Abstract
Hereditary Ferritinopathy (HF) is a neurodegenerative disease characterized by intracellular ferritin inclusion bodies (IBs) and iron accumulation throughout the central nervous system. Ferritin IBs are composed of mutant ferritin light chain as well as wild type light (Wt-FTL) and heavy chain (FTH1) polypeptides. In vitro studies have shown that the mutant light chain polypeptide p.Phe167SerfsX26 (Mt-FTL) forms soluble ferritin 24-mer homopolymers having a specific structural disruption that explains its functional problems of reduced ability to incorporate iron and aggregation during iron loading. However, because ferritins are usually 24-mer heteropolymers and all three polypeptides are found in IBs, we investigated the properties of Mt-FTL/FTH1 and Mt-FTL/Wt-FTL heteropolymeric ferritins. We show here the facile assembly of Mt-FTL and FTH1 subunits into soluble ferritin heteropolymers, but their ability to incorporate iron was significantly reduced relative to Wt-FTL/FTH1 heteropolymers. In addition, Mt-FTL/FTH1 heteropolymers formed aggregates during iron loading, contrasting Wt-FTL/FTH1 heteropolymers and similar to what was seen for Mt-FTL homopolymers. The resulting precipitate contained both Mt-FTL and FTH1 polypeptides as do ferritin IBs in patients with HF. The presence of Mt-FTL subunits in Mt-FTL/Wt-FTL heteropolymers also caused iron loading-induced aggregation relative to Wt-FTL homopolymers, with the precipitate containing Mt- and Wt-FTL polypeptides again paralleling HF. Our data demonstrate that co-assembly with wild type subunits does not circumvent the functional problems caused by mutant subunits. Furthermore, the functional problems characterized here in heteropolymers that contain mutant subunits parallel those problems previously reported in homopolymers composed exclusively of mutant subunits, which strongly suggests that the structural disruption characterized previously in Mt-FTL homopolymers occurs in a similar manner and to a significant extent in both Mt-FTL/FTH1 and Mt-FTL/Wt-FTL heteropolymers.
Keywords: Ferritin, iron, pore, neurodegeneration, hereditary ferritinopathy
1. Introduction
Ferritin is the main cellular iron storage protein and plays a pivotal role in iron homeostasis. There is increasing evidence that iron is involved in the mechanisms that underlie many neurodegenerative diseases[1]. Mammalian ferritin forms an ~ 480 kDa spherical shell (~12 nm of outer and 7 nm inner diameter) from 24 self-assembling subunits creating eight 3-fold pores, which constitute the iron entry pathways, and six 4-fold pores, which are smaller and perhaps sealed [2]. Ferritin oxidizes ferrous iron (Fe(II)) to ferric iron (Fe(III)), which is then sequestered in itsinterior as an iron -oxy biomineral matrix of up to 4,500 iron atoms. In doing so it performs a cellular detoxification role in helping control free iron levels and reducing radical-mediated damage.
Ferritin is usually found as a 24-mer heteropolymer with different ratios of light (FTL) and heavy chain (FTH1) subunits associated with tissue specific roles, although FTL homopolymers also occur in vivo [2]. The FTH1 subunit has ferroxidase activity (initiating the oxidation of Fe(II)), while the FTL subunit enhances iron nucleation (leading to biomineral formation) [2] and increases protein stability against elevated temperature and denaturants [3,4]. The FTL subunit also protects the heteropolymer against aggregation and precipitation that may occur while incubating with Fe(II) during iron incorporation, i.e., iron loading [5]. Interestingly, the homopolymer composed exclusively of FTL subunits can incorporate iron, but more slowly than the heteropolymer [2,5]. FTL and FTH1 polypeptides are 54% identical in sequence with specific replacements related to function. Both polypeptides fold into subunits composed of a bundle of 4 parallel, longer α helices (A, B, C, D) and a C-terminus with a shorter α helix (E). Extensive helix-helix interactions occur between these bundles and between the inwardly pointing E-helicies forming the 4-fold pores [2,6]. Both subunits are so similarly shaped as to be interchangeable in the shell [2,6].
