Abstract
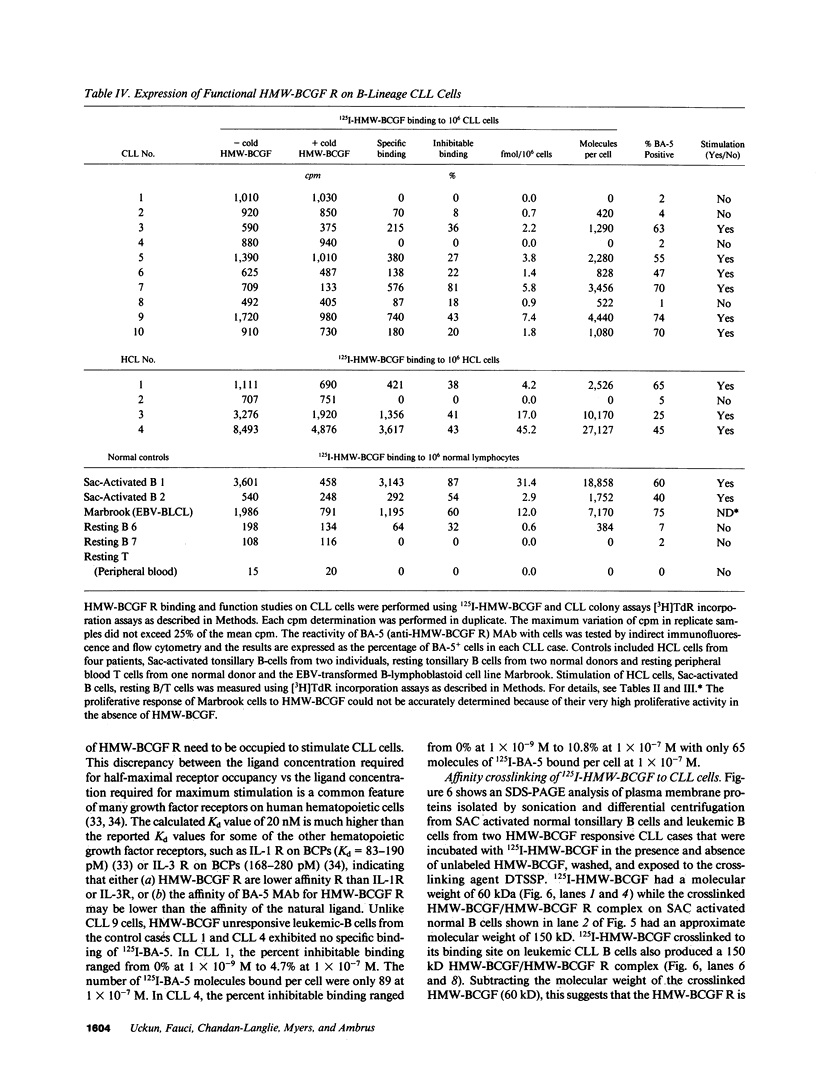
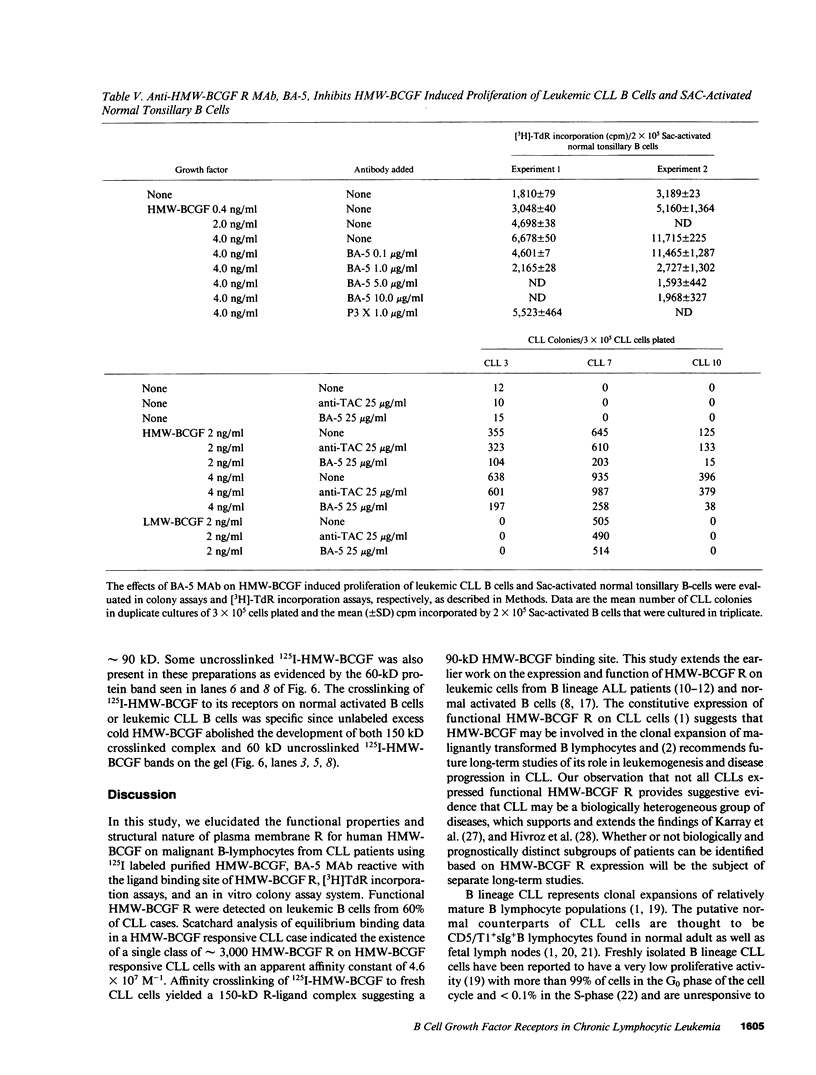
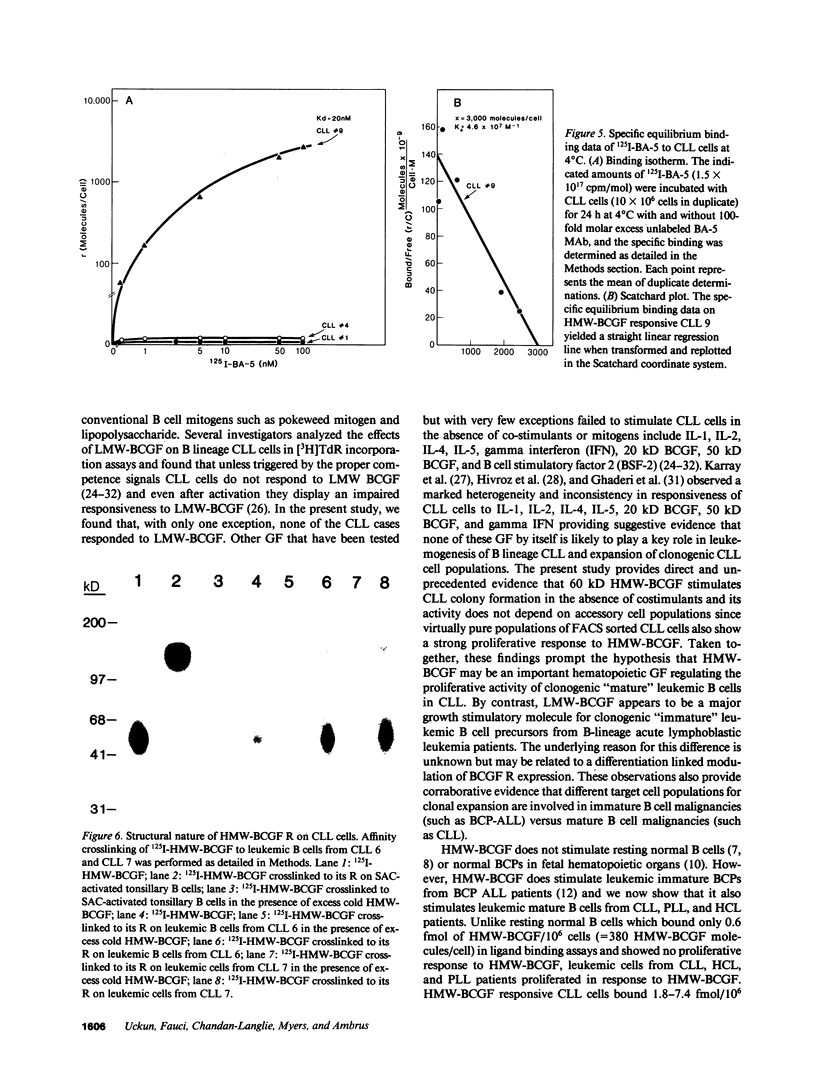
Human high molecular weight-B cell growth factor (HMW-BCGF) (60 kD) stimulates activated normal B cells, B cell precursor acute lymphoblastic leukemia (BCP-ALL) cells, hairy cell leukemia (HCL) cells, prolymphocytic leukemia (PLL) cells, and chronic lymphocytic leukemia (CLL) cells. The expression of human high molecular weight B cell growth factor (HMW-BCGF) receptors (R) on clonal populations of leukemic B cells in CLL was studied by ligand binding assays using 125I-labeled HMW-BCGF as well as by immunofluorescence/flow cytometry and Scatchard analyses using an anti-HMW-BCGF R monoclonal antibody (MAb), designated BA-5. There was a high correlation between