Abstract
Redox cycling compounds (RCCs) generate µM concentrations of hydrogen peroxide (H2O2) in the presence of strong reducing agents, common buffer components used to maintain the catalytic activity and/or folding of target proteins for high throughput screening (HTS) assays. H2O2 generated by RCCs can indirectly inhibit the catalytic activity of proteins by oxidizing accessible cysteine, tryptophan, methionine, histidine or selenocysteine residues, and indeed several important classes of protein targets are susceptible to H2O2-mediated inactivation; protein tyrosine phosphatases, cysteine proteases, and metalloenzymes. The main sources of H2O2 in cells are the Nox enzyme/SOD systems, peroxisome metabolism, and the autoxidation of reactive chemicals by enzyme mediated redox cycling at both the microsomal and mitochondrial sites of electron transport. Given the role of H2O2 as a second messenger involved in the regulation of many signaling pathways it is hardly surprising that compounds which can generate intracellular H2O2 by enzyme mediated redox cycling would have pleiotropic effects. RCCs can therefore have serious negative consequences for the probe and/or lead generation process: primary HTS assay hit rates may be inflated by RCC false positives; critical resources will be diverted to develop and implement follow up assays to distinguish RCCs from real hits; and screening databases will become annotated with the promiscuous activity of RCCs. In an attempt to mitigate the serious impact of RCCs on probe and lead generation, two groups have independently developed assays to indentify RCCs.
Introduction
In the pursuit of small molecules that modulate the activity of isolated protein targets or cellular phenotypes the selection of the screening assay format is arguably one of the most critical factors that influences whether a chemical biology endeavor will be successful [1,2]. The pairing of a particular target or cellular phenotype with an assay technology not only requires the development, optimization and validation of an assay that is biologically appropriate, but also one which provides a robust and reproducible signal window with sufficient throughput and capacity to screen the compound diversity of interest [1–4]. The potential for compound interference with an assay format must also be considered, especially since it typically requires the integration of orthogonal counter screens into the follow up paradigm to identify and eliminate false positives [1,2,5,6]. Assay interference can be attributed to both chemical and physical compound properties [1,5–10]. Compounds that are colored, fluorescent, or that form aggregates have the potential to promiscuously interfere with a wide variety of biochemical and cell based assay formats [1,2,5,6,11]. Alternatively, some inhibitors of the firefly luciferase (FLuc) enzyme that stabilize intracellular FLuc can produce a gain in signal in cell based luciferase reporter assays that may be misconstrued as transcriptional activation, and represent a more restricted example of assay format interference [6,7,12]. Redox cycling compounds (RCCs) generate hydrogen peroxide (H2O2) in the presence of strong reducing agents like dithiothreitol (DTT) (Figure 1A) or tris(2-carboxyethyl)phosphine (TCEP), common buffer components used to maintain the catalytic activity and/or folding of target proteins for high throughput screening (HTS) assays [8–10,13,14]. H2O2 generated by RCCs in HTS assay buffers containing DTT/TCEP can indirectly inhibit the catalytic activity of proteins by oxidizing accessible cysteine, tryptophan, methionine, histidine or selenocysteine residues, and indeed several important classes of protein targets are susceptible to H2O2-mediated inactivation; protein tyrosine phosphatases (PTPs), cysteine proteases (cathepsins and caspases) ,and metalloenzymes (Figure 1A) [8–10,13,14]. The current review focuses on RCCs as nuisance/pan assay interference compounds with the potential to annotate screening databases with promiscuous bioactivity profiles (Figure 1B) [6,8–10].
Figure 1. Redox Cycling Compounds Generate H2O2 in Reducing Environments and Exhibit Promiscuous Bioactivity Profiles in HTS Databases.
A) Aqueous solutions containing dithiothreitol (DTT) and oxygen at neutral to acidic pH generate H2O2 via a chain reaction, and if compounds capable of redox cycling are added to this environment, micromolar (µM) concentrations of H2O2 can be produced. H2O2 generated by placing RCCs like 1-ethyl-6-methyl-3-phenyl-1H-pyrimido[5,4-e][1,2,4]triazine-5,7-dione, PubChem substance identifier (SID) 845167, in HTS assay buffers containing DTT can indirectly inhibit the catalytic activity of proteins by oxidizing accessible cysteine, tryptophan, methionine, histidine or selenocysteine residues. H2O2 generated by RCCs may also inhibit assays by having adverse effects on cofactor binding, or by disrupting disulfide bonds that contribute to the dimerization and/or folding of proteins. B) The PubChem database (http://pubchem.ncbi.nlm.nih.gov/) was queried using the PubChem SID 845167 for the RCC 1-ethyl-6-methyl-3-phenyl-1H-pyrimido [5,4-e][1,2,4]triazine-5,7-dione. The bioassay activity information is captured in two PubChem data fields, the activity outcome and the activity score. A substance may be flagged active in a bioassay data uploaded to PubChem if it meets the active criterion for the assay that has been designated appropriate by the depositor. A substance may be flagged a “confirmed” active in bioassay data uploaded to PubChem if it exhibits a concentration response that has been designated appropriate by the depositor. RCCs represent nuisance/pan assay interference compounds with the potential to annotate screening databases with promiscuous bioactivity profiles. The RCC SID 845167 has been tested in 541 bioassays and was designated active in 173 (32%) of them. This RCC was confirmed active in 77 concentration response assays including a number of protein targets that are susceptible to H2O2-mediated inactivation; protein tyrosine phosphatases (PTPs), and cysteine proteases (cathepsins and caspases).
