Abstract
Olfactory cues can exert priming effects on many mammalian species. Paternally experienced marmosets, Callithrix jacchus, exposed to direct isolated olfactory contact with their own infant's scent show rapid decreases in testosterone levels within 20 minutes, whereas paternally inexperienced males do not. The following study tests whether there is a differential steroid response to exposure of infant scent from dependent infants (own and novel) and independent infants (own and novel). We examined the serum levels of estradiol, estrone, testosterone, dihydrotestosterone (DHT), and combined estrogens and androgens in eight male marmosets 20 minutes after exposure to isolated infant scent. Testosterone and androgen levels combined were significantly lower with exposure to own infant scent than a novel infant scent when the infants were at a dependent age but not at an independent age. Estrogen levels elevated significantly in response to own infant scent when the infants were at a dependent age but not at an independent age. These results suggest that marmoset fathers are more responsive to priming cues from related infants and hormonal responses from fathers are greatest when the infant is at a dependent age.
Keywords: olfactory communication, chemosensory, infant odors, testosterone, androgens, estrogens, marmoset, parenting
INTRODUCTION
Olfactory stimuli play an important role in optimizing sex and reproductive outcome in many species. Odor cues can produce signaling effects, such as signaling the estrous state of females (Vandenbergh, 1994), or primer effects on reproduction, such as changes in the physiological condition of the recipient due to odor cues (Koyama, 2004). Many primer effects have been found including stimuli that can disrupt pregnancy [as in the Bruce affect (Bruce, 1959)], extend female estrous cycles (Lee & van der Boot, 1955), and induce estrus (Whitten, 1956), accelerate the onset of puberty (Vandenbergh, 1969) and increase LH levels in males (Maruniak and Bronson, 1976). However, olfactory cues may play a lesser role in social relationships in primates.
Odor recognition is an important component in the maternal – infant bond. In species such as rats, mice, sheep, goats, and rabbits, as well as in humans, olfaction is involved in the regulation of maternal care to the offspring (Fleming et al., 1993; Lévy et al., 2004; Gonzalex-Mariscal and Poindron, 2002; Numan and Insel, 2003; Stern, 1989). The neonate chemosensory signal is processed differently relative to the hormonal priming that occurs during pregnancy and parturition. The valence of the chemosensory signal from rat neonates can cause an averse or avoidance response in virgin, non-mothers while this signal is a motivational cue for maternal behaviors in mothers (Rosenblatt and Mayer, 1995). At parturition mothers of many mammalian species develop immediate responsiveness to olfactory cues by exhibiting maternal care-taking behaviors (Lévy et al., 2004). However large brained primates can form maternal - infant bonds without relying primarily on odor cues.
Parental recognition of offspring odors plays a role in determining the difference between offspring and non-offspring. Offspring recognition by mothers to their kin has been well characterized and is a function of the MHC odor type (Yamazaki et al., 2000). Kin recognition is an assessment of genetic relatedness, and can infer differential treatment of conspecifics based on cues that correlate with relatedness (Gamboa et al., 1991). Recognition of odor signals from offspring requires the production of the specific label and the recognition of the labels through parent learning of the odor signature (Mateo, 2002). Less is understood about paternal offspring recognition in biparental mammalian species. Males do not undergo pregnancy and parturition where hormonal facilitation of brain plasticity is produced, with neurogenesis occurring to promote olfactory involvement in offspring recognition and facilitation of infant care. However, neuroendocrine changes are recorded from males of biparental species during the gestational phase of their offspring or in response to infants (Berg and Wynne-Edwards, 2001; Brown et al, 1995; Fleming et al., 2002; Reburn and Wynne-Edwards, 1999; Ziegler, 2000).
The New World primates, marmosets and tamarins, are especially reactive to olfactory scents as both stimulating effects on behavior and priming effects on hormones. They have highly developed circumgenital scent glands, which are under hormonal control (Epple, 1986; Savage et al, 1988). Scent secretions can identify individuals and determine sex and dominance status (Belscher et al., 1990) and activate neural pathways for sexual arousal and increase testosterone levels in response to isolated ovulatory scents (Ferris et al., 2004; Ziegler et al., 2005). Marmosets and tamarins have well-defined main and accessory olfactory systems with an intact vomeronasal organ and mature sensory neurons in their vomeronasal organ with marker proteins that indicate their functional state (Dennis et al., 2004).
Marmoset infant odor cues may work as signaling odors in the form of recognition of offspring and as primer odors by affecting paternal hormones. We have shown paternal recognition of infant scent cues where father marmosets show reduced serum testosterone levels within 20 minutes of contact with an isolated scent stimuli (Prudom et al., 2008). Parentally inexperienced males show no changes in testosterone levels. Testosterone responsiveness to infant odor may indicate kin recognition and it's role in the promotion of paternal behaviors and/or the regulation of testosterone production to reduce testosterone-dependent behaviors that are not conducive to paternal care. Testosterone responsiveness in fathers has not been tested with novel infant scents where the contrast between related and novel infants can provide information on kin recognition and whether the priming effect on fathers is specific to related infants.