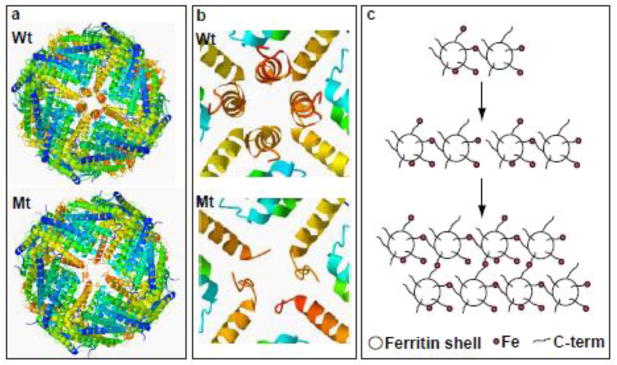
Nucleotide duplications in exon 4 of the FTL gene [7–12] cause the autosomal dominant neurodegenerative disease known as Neuroferritinopathy [7] or Hereditary Ferritinopathy (HF) [8,9]. HF is characterized by the presence of intracellular ferritin inclusion bodies (IBs) and iron accumulation throughout the central nervous and other organ systems [8,9]. The mutations alter the C-terminal residues of the FTL polypeptide, yielding light chains that are changed in length and sequence to differing extents depending on the specific mutation [13]. We have previously shown that the mutant FTL polypeptide p.Phe167SerfsX26 (Mt-FTL) assembled into soluble 24-mer homopolymericshells that were very similar to the Wt -FTL homopolymers in size, spherical shape, and overall structure (Fig. 1a) [14,15]. Relative to Wt-FTL, soluble Mt-FTL homopolymers showed reduced iron incorporation and iron loading-induced precipitation from solution as iron concentration was increased [14,15]. Structurally, we found that all 24 of the subunits in the Mt-FTL homopolymers lost the conformational integrity of their E helices, which in turn caused localized but substantial disruption of all six 4-fold pores making them unstable and leaky (Fig. 1b) [14, 15]. We proposed that this unraveling and extension of the C-terminal portion of the Mt-FTL subunits on one ferritin shell allowed them to link with unraveled C-termini on other shells through iron ion bridging leading to aggregation of the homopolymers (Fig. 1c) [14]. However, mammalian ferritins are almost exclusively heteropolymeric, and all three polypeptides (Mt-FTL, FTH1 and Wt-FTL) are found in IBs of patients with HF and in IBs of a transgenic animal model [8,16]. Thus, the focus of this work is to determine the consequences of (i) substituting Mt-FTL in place of Wt-FTL subunits in FTL/FTH1 24-mers and (ii) forming FTL 24-mers composed of both Mt-FTL and Wt-FTL subunits.
Figure 1. Ferritin structural disruption and aggregation caused by Mt-FTL subunits.
(a) The structure of the spherical shell is maintained in mutant ferritin as seen in thecrystallographic structures of Wt- and Mt-FTL homopolymers viewed down one of their 4-fold axes. (b) Close up views of the 4-fold pore from the interior of the Wt- and Mt-FTL structures show remarkable disruption in Mt-FTL making the pores unstable and leaky. Note that since the last 26 amino acids of Mt-FTL remained unaccounted for crystallographically, mutant C-termini are substantially longer than represented in (b), and if extended could reach as far as the diameter of the ferritin shell. (c) Iron loading-induced aggregation of Mt-FTL homopolymers is consistent with a model in which iron binds to the unraveled and extended portion of the mutant C-termini on two different ferritin shells bridging them and initiating a gradual accumulation of ferritin and iron into a precipitate. Bridging is not necessarily restricted to C-termini and may become more general, e.g., between a C-terminal group and a surface amino acid which both have affinity for iron. Structures were taken from RCSB (code 2FG8 for Wt-FTL and 3HX2 for Mt-FTL) and the precipitation model was modified from [14].
2. Materials and Methods
2.1. Purification, assembly and characterization of ferritin homo- and heteropolymers
Unless specified otherwise, the reagents and buffers used in this study were obtained from Sigma-Aldrich Chemical Company, Inc.