False Positive Hits due to Redox Cycling Compounds
A HTS of > 700,000 compounds to identify inhibitors of caspase-8 produced a high hit rate (~1%), and it was found that 85% of the inhibitors from the initial set of 20,000 compounds were RCCs that inhibited the enzyme through the generation of H2O2 in the presence of DTT [14]. 97% of the actives in a HTS campaign to identify activators of glucokinase (GK) activity were found to be nuisance RCCs that interfered with the resazurin coupled assay format independently of the GK enzyme [9]. A retrospective analysis of screening data revealed that the pyrimidotriazinedione hits indentified in a Cathepsin L cysteine protease inhibitor screen (structurally related to the RCC compound presented in Figure 1A) had been promiscuously active in many historical HTS campaigns, predominantly those conducted against cysteine proteases, metalloenzymes, and other drug targets with an active site cysteine [9]. Furthermore 2.3% of a 10,000 compound library that was used to validate the performance of HTS assays prior to conducting a full diversity screen were found to be redox active at 10 µM [9]. In a HTS to identify inhibitors of the dual-specificity phosphatase mitogen activated protein kinase phosphatase-1 (MKP-1), 10.4% of the concentration dependent MKP-1 inhibitors were found to be RCCs [8,15], and in another HTS to identify inhibitors of the dual-specificity phosphatase cell division cycle 25B (Cdc25B), 55% of the concentration dependent Cdc25B inhibitors were shown to be RCCs [16]. The thirty seven (0.02%) RCCs that were identified in the profiling of ~200,000 compounds from the of the NIH’s Molecular Library Screening Center Network (MLSCN) compound library exhibited a significantly higher number of active flags and “confirmed” active flags in the PubChem database compared to non-RCCs (p-value < 0.01) [10]. Interestingly, the majority (91.9%) of the RCC’s had not been screened against any of the well known oxidation-sensitive targets in the database, suggesting that more targets may be susceptible to oxidation than has previously been recognized [10]. RCCs can therefore have serious negative consequences for the probe and/or lead generation process: primary HTS assay hit rates may be inflated by RCC false positives; critical resources will be diverted to develop and implement follow up assays to distinguish RCCs from real hits; and screening databases will become annotated with the promiscuous activity of RCCs [8–10].
Functional Characteristics of Redox Cycling Compounds
Several hallmark characteristics have been utilized to distinguish the behavior of RCCs from hit compounds that modulate target activities directly [8,13,14,16–18]. The degree of inhibition of the target protein activity by the RCC increases over time, consistent with the time dependent redox cycling generation of H2O2. The inhibition of the target protein activity by the RCC can be abolished by the addition of catalase (CAT) to the assay to degrade any H2O2 produced. RCCs can inhibit target protein activity in buffers containing strong reducing agents like DTT or TCEP, but not in buffers containing weaker reducing agents such as glutathione (GSH), cysteine (Cys) or β-mercaptoethanol (BME). The inhibition of the target protein activity by the RCC depends on the concentrations of both the compound and the reducing reagent. For example, the potency and efficacy of sixteen RCC Cdc25B inhibitors are significantly reduced when the conditions of the Cdc25B assay are modified: if the strong non-physiological reducing agent DTT is replaced with the weaker BME or by the physiologically relevant GSH; if CAT is added to the assay; if the DTT concentration is increased from 1 to 25 mM; or in reactions conducted in the presence of both CAT and high levels of DTT [16].
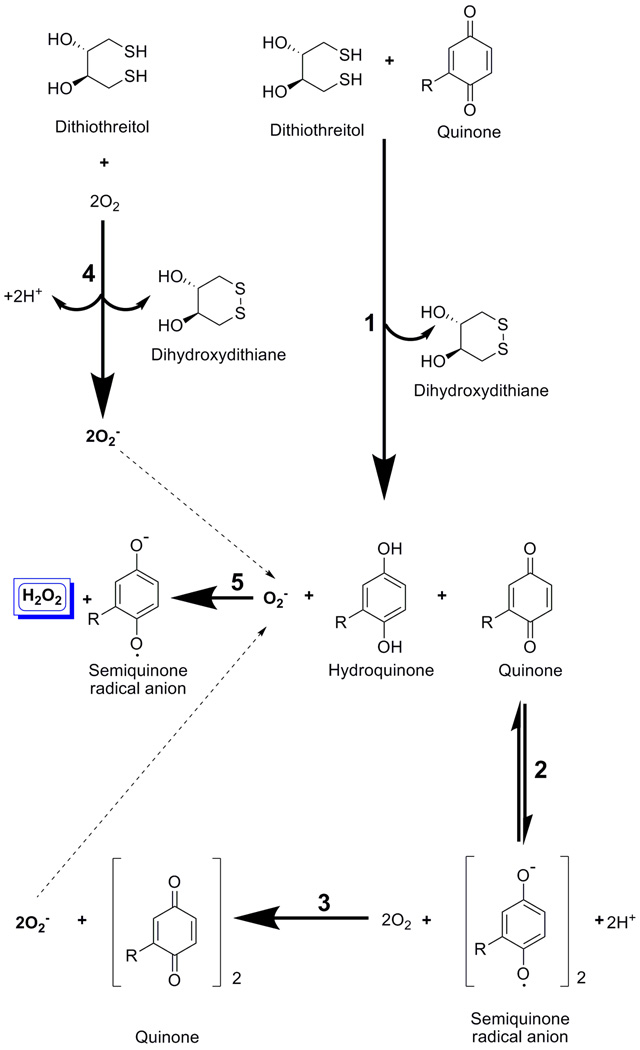
