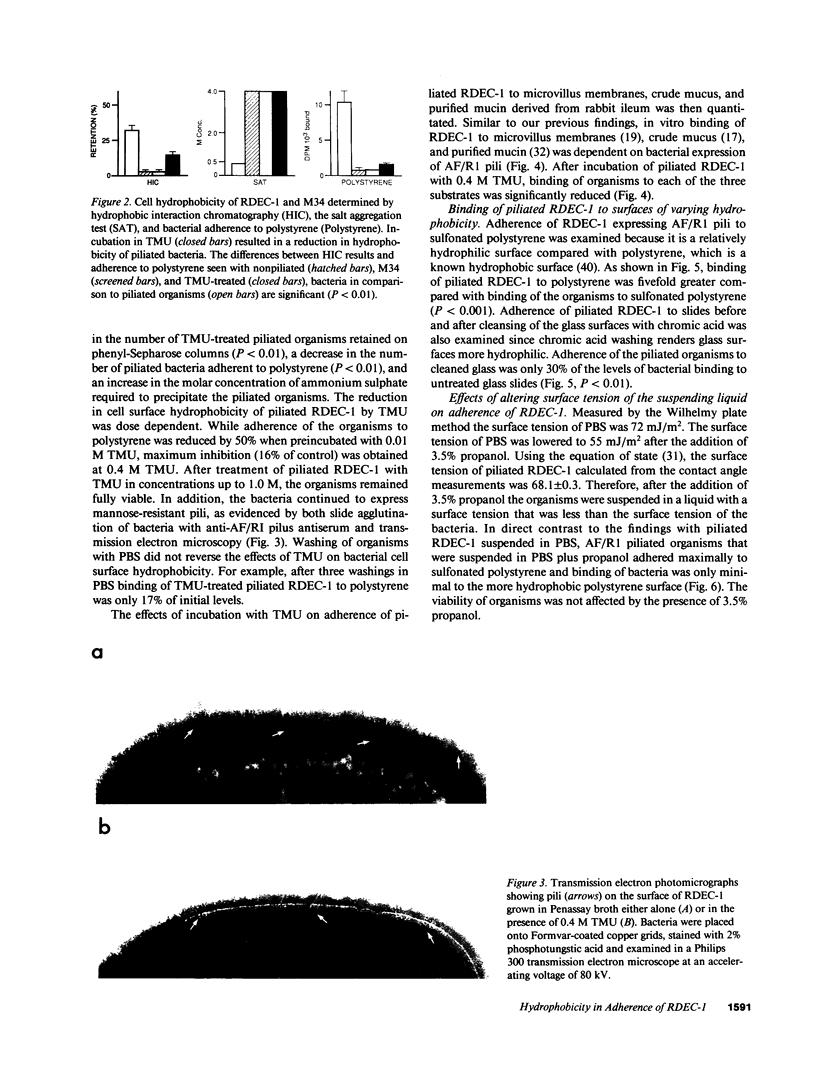
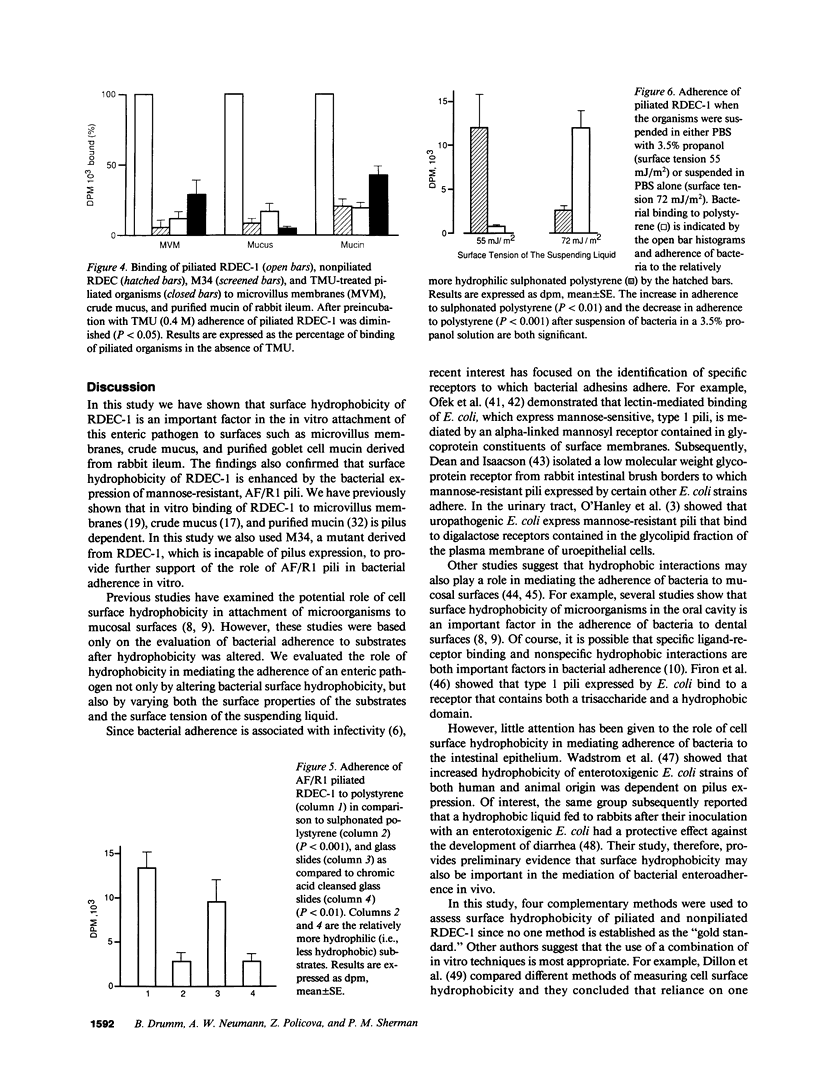


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Absolom D. R., Lamberti F. V., Policova Z., Zingg W., van Oss C. J., Neumann A. W. Surface thermodynamics of bacterial adhesion. Appl Environ Microbiol. 1983 Jul;46(1):90–97. doi: 10.1128/aem.46.1.90-97.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Berendson R., Cheney C. P., Schad P. A., Boedeker E. C. Species-specific binding of purified pili (AF/R1) from the Escherichia coli RDEC-1 to rabbit intestinal mucosa. Gastroenterology. 1983 Oct;85(4):837–845. [PubMed] [Google Scholar]
- Boedeker E. C. Enterocyte adherence of Escherichia coli: its relation to diarrheal disease. Gastroenterology. 1982 Aug;83(2):489–492. [PubMed] [Google Scholar]
- Borkman R. F., Hibbard L. B., Dillon J. The photolysis of tryptophan with 337.1 nm laser radiation. Photochem Photobiol. 1986 Jan;43(1):13–19. doi: 10.1111/j.1751-1097.1986.tb05585.x. [DOI] [PubMed] [Google Scholar]
- Cheney C. P., Formal S. B., Schad P. A., Boedeker E. C. Genetic transfer of a mucosal adherence factor (R1) from an enteropathogenic Escherichia coli strain into a Shigella flexneri strain and the phenotypic suppression of this adherence factor. J Infect Dis. 1983 Apr;147(4):711–723. doi: 10.1093/infdis/147.4.711. [DOI] [PubMed] [Google Scholar]
- Dean E. A., Isaacson R. E. Purification and characterization of a receptor for the 987P pilus of Escherichia coli. Infect Immun. 1985 Jan;47(1):98–105. doi: 10.1128/iai.47.1.98-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm B., Roberton A. M., Sherman P. M. Inhibition of attachment of Escherichia coli RDEC-1 to intestinal microvillus membranes by rabbit ileal mucus and mucin in vitro. Infect Immun. 1988 Sep;56(9):2437–2442. doi: 10.1128/iai.56.9.2437-2442.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firon N., Ofek I., Sharon N. Carbohydrate-binding sites of the mannose-specific fimbrial lectins of enterobacteria. Infect Immun. 1984 Mar;43(3):1088–1090. doi: 10.1128/iai.43.3.1088-1090.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I. Comparative hydrophobicities of oral bacteria and their adherence to salivary pellicles. Infect Immun. 1983 Sep;41(3):1190–1196. doi: 10.1128/iai.41.3.1190-1196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindahl M., Faris A., Wadström T., Hjertén S. A new test based on 'salting out' to measure relative surface hydrophobicity of bacterial cells. Biochim Biophys Acta. 1981 Nov 5;677(3-4):471–476. doi: 10.1016/0304-4165(81)90261-0. [DOI] [PubMed] [Google Scholar]
- Magnusson K. E. Hydrophobic interaction--a mechanism of bacterial binding. Scand J Infect Dis Suppl. 1982;33:32–36. [PubMed] [Google Scholar]
