Abstract
Organophosphates (OPs) pose a constant threat to human health due to their widespread use as pesticides and their potential employment in military and terrorist attacks. The acute toxicity of OPs has been extensively studied; however, the consequences of prolonged or repeated exposure to levels of OPs that produce no overt signs of acute toxicity (i.e., subthreshold levels) are poorly understood. Further, there is clinical evidence that such repeated exposures to OPs lead to prolonged deficits in cognition, although the mechanism for this effect is unknown. In this study, the behavioral and neurochemical effects of repeated, intermittent, and subthreshold exposures to the alkyl OP, diisopropylfluorophosphate (DFP) were investigated. Rats were injected with DFP subcutaneously (dose range, 0.25-1.0 mg/kg) every other day over the course of 30 days, and then given a two week, DFP-free washout period. In behavioral experiments conducted at various times during the washout period, dose dependent decrements in a water maze hidden platform task and a spontaneous novel object recognition (NOR) procedure were observed, while prepulse inhibition of the acoustic startle response was unaffected. There were modest decreases in open field locomotor activity and grip strength (particularly during the DFP exposure period); however, rotarod performance and water maze swim speeds were not affected. After washout, DFP concentrations were minimal in plasma and brain, however, cholinesterase inhibition was still detectable in the brain. Moreover, the 1.0 mg/kg dose of DFP was associated with (brain region-dependent) alterations in nerve growth factor-related proteins and cholinergic markers. The results of this prospective animal study thus provide evidence to support two novel hypotheses: 1) that intermittent, subthreshold exposures to alkyl OPs can lead to protracted deficits in specific domains of cognition and 2) that such cognitive deficits may be related to persistent functional changes in brain neurotrophin and cholinergic pathways.
Keywords: organophosphate, nerve agent, memory, acetylcholine, neurotrophin, cognition
Research Highlights.
Subthreshold exposures to DFP in rats lead to protracted deficits in cognition.
DFP exposures also result in protracted alterations in NGF and cholinergic proteins.
DFP-related cognitive deficits may involve neurotrophin and cholinergic alterations.
The risk of exposure to organophosphate (OP)-based chemicals is significant for humans worldwide given their widespread use as pesticides in household, agricultural, and industrial environments. While less likely for most people, there is also the ever-present risk of intentional (OP-related) poisonings by the militaries of rogue governments and terrorists. The world was reminded of this threat when OP nerve agents were used in the 1980s against Iranian military soldiers by Iraq (Majnoon Island) and against civilians in the Kurdish village of Birjinni. The attacks produced casualties estimated by United Nations specialists to be as high as “tens of thousands” (Barnaby, 1988; Macilwain, 1993). More recently, two terrorist attacks in Matsumoto and Tokyo, Japan in 1994 and 1995, respectively, with the nerve agent sarin resulted in the deaths of 19 people and the injury and/or hospitalization of more than 6000 individuals (reviewed, Yanagisawa et al., 2006). Such incidents clearly indicate that terrorist groups have the desire to use nerve agents on civilian populations and that they are capable of producing and/or acquiring them.
It is important to note that while considerable research has focused on the acute symptoms and long-term consequences of overtly toxic exposures to OPs, relatively little attention has been given to the subject of exposures to levels that are not associated with acute cholinergic symptoms. This type of exposure risk is exemplified by the case where more than 100,000 United States service members participating in the first Gulf War (1991) were potentially exposed to low (i.e., non-acutely toxic) levels of sarin/cyclosarin following the destruction of an Iraqi munitions storage complex at Khamisiyah, Iraq, in March 1991. Subtle, but persistent, central nervous system pathology (i.e., volumetric alterations in white matter and the lateral ventricles) were recently reported in veterans involved in this incident (Heaton et al., 2007). In addition, there is also significant evidence to suggest that chronic exposure to (non-acutely toxic) levels of OPs is associated with persistent impairments in attention, memory, and other domains of cognition (Ray and Richards, 2001).