Mt-FTL (p.Phe167SerfsX26), FTH1 and Wt-FTL recombinant human polypeptides were expressed and purified from BL21 (DE3) Escherichia coli (Invitrogen) as ferritin 24-mer homopolymers as described previously [14]. In order to produce apoferritin, iron was removed through incubation of homopolymers with 1% thioglycollic acid, pH 5.5, and 2,2′-bipyridine, followed by dialysis against 0.1M phosphate buffer, pH 7.4. The resulting apoferritin homopolymers were dissociated into monomers and co-assembled into heteropolymers as follows. Mt-FTL, FTH1, and Wt-FTL homopolymers were equilibrated separately for 6 h with 6 M GdnHCl, pH 3.5, to ensure complete subunit dissociation. After mixing equal molar amounts (1 mM) of FTH1 subunits and either Mt-FTL or Wt-FTL, the solutions were diluted with phosphate buffer (0.1 M, pH 7.4) to a final GdnHCl concentration below 0.6 M. After 12 h incubation at room temperature for subunit reassembly, the solutions were dialyzed overnight against 0.1 M MOPS, pH 6.5. Reassembled 24-mers were electrophoresed on non-denaturing 3–8% polyacrylamide gels (Invitrogen) and stained with Coomasie blue (Pierce). Size exclusion chromatography elution profiles of the heteropolymers were obtained with a Superose 6 10/300 GL column (GE Healthcare) equilibrated with 50 mM Tris, 150 mM NaCl, pH 7.4, using an AKTA purifier system (GE Healthcare) as described previously [14]. Protein concentration was determined using BCA reagent (Pierce) with bovine serum albumin as standard.
2.2. Iron loading of apoferrritin heteropolymers and characterization of resulting heteropolymer solubility
Freshly prepared ferrous ammonium sulphate was added to separate samples of heteropolymeric or homopolymeric apoferritins in 0.1 M MOPS, pH 6.5, and incubated for 2 h at 24°C. The protein concentration was 1 μM and iron concentrations were 0, 1, and 3 mM. Samples were centrifuged for 15 min at 10,000 g in order to separate soluble from aggregated protein. The resulting soluble fractions were electrophoresed on non-denaturing 3–8% polyacrylamide gels and stained with Coomasie blue, and the pellets were resuspended in Laemmili sample buffer, electrophoresed on SDS 12% polyacrylamide gels (Pierce), and blotted with antibodies against FTH1 (Ramco), Mt-FTL (1238), and Wt-FTL (1277), as described previously [8].
2.3. Quantifying iron incorporation into soluble ferritin heteropolymers
Apoferritin heteropolymers were separately incubated with ferrous ammonium sulphate in 0.1 M MOPS, pH 6.5. The protein concentration was 1 μM and iron concentration was 1 mM. After 2 h, incubation was stopped by the addition of HCl and bathophenanthroline, each to a 10 mM final concentration. Unincorporated iron was separated from ferritin by electrophoresis on non-denaturing 3–8% polyacrylamide gels, and the remaining iron biomineral was quantitated by Prussian blue formation within the ferritin bands [15]. The amount of iron not incorporated into ferritin after incubation was determined by spectroscopically measuring the iron remaining in solution. Bathophenantholine was added to the samples after the 2 h incubation with iron, the samples were diluted 10-fold with MOPS buffer (0.1 M, pH 6.5), and their absorbances were read at 535 nm. The amount of chelated Fe(II) was calculated using an extinction coefficient at 535 nm of 22140 M−1cm −1. All data are presented as mean ± S.D. using independent experiments performed in triplicate. *, p<0.01.
