Abstract
The hypothalamic melanocortin system is known for its role in regulating energy homeostasis through it actions within hypothalamic brain centers. However, emerging evidence suggests that this system regulates addictive behaviors through signaling within mesolimbic neurons. Here, we hypothesized the melanocortin system modulates feeding behavior through its actions on mesolimbic neurons. In particular, we predicted that central administration of the melanocortin antagonist agouti-related peptide (AgRP) would activate midbrain dopamine neurons, increase mesolimbic dopamine turnover, and alter food seeking behaviors. We found that intraventricular administration of agouti-related peptide increased neuronal activation within midbrain dopamine neurons in addition to increasing dopamine turnover in the medial prefrontal cortex. Additionally, using the conditioned place preference paradigm to assay food seeking behavior, we report that central injection of agouti-related peptide attenuates the acquisition of a conditioned place preference for sucrose, but not high fat diet. These results suggest that the melanocortin system is capable of regulating mesocorticolimbic activity and food seeking behavior.
Keywords: melanocortins, MC4R, reward, conditioned place preference, Food Seeking Behavior, Mesocorticolimbic, Dopamine
1.Introduction
The melanocortin system has been studied extensively for its role in the regulation of food intake. This system is unique in that it possesses both an endogenous agonist and an inverse agonist with opposite effects on feeding behavior. Alpha-Melanocyte stimulating hormone (α-MSH) and agouti related peptide (AgRP) are the primary effector peptides of the melanocortin system. Increases in α -MSH serve to decrease food intake while increases in AgRP drive food consumption; under basal conditions the expression of each peptide is regulated by caloric status as they are downstream targets of the adiposity hormone leptin (Schwartz M et al., 2000). Traditionally, these peptides are thought to mediate their effects by binding specifically to the melanocortin-4 receptor (MC4R) within a distributed set of nuclei in the hypothalamus. However, emerging evidence suggests that this system is capable of mediating effects outside the hypothalamus, specifically within the mesolimbic system (Alvaro et al., 1996, 2003, Hsu et al., 2005).
Separate from their expression in hypothalamic nuclei, melanocortin receptors (MC3R & MC4R) are also expressed in brain regions that regulate motivated behaviors (Mountjoy et al., 1994; Alvaro et al., 1996; Adan & Gispen, 1997). Importantly, pharmacological studies have outlined functional roles for these receptors in the modulation of drug taking behavior. Specifically, antagonism of these receptors within nucleus accubmens inhibits operant responding for cocaine, while central agonism of this system augments amphetamine related behaviors (Cabeza de Vaca et al., 2002, Hsu et al., 2005). Moreover, the expression of the MC4R is increased in the striatum after repeated exposure to psychostimulants and mutant mice lacking this particular receptor fail to display a sensitized locomotor response to repeated cocaine administration (Hsu et al., 2005). In terms of food reward, central administration of AgRP increases neuronal activation within brain reward circuitry and augments operant responding for palatable food reinforcers (Hagan et al., 2001, Tracy et al., 2008) indicating that the nature by which melanocortins influence reward may differ depending on the particular reinforcer examined, i.e. drug vs. food. Taken together, these results suggest that the MC system is capable of influencing a variety of reward-related behaviors.
Here we hypothesized that the melanocortin antagonist AgRP would activate midbrain dopamine neurons and stimulate dopamine release within the mesolimbic system, in addition to modulating the conditioned place preference for food. In particular, we predicted that central injection of AgRP would increase c-Fos immunoreactivity within dopamine neurons in the ventral tegmental area and facilitate dopamine turnover in the nucleus accumbens (NAcc). We further predicted that antagonism of the MC system would enhance the conditioned place preference (CPP) for food. We found that central injection of AgRP activated dopamine containing neurons in the ventral tegmental area and increased dopamine turnover in the medial Prefrontal Cortex (mPFC). In addition, pharmacological administration of AgRP supported a CPP for high fat diet but attenuated CPP for sucrose. Collectively, these results suggest that melanocortin antagonists alter mesocortical dopamine neurochemistry, and modulate food seeking behavior.