HMW-BCGF R expression and responsiveness to HMW-BCGF. 60% of CLL cases constitutively expressed HMW-BCGF R and showed a marked proliferative response to HMW-BCGF in [3H]TdR incorporation assays as well as colony assays. Similarly, HCL cells, PLL cells, and activated normal B cells expressed functional HMW-BCGF R, as determined by ligand binding assays using 125I-HMW-BCGF, [3H]TdR incorporation assays, and reactivity with BA-5 MAb. Scatchard analyses indicated the existence of approximately 3,000 HMW-BCGF R/cell on HMW-BCGF responsive CLL cells with an apparent Ka value of 4.6 X 10(7) M-1. The concentrations of HMW-BCGF required for maximum stimulation of CLL cells were two to three orders of magnitude lower than those needed for half maximal receptor occupancy, indicating that only a small fraction of HMW-BCGF R need to be occupied to stimulate leukemic CLL B cells. Crosslinking of surface bound 125I-HMW-BCGF (60 kD) with the bivalent crosslinker DTSSP to its binding site on fresh CLL cells identified a 150-kD HMW-BCGF/HMW-BCGF R complex, suggesting an apparent molecular weight of 90 kD for the receptor protein. The growth stimulatory effects of HMW-BCGF on clonogenic CLL cells did not depend on accessory cells or costimulant factors. The anti-HMW-BCGF R monoclonal antibody BA-5 disrupted HMW-BCGF/HMW-BCGF R interactions at the level of clonogenic CLL cells and inhibited HMW-BCGF-stimulated CLL colony formation in vitro. To our knowledge, this study represents the first detailed analysis of expression, function, and structure of HMW-BCGF R on B lineage CLL cells.