Diisopropylfluorophosphate (DFP) is a prototypical alkylphosphate OP (first described in 1941) that was originally synthesized by British researchers as a potential chemical warfare agent (see Saunders, 1957). It possesses a great deal of structural homology with other highly toxic nerve agents such as sarin and soman. However, it exhibits a markedly reduced potency (in terms of lethality) compared to these OPs (Hobbiger, 1972) and as a consequence is probably the most extensively studied alkylphosphate OP in the research laboratory. DFP is known to bind to the active site of acetylcholinesterase and neurotoxic esterase and to produce an irreversibly inhibited enzyme by a mechanism known as “aging”. These factors may contribute to the histopathologic lesions in the CNS, profound ataxia, and neurologic difficulties produced by DFP. However, these interactions (in particular, the degree of cholinesterase inhibition) are not necessarily predictive of the level of cognitive impairment observed in animals chronically exposed to DFP (Bushnell et al., 1991; Prendergast et al., 1998). Further, we have observed prolonged cognitive deficits in rats after exposure to doses of DFP for 14 days not associated with acute signs of toxicity (Prendergast et al., 1998).
In the experiments described here, we specifically focused on persistent effects (i.e., during and after an extended drug-free washout) of repeated, intermittent, and subthreshold exposures to DFP on behavior, nerve growth factor-related proteins, and cholinergic proteins. In previous studies we have observed alterations in these proteins using this type of exposure protocol with insecticide OPs (Terry et al., 2003. Terry et al., 2007), but had not yet evaluated alkylphosphate OPs. We have operationally defined “subthreshold exposures” as doses that do not produce overt signs of cholinergic toxicity such as muscle fasciculations, seizures, diarrhea, excessive urination, and salivation (see reviews, Rusyniak and Nanagas, 2004; Sungurtekin et al., 2006). The intermittent dosing regimen was used to provide a model for the types of environmental exposures that might be experienced by individuals who live in and around areas where OP nerve agents have been released or by soldiers who are deployed in these areas.
2. Experimental Procedures
2.1 Test Subjects
Male albino Wistar rats (Harlan, Indianapolis, IN) 2-3 months old were doubly housed in a temperature controlled room (25°C), maintained on a reversed 12-hour light/dark cycle with free access to food (Teklad Rodent Diet 8604 pellets, Harlan, Madison, WI). Table 1 provides the details for all study cohorts, the numbers of animals tested per group, and the experiments conducted in each group at different time points. All procedures employed during this study were reviewed and approved by the Medical College of Georgia Institutional Animal Care and Use Committee and are consistent with AAALAC guidelines. Measures were taken to minimize pain or discomfort in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996. Significant efforts were also made to minimize the total number of animals used while maintaining statistically valid group numbers.
Table 1.
Rat Testing Protocol
| Cohort Description | Group | N | Treatment | 30 Day DFP Exposure Period Procedure/Testing Days | 14 Day Drug-Free Washout Period Procedure/Testing Days | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Behavioral Cohort | Grip Strength | Open Field | Rotarod | Grip Strength | Open Field | Rotarod | NOR | WM | PPI | |||
| A | 26 | VEH | 7,14,21,28 | 8,22 | 16,30 | 35,42 | 37 | 34,44 | 33-37 | 38-44 | 41-43 | |
| B | 26 | DFP 0.25 | 7,14,21,28 | 8,22 | 16,30 | 35,42 | 37 | 34,44 | 33-37 | 38-44 | 41-43 | |
| C | 15 | DFP 0.5 | 7,14,21,28 | 8,22 | 16,30 | 35,42 | 37 | 34,44 | 33-37 | 38-44 | 41-43 | |
| D | 19 | DFP 0.75 | 7,14,21,28 | 8,22 | 16,30 | 35,42 | 37 | 34,44 | 33-37 | 38-44 | 41-43 | |
| E | 11 | DFP 1.0 | 7,14,21,28 | 8,22 | 16,30 | 35,42 | 37 | 34,44 | 33-37 | 38-44 | 41-43 | |
| Blood Draw | Blood Draw | |||||||||||
| Plasma Cohort | A | 4 | VEH | 0,7,14,21,30 | 37, 44 | |||||||
| B | 4 | DFP 0.25 | 0,7,14,21,30 | 37, 44 | ||||||||
| C | 4 | DFP 0.5 | 0,7,14,21,30 | 37, 44 | ||||||||
| D | 4 | DFP 0.75 | 0,7,14,21,30 | 37, 44 | ||||||||
| E | 4 | DFP 1.0 | 0,7,14,21,30 | 37, 44 | ||||||||