2. General Method
2.1 Subjects
Thirty-two Long-Evans rats (Harlan, IN) weighing 200-250 g were housed individually in a vivarium with a 12:12 light/dark schedule. The temperature of the room was maintained at 25° C. All animals had ad libitum access to food and water and were maintained on standard rodent chow. The temperature of the room was maintained at 25° C. All animals had ad libitum access to food and water and were maintained on standard rodent chow. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati and are in accordance with the guidelines set forth by the American Psychological Association.
2.2 Diets
During the conditioned place preference experiment, rats received either high fat diet (HFD; Dyets, Inc., Bethlehem, PA, 4.41 kcal/gm, 1.71 kcal/gm from fat) or thirty 45mg sucrose pellets (TestDiet, Richmond, IN).
2.3 Stereotaxic Surgery
After a one week habituation period, animals in experiment 1& experiment 2 were deeply anesthetized with a 1-ml/kg dose of (0.22g Ketamine/0.03g Xylazine) and placed into a sterotaxic apparatus with the incisor bars set at +1.0. Subsequently, an indwelling cannula was lowered into the third ventricle using the following coordinates, AP= -2.2, ML=0, DV= -7.0. All animals were allowed to recover for two weeks during which time they regained their pre-surgical body weight. To verify cannula placement, angiotensin II (10ng/μl) was injected into the third ventricle and water consumption was measured over a 1 hour period. To be included animals had to drink more than 7ml.
2.4 Drugs
For the conditioned place preference experiment (experiment 1) rats were given a 10pmole injection of (83-132) Agouti related peptide (AgRP) fragment (Phoneix Pharmaceuticals, Phoenix, AZ) thirty minutes prior to each conditioning session. To assess neuronal activation within midbrain dopamine neurons rats were given 1nmol of AgRP and sacrificed sixty minutes following injection.
2.5 Immunohistochemistry
To determine if midbrain dopaminergic neurons become activated following AgRP injection male rats (n=8/group) received central injections of saline or AgRP (1nmol) into the third ventricle. Sixty minutes following the injection, rats were injected with an overdose of pentobarbital and perfused transcardially with ice cold saline for 1 min followed by 4% paraformaldehyde in 1x PBS for 20 min. The brains were postfixed overnight in 4% paraformaldehyde with 1x PBS and then cryoprotected in 1x PBS with 30% sucrose for a minimum of 24 h. Brains were frozen and sectioned at 35 mm intervals and collected in cryoprotectant. A full series of sections was double-labeled for c-Fos and tyrosine hydroxylase (TH) immunohistochemistry. After washes in 1% sodium hydroxide + 1% hydrogen peroxide/PBS; 0.3% glycine/PBS; and 0.03% sodium dodecyl sulfate/PBS; sections were blocked in 4% horse serum with 0.4% Triton X-100. Rabbit anti-c-Fos antibody (1:500) was applied to the sections for overnight incubation at RT. The secondary antibody, biotinylated goat anti-rabbit IgG (1:300; Vector Laboratories; Burlingame, CA), was applied to the sections at RT followed with incubation in avidin-biotin complex (1:500; Vectastain ABC Elite Standard; Vector Laboratories; Burlingame, CA) and an incubation in biotinyl tyramide signal amplification solution (1:250; PerkinElmer LAS; Boston, MA). Cyanine 3 (Cy3; 1:200; Jackson Immuno Research; West Grove, PA) was then applied to the sections. The sections were then washed with PBS followed by incubation in 4% goat serum + 0.4% Triton X-100 blocking solution followed by mouse anti-TH antibody (1:500 Millipore, Temecula CA, catalog # MAB 318) formulated in the same blocking solution overnight at RT followed by incubation in Alexa-488 goat anti-mouse IgG diluted (1:200; Invitrogen, Carlsbad CA, catalog # A11001). Slides were cover slipped with gelvatol mounting media.