The perception of the infant odor signal may change depending upon the hormonal priming, as occurs in mothers for whom the signal is a motivational cue for maternal behaviors (Rosenblatt and Mayer, 1995). Older infants who are no longer dependent upon the father to care for them may be recognized as kin but do not exert a priming effect on the father. Chemical signals found in the scent secretions may only be relevant when the infant is totally dependent upon being carried.
Estrogen levels are elevated in expectant marmoset and tamarin fathers, especially during the last month of their mate's pregnancy (Ziegler et al., 2004a; Ziegler et al., 2009). Male cotton-top tamarins have high levels of estrogens at times and these are derived from gonadal testosterone (Ziegler et al., 2000). Our infant scent tests indicate that testosterone levels decline within 20 minutes of exposure in marmoset fathers. The mechanism is unknown but could be due to a rapid conversion. Estrogens could act to reduce the behavioral effects of elevated testosterone at a time when males need to be parental. In the California mouse testosterone is aromatized to estradiol in the brain to facilitate parental behaviors (Trainor and Marler, 2002). Aromatase activity can increase with singing in songbirds within 30 minutes of the singing onset (Remage-Healey et al., 2009). Rapid changes in circulating testosterone levels have been observed in males of various species following social encounters (see Cornil et al., 2009 for review). These short-acting effects are thought to be due to the non-genomic effects of estrogens on behavior in the brain. Yet, the rapid changes in circulating testosterone might also implicate peripheral aromatase changes of testosterone to estrogens.
The following study was designed to examine the changes in paternal gonadal steroids due to olfactory stimuli from isolated infant scent cues. Levels of the major estrogens, estradiol and estrone, as well as the major androgens, testosterone and dihydrotestosterone, were examined in parentally-experienced male marmosets to address the following questions: 1) does relatedness influence the priming effect of infant odors on fathers 2) does age of the infant odor influence the male testosterone levels and, 3) does relatedness or age affect estrogen levels in fathers?
METHODS
Animals
Eight paternally experienced male common marmosets were used for this study. They were socially housed with their mate and offspring in the marmoset colony at the Wisconsin National Primate Research Center. The males were between the ages of 4 and 12 years. These males are part of the breeding colony and had sired one to eight previous offspring prior to their current infants. Only one of the fathers had been used in our previous study on scent discrimination. Details of male age, the family composition, and mate's pregnancy state at the times of the studies are shown in Table 1.
Table 1.
Age of marmoset fathers, number of offspring present and pregnancy state of his mate at the time of infant scent presentation.
| Father age at first presentation (years) | Offspring present at first presentation | Offspring present at second presentation | Female pregnancy condition |
|---|---|---|---|
| 7.3 yr | 3, inc. 1 infant | 3, inc. 1 infant | pregnant ~2 month |
| 12.6 yr | 4, inc. 2 infants | 4, inc. 2 infants | pregnant ~3 month |
| 4.4 yr | 4, inc. 1 infant | 3, inc. 1 infant | pregnant ~10 days aborted 3 wks earlier |
| 6.0 yr | 7, inc. 2 infants | 6, inc. 2 infants | pregnant ~3 months |
| 4.4 yr | 5, inc. 2 infants | 4, inc. 1 infant | pregnant ~3 months |
| 4.2 yr | 1 infant | 1 infant | nonpregnant aborted 3 wks earlier |
| 7.5 yr | 8, inc. 2 infants | 8, inc. 2 infants | pregnant ~3 months |
| 5.2 yr | 3, inc. 1 infant | 3, inc. 1 infant | pregnant ~3 months |
Marmoset families were housed in cages that measured either 122 × 61 × 183 or 61 × 91 × 183 cm. Diet and husbandry have been reported previously for this colony (Saltzman et al., 1997). Marmosets were fed twice daily, between 07:00 and 08:30 am, and between 12:30 and 14:00. Water was provided ad libitum. Lighting was regulated on a 12:12 hours light/dark cycle and the humidity was maintained at approximately 40%. Housing conditions and olfactory testing met the guidelines for nonhuman primate and were approved by the Animal Care and Use Committee (IACUC) at the University of Wisconsin.
Scent collection
To collect a scent from an infant, the infant was removed from its family and isolated for the short time it takes to collect the sample. As reported in Prudom et al. (2008), a ground glass laboratory stopper was gently rubbed in the anogenital area of the infant to remove the scent secretions that were usually mixed with urine. The stopper was washed with 300 μl of a mixture of deoxygenated ethanol/distilled water (50:50) to remove the scent. Scent samples were pipetted off the glass stopper and into a micro centrifuge tube and stored at -80°C until testing.
Infant scent was collected several days prior to testing the male with the scent. More than one scent sample was collected from each of the infant marmosets so that each infant could provide a scent stimulus for it's own father (own) and for an unknown father (novel).
Experimental design
Eight father marmosets were tested with 3 different scent stimuli at two different ages of their offspring in a randomized design. For the first test, infant scent was collected at infant age 5-10 days. Males were presented with the vehicle, own infant scent, or novel infant scent. The vehicle odor was ethanol/water presented at the same volume as the infant scents. Males were removed from their home cages in a metal nest box and then once in the testing room were transferred to an identical but clean nest box without the odors associated with his family. The testing room was isolated from the marmoset housing rooms so males would be separated from olfactory and auditory cues from other marmosets. Males would sit in the nest box for 10 minutes to allow their olfactory system to clear of the family's odors and then testing would begin. Testing consisted of applying the scent onto a small wooden dowel, 3 cm in diameter, placing the dowel into the male's nest box and allowing him to sit with the dowel for 20 minutes. Males were allowed to touch, lick, and smell the disc. Twenty minutes after initial presentation of scent stimuli males were bled within three minutes to obtain 0.6 ml of blood, received a liquid treat, and were taken back to their families. Odor testing and blood sample collection occurred between 11:00 and 13:00. Males were tested with the three odors within a week's time. Three to four months’ later males were tested again for all three scents in a randomized design where the scents were collected from the same infants after the infants were weaned and independent of constant care.