| Sacrifice | ||||||||||||
| Brain AChE & ChAT Cohort 1 | A | 4 | VEH | 30 | ||||||||
| B | 4 | DFP 0.25 | 30 | |||||||||
| C | 4 | DFP 0.5 | 30 | |||||||||
| D | 4 | DFP 0.75 | 30 | |||||||||
| E | 4 | DFP 1.0 | 30 | |||||||||
| Sacrifice | ||||||||||||
| Brain AChE & ChAT Cohort 2 | A | 4 | VEH | 37 | ||||||||
| B | 4 | DFP 0.25 | 37 | |||||||||
| C | 4 | DFP 0.5 | 37 | |||||||||
| D | 4 | DFP 0.75 | 37 | |||||||||
| E | 4 | DFP 1.0 | 37 | |||||||||
| Brain AChE & ChAT Cohort 3 | A | 4 | VEH | Sacrifice | ||||||||
| B | 4 | DFP 0.25 | 44 | |||||||||
| C | 4 | DFP 0.5 | 44 | |||||||||
| D | 4 | DFP 0.75 | 44 | |||||||||
| E | 4 | DFP 1.0 | 44 | |||||||||
VEH, vehicle; DFP, diisopropylfluorophosphate; NOR, novel object recognition, WM, water maze, PPI, prepulse inhibition; ChAT, choline acetyltransferase, AChE, acetylcholinesterase
2.2 DFP Administration and Observational Studies
Each experimental group received subcutaneous injections of vehicle (Kroger® Pure Peanut Oil) or DFP, CAS 55-91-4 (Sigma Aldrich D0879-1G Lot: 126K1306, St. Louis, MO) 0.25, 0.5, 0.75, and 1.0 mg/kg dissolved in vehicle in a volume of 0.7 ml/kg body weight every other day over a 30 day treatment period. Individual rats were weighed and monitored (in their home cages for a period of approximately 5 minutes each day) for visible cholinergic symptoms (diarrhea, excessive salivation or lacrimation, respiratory difficulties, muscle fasciculations) or other signs of distress throughout the study.
2.3 Behavioral Experiments
All behavioral experiments were conducted in rooms equipped with white noise generators (San Diego Instruments, San Diego, CA) set to provide a constant background level of 70 dB, and ambient lighting of approximately 25-30 Lux (lumen/m2). Animals were transferred (in their home cages) to the behavioral testing rooms each morning approximately 30 min before the beginning of experiments. Due to the large number of subjects tested in the behavioral studies (see Table 1), the cohorts were divided into groups (with representatives from each DFP dose) so that no more than 24 total animals were tested in a given day. Group sizes were based on previous OP studies (e.g., Terry et al., 2007a) in our laboratories where significant effects on water maze performance and other behavioral measures (e.g., prepulse inhibition) were detected. In a few cases animals were injected with DFP on the same day as a behavioral test was conducted (odd days in the grip strength test during the DFP exposure period, see Table 1 and the text below). On these days the grip strength tests were conducted a minimum of 4 hours after drug injection.
2.3.1 Motor Function Tests
Open Field Activity
Rat open field activity monitors (43.2 × 43.2 cm, Med Associates, St. Albans, VT) were used for these experiments. The following parameters were recorded for each 30 min test session: spontaneous locomotor activity assessed by the number of ambulatory counts (horizontal photobeam breaks), rearing and sniffing movements assessed by the number of vertical counts (photobeam breaks associated with rearing), and the number of stereotypical movements (repeated photobeam breaks). The time spent in the central and peripheral zones of the apparatus (defined areas represented approximately 75% and 25% of the total floor area, respectively) was also recorded as an anxiety-related behavioral assessment.
Accelerating Rotarod
Motor coordination, balance, and motor learning were evaluated with an accelerating rotarod (Rotor-Rod System®, San Diego Instruments, San Diego, CA). Individual rats were assessed for their ability to maintain balance on a rotating bar that accelerated from 4 to 40 rpm over a 5-min period. The amount of time elapsed before each subject fell from the rod was recorded. Each test subject was given four trials per day for two consecutive days with an intertrial interval of 30 min.
Grip Strength
Forelimb grip strength was measured with a digital grip strength meter (Animal Grip Strength System®, San Diego Instruments, San Diego, CA) by holding the rat by the nape of the neck and by the base of the tail. The forelimbs were placed on the tension bar and the rat was pulled back gently until it released the bar. Each animal was assessed three times and mean grip strength (measured in kg of resistance ± S.E.M) was calculated.