3. Results
3.1. Mt-FTL formed heteropolymers with either FTH1 or Wt-FTL under routine conditions
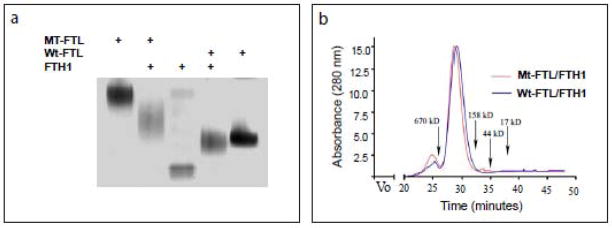
To determine whether Mt-FTL subunits are capable of co-assembly with FTH1 subunits into 24-mer heteropolymers, recombinant ferritin subunits were mixed in a 1:1 ratio and put through a routine reassembly procedure [14,15]. Native PAGE showed that Mt-FTL/FTH1 and Wt-FTL/FTH1 heteropolymers have intermediate electrophoretic mobilities between Mt-FTL, FTH1 and Wt-FTL homopolymers, which confirmed that the individual subunits had co-assembled into heteropolymers (Fig. 2a). By gel chromatography, the two heteropolymers showed elution profiles that were almost identical with clear, overlapping peaks indicative of ferritin 24-mer formation (Fig. 2b). Importantly, we did not observe a peak at ~20 kDa in the chromatograms, which would be indicative of the presence of non-assembled subunits. In a parallel set of experiments, Mt-FTL subunits were found to assemble in a very similar manner with Wt-FTL subunits producing Mt-FTL/Wt-FTL heteropolymers (data not shown).
Figure 2. Formation of ferritin heteropolymers from Mt-FTL and FTH1 polypeptides.
Recombinant Mt-FTL and FTH1 (or Wt-FTL and FTH1) polypeptides (1 mM) were mixed in a 1:1 ratio and allowed to co-assemble into heteropolymers by gradually removing GdnHCl. (a) Five g of reassembled Mt-FTL/FTH1 or Wt-FTL/FTH1 apoferritin heteropolymers were resolved by 3–8% native PAGE and stained with Coomasie blue. Mt-FTL, FTH1, and Wt-FTL apoferritin homopolymers were run as controls. (b) Size exclusion chromatographic elution profiles of the heteropolymers were obtained with a Superose 6 10/300 GL column with elution time markers for molecular weights indicated with arrows.
3.2. Iron loading induced the precipitation of both Mt-FTL/FTH1 and Mt-FTL/Wt-FTL heteropolymers
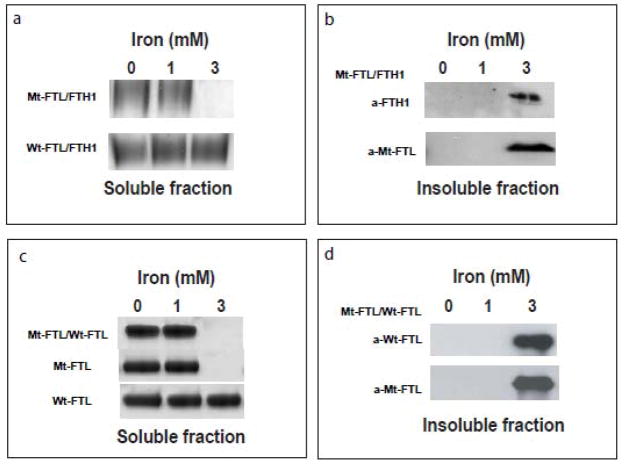
To test if the substitution of Mt-FTL for Wt-FTL subunits in heteropolymeric ferritin can enhance susceptibility to precipitation, we compared the solubility of Mt-FTL/FTH1 and Wt-FTL/FTH1 heteropolymers during iron loading by increasing the concentration of iron in increments of 1000 iron atoms per ferritin molecule as described previously [5,14,15]. Mt-FTL/FTH1 heteropolymers began to precipitate at iron loading over 1,000 atoms of iron per 24-mer, as previously reported for Mt-FTL homopolymers [14]. Native PAGE showed that at an iron loading of 3,000 atoms of iron per 24-mer, Mt-FTL/FTH1 heteropolymers were removed from solution by centrifugation, but Wt-FTL/FTH1 heteropolymers remained soluble (Fig. 3a). Western blot analysis of the precipitates showed the presence of Mt-FTL and FTH1 polypeptides in the insoluble Mt-FTL/FTH1 heteropolymeric fraction (Fig. 3b). In a parallel experiment, iron loading of Mt-FTL/Wt-FTL heteropolymers under the same conditions showed precipitation by iron in a manner similar to that observed for Mt-FTL/FTH1 heteropolymers (Fig. 3c). Western blot analysis of the resulting insoluble fraction showed the presence of both polypeptides in the precipitate (Fig. 3d).
Figure 3. Iron loading-induced precipitation of ferritin heteropolymers containing Mt-FTL subunits.