2.6 Dopamine Neurochemistry
The nucleus accumbens (NAcc) and medial Prefrontal Cortex (mPFC) were collected from each group of animals (vehicle, AgRP) respectively. Ninety minutes after injection, tissue was collected and stored at -80°C until processing. For high-performance liquid chromatography (HPLC) analysis, an antioxidant solution (0.4 N perchlorate, 1.343 mM ethylenediaminetetraacetic acid (EDTA) and 0.526 mM sodium metabisulfite) was added to the samples followed by homogenization using an ultrasonic tissue homogenizer (Biologics, Gainesville, VA). A small portion of the tissue homogenate was dissolved in 2% sodium dodecyl sulfate (SDS) (w/v) for protein determination (Pierce BCA Protein Reagent Kit, Rockford, IL). The remaining suspension was spun at 14,000g for 20 min in a refrigerated centrifuge. The supernatant was reserved for HPLC. Samples were separated on a Microsorb MV C-18 column (5 Am, 4.6_250 mm, Varian, Walnut Creek, CA) and simultaneously examined for DA, its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), 5-HT and 5-HIAA. Compounds were detected using a 12-channel coulometric array detector (CoulArray 5200, ESA, Chelmsford, MA) attached to a Waters 2695 Solvent Delivery System (Waters, Milford, MA) under the following conditions: flow rate of 1 ml/min; detection potentials of 50, 175, 350, 400 and 525 mV, and; scrubbing potential of 650 mV. The mobile phase consisted of a 10% methanol solution in distilled H2O containing 21 g/l (0.1 M) citric acid, 10.65g/l (0.075 M) Na2HPO4, 176 mg/l (0.8 M) heptanesulfonic acid and 36 mg/l (0.097 mM) EDTA at a pH of 4.1. Unknown samples were quantified against a 6-point standard curve with a minimum R2 of 0.97. Quality control samples were interspersed with each run to ensure HPLC calibration. Results were expressed as ng of analyte per mg protein.
2.7 Conditioned Place Preference
The conditioned place preference (CPP) apparatus used in this study was partitioned into three chambers with a neutral center chamber. One side of the chamber had white walls and a grid flooring, while the other side was black with stainless steel rods as flooring, the center chamber was grey with plexiglass flooring (Med Associates, St. Albans, VT). In order to reduce neophobia to the CPP chamber, all rats were placed into the center chamber with free access to all chambers for fifteen minutes prior to any conditioning session. During this habituation session the time spent in each chamber was monitored using a video tracking system (Cleversystems, Reston VA) to determine a natural preference for each rat. In experiment 2, rats were given a central injection of AgRP (10pmole/μl) or isotonic saline and restricted to one side of the chamber for thirty minutes with 5gm of HFD, which served as the rewarding stimuli. Both injection schedule and receipt of food reward were counterbalanced across groups and this conditioning paradigm was repeated for 12 consecutive days totaling six conditioning sessions before the final test day. On the test day, animals were placed in the center chamber and allowed free access to all chambers for fifteen minutes and the total time in each chamber was again recorded in order to establish a post-test preference value. Experiment 3 utilized the same conditioning strategy but used 30 45mg sucrose pellets as a reward rather than HFD. All rats in each experiment were maintained on standard rodent chow throughout the CPP conditioning sessions; food was removed during the thirty minute period following AgRP or saline administration and re-fed following testing.
2.8 Statistical analysis
Data were analyzed using STATISTICA version 6.0 for PC's. All data were analyzed using analysis of variance (ANOVA) and LSD post-hoc comparisons were used to asses the source of significant main effects.