2.3.2 Memory-Related Behavioral Tasks
Water Maze
Water maze experiments were conducted as described in detail previously (Terry et al., 2006). Briefly, for the hidden platform test, rats were given 2 trials per day for 6 consecutive days to locate and climb on to the hidden platform. Probe trials in the water maze were conducted twenty-four hours following the last hidden platform trial to measure spatial bias for the previous platform location. Visible platform tests were subsequently conducted after probe trials (as a gross estimate of visual acuity) using a highly visible (white) cover fitted with a small white flag attached to the platform.
Spontaneous Novel Object Recognition Test (NOR)
NOR tests were conducted as described in detail previously (Terry et al., 2007). Briefly, habituation to the test apparatus consisted of two daily 10-min sessions in which the animals were allowed to freely explore the open field box. Video-recorded NOR testing began on the third day and ended on day 5. Each test day began with a 3-minute information session (i.e., the A/A session with identical objects) followed by a 15 or 60 min delay period (administered in a pseudorandom order), and a subsequent a 3-minute dissimilar stimuli (A/B) session. The objects discriminated were made of glass, ceramic, clay, or plastic. The total amount of time spent with each object was recorded, where “time spent” was operationally defined as the animal directing its nose to the object at a distance of less than 2.0 cm and/or by the animal touching the object with its nose or mouth. A discrimination index (d2) was calculated on each A/B trial in the NOR task and was defined as the difference in time spent exploring the novel and familiar objects divided by the total exploration time for both objects: d2 index = (novel-familiar)/(novel + familiar). This measure is considered an index of recognition memory and takes into account individual differences in the total amount of object exploration time.
Prepulse Inhibition (PPI)
To assess the effects of prior DFP exposure on sensorimotor gating, a PPI procedure was conducted as described in detail previously (Hohnadel et al., 2007). Briefly, four startle chambers (San Diego Instruments, San Diego, CA) were used, the background white noise was set at 70 dB, and the PPI trials consisted of a prepulse (20 ms burst of white noise with intensities of 75, 80, or 85 dB) followed, 100 ms later, by a startle stimulus (120 dB, 20 ms white noise). PPI was calculated according to the formula: [100 - (startle amplitude on prepulse-pulse trials ÷ startle amplitude on pulse alone trials) × 100]. The mean level of PPI (i.e., averaged across the 3 prepulse intensities) was also analyzed.
2.4 Blood Collection for Plasma Assays and Brain Harvest
Blood sampling occurred weekly throughout the DFP treatment regimen and during a subsequent two-week washout period. Rats were anesthetized by intraperitoneal injection (1 ml/kg body weight) of a cocktail containing ketamine (40 mg/ml) and xylazine (8 mg/ml). Blood was collected from the jugular vein using a 1.0 cc syringe fitted with a 25 G needle; 0.7 ml of blood was immediately added to a Microtainer® Plasma Separator Tube containing lithium heparin (BD catalog #365958). This tube was inverted eight times, and then centrifuged according to the BD protocol. A small amount of the resulting plasma (40ul) was aliquoted into 0.5 ml tubes while the remaining plasma was added to 1.5 ml tubes. The aliquots were snap frozen in liquid nitrogen, and stored at −70° C until analyzed. At the end of the washout period, rats used in the blood sampling and behavioral studies were anesthetized with isoflurane; brains were harvested and snap frozen in dry ice-chilled isopentane, and then cut in half (sagittally) before storage at −70°C. Detailed methods for the dissection of brain regions, preparation of brain lysates have been published previously (Gearhart et al. 2006). One-half of each brain was used in enzyme activity assays (choline acetyltransferase and cholinesterase) and for immunoblots, and the other half of the brain was analyzed for levels of DFP.