Ferrous ammonium sulphate was added to separate samples of heteropolymeric (Mt-FTL/FTH1, Wt-FTL/FTH1, and Mt-FTL/Wt-FTL) or homopolymeric (Mt-FTL and Wt-FTL) apoferritins, and incubated for 2 h. The protein concentration was 1 μM and iron concentrations were 0, 1, and 3 mM. Samples were centrifuged for 15 min at 10,000 g in order to separate soluble from aggregated protein. In contrast to the solubility of Wt-FTL/FTH1 heteropolymers, precipitation of Mt-FTL/FTH1 and Mt-FTL/Wt-FTL heteropolymers was observed at 3 mM iron. (a) Soluble fractions of Mt-FTL/FTH1 and Wt-FTL/FTH1 heteropolymers were characterized by 3–8% native PAGE and stained with Coomasie blue. (b) The insoluble fraction was resuspended in Laemmli sample buffer, resolved by SDS 12% PAGE, and blotted with antibodies against FTH1 and Mt-FTL. (c) Mt-FTL/Wt-FTL heteropolymers and the corresponding homopolymers were iron loaded and electrophoresed as in (a) above, showing precipitation for hetero- or homopolymers containing Mt-FTL. (d) The insoluble fraction from Mt-FTL/Wt-FTL was resuspended, electrophoresed, and blotted as in (b) above, with antibodies against Wt-FTL and Mt-FTL.
3.3. Soluble Mt-FTL/FTH1 heteropolymers exhibited reduced iron incorporation
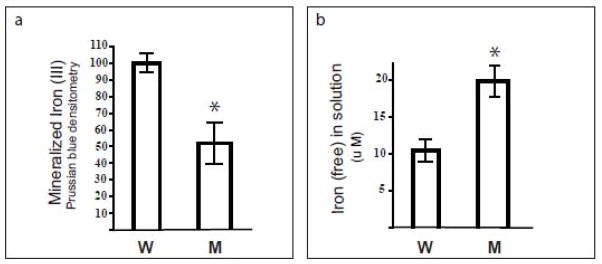
To determine if the substitution of Mt-FTL for Wt-FTL subunits in ferritin heteropolymers can reduce net iron incorporation, soluble Mt-FTL/FTH1 and Wt-FTL/FTH1 heteropolymers were separately incubated in a 1000:1 iron:heteropolymer ratio as described previously [15]. After non-denaturing electrophoresis and Prussian blue staining, we observed an ~50% reduction in the amount of biomineralized iron within Mt-FTL/FTH1 heteropolymers compared to that of Wt-FTL/FTH1 heteropolymers (Fig. 4a). Moreover, the amount of free iron remaining in solution for Mt-FTL/FTH1 heteropolymers was approximately double of that measured for Wt-FTL/FTH1 heteropolymers (Fig. 4b) in agreement with the biomineralized iron data.
Figure 4. Iron incorporation by soluble ferritin heteropolymers.
Mt-FTL/FTH1 (M) and Wt-FTL/FTH1 (W) apoferritin heteropolymers were separately incubated with ferrous ammonium sulphate at concentrations of 1 μM and 1 mM, respectively. After 2 h, incubation was stopped by the addition of HCl and bathophenanthroline. (a) After separating unincorporated iron from the protein by electrophoresis on non-denaturing gels (3–8% native PAGE), iron biomineral within the heteropolymer interior was quantitated as density of Prussian blue formed in the protein bands. (b) Iron incorporation by the Mt-FTL/FTH1 heteropolymers relative to Wt-FTL/FTH1 was determined through measurement of residual ferrous iron in solution. Bathophenantholine was added to the samples after the 2 h incubation with iron, the samples were diluted 10-fold with buffer, their absorbances were read at 535 nm, and the amount of chelated Fe(II) was calculated using an extinction coefficient of 22140 M−1cm −1.