Non-enzymatic Redox Cycling Generation of H2O2
A reaction scheme for the non-enzymatic generation of H2O2 by redox cycling between a quinone RCC and DTT in aqueous solutions containing oxygen has been proposed (Figure 2) [8,10,13]. (1) DTT reacts with the quinone RCC to form dihydroxydithiane (ox-DTT) and a hydroquinone. (2) When the hydroquinone and quinone RCC are present together they undergo a synproportionation to form a transient semiquinone radical anion species (RCC.-) and 2H+. (3) The semiquinone radical anion reacts with O2 to form superoxide anion (O2.-) and regenerate the quinone RCC. (4) In neutral to acidic pH DTT may also react with O2 in aqueous buffers to generate O2.- and ox-DTT [19]. (5) O2.- produced in reactions 3 and 4 can oxidize a hydroquinone resulting in the production of H2O2 and the regeneration of the RCC.- semiquinone radical anion. In aqueous buffers containing a strong reducing agent and molecular oxygen, quinone RCCs cycle between the quinone, hydroquinone and semiquinone radical anion species to generate the reactive oxygen species O2.- and H2O2.
Figure 2. Redox Cycling Reaction Scheme for a Quinone RCC in DTT.
A reaction scheme for the generation of H2O2 by redox cycling between a quinone RCC and DTT in the presence of oxygen is presented. (1) DTT reacts with the quinone RCC to form dihydroxydithiane (ox-DTT) and a hydroquinone. (2) When the hydroquinone and quinone RCC are present together they undergo a synproportionation to form a transient semiquinone radical anion species (RCC*-) and 2H+. (3) The semiquinone radical anion reacts with O2 to form superoxide anion (O2*-) and regenerate the quinone RCC. (4) In neutral to acidic pH DTT may also react with O2 in aqueous buffers to generate O2.- and ox-DTT. (5) O2.- produced in reactions 3 and 4 can oxidize a hydroquinone resulting in the production of H2O2 and the regeneration of the RCC.- semiquinone radical anion. In aqueous buffers containing a strong reducing agent and molecular oxygen, quinone RCCs cycle between the quinone, hydroquinone and semiquinone radical anion species to generate the reactive oxygen species O2.- and H2O2.
H2O2 and Signal Transduction
Of all the reactive oxygen species produced by cells, H2O2 best meets the criteria of an intracellular second messenger involved in the transduction of external signals [20–23]. H2O2 is a small molecule that diffuses rapidly, can cross membranes, and is rapidly synthesized and destroyed in cells in response to external stimuli [20–24]. Many cell types generate low levels of H2O2 in response to a variety of extracellular stimuli including; cytokines (TNF-α, IL-1, GM-CSF, IL-3, IFN-γ, & TGF-β1), growth factors (PDGF, EGF, bFGF,& insulin) and GPCR agonists (angiotnesin II, thrombin, thyrotrophin, parathyroid hormone, lysophosphatidic acid, sphingosine-1-phosphate, serotonin, acetyl choline, platlet activating factor, and bradykinin) [20,22,23].
The NADPH oxidase (Nox) family of proteins are membrane associated multi-unit enzymes that catalyze the reduction of oxygen using NADPH to form the superoxide anion radical (O2.- ) that is in turn dismutated to H2O2 by cellular SOD enzymes [20–23]. Five Nox family members (Nox 1-5) and two dual oxidase enzymes (Duox 1-2) are expressed in many cell types and tissues [20]. Stimuli that activate H2O2 production in cells may promote Nox-complex formation with regulatory subunits by altering the activity or expression of Nox enzymes and their regulatory subunits, or by increasing intracellular Ca2+ concentrations [20]. Nox proteins are targeted to different subcellular localizations, and signaling specificity may be achieved by the coupling of an external stimulus to H2O2 production by a specific Nox homologue in a distinct cellular location [20]. H2O2 derived from Nox enzymes can react with cellular proteins to alter their activity, localization and half-life [20–23].