2.5 Plasma and Brain DFP Concentrations
DFP concentrations in rat plasma and brain tissue were determined by headspace solid-phase microextraction gas chromatography/mass spectrometry as we have described in detail previously (Xu et al., 2008). Briefly, plasma or brain homogenate samples were added to sample vials that had 10 mg of solid sodium fluoride previously added. Samples were vortexed for 5 min and placed into the autosampler. HS-SPME sampling was performed using a 65mm polydimethylsiloxane/ divinylbenzene (PDMS/DVB) fiber (Sulpelco, Bellefonte, PA, USA) mounted on a Combi/Pal System autosampler (CTC Analytics, Zwingen, Switzerland). Fibers were conditioned at 250°C for 30 min prior to use. Sample vials were preheated in the agitator for 10 min before analysis and the SPME fiber was then exposed to the headspace by piercing the septum with the needle of the fiber assembly. After extraction for 15 min at 30°C under agitation, the fiber was withdrawn into the needle and immediately desorbed at 250°C for 1 min into the injection port of the gas chromatograph. The analyses were carried out on an Agilent 6890 gas chromatograph coupled with an Agilent model 5973 mass selective detector mass spectrometer (Agilent Technologies, Palo Alto, CA, USA). The gas chromatograph was equipped with a 0.75mm i.d. SPME liner. Separation of the analytes was obtained on a ZB-5MS column (30m × 0.25mm i.d., 0.25mm film thickness; Phenomenex, Torrance, CA, USA) using helium as a carrier gas (flow rate: 1 mL/min). The gas chromatograph injection port and interface transfer line were maintained at 250 and 280°C, respectively. During the fiber desorption process, the splitless mode of injection was used. The oven temperature was initially held at 60°C for 1 min, then increased to 100°C at 5°C/min, and held for 1 min. The mass spectrometer was operated in electron ionization (EI) mode with an electron energy of 70 eV. Quantitation of DFP was performed using selected-ion monitoring (SIM) of m/z 101 (quantitation ion) and m/z 127 (confirmation ion).
2.6 Homogenization of Brain for Enzyme Assays
The dissection protocol described by Gearhart et al. (2006) was used to isolate five brain regions: basal forebrain, hippocampus, striatum, prefrontal cortex, and the remaining cortex — from frozen brains (collected above). Brain tissues were manually homogenized using a polypropylene pestle in a 1.5 ml microcentrifuge tube (Scienceware® catalog #19923-0000; Bel-Art Products, Pequannock, NJ). Homogenation buffer contained sucrose (0.25 M), EDTA (10 mM), Triton-X 100 (0.5% v/v), and 0.01 M sodium phosphate (pH 7.4). After manual homogenation in five volumes (5 μl of buffer per mg tissue) of ice-cold homogenation buffer, the crude homogenate was briefly sonicated (on ice) using a Sonic Dismemberator™ (Model #100; set at level 1; Fisher Scientific). Sonicated homogenates were aliquoted into 0.5 ml tubes (20 μl/tube), and stored at −20°C. Within two weeks of freezing, the homogenates from all five brain regions were analyzed for total protein (Coomassie Plus Assay; catalog #23236; Pierce Biotechnology, Rockford, IL), cholinesterase activity, and choline acetyltransferase activity.
2.7 Plasma and Brain Cholinesterase Activities
Cholinesterase activity in plasma samples and brain homogenates were measured according to Ellman et al. (1961) in a 96-well plate format at room temperature. Five microliters (5 μl) of plasma (100-130 μg protein/μl) or brain homogenate (20-50 μg protein/μl) were dispensed into the bottom of the wells of the 96-well plate (Fisher Scientific #12-565-501). An 8- or 12-channel pipeter was used to quickly add 310 μl of reaction mixture to the wells. The reaction mixture contained acetylthiocholine (0.48 mM; # D-8130, Sigma-Aldrich, Inc., St. Louis, MO) and dithiobisnitrobenzoic acid (0.52 mM; Acros # 102710050) in 0.1 M sodium phosphate buffer (pH 8.0). The microplate was shaken for ~30 seconds using a Jitterbug™ plate shaker (Boekel Scientific; Feasterville, PA), before placing the microplate in a μQuant™ Microplate Spectrophotometer (BioTek Instruments Inc.; Winooski, VT). The formation of reaction product (yellow color) was monitored by measuring absorbance at 412 nm every 2 min for 16 min. The cholinesterase-mediated reaction rate (moles/liter per min) was calculated by dividing the change in absorbance per minute by 13,600 (for details, see Ellman et al., 1961). Each plasma sample or brain homogenate was assayed in triplicate.