4. Discussion
Although the role of ferritin in iron homeostasis has been extensively studied, the mechanism(s) by which mutant FTL polypeptides cause neurodegeneration is still unclear. Herein, we show that the non-native structure of Mt-FTL subunits [14,15] does not prevent their co-assembly into heteropolymers with either FTH1 or Wt-FTL subunits. However, substitution of Mt-FTL for Wt-FTL subunits in ferritin can cause compromised function through reduced ability of soluble ferritin to incorporate iron (a loss of normal function) and through iron-induced aggregation during the iron loading process (a gain of toxic function). The reduction of net iron incorporation into soluble heteropolymers of Mt-FTL/FTH1 relative to Wt-FTL/FTH1 is consistent with increased iron levels in HF and an HF animal model [13, 16, 17], and is most likely due to structural disruption of the normally small (or sealed) 4-fold pores in ferritin heteropolymers, as was demonstrated crystallographically in Mt-FTL homopolymers (Fig. 1b) [15]. In HF, loss of normal ferritin function may lead to altered iron homeostasis and free radical formation, as suggested by multiple cellular markers for damage by reactive oxygen species (ROS) found in transgenic mice overexpressing Mt-FTL [17]. Increased free iron levels would also lead to iron-dependent upregulation of cytosolic ferritin by activation of translation of ferritin mRNAs, culminating in intracellular accumulation of ferritin as aggregates in the presence of iron [13].
The iron loading-induced aggregation and precipitation from solution of Mt-FTL/FTH1 and Mt-FTL/Wt-FTL heteropolymeric ferritins are most likely due to both these 24-mers having some level of structural disruption similar to that found in the Mt-FTL homopolymers with their unraveled and extended C-terminal E helices [15]. Precipitation of the mutant-containing heteropolymers would follow from our model for the Mt-FTL homopolymers of iron bridging initiating at unraveled C-termini (Fig.1c) [14]. However, the Mt-FTL/FTH1 and Mt-FTL/Wt-FTL heteropolymeric precipitation observed here indicates that aggregation does not require all 24 or even a majority of the 24 subunits to be Mt-FTL. Clearly, the presence of half Wt-FTL subunits in the Mt-FTL/Wt-FTL heteropolymers was insufficient to prevent iron loading-induced precipitation from solution, even though Wt-FTL subunits stabilize ferritin against elevated temperature and denaturants [3,4] and can protect against aggregation during the iron loading process [5]. It remains to be systematically determined what is the minimal number (per 24-mer) of Mt-FTL subunits that can make the heteropolymers susceptible to precipitation by iron. The presence of either FTH1 or Wt-FTL polypeptides in combination with Mt-FTL polypeptides in the iron loading-induced precipitates in vitro parallels their presence in IBs of patients with HF and in IBs from a transgenic mouse model [8,16].
The finding that the functional problems (aggregation and reduced iron incorporation) caused by the presence of the Mt-FTL subunits in heteropolymeric ferritins parallel those found when examining the difference between Mt- and Wt-FTL homopolymers is significant. The parallel functional problems strongly support the proposal that the structural disruption previously characterized in Mt-FTL homopolymers occurs in a similar manner and to a significant extent in the Mt-FTL/FTH1 and Mt-FTL/Wt-FTL heteropolymers leading to their compromised function. Functional problems with FTH1-containing heteropolymers may take on added importance in that they exhibit a larger iron incorporation rate than Wt-FTL homopolymers, although the potential importance to cellular dysfunction of cumulative aggregation of all forms of ferritin enhanced by the mutant should not be discounted.
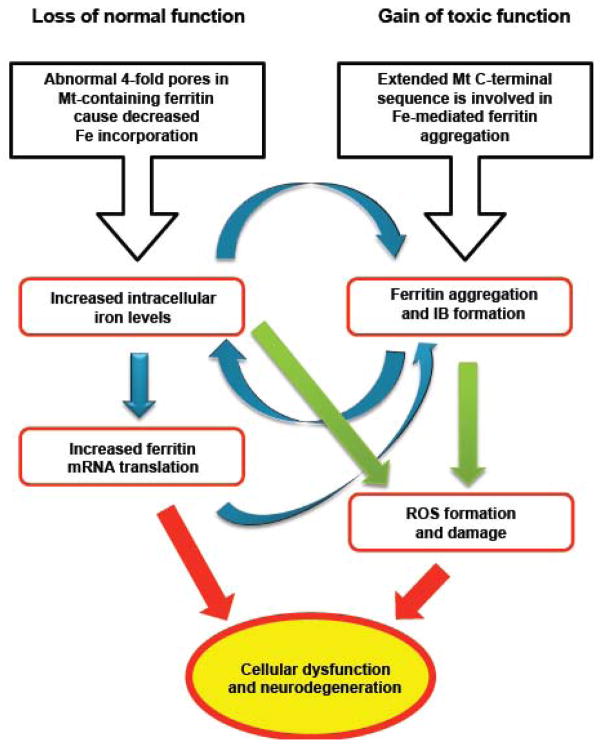
The in vitro functional problems characterized here in mutant-containing ferritin heteropolymers support a connection to demonstrated cellular abnormalities in patients with HF and in an animal model [8, 16–18] consistent with both a loss of normal function and a gain of toxic function of ferritin (Fig. 5). Further study of the molecular basis of altered iron homeostasis, ferritin aggregation, and resulting cellular dysfunction may lead to a better understanding of neurodegenerative disorders and point toward the development of forms of therapeutic strategy.
