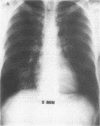
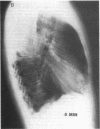
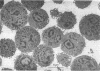
Abstract
Leukotriene B4 (LTB4) is a major product of human alveolar macrophages and has potent chemotactic activity for neutrophils (PMN) in vitro. To evaluate the effects of LTB4 in the normal human lung, we instilled LTB4 (5 X 10(-7)M, 10 ml) into a subsegment of the right middle lobe and 0.9% NaCl (10 ml) into a subsegment of the lingula using a fiberoptic bronchoscope in 12 healthy human volunteers. 4 h later, we performed bronchoalveolar lavage of the same subsegments. Compared with the NaCl instillation, LTB4 caused a large increase in lavage total cells (NaCl = 6.8 +/- 1.0 X 10(6) vs. LTB4 = 26.4 +/- 5.0 X 10(6), P less than 0.01), most of which were PMN (NaCl = 12.2 +/- 4.6% vs. LTB4 = 55.7 +/- 6.0%, P less than 0.001). In contrast, there was only a small increase in lavage total protein, and the lavage total protein correlated weakly with lavage total cells and PMN. The production of superoxide anion by the lavage PMN in response to phorbol myristate acetate was similar to that of peripheral blood PMN. The migration of lavage PMN was normal toward the chemotactic peptide FMLP, but reduced toward LTB4 and zymosan-activated human serum. Morphometric analysis using transmission electron microscopy indicated a selective loss of small granules in the lung neutrophils as compared with peripheral blood neutrophils. The data indicate that in the normal human lung, LTB4 can recruit active PMN into the airspaces without causing a significant change in the protein permeability of the epithelial barrier.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton D. F., Miller L. J., Kishimoto T. K., Springer T. A. Leukocyte adhesion receptors are stored in peroxidase-negative granules of human neutrophils. J Exp Med. 1987 Dec 1;166(6):1641–1653. doi: 10.1084/jem.166.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty P. G., Ledbetter J. A., Martin P. J., Price T. H., Hansen J. A. Definition of a common leukocyte cell-surface antigen (Lp95-150) associated with diverse cell-mediated immune functions. J Immunol. 1983 Dec;131(6):2913–2918. [PubMed] [Google Scholar]
- Björk J., Hedqvist P., Arfors K. E. Increase in vascular permeability induced by leukotriene B4 and the role of polymorphonuclear leukocytes. Inflammation. 1982 Jun;6(2):189–200. doi: 10.1007/BF00916243. [DOI] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Metabolism of arachidonic acid in polymorphonuclear leukocytes. Structural analysis of novel hydroxylated compounds. J Biol Chem. 1979 Aug 25;254(16):7865–7869. [PubMed] [Google Scholar]