2.8 Choline Acetyltransferase Activity
Brain homogenates (prepared above) were analyzed for choline acetyltransferase activity using a modification of the method described by Fonnum (1969). Typically a set of 24-30 samples were processed at once—for example, cortex homogenates from the four treatment groups (N=5-6 samples per group). Each assay (20 μl total volume) was prepared in a 0.5 ml tube. For each set of assays, brain homogenate (8 μl; 20-50 μg protein/μl) was added to every tube, and then 12 μl of “reagent master mix” were quickly added to start the reaction. The “reagent master mix” contained (per assay tube): 0.2 μCi of tritium-labeled acetyl coenzyme A (Perkin Elmer #NET290); non-tritiated acetyl coenzyme A 0.4 mM; choline chloride 10 mM; eserine 0.2 mM; EDTA 10 mM; sodium chloride 0.3 mM; and Triton X-100 0.5% v/v in sodium phosphate 50 mM, pH 7.4. Tubes were maintained at 37°C for 30 min. The reaction was quenched by placing the assay tubes in a pre-chilled (−20°C) microcentrifuge rack and adding 100 μl of cold ultrapure water to each tube. Liquid-liquid extraction was used to isolate the acetylcholine formed during the reaction: 300 μl of an organic solution containing 20 mg/ml sodium tetraphenylborate in 3-heptanone was added to the quenched reaction. The mixture was vortexed for 30 sec, and then centrifuged for 5 min at 7,000xg. Two-hundred microliters (200 μl) of the upper organic layer was carefully pipeted out of the assay tube, and then transferred to a 20 ml glass scintillation vial that contained 10 ml of Scintiverse BD™ (Fisher Scientific; Waltham, MA) liquid scintillation cocktail. A Beckman LS6000TA (Beckman Instruments, Inc; Fullerton, CA) was used for liquid scintillation counting.
2.9 Immunoblots for Neurotrophin and Cholinergic Proteins
At the end of the 14-day washout, relative levels of five proteins (proNGF, NGF, p75NTR, TrkA, and α7-nAChRs) were measured in tissue lysates from three brain regions (basal forebrain, hippocampus, prefrontal cortex) prepared from vehicle- and DFP (1.00 mg/kg)-treated rats from the behaviorally tested cohorts. These rats were sacrificed approximately one hour after the last visible platform test in the water maze. Protein concentrations were determined by the bicinchoninic acid method (BCA Protein Assay Kit, Pierce/Thermo Scientific, USA).
Equal amounts of protein were resolved in SDS-polyacrylamide gels (ranging from 7-12%) and transferred electrophoretically onto a nitrocellulose membrane (Bio-Rad). Membranes were blocked for 1 h in PBST (3.2 mM Na2HPO4,. 0.5 mM KH2PO4, 1.3 mM KCl, 135 mM NaCl, 0.05% Tween-20) and 5% non-fat milk (or BSA as per the manufacturers recommendations) and incubated overnight with the indicated antibodies. The primary antibodies used were anti-NGF (1:250; #AN-240; Almone Labs, Jerusalem, Israel), anti-TrkA (1:300; #sc-118; Santa Cruz Biotech, CA, USA), anti-p75NTR (1:1000; #07-476; Upstate, Lake Placid, NY), anti-α7-nAChR (1:2000 #ab23832 Abcam Inc., Cambridge, MA), or anti-β-actin (1:1500; #A-5441; Sigma-Aldrich, Inc., St. Louis, MO). After washing with PBST, the membranes were incubated for 1 hr with horseradish peroxidase-conjugated anti-rabbit or anti-mouse anti-sera in PBST and 3% non-fat milk. The membranes were washed again with PBST, and proteins were visualized by enhanced chemiluminescence (Immobilon Western, Millipore, Billerica, MA, or SuperSignal West Pico Chemiluminescent Substrate, Thermo Scientific, Rockford, IL). The optical density of the immunoreactive bands was measured using NIH ImageJ software. The densitometric values for the proteins of interest were corrected for protein loading using β-actin.