Figure 5. Altered iron metabolism and ferritin aggregation in the pathogenesis of HF.
Iron homeostasis is crucial in providing an optimum amount of iron as a cofactor for proteins while preventing generation of ROS, which can cause damage to proteins, membranes and DNA [1]. The cellular abnormalities associated with the FTL mutation originate in 4-fold pore disruption and enhanced aggregation, and can be understood in terms of a loss of normal function (left) and a gain of toxic function (right) of ferritin. Increased cellular iron levels, up-regulation of ferritin production, and aggregation appear to work synergistically (blue arrows) to form large nuclear and cytoplasmic IBs, perhaps even causing cellular misfunction through mechanical crowding. Increased iron levels may enhance ROS generation at a location separate from ferritin or as part of the compromised iron handling and aggregation of ferritin itself (green arrows). Thus cellular dysfunction may be a consequence of both a decrease in normal function and an increase of toxic function of ferritin leading to neurodegeneration.
Acknowledgments
The work was supported by National Institutes of Health Grants NS050227 and NS063056.
Abbreviations
The abbreviations used are
- FTL
ferritin light chain
- FTH1
(wild type) ferritin heavy chain
- Wt-FTL
wild type ferritin light chain
- Mt-FTL
mutant ferritin light chain
- HF
hereditary ferritinopathy
- IBs
inclusion bodies
- GdnHCl
guanidine hydrochloride
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 2.Crichton R. Iron metabolism from molecular mechanisms to clinical consequences. 3. Wiley; West Sussex, U.K: 2009. [Google Scholar]
- 3.Stefanini S, Cavallo S, Wang CQ, Tataseo P, Vecchini P, Giartosio A, Chiancone E. Thermal stability of horse spleen apoferritin and human recombinant H apoferritin. Arch Biochem Biophys. 1996;325:58–4. doi: 10.1006/abbi.1996.0007. [DOI] [PubMed] [Google Scholar]
- 4.Santambrogio P, Levi S, Arosio P, Palagi L, Vecchio G, Lawson DM, Yewdall SJ, Artymiuk PJ, Harrison PM, Jappelli R, Cesareni G. Evidence that a salt bridge in the light chain contributes to the physical stability difference between heavy and light human ferritins. J Biol Chem. 1992;267:14077–14083. [PubMed] [Google Scholar]
- 5.Levi S, Santambrogio P, Cozzi A, Rovida E, Corsi B, Tamborini E, Spada S, Albertini A, Arosio P. The role of the L-chain in ferritin iron incorporation. Studies of homo and heteropolymers. J Mol Biol. 1994;238:649–654. doi: 10.1006/jmbi.1994.1325. [DOI] [PubMed] [Google Scholar]
- 6.Hempstead PD, Yewdall SJ, Fernie AR, Lawson DM, Artymiuk PJ, Rice DW, Ford GC, Harrison PM. Comparison of the three-dimensional structures of recombinant human H and horse L ferritins at high resolution. J Mol Biol. 1997;268:424–448. doi: 10.1006/jmbi.1997.0970. [DOI] [PubMed] [Google Scholar]
- 7.Curtis AR, Fey C, Morris CM, Bindoff LA, Ince PG, Chinnery PF, Coulthard A, Jackson MJ, Jackson AP, McHale DP, Hay D, Barker WA, Markham AF, Bates D, Curtis A, Burn J. Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nature Genet. 2001;28:350–354. doi: 10.1038/ng571. [DOI] [PubMed] [Google Scholar]