Hormonal Assays
All samples were centrifuged and collected as serum samples and then stored at -80°C until assay. Samples were analyzed in two batches over a year's time and analyzed for testosterone (T), dihydrotestosterone (DHT), estradiol (E2) and estrone (E1) by the method reported in (Ziegler et al., 2000). An aliquot of 200 μl was used for each sample. They were extracted by adding 300 μl water and 5 ml diethyl ether. Samples were reconstituted in 1 ml 96:4 iso-octane:ethyl acetate and the individual steroids were separated by celite chromatography using system I (Abraham et al., 1972). Using this system DHT is eluted in 4 mls of 10% ethyl acetate, E1 and T are eluted in 4 mls 20% ethyl acetate and E2 is eluted in 4 mls of 40% ethyl acetate. The eluted samples were dried and reconstituted back to 200 μl in ethanol and the individual fractions were assayed for T, DHT, E2 and E1.
The method for measuring marmoset serum T as assayed by EIA is described in Ziegler et al. (2005) at a volume of 15 μl and a 90% recovery. The method for DHT is the same assay as for T except with DHT standards and the sample size was 200 μl. Marmoset serum was validated for DHT: serial dilutions of pooled marmoset serum were parallel to the DHT standards (no difference in the slopes of the lines, t=-1.50, P>0.05) and accuracy was 98.65 ± 0.42%, n=8. The recovery of DHT through the procedures was 86%. Coefficient of variation (CV) was 6.24 for intra CV for two assays. Since all samples for T, DHT, E1 and E2 were assayed in three or less assays, we report the lab values for marmoset serum pools: Intra and inter-assay CVs for the T EIA were 3.2 and 12.0%, n=11.
E2 and E1 were validated and assayed with a radioimmunoassay as described in Saltzman et al. (1998) using 200 μl amounts. Recoveries for E2 were 97% and for E1 were 86.5% Intra and inter-assay CVs for the E2 were 3.8 and 15.7% and for E1 were 6.0 and 17%, n=11.
Statistical analysis
Steroid results were recorded for each steroid per male per treatment. Tests for normality were performed and the data had a normal distribution. There were no order effects on the randomized steroid data. Repeated measures ANOVA with post hoc comparisons (least squares differences, LSD) were used to determine differences between hormonal response to own, novel and vehicle for dependent infants and independent infants. Planned comparisons were used to determine the steroid response to infant scent from the same infant with scents taken at different ages. Pair-wise t-tests were used to determine significant differences between responses to own infant scent at dependent and independent ages, 2-tailed, p<0.05.
RESULTS
Dependent age
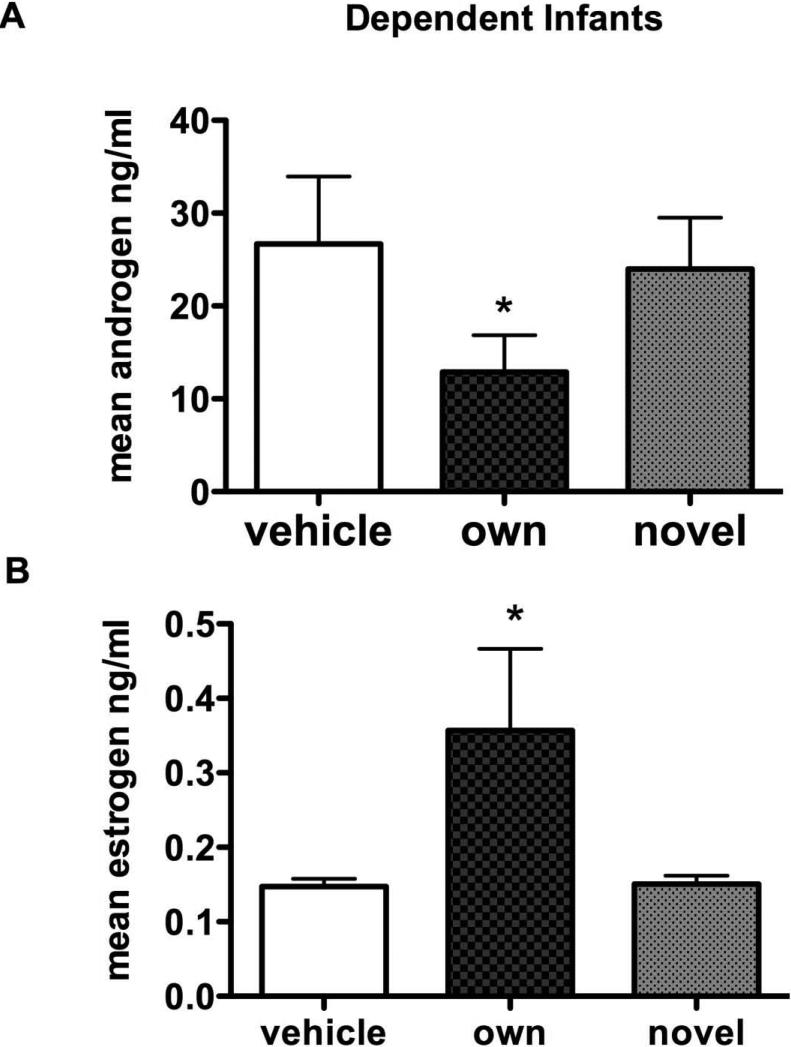
Androgens: Testosterone levels were significantly different between condition at the dependent age (F2,7 = 4.2, p = 0.04) where own infant scent was significantly lower than the vehicle and the control scent (p < 0.05). DHT levels were not different by condition. Combined androgens were significantly different by condition (F2,7 = 4.02, p = 0.04) and levels with own infant scent were significantly lower than the vehicle and the novel infant scent (p < 0.05) (Figure 1A). Estrogens: Estradiol levels were not different by condition at the dependent age but estrone levels were near significance (F2,7 = 2.99, p = 0.07) and own infant was significantly different from both the vehicle and the novel infant (p < 0.05). Total estrogens were significantly different by conditions (F2,7 = 3.41, p = 0.05) and levels with own infant scent were significantly higher than the vehicle and the novel infant (Figure 1B, p < 0.05).