3.Results
3.1 Immunohistochemistry
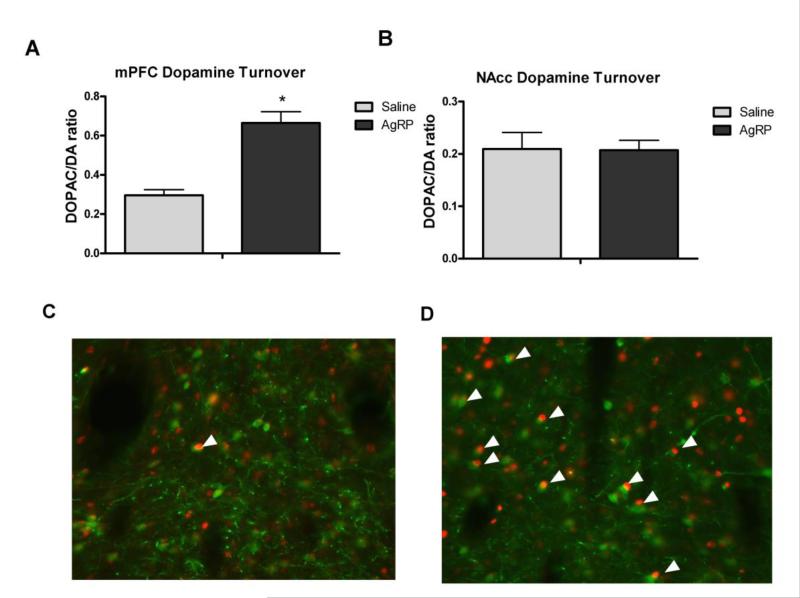
To determine if AgRP was sufficient to activate midbrain dopamine neurons we assessed the colocalization of c-Fos with tyrosine hydroxylase, the rate limiting enzyme for dopamine production, neurons. Compared to saline injected control rats, a 1nmol dose of AgRP led to increased activation of TH neurons in the midbrain tissue (Fig 1 C&D) indicating that melanocortin antagonists are capable of eliciting neuronal activation within midbrain dopaminergic neurons. In addition, this dose of AgRP was sufficient to increase overall c-Fos levels in the VTA compared to controls; however this difference did not reach statistical significance (Supplemental Figure 1).
Figure 1.
Dopamine turnover in the A) medial prefrontal cortex (mPFC) or B) nucleus accumben (NAcc) ninety minutes after a I3vt AgRP injection and c-Fos immunoreactivity in TH positive neurons following C) saline or D) 1nmol AgRP injection. arrows indicate co-localization of c-Fos in TH neurons; c-Fos appears in red, TH positive neurons are labeled in green.* = p<0.05.
3.2 Dopamine Neurochemistry
In experiment 1, we further hypothesized that AgRP would increase dopamine turnover in brain regions that regulate motivated behaviors, namely the mesocorticolimbic system. Animals injected with AgRP displayed increased dopamine turnover ([HVA]/[DA]) within the mPFC relative to animals injected with saline [F(1,6)=26.1670, p<0.01] (Fig 1A). There were no differences in NAcc dopamine between any of the groups tested (ps>0.05), nor did we observe any differences in catecholamine metabolites (5HT, HVA, NE, 5HIAA, DOPAC) in either brain region (Fig 1B).