- Brigham K. L., Meyrick B. Granulocyte-dependent injury of pulmonary endothelium: a case of miscommunication? Tissue Cell. 1984;16(2):137–155. doi: 10.1016/0040-8166(84)90039-9. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Charon J. A., Metzger Z., Hoffeld J. T., Oliver C., Gallin J. I., Mergenhagen S. E. An in vitro study of neutrophils obtained from the normal gingival sulcus. J Periodontal Res. 1982 Nov;17(6):614–625. doi: 10.1111/j.1600-0765.1982.tb01183.x. [DOI] [PubMed] [Google Scholar]
- Chi E. Y., Henderson W. R., Klebanoff S. J. Phospholipase A2-induced rat mast cell secretion. Role of arachidonic acid metabolites. Lab Invest. 1982 Dec;47(6):579–585. [PubMed] [Google Scholar]
- Donabedian H., Gallin J. I. Deactivation of human neutrophil chemotaxis by chemoattractants: effect on receptors for the chemotactic factor f-Met-Leu-Phe. J Immunol. 1981 Sep;127(3):839–844. [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Fels A. O., Pawlowski N. A., Cramer E. B., King T. K., Cohn Z. A., Scott W. A. Human alveolar macrophages produce leukotriene B4. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7866–7870. doi: 10.1073/pnas.79.24.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Gerard C., McPhail L. C., Marfat A., Stimler-Gerard N. P., Bass D. A., McCall C. E. Role of protein kinases in stimulation of human polymorphonuclear leukocyte oxidative metabolism by various agonists. Differential effects of a novel protein kinase inhibitor. J Clin Invest. 1986 Jan;77(1):61–65. doi: 10.1172/JCI112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Pickett W. C. Novel structural determinants of the human neutrophil chemotactic activity of leukotriene B. J Exp Med. 1981 Feb 1;153(2):482–487. doi: 10.1084/jem.153.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin B. J., Weinberg J. B. Receptor-mediated modulation of human monocyte, neutrophil, lymphocyte, and platelet function by phorbol diesters. J Clin Invest. 1982 Oct;70(4):699–706. doi: 10.1172/JCI110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
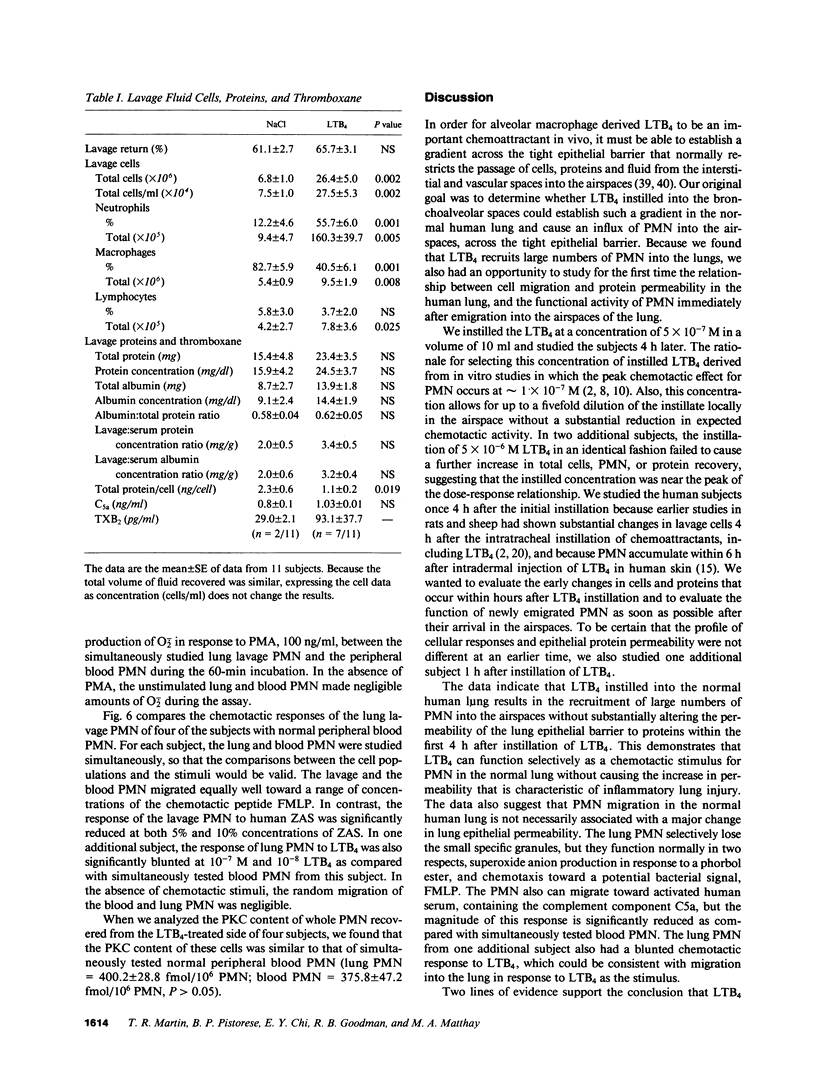
- Henderson W. R., Chi E. Y. Ultrastructural characterization and morphometric analysis of human eosinophil degranulation. J Cell Sci. 1985 Feb;73:33–48. doi: 10.1242/jcs.73.1.33. [DOI] [PubMed] [Google Scholar]
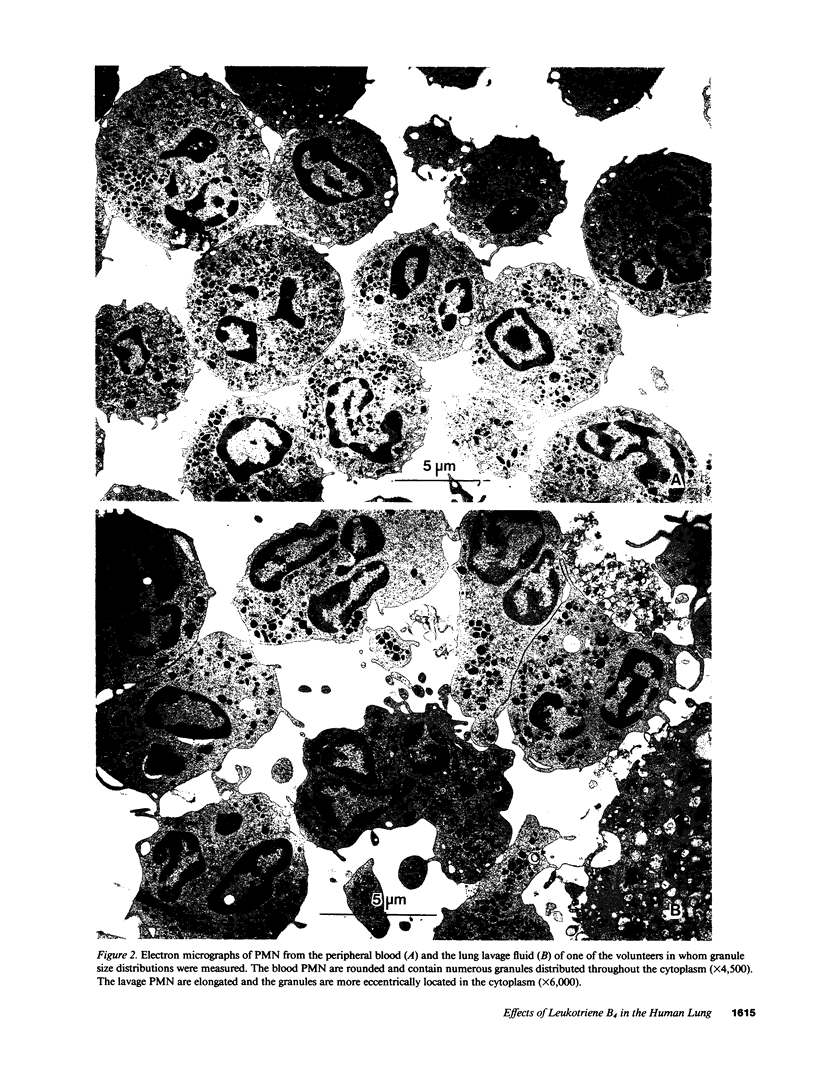
- Henderson W. R., Jr Eicosanoids and lung inflammation. Am Rev Respir Dis. 1987 May;135(5):1176–1185. doi: 10.1164/arrd.1987.135.5.1176. [DOI] [PubMed] [Google Scholar]