Figure 1.
The effect of infant scent exposure on mean hormone levels in marmoset fathers. A. Androgen (testosterone + DHT) levels in fathers were significantly lower from scent collected from own infant aged 5 – 10 days. B. Estrogen (estradiol + estrone) levels in fathers were significantly higher from own infant scent.
Independent age
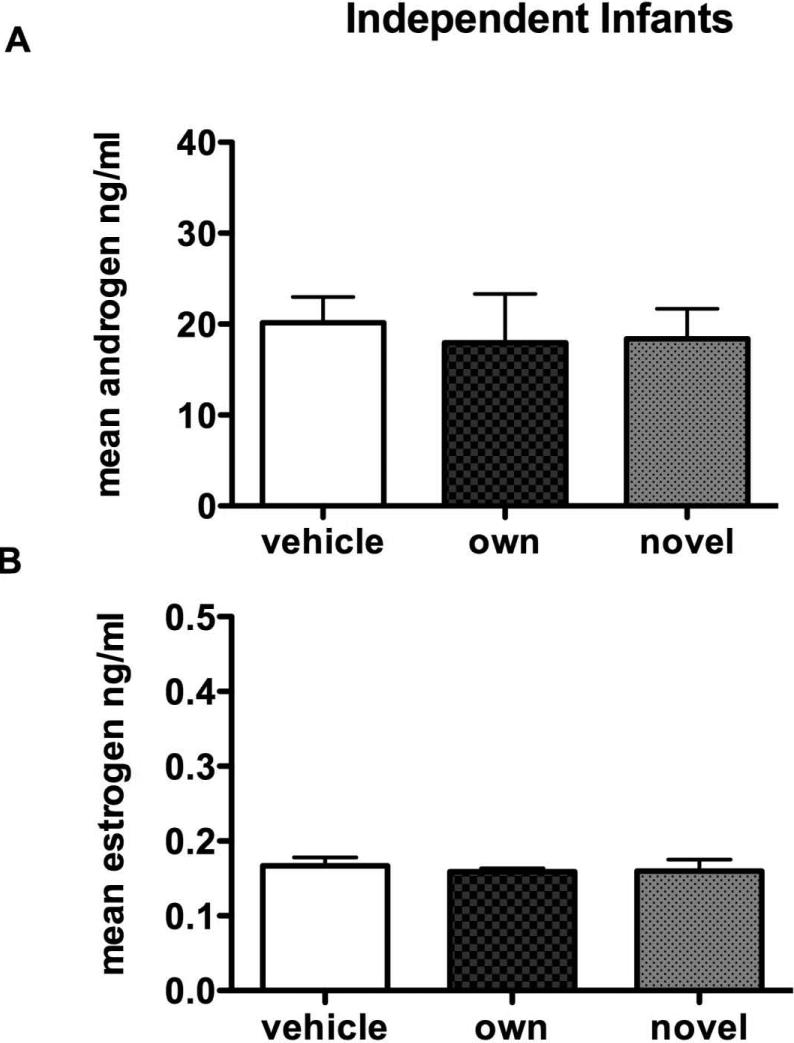
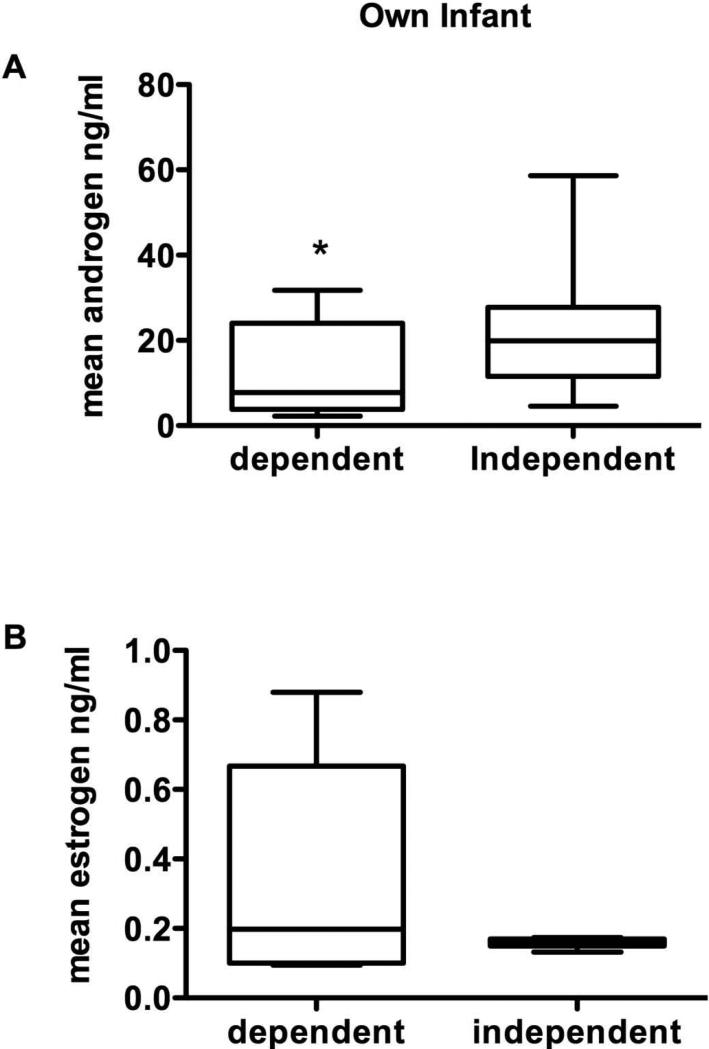
No significant differences were found for any of the hormones or for estrogens or androgens when the infants were of an independent age (Figure 2). Planned comparisons of each infant scent collected from the infant when dependent and when independent showed significant differences for androgen levels where androgens were significantly lower for the dependent infant condition (Figure 3A, t = 2.19, p = 0.03). Estrogen levels were near significance (Figure 3B, t = 1.76, p = 0.06).
Figure 2.
The effect of infant scent exposure on hormone levels in fathers exposed to infant scents collected at an independent age (3 – 4 months). No significant differences are found in A. androgen levels or B. estrogen levels.
Figure 3.
A comparison of marmoset father's hormonal response to his own infant scent collected at a dependent age and again at an independent age. Androgen levels were significantly higher after exposure to own infant scent at the dependent age. Estrogen levels are higher for the dependent own infant versus the independent own infant but not significantly.
Estrogen response
Estrogen levels were significantly higher for own infants in dependent-aged infants. Estrogen levels were similar in fathers during this time for both estrone and estradiol ranging between 0.04 and 0.35 ng/ml and these levels are in the same range as reported for estradiol and estrone in female marmosets during the follicular and periovulatory period (Saltzman et al., 1998).
DISCUSSION
Marmoset fathers showed relatively quick changes in circulating hormone levels following exposure to anogenitally-derived scents of their infant offspring. Olfactory cues clearly distinguished between dependent, pre-weaned related infants and unrelated, novel infants. Dependent aged infant scent lowered testosterone and elevated estrogens