The addition of exogenous H2O2 or its intracellular production in response to external stimuli modulates (activates or inhibits) the activity of a variety of proteins including protein kinases, protein phosphatases, transcription factors, phospholipases, ion channels and G proteins [20,22,23]. The removal of H2O2 in cells is mediated predominantly by CAT, glutathione peroxidase (GPx) and peroxiredoxins (Prxs) [20,22,23]. CAT is localized exclusively in peroxisomes, the major isoform of GPx is located in the cytosol and mitochondria, while the six Prx isoforms are distributed throughout the cytosol, mitochondria and endoplasmic reticulum [22,23]. H2O2 is a relatively mild oxidant that can oxidize the cysteine residues of proteins to cysteine sulfenic acid (Cys-SOH) or to form disulfide (-S-S-) bonds that can be reduced back to cysteine by cellular thiol donors such as GSH, glutaredoxin (Grx) and thioredoxin (Trx) [21–23]. The pKa of sulfhydryl groups of most cysteine residues (Cys-SH) in proteins is ~ 8.5 and they do not react at physiologically significant rates with H2O2 unless the reaction is catalyzed [21–23]. The cysteine thiolate anion (Cys-S-) is much more readily oxidized by H2O2 and for some signaling proteins, most notably the PTP’s, the active site cysteine is preferentially in a thiolate form [21–23]. The 107 PTPs found in the human genome are defined by the active site sequence C(X)5R(S/T), with X being any amino acid, and they are critical regulators of mammalian cell proliferation, differentiation, and apoptosis [21–23,25]. The active site cysteine of PTPs is required for catalytic activity and performs a nucleophilic attack on the phosphotyrosine residues of the substrate to form a covalent thiol-phosphate intermediate followed by hydrolysis and the release of the phosphate [21–23,26–31]. Due to the unique environment and the invariant arginine, PTP active site cysteines have an unusually low pKa of 4.7-5.4 and exist as thiolate anions at neutral pH, which enhances catalytic function as a nucleophile but also increases susceptiblity to inactivation by H2O2 [21–23,26–31]. The PTP cysteine thiolate reacts with H2O2 to yield the sulfenic acid of cysteine which renders the PTP enzyme inactive against phosphorylated substrates, but can be reduced back to the active thiolate species by cellular thiol donors such as GSH, Grx, and Trx [25]. Sulfenic acids are highly reactive and readily undergo further oxidation to sulfinic and sulfonic forms, which are irreversibly oxidized and inactive [26–31]. The specific and reversible oxidation and inactivation of PTPs by H2O2 has now been demonstrated for numerous PTP targets; PTP1B, PTPα, LAR, VHR, PTEN, SHP-2, Cdc25B, MKP-1, and MKP-3 [8,13,16,17,30–32]. Other proteins with critical cysteine residues in the thiolate form that are susceptible to oxidation by H2O2 include AP1 and NFκB transcription factors, caspases and some protein kinase C isoforms [23,27,29,33,34]. The protein serine-threonine phosphatases are metalloenzymes regulated by specific regulatory subunits, but some family members (PP2B, PP1 & PP2A) may be inactivated by the H2O2 mediated formation of disulfide bonds between the redox-sensitive cysteine residues of these proteins [29]. A number of protein serine-threonine kinases and protein tyrosine kinases have been also shown to be activated by H2O2; EGF-receptor, MAPK, c-Ret, Abl, Src and LCK [23,27,29,33,34].
HTS Assays to Identify Redox Cycling Compounds
Until recently, the process to identify and eliminate RCCs from HTS hit lists involved the development and implementation of multiple counter screens and secondary assays: (i) performing a detailed enzyme kinetic analysis to verify the time- and concentration-dependent inhibition of target activity by RCCs in the presence of DTT or TCEP; (ii) testing whether CAT abolishes the target inhibition by RCCs in DTT or TCEP; (iii) investigating RCC inhibition in the presence of weaker reducing agents such as GSH, BME or Cys; (iv) measuring the UV/Vis spectra of the RCC +/− the reducing agent to determine if the RCC is reduced in a time-dependent manner; and (v) liquid chromatography and mass spectrometry analysis to confirm the oxidation of active site cysteines in target proteins [8,9,13,14,16,17]. Such detailed analyses consume critical resources and significantly lengthen the timelines for hit characterization and lead selection. In an attempt to mitigate the serious impact of RCCs on probe and lead generation, two groups have independently developed assays to indentify RCCs (Figure 3) [8–10].
Figure 3. HTS Assays to Identify Compounds that Redox Cycle in Reducing Environments.
A) Surrogate assay using the conversion of resazurin to resorufin to detect small molecule redox activity [9]. 100 nL of compounds are added to the wells of black low volume plates to which is added 10 µL of a mixture containing 5 µM resazurin and 50 µM DTT in 50 mM HEPES, 50 mM NaCl at pH 7.5, and after a 60 minute incubation at ambient temperature the fluorescent intensity of resorufin (Ex 560 nm. Em 590 nm) is captured. B) Colorimetric assay to measure H2O2 generated by RCCs incubated with strong reducing agents based on the H2O2-dependent horseradish peroxidase (HRP) mediated oxidation of phenol red (PR) that produces a change in its absorbance at 610 nm in alkaline pH [8,10]. The assay is performed in 384-well flat bottomed clear polystyrene microtiter plates and involves three liquid transfer steps of 20 µL each of compounds/controls, DTT, and the HRP-PR detection reagent to give a final assay volume of 60 µL. Compounds (0.01 to 50 µM, final), plate controls (100 µM H2O2 or 1 % DMSO), DTT (0.5 to 1.0 mM, final), and the HRP-PR detection reagent (100 µg/mL phenol red and 60 µg/ml HRP, final) are all prepared in Hank’s balanced salt solution (HBSS). Compounds and DTT are incubated together at ambient temperature for a minimum of 15 min prior to the addition of the HRP-PR detection reagent and after an additional incubation period at ambient temperature, minimally 5 min, the assay is terminated by the addition of 10 µL of 1N NaOH to all wells and the absorbance of the phenol red is measured at 610 nm on a plate reader.