- Henderson W. R., Klebanoff S. J. Leukotriene production and inactivation by normal, chronic granulomatous disease and myeloperoxidase-deficient neutrophils. J Biol Chem. 1983 Nov 25;258(22):13522–13527. [PubMed] [Google Scholar]
- Hickstein D. D., Ozols J., Williams S. A., Baenziger J. U., Locksley R. M., Roth G. J. Isolation and characterization of the receptor on human neutrophils that mediates cellular adherence. J Biol Chem. 1987 Apr 25;262(12):5576–5580. [PubMed] [Google Scholar]
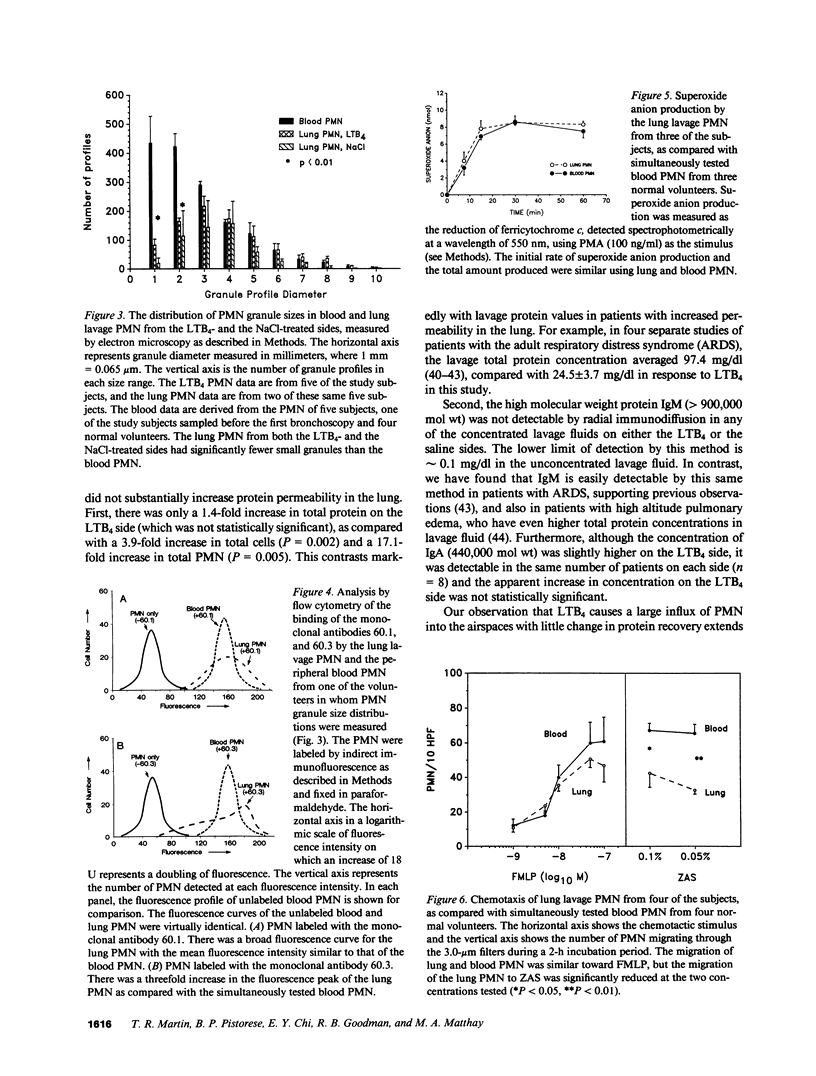
- Holter J. F., Weiland J. E., Pacht E. R., Gadek J. E., Davis W. B. Protein permeability in the adult respiratory distress syndrome. Loss of size selectivity of the alveolar epithelium. J Clin Invest. 1986 Dec;78(6):1513–1522. doi: 10.1172/JCI112743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller H. U., Cottier H. Comparison of locomotion, chemotaxis and adhesiveness of rabbit neutrophils from blood and peritoneal exudates. Blood Cells. 1984;10(1):45–57. [PubMed] [Google Scholar]
- Kouzan S., Brody A. R., Nettesheim P., Eling T. Production of arachidonic acid metabolites by macrophages exposed in vitro to asbestos, carbonyl iron particles, or calcium ionophore. Am Rev Respir Dis. 1985 Apr;131(4):624–632. doi: 10.1164/arrd.1985.131.4.624. [DOI] [PubMed] [Google Scholar]
- Kreisle R. A., Parker C. W., Griffin G. L., Senior R. M., Stenson W. F. Studies of leukotriene B4-specific binding and function in rat polymorphonuclear leukocytes: absence of a chemotactic response. J Immunol. 1985 May;134(5):3356–3363. [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. The biologically active leukotrienes. Biosynthesis, metabolism, receptors, functions, and pharmacology. J Clin Invest. 1984 Apr;73(4):889–897. doi: 10.1172/JCI111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. E., Granger H. J. Diapedesis and the permeability of venous microvessels to protein macromolecules: the impact of leukotriene B4 (LTB4). Microvasc Res. 1988 Jan;35(1):27–47. doi: 10.1016/0026-2862(88)90048-9. [DOI] [PubMed] [Google Scholar]
- Liles W. C., Meier K. E., Henderson W. R. Phorbol myristate acetate and the calcium ionophore A23187 synergistically induce release of LTB4 by human neutrophils: involvement of protein kinase C activation in regulation of the 5-lipoxygenase pathway. J Immunol. 1987 May 15;138(10):3396–3402. [PubMed] [Google Scholar]