while scent from the infant while it was older was less effective. This ability to alter the males’ hormones appears to be related to the dependence of the infant. Scent from older related offspring was more variable in inducing hormonal changes in the fathers. This suggests that males actually perceive the scent as more relevant when the infants are young. Hormonally primed fathers may be more receptive to the odors of their own young infants. Alternatively, there may be a change in the odor signature of older infants. The olfactory-hormonal system in parents may be highly tuned or highly flexible to show hormonal responses to the odor of dependent offspring but less to the odors of independent or non-related offspring.
This study provides a powerful indicator of the olfactory/chemical communication that occurs in this species. As we have seen when males respond to isolated scents of ovulating females (Ziegler et al., 2005), males have a rapid response of altering testosterone production. The chemical/olfactory communication in marmosets provides an important mechanism for maintaining the bonds between individuals with their family groups. Olfactory cues play a primary role in social bonding in small-brained mammals such as rodents but are thought to play a lesser role in large-brained primates (Broad et al., 2006). Odor recognition of offspring in rodents and other small-brained mammals that are regulated by odor cues is short lived compared to essential long-lasting cues in a primate with a long birth to weaning period and continued parental care up to offspring reproduction or longer. While primate species may use all sensory cues to form and maintain parent – infant bonds, odor cues may still be essential for many aspects of parental care. Human mothers have been shown to recognize their newborn infants by olfactory cues (Kaitz et al., 1987). Human fathers are more affectionate toward children whose smell they can identify than towards children smells that they do not recognize (Dubas et al., 2009). As our data indicate, marmoset fathers are highly responsive to infant scent cues and hormonal responses are strongest when the infants are related, pre-weaned and dependent upon parental care for survival.