Full text
PDF


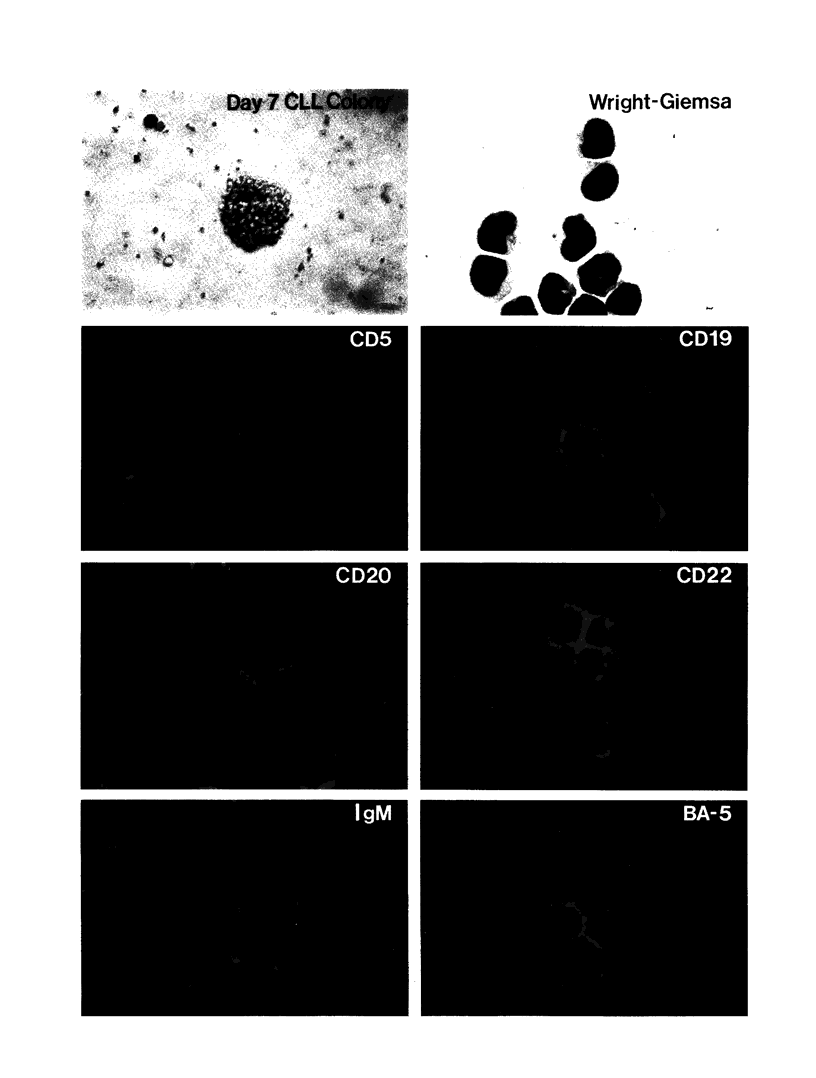
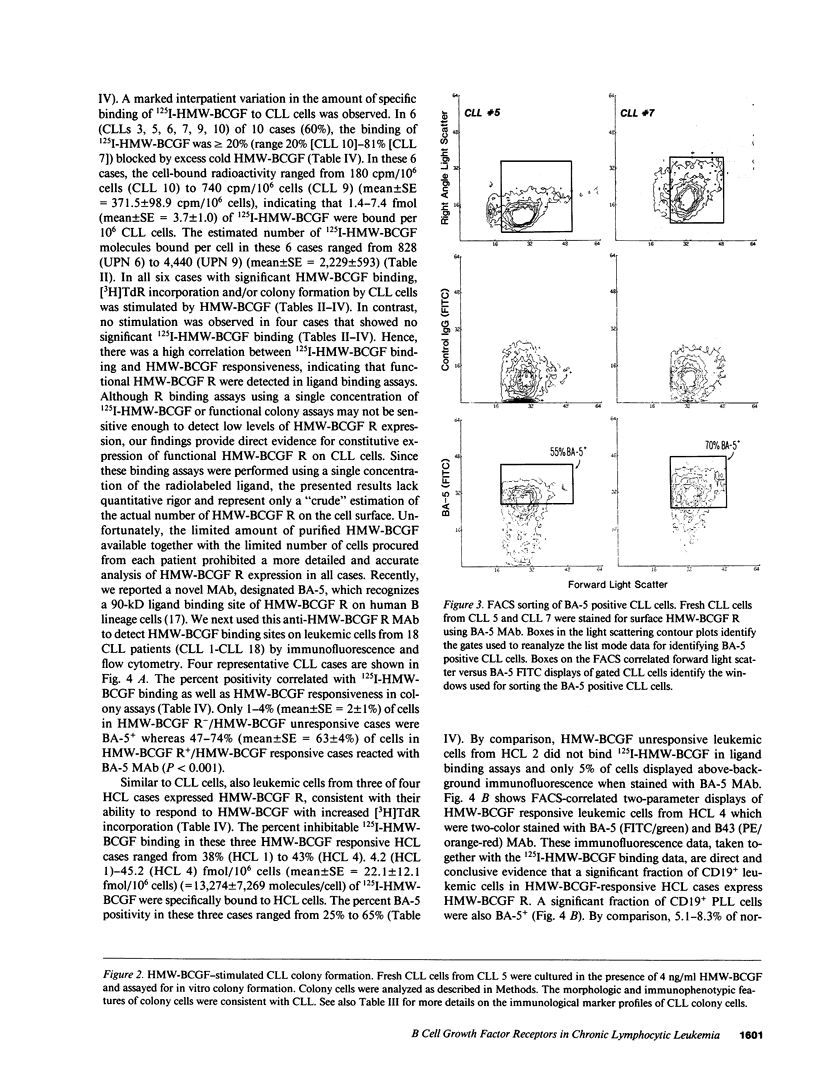
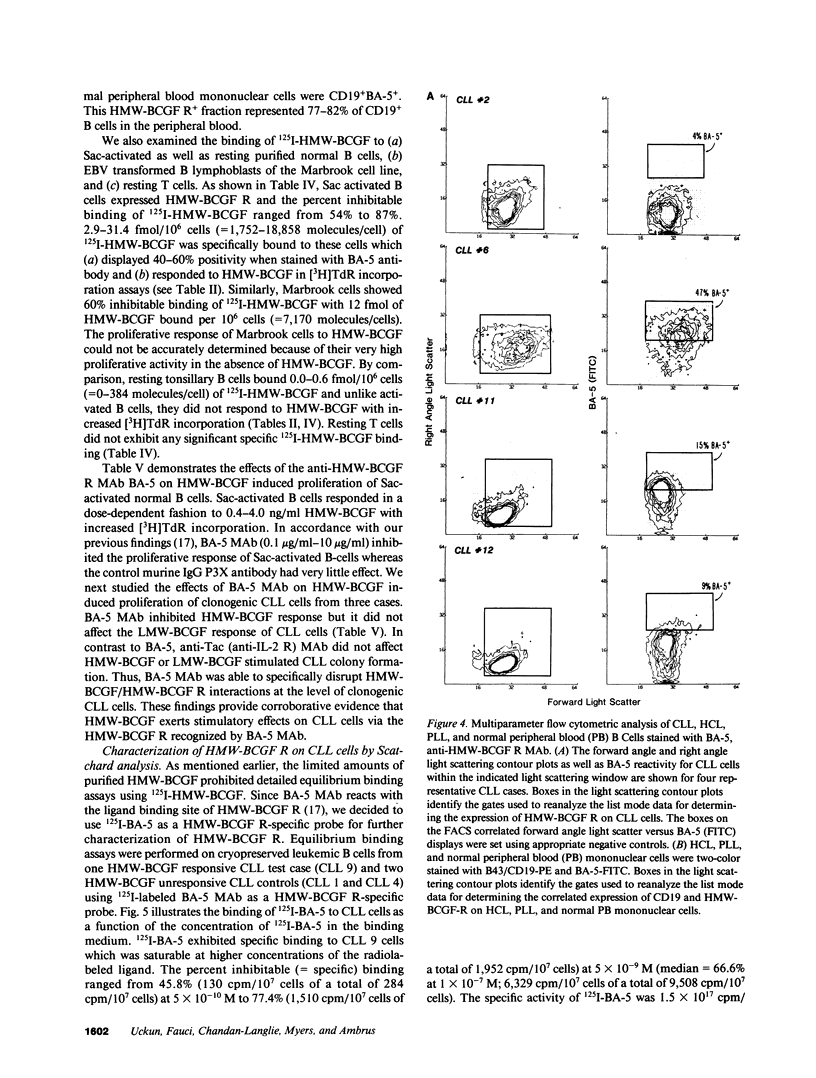
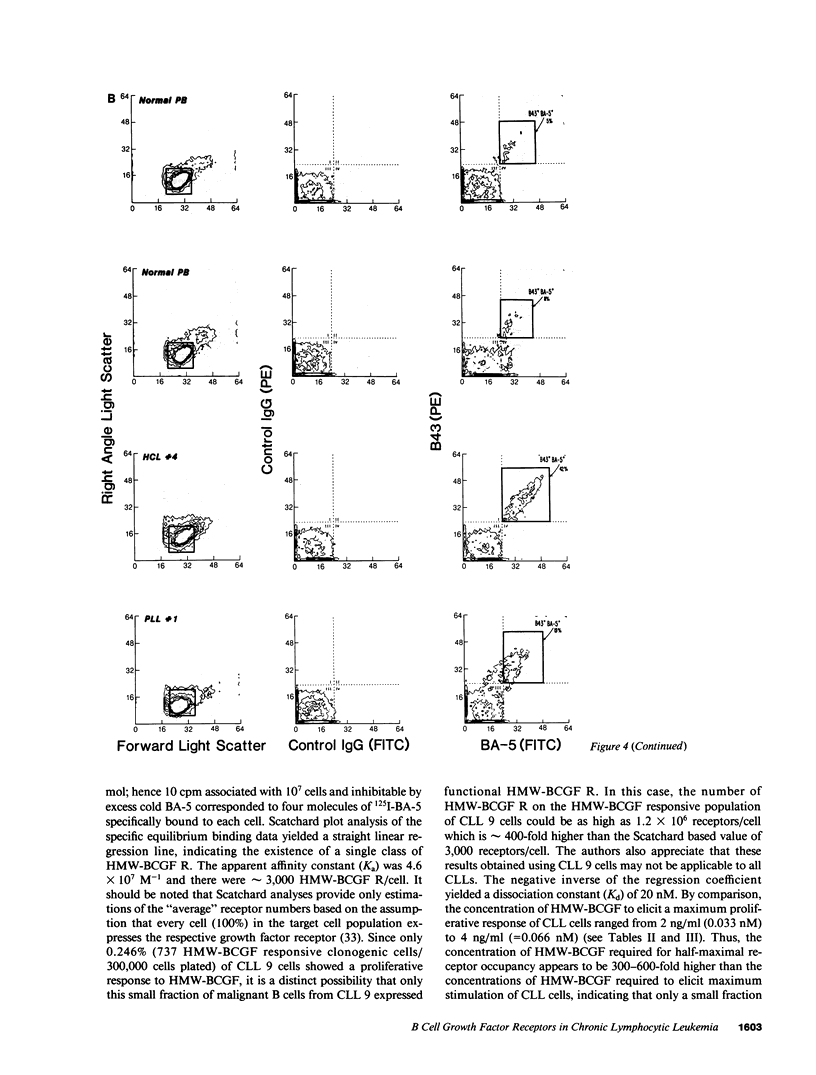


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrus J. L., Jr, Fauci A. S. Human B lymphoma cell line producing B cell growth factor. J Clin Invest. 1985 Feb;75(2):732–739. doi: 10.1172/JCI111754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrus J. L., Jr, Jurgensen C. H., Brown E. J., Fauci A. S. Purification to homogeneity of a high molecular weight human B cell growth factor; demonstration of specific binding to activated B cells; and development of a monoclonal antibody to the factor. J Exp Med. 1985 Oct 1;162(4):1319–1335. doi: 10.1084/jem.162.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreeff M., Darzynkiewicz Z., Sharpless T. K., Clarkson B. D., Melamed M. R. Discrimination of human leukemia subtypes by flow cytometric analysis of cellular DNA and RNA. Blood. 1980 Feb;55(2):282–293. [PubMed] [Google Scholar]
- Benjamin D., Bazar L. S., Wallace B., Jacobson R. J. Heterogeneity of B-cell growth factor receptor reactivity in healthy donors and in patients with chronic lymphatic leukemia: relationship to B-cell-derived lymphokines. Cell Immunol. 1986 Dec;103(2):394–408. doi: 10.1016/0008-8749(86)90099-7. [DOI] [PubMed] [Google Scholar]
- Bofill M., Janossy G., Janossa M., Burford G. D., Seymour G. J., Wernet P., Kelemen E. Human B cell development. II. Subpopulations in the human fetus. J Immunol. 1985 Mar;134(3):1531–1538. [PubMed] [Google Scholar]