- Loomis T. C., Stahl W. L. A rapid, flexible method for biochemical assays using a microtiter plate reader and a microcomputer. Application for assays of protein, Na,K-ATPase and K-p-nitrophenylphosphatase. Int J Biomed Comput. 1986 May;18(3-4):183–192. doi: 10.1016/0020-7101(86)90015-2. [DOI] [PubMed] [Google Scholar]
- Marasco W. A., Phan S. H., Krutzsch H., Showell H. J., Feltner D. E., Nairn R., Becker E. L., Ward P. A. Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J Biol Chem. 1984 May 10;259(9):5430–5439. [PubMed] [Google Scholar]
- Martin T. R., Altman L. C., Albert R. K., Henderson W. R. Leukotriene B4 production by the human alveolar macrophage: a potential mechanism for amplifying inflammation in the lung. Am Rev Respir Dis. 1984 Jan;129(1):106–111. doi: 10.1164/arrd.1984.129.1.106. [DOI] [PubMed] [Google Scholar]
- Martin T. R., Raghu G., Maunder R. J., Springmeyer S. C. The effects of chronic bronchitis and chronic air-flow obstruction on lung cell populations recovered by bronchoalveolar lavage. Am Rev Respir Dis. 1985 Aug;132(2):254–260. doi: 10.1164/arrd.1985.132.2.254. [DOI] [PubMed] [Google Scholar]
- Martin T. R., Raugi G., Merritt T. L., Henderson W. R., Jr Relative contribution of leukotriene B4 to the neutrophil chemotactic activity produced by the resident human alveolar macrophage. J Clin Invest. 1987 Oct;80(4):1114–1124. doi: 10.1172/JCI113168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay M. A., Berthiaume Y., Staub N. C. Long-term clearance of liquid and protein from the lungs of unanesthetized sheep. J Appl Physiol (1985) 1985 Sep;59(3):928–934. doi: 10.1152/jappl.1985.59.3.928. [DOI] [PubMed] [Google Scholar]
- McGuire W. W., Spragg R. G., Cohen A. B., Cochrane C. G. Studies on the pathogenesis of the adult respiratory distress syndrome. J Clin Invest. 1982 Mar;69(3):543–553. doi: 10.1172/JCI110480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensing H., Czarnetzki B. M. Leukotriene B4 induces in vitro fibroblast chemotaxis. J Invest Dermatol. 1984 Jan;82(1):9–12. doi: 10.1111/1523-1747.ep12258678. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Molski T. F., Borgeat P., Sha'afi R. I. Mechanism of action of leukotriene B4: intracellular calcium redistribution in rabbit neutrophils. J Cell Physiol. 1984 Jan;118(1):13–18. doi: 10.1002/jcp.1041180104. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Arachidonic acid induced degranulation of rabbit peritoneal neutrophils. Biochem Biophys Res Commun. 1979 Mar 15;87(1):292–299. doi: 10.1016/0006-291x(79)91678-4. [DOI] [PubMed] [Google Scholar]