Marmoset fathers are very responsive to their social environment. They are also hormonally primed for paternal care. Marmoset and tamarin fathers have hormonal and physical changes that occur prior to and following birth of their infants (Ziegler et al., 2004a; Ziegler et al., 2009). Males increase their weight prior to infant birth and have hormonal changes. Following birth, prolactin increases and testosterone decreases during weight loss. The prolactin/testosterone inverse relationship has been well documented in biparental birds during the breeding season versus the parenting season (Buntin, 1996; Wingfield and Goldsmith, 1990). Prolactin regulates neurogenesis in the olfactory bulb and hippocampus in recognition of offspring in mice (Mak and Weiss, 2010). Marmoset fathers should be especially receptive to infant cues.
Socially relevant infant scents can alter the levels of testosterone and estradiol within 20 minutes of exposure. This mechanism of increased estrogens and lowered androgens would favor more “maternal” type behaviors from the males. It is highly likely that males need to be responsive to many stimuli during this period since their mates usually ovulate within 10 days following birth (McNeilly et al., 1981) and fathers are also responsible for guarding their territory from other unrelated males (Lazaro-Perea, 2001). In fact, cotton-top tamarin (Saguinus oedipus) parentally experienced fathers show lower testosterone levels during the infant dependence period but also increased testosterone during their mate's periovulatory period while the infants are still being carried (Ziegler et al., 2004b).
Estrogen levels were elevated following infant scent exposure in our fathers. Males in this study have comparable levels to fertile female marmosets during follicular and periovulatory periods for both estradiol and estrone (Saltzmann et al., 1998). Since males have such high levels of circulating estrogens, the gonads are the most likely source. In the cotton-top tamarin we have found that high levels of urinary estrogens are from the gonads since treatment with a gonadotropin-releasing hormone antagonist, Antide, which blocks LH stimulation of gonadal steroidogenesis, significantly lowers estradiol levels and intramuscular injection of testosterone increases urinary estrogens (Ziegler et al., 2000). This would most likely be the situation for the common marmoset.
The mechanism of olfactory stimulation causing decreased androgens and increased estrogens in response to infant scent is unknown. In rodents an increase in testosterone occurs through chemosensory input from the main olfactory and vomeronasal systems (VNO) where the pathways contain gonadotropin-releasing hormone (GnRH) neurons (Blake and Meredith, 2010). Male marmoset increased testosterone response to an ovulatory scent works through the medial preoptic area (MPOA)/ anterior hypothalamus (AH) as shown by functional magnetic resonance imaging (Ferrris et al., 2004). These hypothalamic areas are the location of the GnRH neurons that activate the LH induced increase in testosterone. GnRH neuronal activity is controlled by VNO – mediated chemosensory input in rodents via the medial and posteromedial cortical nuclei of the amygdala where information is relayed to the MPOA/AH influencing behavioral and endocrine responses (Choi and Anderson, 2005; Touhara and Vosshall for review, 2009). Since scent from related dependent infant does the opposite and lowers testosterone, it is unlikely that the chemosensory activation originates from this route unless there is a negative input to GnRH neurons.
It would be useful to determine aromatase activity in the gonads of parentally experienced compared to inexperienced males. The rapid regulation of aromatase activity is known to work in the brain to cause estrogen actions on behaviors (Charlier et al., 2010). Aromatase catalyzes estrogen biosynthesis and is expressed in many tissues such as the gonads, brain and adipose tissue (Boon et al., 2010). Since estrogens are so high in circulation they may be derived more from a peripheral source than from brain aromatase. Brain and circulating estrogens may work to promote both acute and more long-term changes in the male's behavior.
Research Highlights
Common marmoset fathers have olfactory primer effects in response to isolated infant scents.
Androgen declines and estrogen elevations in fathers are related to the age of their own related infant.
Fathers are significantly more hormonally responsive to their own infant than a novel infant.
Olfactory cues from infants play a role in influencing paternal behaviors
Acknowledgements
We would like to acknowledge the expertise of the animal care staff for their special care of the marmosets. We thank Dan Wittwer for his consultation with steroid assays. This work was funded by the NIH grant: HD057684 To T.E.Z. Support was also provided by Wisconsin National Primate Research Center, RR000167 and the University of Wisconsin's Institute of Clinical and Translational Research, 1UL1RR025011, both from the NIH National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham GE, Buster JE, Lucas LA, Corrales PC, Teller RC. Chromatographic separation of steroid hormones for use in radioimmunoassay. Anal. Lett. 1972;5:509–517. [Google Scholar]