- Mantle M., Allen A. Isolation and characterization of the native glycoprotein from pig small-intestinal mucus. Biochem J. 1981 Apr 1;195(1):267–275. doi: 10.1042/bj1950267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Argenzio R. A., Levine M. M., Giannella R. A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983 Sep;41(3):1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann A. W., Absolom D. R., van Oss C. J., Zingg W. Surface thermodynamics of leukocyte and platelet adhesion to polymer surfaces. Cell Biophys. 1979 Mar;1(1):79–92. doi: 10.1007/BF02785058. [DOI] [PubMed] [Google Scholar]
- O'Hanley P., Low D., Romero I., Lark D., Vosti K., Falkow S., Schoolnik G. Gal-Gal binding and hemolysin phenotypes and genotypes associated with uropathogenic Escherichia coli. N Engl J Med. 1985 Aug 15;313(7):414–420. doi: 10.1056/NEJM198508153130704. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Sadoff J. C., Pyle M., Silipigni J. D. Role of pili in the adherence of Pseudomonas aeruginosa to injured tracheal epithelium. Infect Immun. 1984 Apr;44(1):38–40. doi: 10.1128/iai.44.1.38-40.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M. Bacterial adherence to polystyrene: a replica method of screening for bacterial hydrophobicity. Appl Environ Microbiol. 1981 Aug;42(2):375–377. doi: 10.1128/aem.42.2.375-377.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum R., McAdams A. J., Giannella R., Partin J. C. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology. 1982 Aug;83(2):441–454. [PubMed] [Google Scholar]
- Sherman P. M., Boedeker E. C. Pilus-mediated interactions of the Escherichia coli strain RDEC-1 with mucosal glycoproteins in the small intestine of rabbits. Gastroenterology. 1987 Oct;93(4):734–743. doi: 10.1016/0016-5085(87)90435-5. [DOI] [PubMed] [Google Scholar]
- Sherman P. M., Boedeker E. C. Regional differences in attachment of enteroadherent Escherichia coli strain RDEC-1 to rabbit intestine: luminal colonization but lack of mucosal adherence in jejunal self-filling blind loops. J Pediatr Gastroenterol Nutr. 1987 May-Jun;6(3):439–444. doi: 10.1097/00005176-198705000-00022. [DOI] [PubMed] [Google Scholar]
- Sherman P. M., Houston W. L., Boedeker E. C. Functional heterogeneity of intestinal Escherichia coli strains expressing type 1 somatic pili (fimbriae): assessment of bacterial adherence to intestinal membranes and surface hydrophobicity. Infect Immun. 1985 Sep;49(3):797–804. doi: 10.1128/iai.49.3.797-804.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P. M., Soni R. Adherence of Vero cytotoxin-producing Escherichia coli of serotype O157:H7 to human epithelial cells in tissue culture: role of outer membranes as bacterial adhesins. J Med Microbiol. 1988 May;26(1):11–17. doi: 10.1099/00222615-26-1-11. [DOI] [PubMed] [Google Scholar]