3.3 AgRP Conditioned Place Preference
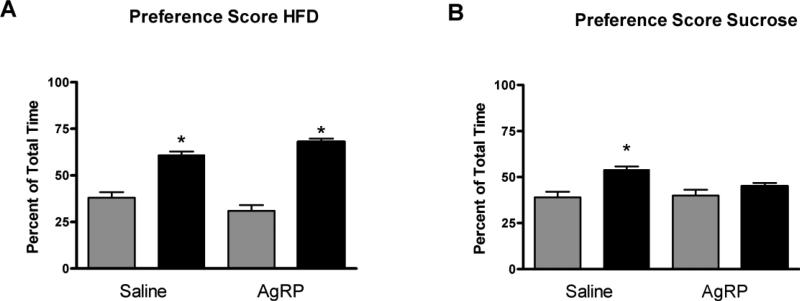
This experiment evaluated the effect of centrally administered AgRP on CPP for HFD. Both AgRP and saline treated rats ingested the entire ration of HFD during each conditioning session (AgRP = 5.4 g vs. Saline = 5.4 g), demonstrating that central administration of AgRP did not effect the consumption of HFD during the acquisition of the CPP. As expected, there was an effect of HFD on the expression of CPP (F (1, 18)=23.97; p < 0.01) with rats spending significantly more time in the compartment that was paired with HFD when compared to that of pre-conditioning (Fig 2A). However, there was no interaction between treatments and the amplitude of CPP formed to HFD did not differ between rats injected with AgRP or saline. The next experiment assessed whether central AgRP administration would support the acquisition of a CPP to sucrose. Similar to the previous experiment, rats given AgRP or saline consumed all of the sucrose pellets provided during training (30 pellets). Control rats injected with isotonic saline throughout training and conditioned with sucrose pellets spent significantly more time in the sucrose paired chamber on test day (F (1, 14)=5.91; p=0.029081) when compared to pre-conditioning values. However, in contrast to the HFD conditioning paradigm, rats given central injections of AgRP failed to form a CPP for sucrose after 12 consecutive conditioning sessions (F (1, 16)=0.609 p>0.05; Fig 2B).
Figure 2.
A) Conditioned place preference score after 6 conditioning sessions with HFD or B) sucrose in rats injected with I3vt AgRP during training. CPP score is expressed as percentage of total time spent in paired chamber on test day only * = p<0.05.
4.Discussion
The goal of the current study was to determine if the melanocortin system was capable of activating midbrain dopamine neurons and modulating mesolimbic dopamine flux. A separate goal was to determine the ability of AgRP to affect food seeking behavior using the conditioned place preference procedure. From these efforts several significant findings emerged: 1) Central administration of AgRP enhanced neuronal activation within dopaminergic neurons in the ventral tegmental area; 2) AgRP administration increased mesocortical but not mesolimbic dopamine turnover and 3) AgRP modulated the acquisition of CPP in a macronutrient dependent fashion. Taken together, these data suggest that one way in which melanocortin antagonists affect feeding behavior is through modulation of brain reward circuitry and that melanocortins are capable of influencing the acquisition of learned associations with food reinforcers.
AgRP increases mesocortical dopamine turnover
Dopamine is known to be a subsecond modulator of food seeking behavior in rodents (Roitman et al., 2004). Importantly, increases in mesolimbic dopamine precede food delivery in classically conditioned animals (Day et al., 2007). In the first experiment, we tested the hypothesis that melanocortins modulate mesolimbic dopamine activity by measuring dopamine turnover in the nucleus accumbens (NAcc) and medial prefrontal cortex (mPFC) after AgRP administration. Central administration of AgRP did not alter NAcc dopamine levels or dopamine turnover relative to saline injected control rats. Previous studies which have investigated the nature of melanocortins to induce alterations in mesolimbic dopamine concentrations have yielded mixed results. For example, Torre & Celis reported that direct injection of α-MSH into the ventral tegmental area (VTA), the primary source of central dopamine, yielded increased NAcc dopamine (Torre & Celis et al., 1988) an effect that is dependent on MC4R activation (Lindbloom et al., 2001) suggesting that agonism of VTA MC4R receptors is sufficient to activate dopaminergic projection neurons resulting in increased NAcc dopamine. . However an earlier report by the same group reported decreased NAcc dopamine following direct application of α-MSH into the third ventricle or VTA (Torre & Celis, 1986). Thus, it is difficult to predict the direction of NAcc levels following application of the melanocortin antagonist, AgRP. In the current study, third ventricular delivery of AgRP did not alter NAcc dopamine. Interestingly, this data is inconsistent with functional anatomical studies which report that AgRP elicits neuronal activation within mesolimbic neurons, notably the NAcc (Hagan et al., 2002). As NAcc neurons also express MC4R receptors (Mountjoy et al., 1994; Alvaro et al., 1996; Adan & Gispen, 1997) it is possible that once released, AgRP may act directly on NAcc neurons to elicit NAcc neuronal activation.