- Caligaris-Cappio F., Gobbi M., Bofill M., Janossy G. Infrequent normal B lymphocytes express features of B-chronic lymphocytic leukemia. J Exp Med. 1982 Feb 1;155(2):623–628. doi: 10.1084/jem.155.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligaris-Cappio F., Schena M., Bergui L., Tesio L., Riva M., Rege-Cambrin G., Funaro A., Malavasi F. C3b receptors mediate the growth factor-induced proliferation of malignant B-chronic lymphocytic leukemia lymphocytes. Leukemia. 1987 Nov;1(11):746–752. [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Fu S. M., Chiorazzi N., Kunkel H. G. Differentiation capacity and other properties of the leukemic cells of chronic lymphocytic leukemia. Immunol Rev. 1979;48:23–44. doi: 10.1111/j.1600-065x.1979.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Gale R. P., Foon K. A. Chronic lymphocytic leukemia. Recent advances in biology and treatment. Ann Intern Med. 1985 Jul;103(1):101–120. doi: 10.7326/0003-4819-103-1-101. [DOI] [PubMed] [Google Scholar]
- Ghaderi A. A., Richardson P., Cardona C., Millsum M. J., Ling N., Gillis S., Ledbetter J., Gordon J. Stimulation of B-chronic lymphocytic leukemia populations by recombinant interleukin-4 and other defined growth-promoting agents. Leukemia. 1988 Mar;2(3):165–170. [PubMed] [Google Scholar]
- Greaves M. F. Differentiation-linked leukemogenesis in lymphocytes. Science. 1986 Nov 7;234(4777):697–704. doi: 10.1126/science.3535067. [DOI] [PubMed] [Google Scholar]
- Hivroz C., Grillot-Courvalin C., Brouet J. C., Seligmann M. Heterogeneity of responsiveness of chronic lymphocytic leukemic B cells to B cell growth factor or interleukin 2. Eur J Immunol. 1986 Aug;16(8):1001–1004. doi: 10.1002/eji.1830160821. [DOI] [PubMed] [Google Scholar]