- Belcher AM, Epple G, Greenfield KL, Richards LE, Kuderling I, Smith AB., III Proteins: biologically relevant components of the scent marks of a primate (Saguinus fuscicollis). Chem. Senses. 1990;15:431–446. [Google Scholar]
- Berg SJ, Wynne-Edwards KE. Salivary hormone concentrations in mothers and fathers becoming parents are not correlated. Horm. Behav. 2002;42:424–436. doi: 10.1006/hbeh.2002.1841. [DOI] [PubMed] [Google Scholar]
- Blake CB, Meredith M. Selective enhancement of main olfactory input to the medial amygdala. 2010. [DOI] [PMC free article] [PubMed]
- Boon WC, Chow JD, Simpson ER. The multiple roles of estrogens and the enzyme aromatase. Prog. Brain Res. 2010;181:209–232. doi: 10.1016/S0079-6123(08)81012-6. [DOI] [PubMed] [Google Scholar]
- Broad KD, Curley JP, Keverne EB. Mother – infant bonding and the evolution of mammalian social relationships. Royal Soc. B. 2006;361:2199–2214. doi: 10.1098/rstb.2006.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Murdoch T, Murphy PR, Moger WH. Hormonal responses o male gerbils to stimuli from mate and pups. Horm. Behav. 1995;29:474–491. doi: 10.1006/hbeh.1995.1275. [DOI] [PubMed] [Google Scholar]
- Bruce HM. An exteroceptive block to pregnancy in the mouse. Nature. 1959;184:105. doi: 10.1038/184105a0. [DOI] [PubMed] [Google Scholar]
- Buntin JD. Neural and hormonal control of parental behavior in birds. In: Rosenblatt JS, Snowdon CT, editors. Advances in the Study of Behavior, Vol. 25. Parental Care: Evolution, Mechanisms, and Adaptive Significance. Academic Press; San Diego, CA: 1996. pp. 161–213. [Google Scholar]
- Charlier TD, Sornil CA, Ball GF, Balthazart J. Diversity of mechanisms involved in aromatase regulation and estrogen action in the brain. Biochim. Biophys. Acta. 2010 doi: 10.1016/j.bbagen.2009.12.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GB, Anderson DJ. A nose by any other name (should smell as sweetly). Cell. 11:550–553. doi: 10.1016/j.cell.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Stevenson TJ, Ball GF. Are rapid changes in gonadal testosterone release involved in the fast modulation of brain estrogen effects? Gen. Compar. Endocrinol. 2009;163:298–305. doi: 10.1016/j.ygcen.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis JC, Smith TD, Bhatnagar KP, Bonar CJ, Burrows AM, Morrison EE. Expression of neuron-specific markers by the vomeronasal neuroepithelium in six species of primates. Anatom. Record, Part A. 2004;281A:1190–1200. doi: 10.1002/ar.a.20124. [DOI] [PubMed] [Google Scholar]
- Dubas JS, Heijkoop M, van Aken MAG. A preliminary investigation of parent-progeny olfactory recognition and parental investment. Hum. Nat. 2009;20:80–92. [Google Scholar]
- Epple G. Communication by chemical signals. In: Mitchell G, Erwin J, editors. Comparative Primate Biology, Vol. 2, Part A: Behavior, Conservation and Ecology. Alan R. Liss; New York, New York: 1986. pp. 531–580. [Google Scholar]
- Ferris CF, Snowdon CT, King JA, Sullivan JM, Jr., Ziegler TE, Olson DP, Schultz-Darken NJ, Tannenbaum PL, Ludwig T, Wu Z, Einspanier A, Vaughan JT, Duong TQ. Imaging neural pathways associated with stimuli for sexual arousal in non-human primates. J. Neuroimaging. 2004;19:168–175. doi: 10.1002/jmri.10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, Corter C, Franks P, Surbey M, Schneider B, Steiner M. Postpartum factors related to mother's attraction to newborn infant odors. Dev. Psychobiol. 1993;26:115–132. doi: 10.1002/dev.420260204. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Corter C, Stallings J, Steiner M. Testosterone and prolactin are associated with emotional response to infant cries in new fathers. Horm. Behav. 2002;42:399–413. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- Gamboa GJ, Reeve HK, Holmes WG. Conceptual issues and methodology in kin-recognition research: a critical discussion. Ethol. 1991;88:109–127. [Google Scholar]
- Gonzalez-Mariscal G, Poindron P. Parental Care in Mammals: Immediate Internal and Sensory Factors of Control, Hormones, Brain and Behavior. Vol. 1. Elsevier; San Diego: 2002. pp. 215–298. [Google Scholar]
- Kaitz M, Good A, Rokem AM, Eidelman AI. Mothers’ recognition of their newborns by olfactory cues. Dev. Psych. 1987;20:587–591. doi: 10.1002/dev.420200604. [DOI] [PubMed] [Google Scholar]
- Koyama S. Primer effects by conspecific odors in house mice: a new perspective in the study of primer effects on reproductive activities. Horm. Behav. 2004;46:303–310. doi: 10.1016/j.yhbeh.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Lazaro-Perea C. Intergroup interactions in wild common marmosets, Callithrix jacchus: territorial defense and assessment of neighbors. Anim. Behav. 2001;62:11–21. [Google Scholar]
- Lazaro-Perea C, Snowdon CT, Arruda MF. Scent-marking behavior in wild groups of common marmosets (Callithrix jacchus). Behav. Ecol. Sociobiol. 1999;46:313–324. [Google Scholar]
- Lee S, van der Boot LM. Spontaneous pseudopregnancy in mice. Acta Physiol. Pharmacol. Neerl. 1955;4:442–443. [PubMed] [Google Scholar]
- Levy F, Keller M, Poindron P. Olfactory regulation of maternal behavior in mammals. Horm. Behav. 2004;46:284–302. doi: 10.1016/j.yhbeh.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Mak GK, Weiss S. Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat. Neurosci. 2010;13:753–758. doi: 10.1038/nn.2550. [DOI] [PubMed] [Google Scholar]