Another major target of dopaminergic projection neurons in the VTA is the mPFC. In the current study, central administration of AgRP yielded increases in dopamine turnover within the mPFC ninety minutes after injection. In the context of feeding behavior, it is interesting to note that neurons in the mPFC selectively respond to the positive hedonic aspects of palatable foods (Bassareo and Di Chiara 1997, Rolls 2000). Importantly, cue induced anticipation of feeding activates medial prefrontal cortex (mPFC) neurons (Mendoza et al., 2005, Petrovich et al., 2005) a process that is modified by AgRP (Tracy et al., 2008). It is important to note here that melanocortin receptors are widely distributed throughout the hypothalamus and brainstem (Tatro et al., 1994, Mountjoy et al., 1994). Interestingly, periaqueductal grey matter in the brainstem contain dopamine neurons which project to the mPFC (Lu et al., 2006) raising the possibility that the increased mPFC dopamine observed in this study may be derived from AgRP action within brainstem nuclei. In contrast, melanocortin receptors are also expressed within the lateral hypothalamus (LH) an area known for its ability to integrate rewarding stimuli (Aston-Jones et al., 2009, Choi & Davis et al., 2010). In particular, orexin neurons in the LH are activated by AgRP (Zheng et al., 2002) an area that sends direct projections to the VTA (Balcita-Pedicino & Sesack 2007) thus raising the possibility that the increased mPFC dopamine may reflect actions of AgRP within hypothalamic regions.
It is also possible that once released, AgRP activates MC4R receptors within hypothalamic structures which stimulate VTA neurons with direct projections to the mPFC but not the NAcc. In fact, anatomical evidence supports the contention that dopamine neurons which activate mesoaccumbens and mesoprefrontal pathways can be activated by different sources of excitatory drive (Carr & Sesack, 2000, Sesack et al., 2003). To determine if AgRP injection would stimulate dopamine neurons in the VTA we measured neuronal activation in dopaminergic neurons following AgRP injection. Rats injected with AgRP displayed increased c-Fos immunoreactivity within TH-positive neurons relative to saline injected controls suggesting that central AgRP administration is sufficient to activate mesolimbic dopamine neurons which project to forebrain centers. Collectively, the current data provide new evidence that melanocortins are capable of eliciting changes in dopamine turnover within a region known to mediate reward based decision making (Rogers et al., 2003, Xue et al., 2008) and suggest that AgRP's ability to modulate food intake through anticipatory state may occur by altering dopamine neurochemistry within cortical reward circuitry.
AgRP blocks CPP for sucrose
Animals given third ventricle injections of AgRP formed a strong place preference to a nutritionally complete high fat diet. When injected centrally, AgRP causes animals to ingest more calories from fat when given a choice between high fat diet and chow (Hagan et al 2001). Thus, the ability of AgRP to support a place preference for a diet that is rich in fat is consistent with previous data and suggests that one way in which AgRP induces consumption of fat rich foods is by modulating the reinforcing efficacy of palatable food consumption. Perhaps more important is the finding that central administration of AgRP blocked the acquisition of a place preference to sucrose. Animals injected with AgRP ate all of the sucrose pellets during the conditioning regimen as control animals did, but only saline injected controls formed a place preference to the sucrose. Previous studies have reported that animals injected with AgRP prefer to eat chow diet rather than sucrose when given a choice between sucrose and chow (Hagan et al 2001). In our place preference protocol animals were only exposed to sucrose in the place preference chamber after injection with AgRP, thus, it is possible that they ate all of the pellets because this was a forced choice paradigm that is only sucrose pellets were available. In this circumstance, consumption of the sucrose pellets in the AgRP injected animals mimicked that of the control group but was likely not reinforcing. This could be due to the ability of AgRP to orient animals towards fat rich foods. In support of this notion, a recent study from our lab indicates that AgRP has the capacity to increase appetitive responding for fat but not sucrose (Tracy et al., 2008). Therefore, it is possible that the effect on food reinforcement observed with central AgRP injection is dependent on the macronutrient content of the diet available. When viewed collectively, these data raise the possibility that in times in which endogenous levels of AgRP are high, such as caloric deprivation, AgRP increases the drive to feed from calorically dense foods in order to liberate energy quickly as well as to replenish lost energy stores. Our data indicate that one way this is achieved is through modulating the reinforcing properties associated with these foods.