Lor et al. [9] describe a surrogate assay using the conversion of resazurin to resorufin to detect small molecule redox activity. In a coupled enzyme assay format to screen for glucokinase (GK) inhibitors, 97% of the primary screening actives generated the resorufin product in a process that was independent of GK activity and required only compound, resazurin and DTT [9]. These observations led to the development of a surrogate assay to identify redox active compounds; 100 nL of compounds are added to the wells of black low volume plates to which is added 10 µL of a mixture containing 5 µM resazurin and 50 µM DTT in 50 mM HEPES, 50 mM NaCl at pH 7.5, and after a 60 minute incubation at ambient temperature the fluorescent intensity of resorufin (Ex 560 nm. Em 590 nm) is captured (Figure 3A). Although the homogeneous mix and read format and robust fluorescent assay signal window are attractive features of the resazurin surrogate assay, there are inconsistencies between the performance of the assay and the behavior of RCCs [8,13,14,16–18]. A pyrimidotriazinedione RCC (5 µM) generated O2.- in the presence of DTT (10 µM) that was readily detected in a cytochrome C assay and was prevented by the inclusion of SOD to scavenge the O2.- and convert it to H2O2 [9]. However the addition of SOD and/or CAT to the resazurin surrogate assay reaction does not prevent the generation of resorufin by RCCs in DTT, indicating that the reaction is not mediated by either O2.- or H2O2 [9]. Also, the amount of resorufin generated is limited by the amount of resazurin and DTT, but not the compound concentration. It was proposed that the compound is not a substrate but behaves as a catalyst facilitating the 2e- reduction of resazurin by DTT [9]. There was also no indication whether weak reducing agents such as GSH or Cys can substitute for DTT in the surrogate assay. Despite these inconsistencies the surrogate assay successfully identified previously described RCCs and several other structural classes of RCCs in the hits from historical HTS campaigns conducted at GlaxoSmithKline (Figure 4) [9]. The reduction of resazurin to resorufin is a popular assay format used to measure cell viability (mammalian cells, bacteria and parasites), and is often used in coupled enzyme assays to measure the activity of target enzymes that reduce NAD to NADH [9,35,36]. During the initial planning and design phase of an HTS campaign it therefore might be advisable to consider the potential for RCCs to interfere with and greatly inflate the hit rates of screening assays that use a resazurin-resorufin format.
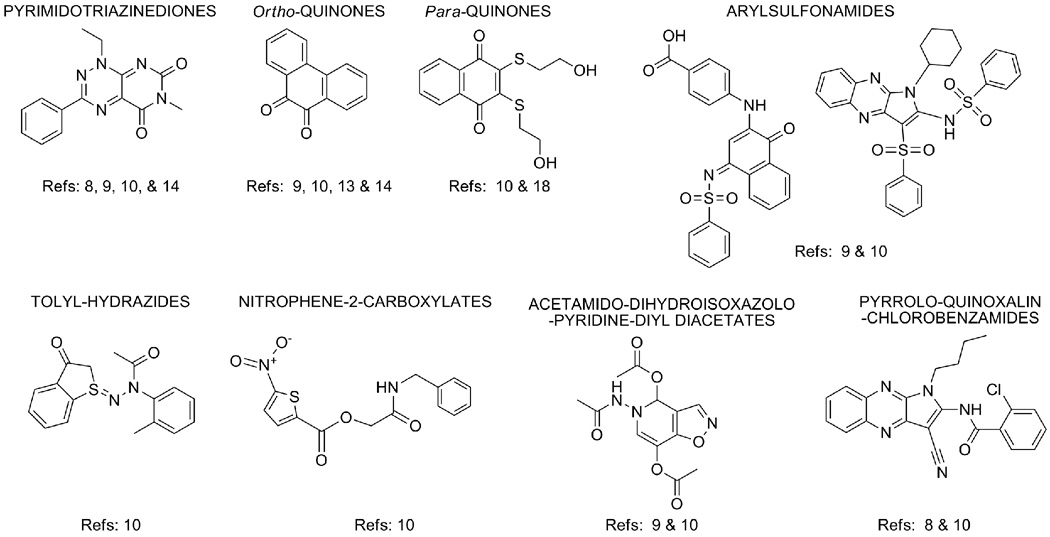
Figure 4. Representative Structural Classes of Redox Cycling Compounds.
Representative RCCs reported in the literature [8–10,13,14,16–18] were subjected to structure based clustering and classification techniques based on recursive partitioning using the Leadscope Enterprise 2.4.6-1 software as described previously [8,10,15,16].