- Sherman P., Soni R., Yeger H. Characterization of flagella purified from enterohemorrhagic, vero-cytotoxin-producing Escherichia coli serotype O157:H7. J Clin Microbiol. 1988 Jul;26(7):1367–1372. doi: 10.1128/jcm.26.7.1367-1372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Jonsson P., Olsson E., Soderlind O., Rosengren J., Hjertén S., Wadström T. Differences in hydrophobic surface characteristics of porcine enteropathogenic Escherichia coli with or without K88 antigen as revealed by hydrophobic interaction chromatography. Infect Immun. 1978 Nov;22(2):462–472. doi: 10.1128/iai.22.2.462-472.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Inman L. R., O'Hanley P. D., Cantey J. R., Lushbaugh W. B. Scanning and transmission electron microscopic study of Escherichia coli O15 (RDEC-1) enteric infection in rabbits. Infect Immun. 1978 Feb;19(2):686–694. doi: 10.1128/iai.19.2.686-694.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulshen M. H., Rollo J. L. Pathogenesis of escherichia coli gastroenteritis in man--another mechanism. N Engl J Med. 1980 Jan 10;302(2):99–101. doi: 10.1056/NEJM198001103020207. [DOI] [PubMed] [Google Scholar]
- Wadström T., Faris A., Freer J., Habte D., Hallberg D., Ljungh A. Hydrophobic surface properties of enterotoxigenic E. coli (ETEC) with different colonization factors (CFA/i, CFA/ii, K88 and K99) and attachment to intestinal epithelial cells. Scand J Infect Dis Suppl. 1980;Suppl 24:148–153. [PubMed] [Google Scholar]
- Wadström T., Faris A., Lindahl M., Hjertén S., Agerup B. A new principle for prevention of diarrhoea caused by enterotoxigenic Escherichia coli (ETEC) possessing colonization factor antigen (CFA/I). Scand J Infect Dis. 1981;13(2):129–132. doi: 10.3109/inf.1981.13.issue-2.09. [DOI] [PubMed] [Google Scholar]
- Weerkamp A. H., van der Mei H. C., Slot J. W. Relationship of cell surface morphology and composition of Streptococcus salivarius K+ to adherence and hydrophobicity. Infect Immun. 1987 Feb;55(2):438–445. doi: 10.1128/iai.55.2.438-445.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M. K., Andrews G. P., Fritz D. L., Sjogren R. W., Jr, Boedeker E. C. Characterization of the plasmid from Escherichia coli RDEC-1 that mediates expression of adhesin AF/R1 and evidence that AF/R1 pili promote but are not essential for enteropathogenic disease. Infect Immun. 1988 Aug;56(8):1846–1857. doi: 10.1128/iai.56.8.1846-1857.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Schraa G., Zehnder A. J. The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol. 1987 Aug;53(8):1893–1897. doi: 10.1128/aem.53.8.1893-1897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]