- Howard M., Nakanishi K., Paul W. E. B cell growth and differentiation factors. Immunol Rev. 1984 Apr;78:185–210. doi: 10.1111/j.1600-065x.1984.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Karray S., Merle-Béral H., Vazquez A., Gerard J. P., Debre P., Galanaud P. Functional heterogeneity of B-CLL lymphocytes: dissociated responsiveness to growth factors and distinct requirements for a first activation signal. Blood. 1987 Oct;70(4):1105–1110. [PubMed] [Google Scholar]
- Kawamura N., Muraguchi A., Hori A., Horii Y., Mutsuura S., Hardy R. R., Kikutani H., Kishimoto T. A case of human B cell leukemia that implicates an autocrine mechanism in the abnormal growth of Leu 1 B cells. J Clin Invest. 1986 Nov;78(5):1331–1338. doi: 10.1172/JCI112719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Rabinovitch P. S., June C. H., Song C. W., Clark E. A., Uckun F. M. Antigen-independent regulation of cytoplasmic calcium in B cells with a 12-kDa B-cell growth factor and anti-CD19. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1897–1901. doi: 10.1073/pnas.85.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. T., Conrad D. H. The murine lymphocyte receptor for IgE. III. Use of chemical cross-linking reagents to further characterize the B lymphocyte Fc epsilon receptor. J Immunol. 1985 Jan;134(1):518–525. [PubMed] [Google Scholar]
- Mehta S. R., Conrad D., Sandler R., Morgan J., Montagna R., Maizel A. L. Purification of human B cell growth factor. J Immunol. 1985 Nov;135(5):3298–3302. [PubMed] [Google Scholar]