2.10 Statistical Analyses
All statistical analyses were performed using SigmaPlot Version 11 (SPSS Inc., Chicago, IL) or JMP™ version 5 (Cary, NC). One, two- or three-way analysis of variance (with repeated measures when indicated) was used for treatment group comparisons. Student Newman Keuls multiple comparison procedures (SigmaPlot) and orthogonal t-tests (JMP) corrected for multiple comparisons via the method of Bonferroni were used to examine post hoc differences when indicated. Statistical significance was assessed using an alpha level of 0.05
3. Results
3.1 Body Weight and Observational Studies
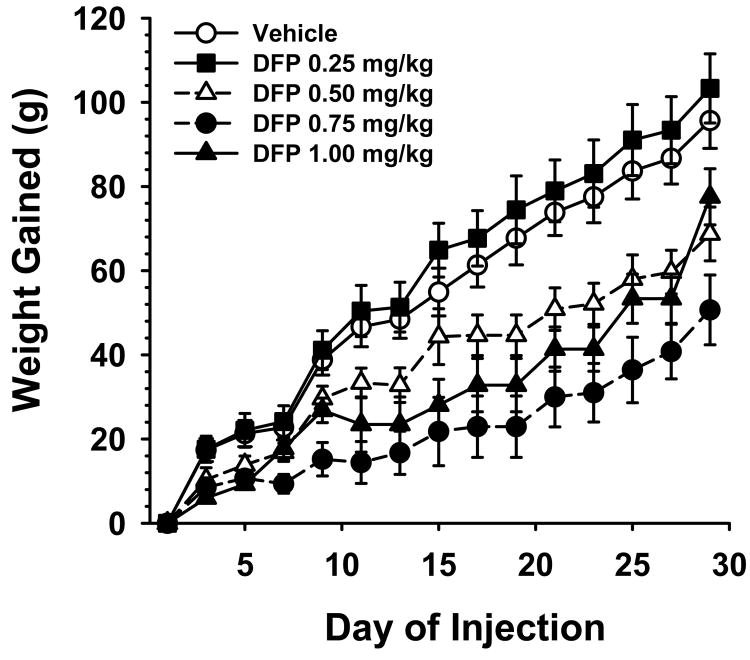
The effect of repeated (i.e., every other day) exposures to DFP on body weight gained over the 30 day treatment period is illustrated in Fig 1. As indicated, the rats in all treatment groups gained significant weight over the 30 day period (range approximately 64-103 grams gained). There were significant dose-related differences between the treatment groups, F4,92=7.06, p<0.001. Post hoc comparisons indicated that the groups treated with 0.5, 0.75, and 1.0 mg/kg (but not the 0.25 mg/kg) dose of DFP gained significantly less weight (p<0.05) than vehicle-treated controls. There were no observations of acute cholinergic signs at any point in the study.
Fig 1.
Effect of repeated exposure (subcutaneous injections every other day) to subthreshold doses of DFP on weight gain over a 30 day period. Each symbol represents the mean ± S.E.M. N=11-26.
3.2 Assessments of Exploratory Activity and Motor Function
In these experiments we were interested in determining whether DFP exposure had significant effects on exploratory activity or motor function (i.e., effects that might have influenced performance in the memory-related tests). The results of these experiments are provided in Figs 2-4.
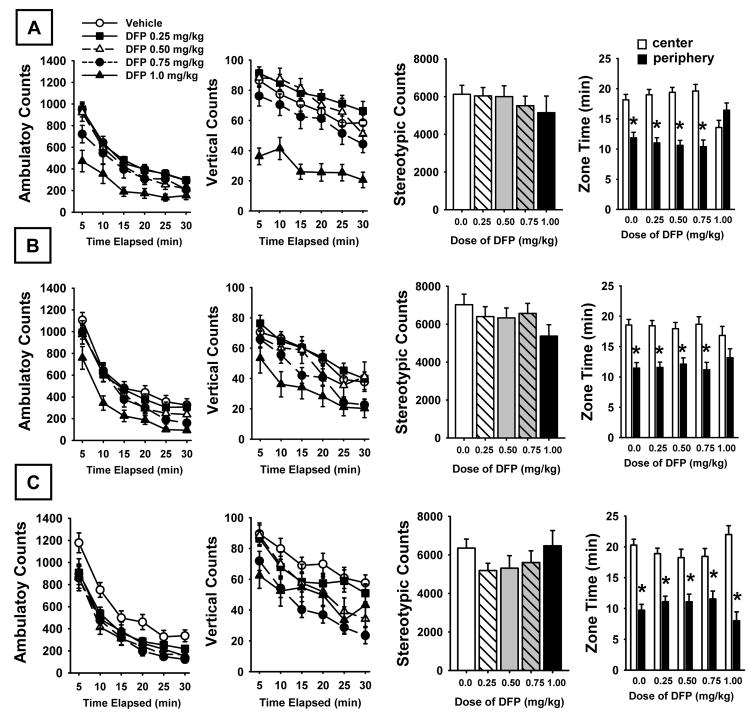
Fig 2.
Open Field Activity on day 8 (A) and day 22 (B) of DFP exposure and on day 7 of a DFP-free washout period (C). Left to right: horizontal activity measured as the number of ambulatory counts (photobeam breaks) at each time point over a 30 min evaluation period; vertical activity (photobeam breaks activated by rearing activity); stereotypical movements (repetitive photobeam breaks); fear/anxiety related behavior measured as the time spent in the central versus the peripheral zone of the activity monitor. N=11-26. * = significant difference (p<0.05) in time spent in the center versus peripheral zone.