Summary
Historically the melanocortin system has been studied for its ability to regulate the duration of the feeding response, and viewed as a downstream effector system of the adiposity signal leptin. However, recent studies from our lab suggest that antagonism of this system is capable of modifying the appetitive aspects of feeding behavior. In the context of food reinforcement, the present results reveal a number of important findings regarding the involvement of the inverse agonist AgRP and the melanocortin system in food seeking behavior. First, AgRP is capable of eliciting mesocortical dopamine activity, specifically within brain regions associated with reward based decision making and cue induced feeding. The ability of AgRP to modulate the conditioned place preference of sucrose suggests at lest two possibilities; 1) similar to melanocortin agonists which block feeding, melanocortin antagonists which increase feeding responses after caloric deprivation are also capable of affecting food reinforcement and 2) that this affect may be achieved, in part, by inhibiting the pleasure associated with consuming low calorie foods in times of caloric deficit. Overall, the data reported here provide new insight into the nature of the melanocortin system's ability to augment energy intake in addition to the psychological processes that accompany feeding responses.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adan RA, Gipsen WH. Brain melanocortin receptors: from cloning to function. Peptides. 1997;8:1279–1287. doi: 10.1016/s0196-9781(97)00078-8. [DOI] [PubMed] [Google Scholar]
- Alvaro JD, Tatro J, Quillan JM, Fogliano M, Eisenhard M, Lerner MR, Nestler EJ, Duman RS. Morphine down regulates melanocortin-4 receptor expression in brain regions that mediate opiate addiction. Mol Pharmacol. 1996;3:583–591. [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci. 1999;11:4389–4397. doi: 10.1046/j.1460-9568.1999.00843.x. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential influence of associative and nonassociative learning mechanism on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J Neurosci. 1997;17:851–861. doi: 10.1523/JNEUROSCI.17-02-00851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Hao J, Afroz T, Krahnne LL, Carr KD. Feeding, body weight and non-ingestive reward stimuli during and after 12 day continuous central infusions of melanocortin receptor ligands. Peptides. 2005;11:2314–2321. doi: 10.1016/j.peptides.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Kim G, Carr KD. The melanocortin receptor agonist MTII augments the rewarding effects of amphetmaines in ad-libitum fed and food restricted rats. Psychopharmacology. 2002;1:77–85. doi: 10.1007/s00213-002-0998-1. [DOI] [PubMed] [Google Scholar]
- Day J, Roitman M, Wightman M, Carelli R. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;8:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Benoit SC, Rushing PA, Pritchard LM, Woods SC, Seeley RJ. Immediate and prolonged patterns of Agouti related peptide-(83-132) induced c-fos activation in hypothalamic and extrahypothalamic sites. Endocrinology. 2001a;3:1050–1056. doi: 10.1210/endo.142.3.8018. [DOI] [PubMed] [Google Scholar]
- Hagan M, Rushing P, Benoit SC, Woods SC, Seeley RJ. Opioid receptor involvement in the effect of AgRP-(83-132) on food intake and food selection. Am J Physiol Reg Integr Comp Physiol. 2001b;1:R814–821. doi: 10.1152/ajpregu.2001.280.3.R814. [DOI] [PubMed] [Google Scholar]