- Maruniak JA, Bronson FH. Gonadotropic responses of male mice to female urine. Endocrinology. 1976;99:963–969. doi: 10.1210/endo-99-4-963. [DOI] [PubMed] [Google Scholar]
- Mateo JM. Kin-recognition abilities and nepotism as a function of sociality. Proc. R. Soc. Lond. B. 2002;269:721–727. doi: 10.1098/rspb.2001.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly AS, Abbott DH, Lunn SF, Chambers PC, Hearn JP. Plasma prolactin concentrations during the ovarian cycle and lactation and their relationship to return of fertility post partum in the common marmoset (Callithrix jacchus). J. Reprod. Fertil. 1981;62:353–360. doi: 10.1530/jrf.0.0620353. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The Neurobiology of Parental Behavior. Springer; New York: 2003. [Google Scholar]
- Prudom SL, Broz CA, Schultz-Darken NJ, Ferris CT, Snowdon CT, Ziegler TE. Exposure to infant scent lowers serum testosterone in father common marmosets (Callithrix jacchus). Biology Letters. 2008;6:603–605. doi: 10.1098/rsbl.2008.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reburn CJ, Wynne-Edwards KE. Hormonal changes in males of a naturally parental and a uniparental mammal. Horm. Behav. 1999;35:163–176. doi: 10.1006/hbeh.1998.1509. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Oyama RK, Schlinger BA. Elevated aromatase activity in forebrain synaptic terminals during song. J. Neuroendocrinol. 2009;21:191–199. doi: 10.1111/j.1365-2826.2009.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt JS, Mayer AD. An Analysis of approach/withdrawal processes in the initiation of maternal behavior in the laboratory rat. In: Hood KE, Greenberg G, Tobach E, editors. Handbook of Behavioral Neurobiology. Vol. 7. Plenu Press; New York: 1995. pp. 229–298. [Google Scholar]
- Saltzman W, Schultz-Darken NJ, Wegner FH, Wittwer DJ, Abbott DH. Suppression of cortisol levels in subordinate female marmosets: Reproductive and Social contributions. Horm. Behav. 1998;33:58–74. doi: 10.1006/hbeh.1998.1436. [DOI] [PubMed] [Google Scholar]
- Savage A, Ziegler TE, Snowdon CT. Sociosexual development, pair bond formation, and mechanisms of fertility suppression in female cotton-top tamarins (Saguinus oedipus oedipus) Amer. J. Primatol. 1988;14:345–359. doi: 10.1002/ajp.1350140404. [DOI] [PubMed] [Google Scholar]
- Stern JM. Maternal behavior: sensory, hormonal, and neural determinants. In: Levine S, Brush FR, editors. Psychoendocrinology. Academic Press; New York: 1989. pp. 104–225. [Google Scholar]
- Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Ann. Rev. Physiol. 2009;71:307–332. doi: 10.1146/annurev.physiol.010908.163209. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Marler CA. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to estrogen. Proc. R. Soc. B. 2002;269:823–829. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh JG. Male odor accelerates female sexual maturation in mice. Endocrinol. 1969;84:658–660. doi: 10.1210/endo-84-3-658. [DOI] [PubMed] [Google Scholar]
- Vandenbergh J. Pheromones and mammalian reproduction. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. second ed. Raven Press; New York: 1994. pp. 343–359. [Google Scholar]
- Whitten WK. Modification of the oestrus cycle of the mouse by external stimuli associated with the male. J. Endocrinol. 1956;13:399–404. doi: 10.1677/joe.0.0130399. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Goldsmith AR. Plasma levels of prolactin and gonadal steroids in relation to multiple-brooding and re-nesting in free-living populations of the song sparrow, Melospiza melodia. Horm. Behav. 1990;24:89–103. doi: 10.1016/0018-506x(90)90029-w. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Beauchamp GK, Curran M, Bard J, Boyse EA. Parent-progeny recognition as a function of MHC odor type identity. Proc. Natl. Acad. Sci. 2000;97:10500–10502. doi: 10.1073/pnas.180320997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE. Hormones associated with non-maternal infant care: A review of mammalian and avian studies. Folia Primatol. 2000;71:6–21. doi: 10.1159/000021726. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Carlson AA, Ginther AJ, Snowdon CT. Gonadal source of testosterone metabolites in urine of male cotton-top tamarin monkeys (Saguinus Oedipus). Gen. Comp. Endocrin. 2000;118:332–343. doi: 10.1006/gcen.2000.7476. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Washabaugh KF, Snowdon CT. Responsiveness of expectant male cotton-top tamarins, Saguinus Oedipus, to mate's pregnancy. Horm. Behav. 2004a;45:84–92. doi: 10.1016/j.yhbeh.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Jacoris S, Snowdon CT. Sexual communication between breeding male and female cotton-top tamarins (Sagunius oedipus) and its relationship to infant care. Amer. J. Primatol. 2004b;61:57–69. doi: 10.1002/ajp.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Schultz-Darken NJ, Scott JJ, Snowdon CT, Ferris CF. Neuroendocrine response to female ovulatory odors depends upon social condition in male common marmosets, Callithrix jacchus. Horm. Behav. 2005;47:56–64. doi: 10.1016/j.yhbeh.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Prudom SL, Zahed SR, Parlow AF, Wegner FW. Prolactin's mediative role in male parenting in parentally experienced marmosets (Callithrix jaccus). Horm. Behav. 2009;56:463–443. doi: 10.1016/j.yhbeh.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]