Recently, a different group reported the development and optimization of a rapid, economical, 384-well colorimetric HTS compatible assay to measure H2O2 generated by RCCs incubated with strong reducing agents (Figure 3B) [8]. The assay is based on the H2O2-dependent horseradish peroxidase (HRP) mediated oxidation of phenol red (PR) that produces a change in its absorbance at 610 nm in alkaline pH, and readily detects H2O2 in the 1–100 µM range [8,10,37]. The assay is performed in 384-well flat bottomed clear polystyrene microtiter plates and involves three liquid transfer steps of 20 µL each of compounds/controls, DTT, and the HRP-PR detection reagent to give a final assay volume of 60 µL. Compounds (0.01 to 50 µM, final), plate controls (100 µM H2O2 or 1 % DMSO), DTT (0.5 to 1.0 mM, final), and the HRP-PR detection reagent (100 µg/mL phenol red and 60 µg/ml HRP, final) are all prepared in Hank’s balanced salt solution (HBSS). Compounds and DTT are incubated together at ambient temperature for a minimum of 15 min prior to the addition of the HRP-PR detection reagent and after an additional incubation period at ambient temperature, minimally 5 min, the assay is terminated by the addition of 10 µL of 1N NaOH to all wells and the absorbance of the phenol red is measured at 610 nm on a plate reader (Figure 3B). For this assay, the redox cycling generation of H2O2 depends upon the concentrations of both the RCC and the reducing agent, is abolished by the addition of CAT, and whereas both DTT and TCEP support H2O2 generation by RCCs, weaker reducing agents (BME, GSH and Cys) do not [8,10]. The HRP-PR assay has been utilized to identify RCCs in the hits from two PTP inhibitor screens [8,16], to evaluate the redox regulation of Cdc25B by para-quinone-based inhibitors[18], and to profile ~ 200,000 compounds from the LOPAC and NIH MLSCN compound libraries for RCC activity [10]. Although ~ 50% of the RCCs that were identified in the RCC profiling screen are structurally similar to previously described pyrimidotriazinedione, ortho-quinone and para-quinone RCCs, several other RCC pharmacophores were identified (Figure 4).
Activities of Redox Cycling Compounds in Cells
The main sources of H2O2 in cells are the Nox enzyme/SOD systems described above, peroxisome metabolism, and the autoxidation of reactive chemicals by enzyme mediated redox cycling [20–23,38–40]. Within the cellular environment and in the presence of molecular oxygen and metal ions, phenols, catechols and hydoquinones are converted to quinones by intracellular monoxygenase and peroxidase enzymes [38]. In cells, quinones undergo enzymatic redox cycling at both the microsomal and mitochondrial sites of electron transport to generate O2.- that is dismutated to H2O2 by cellular SOD enzymes [38–40]. For example, quinones undergo an enzymatic one-electron reduction catalyzed by microsomal NADPH cytochrome P450 reductase to the semiquinone radical, and in the presence of molecular oxygen, the semiquinone radical can transfer an electron to generate O2.- and ultimately via SOD H2O2 [38,40]. Quinones cause toxicity in vivo through a variety of mechanisms including; GSH and/or ATP depletion, damage to DNA and/or mitochondria, and by the oxidation or alkylation of critical cellular proteins [18,38]. Quinones are also Michael acceptors that may covalently bind to cellular nucleophiles including GSH, Cys residues on proteins, and amino groups on proteins and DNA [18,38]. It has been proposed that the ability of quinone RCCs to undergo redox cyling and to generate H2O2 within cells may be the basis of their widespread cellular activities [13,17,38,40,41]. Given the role of H2O2 as a second messenger involved in the regulation of many signaling pathways it is hardly surprising that compounds which can generate intracellular H2O2 by enzyme mediated redox cycling would have pleiotropic effects.
Conclusions
To illustrate the complexities and challenges that RCCs represent to the chemical biology approach, a recent case history is discussed [10,41–43]. Seven pyrimidotriazinedione hits, structurally related to the RCCs presented here (Figures 1A and 4) or that have been characterized previously [8–10,14], were identified in an HTS campaign to identify small molecules that disrupt the interaction between the C-terminal peptide of heat shock protein 90 (Hsp90) and the TPR2A domain of the Hsp organizing protein (HOP) (PubChem assay identifiers (AIDs) 595, 632 and 1400) [42,43]. The pyrimidotriazinedione hits all exhibited cytotoxicity against the BT474 human breast cancer cell line [42]. One of the pyrimidotriazinedione hits was shown to bind to TPR2A protein by isothermal titration calorimetry [42]. Although the hsp90 ATPase inhibitor 17-AAG exhibited selective killing for human breast cancer cell lines versus a normal mammary epithelial cell line, the pyrimidotriazinedione hit exhibited equivalent IC50s (~ 1µM) against all three cell lines [42]. Unlike 17-AAG which induces hsp70 expression, the pyrimidotriazinedione hit had no apparent effect on hsp70 expression levels [42]. In contrast, treatment with both 17-AAG and the pyrimidotriazinedione hit reduced the levels of HER2 in the human breast cancer cell lines relative to untreated cells [42]. The authors concluded that the pyrimidotriazinedione hits represented a novel class of small molecule hsp90 inhibitors [42]. The TPR2A domain of HOP contains two Cys residues and binding to the C-terminal peptide of Hsp90 is better in the presence of DTT [43]. Both the primary HTS and secondary fluorescence polarization assays to measure Hsp90-TPR2A protein-protein interactions were conducted in the presence of DTT [42,43]. However the potential role of H2O2 generated by the pyrimidotriazinedione RCCs in the observed disruption of the Hsp90-TPR2A interaction assays was neither considered nor excluded [10,41]. Given the ability of pyrimidotriazinedione RCCs to generate µM concentrations of H2O2 in buffers containing DTT, the apparent biological promiscuity of this class of compounds, and the role of H2O2 as an intracellular messenger [8–10,14–16,20–23], it would seem prudent to consider the redox cycling capabilities of these hits as they are further characterized and their mechanism of action explored.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Inglese J, Johnson RL, Simeonov A, Xia M, Zheng W, Austin CP, Auld DSZ. High-throughput screening assays for the identification of chemical probes. Nat Chem Biol. 2007;3:466–479. doi: 10.1038/nchembio.2007.17. [DOI] [PubMed] [Google Scholar]
- 2.Johnston PA, Johnston PA. Cellular platforms for HTS: three case studies. Drug Discov Today. 2002;7:353–363. doi: 10.1016/s1359-6446(01)02140-7. [DOI] [PubMed] [Google Scholar]
- 3.Brideau C, Gunter B, Pikounis B, Liaw A. Improved statistical methods for hit selection in high-throughput screening. J Biomol Screen. 2003;8:634–647. doi: 10.1177/1087057103258285. [DOI] [PubMed] [Google Scholar]
- 4.Malo N, Hanley JA, Cerquozzi S, Pelletier J, Nadon R. Statistical practice in high-throughput screening data analysis. Nat Biotechnol. 2006;24:167–175. doi: 10.1038/nbt1186. [DOI] [PubMed] [Google Scholar]
- 5. Baell J, Holloway GA. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem. 2010;53:2719–2740. doi: 10.1021/jm901137j. **A comprehensive analysis of the structures of the promiscuous hits from AlphaScreen assays and an effort to generate substructure filters for the identification and removal of Pan Assay Interference Compounds (PAINS).