- O'Garra A., Umland S., De France T., Christiansen J. 'B-cell factors' are pleiotropic. Immunol Today. 1988 Feb;9(2):45–54. doi: 10.1016/0167-5699(88)91259-5. [DOI] [PubMed] [Google Scholar]
- Ostlund L., Einhorn S., Robèrt K. H., Juliusson G., Biberfeld P. Chronic B-lymphocytic leukemia cells proliferate and differentiate following exposure to interferon in vitro. Blood. 1986 Jan;67(1):152–159. [PubMed] [Google Scholar]
- Perri R. T. Impaired expression of cell surface receptors for B cell growth factor by chronic lymphocytic leukemia B cells. Blood. 1986 Apr;67(4):943–948. [PubMed] [Google Scholar]
- Perri R. T., Kay N. E. Monoclonal CLL B-cells may be induced to grow in an in vitro B-cell colony assay system. Blood. 1982 Feb;59(2):247–249. [PubMed] [Google Scholar]
- Uckun F. M., Fauci A. S., Heerema N. A., Song C. W., Mehta S. R., Gajl-Peczalska K., Chandan M., Ambrus J. L. B-cell growth factor receptor expression and B-cell growth factor response of leukemic B cell precursors and B lineage lymphoid progenitor cells. Blood. 1987 Oct;70(4):1020–1034. [PubMed] [Google Scholar]
- Uckun F. M., Gajl-Peczalska K. J., Kersey J. H., Houston L. L., Vallera D. A. Use of a novel colony assay to evaluate the cytotoxicity of an immunotoxin containing pokeweed antiviral protein against blast progenitor cells freshly obtained from patients with common B-lineage acute lymphoblastic leukemia. J Exp Med. 1986 Feb 1;163(2):347–368. doi: 10.1084/jem.163.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Gesner T. G., Song C. W., Myers D. E., Mufson A. Leukemic B-cell precursors express functional receptors for human interleukin-3. Blood. 1989 Feb;73(2):533–542. [PubMed] [Google Scholar]