- Palmblad J., Malmsten C. L., Udén A. M., Rådmark O., Engstedt L., Samuelsson B. Leukotriene B4 is a potent and stereospecific stimulator of neutrophil chemotaxis and adherence. Blood. 1981 Sep;58(3):658–661. [PubMed] [Google Scholar]
- Peters-Golden M., Thebert P. Inhibition by methylprednisolone of zymosan-induced leukotriene synthesis in alveolar macrophages. Am Rev Respir Dis. 1987 May;135(5):1020–1026. doi: 10.1164/arrd.1987.135.5.1020. [DOI] [PubMed] [Google Scholar]
- Petrequin P. R., Todd R. F., 3rd, Devall L. J., Boxer L. A., Curnutte J. T., 3rd Association between gelatinase release and increased plasma membrane expression of the Mo1 glycoprotein. Blood. 1987 Feb;69(2):605–610. [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Rollins T. E., Zanolari B., Springer M. S., Guindon Y., Zamboni R., Lau C. K., Rokach J. Synthetic leukotriene B4 is a potent chemotaxin but a weak secretagogue for human PMN. Prostaglandins. 1983 Feb;25(2):281–289. doi: 10.1016/0090-6980(83)90110-7. [DOI] [PubMed] [Google Scholar]
- Schoene R. B., Swenson E. R., Pizzo C. J., Hackett P. H., Roach R. C., Mills W. J., Jr, Henderson W. R., Jr, Martin T. R. The lung at high altitude: bronchoalveolar lavage in acute mountain sickness and pulmonary edema. J Appl Physiol (1985) 1988 Jun;64(6):2605–2613. doi: 10.1152/jappl.1988.64.6.2605. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Vanbenthuysen K. M., Tate R. M., Shasby S. S., McMurtry I., Repine J. E. Granulocytes mediate acute edematous lung injury in rabbits and in isolated rabbit lungs perfused with phorbol myristate acetate: role of oxygen radicals. Am Rev Respir Dis. 1982 Apr;125(4):443–447. doi: 10.1164/arrd.1982.125.4.443. [DOI] [PubMed] [Google Scholar]
- Silbaugh S. A., Stengel P. W., Williams G. D., Herron D. K., Gallagher P., Baker S. R. Effects of leukotriene B4 inhalation. Airway sensitization and lung granulocyte infiltration in the guinea pig. Am Rev Respir Dis. 1987 Oct;136(4):930–934. doi: 10.1164/ajrccm/136.4.930. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Goetzl E. J. Molecular and cellular mechanisms of leukocyte chemotaxis. Science. 1981 Aug 21;213(4510):830–837. doi: 10.1126/science.6266014. [DOI] [PubMed] [Google Scholar]
- Soter N. A., Lewis R. A., Corey E. J., Austen K. F. Local effects of synthetic leukotrienes (LTC4, LTD4, LTE4, and LTB4) in human skin. J Invest Dermatol. 1983 Feb;80(2):115–119. doi: 10.1111/1523-1747.ep12531738. [DOI] [PubMed] [Google Scholar]
- Staub N. C., Schultz E. L., Koike K., Albertine K. H. Effect of neutrophil migration induced by leukotriene B4 on protein permeability in sheep lung. Fed Proc. 1985 Jan;44(1 Pt 1):30–35. [PubMed] [Google Scholar]