- 8.Vidal R, Ghetti B, Takao M, Brefel-Courbon C, Uro-Coste E, Glazier BS, Siani V, Benson MD, Calvas P, Miravalle L, Rascol O, Delisle MB. Intracellular ferritin accumulation in neural and extraneural tissue characterizes a neurodegenerative disease associated with a mutation in the ferritin light polypeptide gene. J Neuropathol Exp Neurol. 2004;63:363–380. doi: 10.1093/jnen/63.4.363. [DOI] [PubMed] [Google Scholar]
- 9.Mancuso M, Davidzon G, Kurlan RM, Tawil R, Bonilla E, Di Mauro S, Powers JM. Hereditary ferritinopathy: a novel mutation, its cellular pathology, and pathogenetic insights. J Neuropathol Exp Neurol. 2005;64:280–294. doi: 10.1093/jnen/64.4.280. [DOI] [PubMed] [Google Scholar]
- 10.Ohta E, Nagasaka T, Shindo K, Toma S, Nagasaka K, Ohta K, Shiozawa Z. Neuroferritinopathy in a Japanese family with a duplication in the ferritin light chain gene. Neurology. 2008;70:1493–1494. doi: 10.1212/01.wnl.0000310428.74624.95. [DOI] [PubMed] [Google Scholar]
- 11.Kubota A, Hida A, Ichikawa Y, Momose Y, Goto J, Igeta Y, Hashida H, Yoshida K, Ikeda S, Kanazawa I, Tsuji S. A novel ferritin light chain gene mutation in a Japanese family with neuroferritinopathy: description of clinical features and implications for genotype-phenotype correlations. Mov Disord. 2009;24:441–445. doi: 10.1002/mds.22435. [DOI] [PubMed] [Google Scholar]
- 12.Devos D, Jissendi Tchofo P, Vuillaume I, Destée A, Batey S, Burn J, Chinnery PF. Clinical features and natural history of neuroferritinopathy caused by the 458dupA FTL mutation. Brain. 2008;132(Pt 6):e109. doi: 10.1093/brain/awn274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vidal R, Delisle MB, Ghetti B. Neurodegeneration caused by proteins with an aberrant carboxyl-terminus. J Neuropathol Exp Neurol. 2004;63:787–800. doi: 10.1093/jnen/63.8.787. [DOI] [PubMed] [Google Scholar]
- 14.Baraibar MA, Barbeito AG, Muhoberac BB, Vidal R. Iron-mediated aggregation and a localized structural change characterize ferritin from a mutant light chain polypeptide that causes neurodegeneration. J Biol Chem. 2008;283:31679–31689. doi: 10.1074/jbc.M805532200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baraibar MA, Muhoberac BB, Garringer HJ, Hurley TD, Vidal R. Unraveling of the E helices and disruption of 4-fold pores are associated with iron mishandling in a mutant ferritin causing neurodegeneration. J Biol Chem. 2010;285:1950–1956. doi: 10.1074/jbc.M109.042986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vidal R, Miravalle L, Gao X, Barbeito AG, Baraibar MA, Hekmatyar SK, Widel M, Bansal N, Delisle MB, Ghetti B. Expression of a mutant form of the ferritin light chain gene induces neurodegeneration and iron overload in transgenic mice. J Neurosci. 2008;28:60–67. doi: 10.1523/JNEUROSCI.3962-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbeito AG, Garringer HJ, Baraibar MA, Gao X, Arredondo M, Núñez MT, Smith MA, Ghetti B, Vidal R. Abnormal iron metabolism and oxidative stress in mice expressing a mutant form of the ferritin light polypeptide gene. J Neurochem. 2009;109:1067–1078. doi: 10.1111/j.1471-4159.2009.06028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng X, Vidal R, Englander EW. Accumulation of oxidative DNA damage in brain mitochondria in mouse model of hereditary ferritinopathy. Neurosci Lett. 2010;479:44–48. doi: 10.1016/j.neulet.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]