- Uckun F. M., Jaszcz W., Ambrus J. L., Fauci A. S., Gajl-Peczalska K., Song C. W., Wick M. R., Myers D. E., Waddick K., Ledbetter J. A. Detailed studies on expression and function of CD19 surface determinant by using B43 monoclonal antibody and the clinical potential of anti-CD19 immunotoxins. Blood. 1988 Jan;71(1):13–29. [PubMed] [Google Scholar]
- Uckun F. M., Kersey J. H., Gajl-Peczalska K. J., Heerema N. A., Provisor A. J., Haag D., Gilchrist G., Song C. W., Arthur D. C., Roloff J. Heterogeneity of cultured leukemic lymphoid progenitor cells from B cell precursor acute lymphoblastic leukemia (ALL) patients. J Clin Invest. 1987 Sep;80(3):639–646. doi: 10.1172/JCI113116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Ledbetter J. A. Immunobiologic differences between normal and leukemic human B-cell precursors. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8603–8607. doi: 10.1073/pnas.85.22.8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Myers D. E., Fauci A. S., Chandan-Langlie M., Ambrus J. L. Leukemic B-cell precursors constitutively express functional receptors for human interleukin-1. Blood. 1989 Aug 1;74(2):761–776. [PubMed] [Google Scholar]
- Uckun F. M., Myers D. E., Ledbetter J. A., Swaim S. E., Gajl-Peczalska K. J., Vallera D. A. Use of colony assays and anti-T cell immunotoxins to elucidate the immunobiologic features of leukemic progenitor cells in T-lineage acute lymphoblastic leukemia. J Immunol. 1988 Mar 15;140(6):2103–2111. [PubMed] [Google Scholar]