- 6. Thorne N, Auld DS, Inglese J. Apparent activity in high-throughput screening: origins of compound-dependent assay interference. Curr Opin Chem Biol. 2010;14:315–324. doi: 10.1016/j.cbpa.2010.03.020. ** A good overview of the sources of compound interference in HTS assays.
- 7.Auld DS, Southall NT, Jadhav Ajit, Johnson RL, Diller DJ, Simeonov A, Austin CP, Inglese J. Characterization of Chemical Libraries for Luciferase Inhibitory Activity. J. Med. Chem. 2008;51:2372–2386. doi: 10.1021/jm701302v. [DOI] [PubMed] [Google Scholar]
- 8.Johnston P, Soares KM, Shinde SN, Foster CA, Shun TY, Takyi HK, Wipf P, Lazo JS. Development of a 384-well colorimetric assay to quantify hydrogen peroxide generated by the redox cycling of compounds in the presence of reducing agents. Assay Drug Dev Technol. 2008;6:505–518. doi: 10.1089/adt.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lor L, Schneck J, McNulty DE, Diaz E, Brandt M, Thrall SH, Schwartz B. A simple assay for detection of small-molecule redox activity. J Biomol Screen. 2007;12:881–890. doi: 10.1177/1087057107304113. [DOI] [PubMed] [Google Scholar]
- 10. Soares K, Blackmon N, Shun TY, Shinde SN, Takyi HK, Wipf P, Lazo JS, Johnston PA. Profiling the NIH Small Molecule Repository for Compounds That Generate H(2)O(2) by Redox Cycling in Reducing Environments. Assay Drug Dev Technol. 2010;8:152–174. doi: 10.1089/adt.2009.0247. **First profiling effort for the discovery of redox cycling compounds and discussion of their potential impact on HTS databases.
- 11.Simeonov A, Jadhav A, Thomas C, Wang Y, Huang R, Southall N, Shinn P, Smith J, Austin C, Auld D, et al. Fluorescence spectroscopic profiling of compound libraries. J Med Chem. 2008;51 doi: 10.1021/jm701301m. [DOI] [PubMed] [Google Scholar]
- 12.Thorne N, Inglese J, Auld DS. Illuminating insights into firefly luciferase and other bioluminescent reporters used in chemical biology. Chem Biol. 2010;17:646–657. doi: 10.1016/j.chembiol.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bova MP, Mattson MN, Vasile S, Tam D, Holsinger L, Bremer M, Hui T, McMahon G, Rice A, Fukuto JM. The oxidative mechanism of action of ortho-quinone inhibitors of protein-tyrosine phosphatase [alpha] is mediated by hydrogen peroxide. Archives of Biochemistry and Biophysics. 2004;429:30–41. doi: 10.1016/j.abb.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Smith GK, Barrett DG, Blackburn K, Cory M, Dallas WS, Davis R, Hassler D, McConnell R, Moyer M, Weaver K. Expression, Preparation, and High-Throughput Screening of Caspase-8: Discovery of Redox-Based and Steroid Diacid Inhibition. Archives of Biochemistry and Biophysics. 2002;399:195–205. doi: 10.1006/abbi.2002.2757. [DOI] [PubMed] [Google Scholar]
- 15.Johnston P, Foster CA, Shun TY, Skoko JJ, Shinde S, Wipf P, Lazo JS. Development and implementation of a 384-well homogeneous fluorescence intensity high-throughput screening assay to identify mitogen-activated protein kinase phosphatase-1 dual-specificity protein phosphatase inhibitors. Assay Drug Dev Technol. 2007;5:319–332. doi: 10.1089/adt.2007.066. [DOI] [PubMed] [Google Scholar]
- 16. Johnston PA, F C, Tierno MB, Shun TY, Brummond KM, Wipf P, Lazo JS. Characterization of the Cdc25B Dual Specificity Phosphatase Inhibitor Hits Identified in a High Throughput Screen of the NIH Compound Library. Assays and Drug Development Technologies. 2009;7:250–265. doi: 10.1089/adt.2008.186. * Good discussion on the characterization of redox cycling compounds identified in a HTS screen for inhibitors of the PTP Cdc25B.