- Hsu R, Taylor J, Newton SS, Alvaro JD, Haile C, Han G, Hruby VJ, Nestler EJ, Duman RS. Blockade of melanocortin transmission inhibits cocaine reward. Eur J Neurosci. 2005;8:2233–2242. doi: 10.1111/j.1460-9568.2005.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindbloom J, Opmane B, Mutulis F, Mutule I, Petrovska R, Klusa V, Bergstrom L, Wikberg J. The MC4 receptor mediates α-MSH induced release of nucleus accumbens dopamine. Neuroreport. 2001;12:2155–2158. doi: 10.1097/00001756-200107200-00022. [DOI] [PubMed] [Google Scholar]
- Lu J, Thomas CJ, Saper CB. Identification of Wake-Active Dopaminergic Neurons in the Ventral Periaqueductal Grey Matter. J Neurosci. 2006;26:192–203. doi: 10.1523/JNEUROSCI.2244-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy K, Robbins L, Mortrud M, Cone R. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- Mountjoy K, Mortrud M, Low M, Simerly R, Cone R. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8(10):1298–308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- Petrovich G, Ross C, Holland P, Gallagher M. Medial Prefrontal Cortex Is necessary for an Appetitive Contextual Conditioned Stimulus to Promote Eating in Sated Rats. J Neurosci. 2007;24:6436–41. doi: 10.1523/JNEUROSCI.5001-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich G, Holland P, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci. 2005;25:8295–8302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R, Ramnani N, Mackay C, Wilson J, Jezzard P, Carter C, Smith S. Distinct Portions of Anterior Cingulate Cortex and Medial Prefrontal Cortex Are Activated by Reward Processing in Separable Phases of Decision-Making Cognition. Biol Psychiatry. 2003;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Precis of the brain and emotion. Behav Brain Sci. 2000;23:177–91. doi: 10.1017/s0140525x00002429. [DOI] [PubMed] [Google Scholar]
- Sesack S, Carr D, Omelchenko N, Pinto A. Anatomical Substrates for Glutamate-Dopamine Interactions. Ann. NY Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods S, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;6778:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Tatro JB, Entwistle ML. Distribution of melanocortin receptors in the lower brainstem of the rat. Ann N Y Acad Sci. 1994;739:311–4. doi: 10.1111/j.1749-6632.1994.tb19833.x. [DOI] [PubMed] [Google Scholar]
- Torre E, Celis ME. Cholinergic mediation in the ventral tegmental area of alpha-melanotropin induced excessive grooming: changes of the dopamine activity in the nucleus accumbens and caudate putamen. Life Sci. 1988;42:1651–57. doi: 10.1016/0024-3205(88)90444-4. [DOI] [PubMed] [Google Scholar]
- Torre E, Celis ME. Alpha-MSH injected into the substantia nigra or intraventricularly alters behavior and the striatal dopaminergic activity. Neurochem Int. 1986;9(1):85–9. doi: 10.1016/0197-0186(86)90035-5. [DOI] [PubMed] [Google Scholar]
- Tracy A, Clegg D, Johnson J, Davidson T, Benoit S. The melanocortin antagonist AgRP (83-132) increases appetitive responding for a fat, but not a carbohydrate, reinforcer. Pharmacol Biochem Behav. 2008;89:263–71. doi: 10.1016/j.pbb.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin I, Weller J, Li X, Bechara A. Functional Dissociations of Risk and Reward Processing in the Medial Prefrontal Cortex. Cerebral Cortex. 2008:147. doi: 10.1093/cercor/bhn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Corkern M, Crousillac S, Patterson L, Phifer C, Berthoud H. Neurochemical Phenotype of Hypothalamic Neurons Showing Fos Expression 23h after Intracranial AgRP. Am J Physio Regulatory Integrative Comp Physiol. 2002;282:R1773–R1781. doi: 10.1152/ajpregu.00019.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.