- Todd R. F., 3rd, Arnaout M. A., Rosin R. E., Crowley C. A., Peters W. A., Babior B. M. Subcellular localization of the large subunit of Mo1 (Mo1 alpha; formerly gp 110), a surface glycoprotein associated with neutrophil adhesion. J Clin Invest. 1984 Oct;74(4):1280–1290. doi: 10.1172/JCI111538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. A., Schumacher R., Myers A. R. Phagocytic function of polymorphonuclear leukocytes in rheumatic diseases. J Clin Invest. 1973 Jul;52(7):1632–1635. doi: 10.1172/JCI107342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedder N. B., Harlan J. M. Increased surface expression of CD11b/CD18 (Mac-1) is not required for stimulated neutrophil adherence to cultured endothelium. J Clin Invest. 1988 Mar;81(3):676–682. doi: 10.1172/JCI113372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis W. J., Hickstein D. D., Schwartz B. R., June C. H., Ochs H. D., Beatty P. G., Klebanoff S. J., Harlan J. M. Monoclonal antibody-defined functional epitopes on the adhesion-promoting glycoprotein complex (CDw18) of human neutrophils. Blood. 1986 Apr;67(4):1007–1013. [PubMed] [Google Scholar]
- Weiland J. E., Davis W. B., Holter J. F., Mohammed J. R., Dorinsky P. M., Gadek J. E. Lung neutrophils in the adult respiratory distress syndrome. Clinical and pathophysiologic significance. Am Rev Respir Dis. 1986 Feb;133(2):218–225. doi: 10.1164/arrd.1986.133.2.218. [DOI] [PubMed] [Google Scholar]
- West B. C., Rosenthal A. S., Gelb N. A., Kimball H. R. Separation and characterization of human neutrophil granules. Am J Pathol. 1974 Oct;77(1):41–66. [PMC free article] [PubMed] [Google Scholar]
- Wilton J. M., Renggli H. H., Lehner T. A functional comparison of blood and gingival inflammatory polymorphonuclear leucocytes in man. Clin Exp Immunol. 1977 Jan;27(1):152–158. [PMC free article] [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. Secretory responses of human neutrophils: exocytosis of specific (secondary) granules by human neutrophils during adherence in vitro and during exudation in vivo. J Immunol. 1979 Jul;123(1):285–294. [PubMed] [Google Scholar]
- Zimmerli W., Seligmann B., Gallin J. I. Exudation primes human and guinea pig neutrophils for subsequent responsiveness to the chemotactic peptide N-formylmethionylleucylphenylalanine and increases complement component C3bi receptor expression. J Clin Invest. 1986 Mar;77(3):925–933. doi: 10.1172/JCI112391. [DOI] [PMC free article] [PubMed] [Google Scholar]