- 17.Brisson M, Nguyen T, Wipf P, Joo B, Day BW, Skoko JS, Schreiber EM, Foster C, Bansal P, Lazo JS. Redox Regulation of Cdc25B by Cell-Active Quinolinediones. Mol Pharmacol. 2005;68:1810–1820. doi: 10.1124/mol.105.016360. [DOI] [PubMed] [Google Scholar]
- 18.Keinan SP, W D, Skoko JJ, Beratan DN, Yang W, Shinde S, Johnston PA, Lazo JS, Wipf P. Computational design, synthesis and biological evaluation of para-quinone-based inhibitors for redox regulation of the dual-specificity phosphatase Cdc25B. Org Biomol Chem. 2008;6:3256–3263. doi: 10.1039/b806712k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lal M, Rao R, Fang X, Schuchmann HP, Sonntag CV. Radical-Induced oxidation of dithiothreitol in acidic oxygenated solution: a chain reaction. J Am Chem Soc. 1997;119:5735–5739. [Google Scholar]
- 20. Brown D, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. ** Very good overview of the Nox/Duos protein field; tissue distribution & physiological function, mechanisms of activation, subcellular localization, and role in signal transduction.
- 21. Forman H, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–843. doi: 10.1021/bi9020378. ** Excellent review on the chemistry and biochemistry of reactive oxygen species and their role as signaling molecules.
- 22.Rhee S, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Rhee SG, C T, Bae YS, Lee SR, Kang SW. Cellular regulation by hydrogen peroxide. J Am Soc Nephrol. 2003;14:S211–S215. doi: 10.1097/01.asn.0000077404.45564.7e. [DOI] [PubMed] [Google Scholar]
- 24. Miller E, Dickinson BC, Chang CJ. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci U S A. 2010;107:15681–15686. doi: 10.1073/pnas.1005776107. *Good demonstration that the water channel Aquaporin-3 can facilitate the uptake of H2O2 across mammalian cell membranes to amplify or diminish downstream signaling cascades.
- 25.Ostman A, Hellberg C, Bohmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006;6:307–320. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- 26.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 27.Forman HJ, F J, Torres M. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol. 2004;287:C246–C256. doi: 10.1152/ajpcell.00516.2003. [DOI] [PubMed] [Google Scholar]
- 28.Lee SR, Y K, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 29.Rhee SG, B Y, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;2000:PE1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 30.Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 31.Seth D, Rudolph J. Redox regulation of MAP kinase phosphatase 3. Biochemistry. 2006;45:8476–8487. doi: 10.1021/bi060157p. [DOI] [PubMed] [Google Scholar]
- 32.Lee K, Esselman WJ. Inhibition of PTPs by H2O2 regulates the activation of distinct MAPK pathways. Free Radical Biology and Medicine. 2002;33:1121–1132. doi: 10.1016/s0891-5849(02)01000-6. [DOI] [PubMed] [Google Scholar]
- 33.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 34.Rhee SG, K S, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Bembenek M, Kuhn E, Mallender WD, Pullen L, Li P, Parsons T. A fluorescence-based coupling reaction for monitoring the activity of recombinant human NAD synthetase. Assay Drug Dev Technol. 2005;3:533–541. doi: 10.1089/adt.2005.3.533. [DOI] [PubMed] [Google Scholar]
- 36.Riss T, Moravec RA. Use of multiple assay endpoints to investigate the effects of incubation time, dose of toxin, and plating density in cell-based cytotoxicity assays. Assay Drug Dev Technol. 2004;2:51–62. doi: 10.1089/154065804322966315. [DOI] [PubMed] [Google Scholar]
- 37.Pick E, Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38:161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- 38.Bolton J, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- 39.Goetz M, Luch A. Reactive species: a cell damaging rout assisting to chemical carcinogens. Cancer Lett. 2008;266:73–83. doi: 10.1016/j.canlet.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 40.Villamil S, Stoppani AOM, Dubin M. Redox Cycling of -Lapachone and Structural Analogues in Microsomal and Cytosol Liver Preparations. Methods Enzymol. 2004;378:67–87. doi: 10.1016/S0076-6879(04)78004-0. [DOI] [PubMed] [Google Scholar]
- 41.Auld D, Simeonov A, Thomas C. Literature search and review. Assay Drug Dev Technol. 2008;6:621–622. doi: 10.1089/adt.2011.0903.lr. [DOI] [PubMed] [Google Scholar]
- 42.Yi F, Regan L. A novel class of small molecule inhibitors of Hsp90. ACS Chem Biol. 2008;3:645–654. doi: 10.1021/cb800162x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi F, Zhu P, Southall N, Inglese J, Austin CP, Zheng W, Regan L. An AlphaScreen-based high-throughput screen to identify inhibitors of Hsp90-cochaperone interaction. J Biomol Screen. 2009;14:273–281. doi: 10.1177/1087057108330114. [DOI] [PMC free article] [PubMed] [Google Scholar]