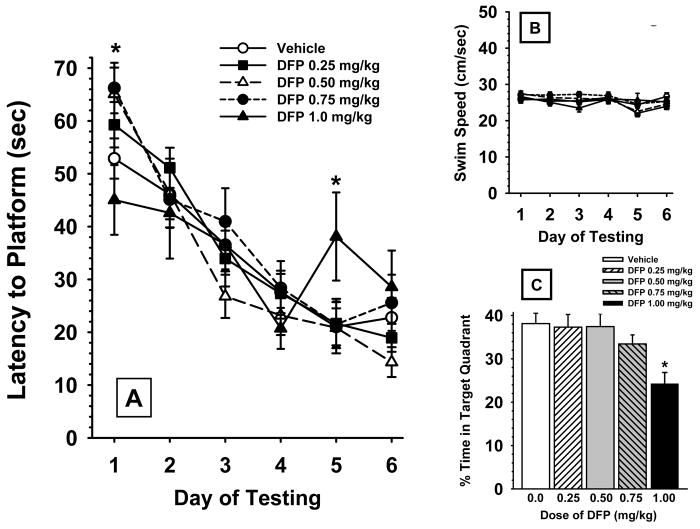
Fig 4.
Effects of prior chronic exposure to DFP (i.e., testing beginning day 7 of a DFP-free washout period) on a water maze spatial learning procedure. (A) Hidden platform test (mean ± S.E.M), 2 trials/day over 6 consecutive days of testing. (B) Daily swim speeds (mean ± S.E.M. cm/sec) during water maze hidden platform trials. (C) Water maze probe trials (mean platform area crossings ± S.E.M.) conducted on day 14 after the last day of a 30 day exposure period to DFP or vehicle. * = significantly different from vehicle controls (p<0.05). N=11-26.
Open Field Activity
Fig 2 illustrates the effects of DFP on open field locomotor activity at two time points during exposure and one time point during the DFP-free washout period. Horizontal locomotor activity (ambulatory counts), vertical activity (rearing), and stereotypical movements are depicted. The time spent in the peripheral versus central zones of the test apparatus was also assessed. There were significant dose-related effects on both horizontal and vertical activity (but not stereotypical movements) that persisted throughout the study. For horizontal activity (ambulatory counts) statistical analysis provided the following results: main effect of dose, F4,92 = 3.47, p<0.01; time Point, F5,1564 =296.60, p<0.0001; session, F2,1564 =10.17, p<0.0001; dose × time point, F20,1564 =3.51, p<0.0001; dose × session, F8,1564 =17.72, p<0.0001; time point × session, F10,1564 =0.25, p=0.991; dose × time point × session, F40,1564 =0.50, p=0.99. For the vertical activity comparison the following statistical results were obtained, main effect of dose, F4,92 = 7.51, p<0.0001; time point, F5,1564 =455.60, p<0.0001; session, F2,1564 =59.90, p<0.0001; dose × time point, F20,1564 =4.79, p<0.0001; dose × session, F8,1564 =13.49, p<0.0001; time point × session, F10,1564 =3.86, p<0.0001; dose × time point × session, F40,1564 =0.80, p=0.81. In each of the test sessions, there was clear evidence of habituation to the open field environment in all test groups as indicated by the diminishing horizontal and vertical counts over time (see time point effects above). In the zone time assessment, the rats generally spent more time in the center of the open field as expected (since it represented 75% of the test arena) with the exception of the rats administered the higher dose of DFP (1.0 mg/kg) during the drug exposure periods. In these subjects there was no significant difference in the time spent in the central versus peripheral zone, an indication of thigmotaxis and/or elevated anxiety. This effect, however, disappeared during the drug-free washout period. The following statistical results were obtained in the zone analysis, main effect of dose, F4,92 = 0.01, p=0.99 ; session, F2,460 = 0.01, p=0.99 ; zone, F1,460 = 318.21, p<0.0001 ; dose × session, F8,460 = 0.01, p=0.99 ; dose × zone, F4,460 = 1.46, p=0.21 ; session × zone, F2,460 = 7.40, p<0.001 ; dose × session × zone, F8,460 = 4.91, p<0.0001.
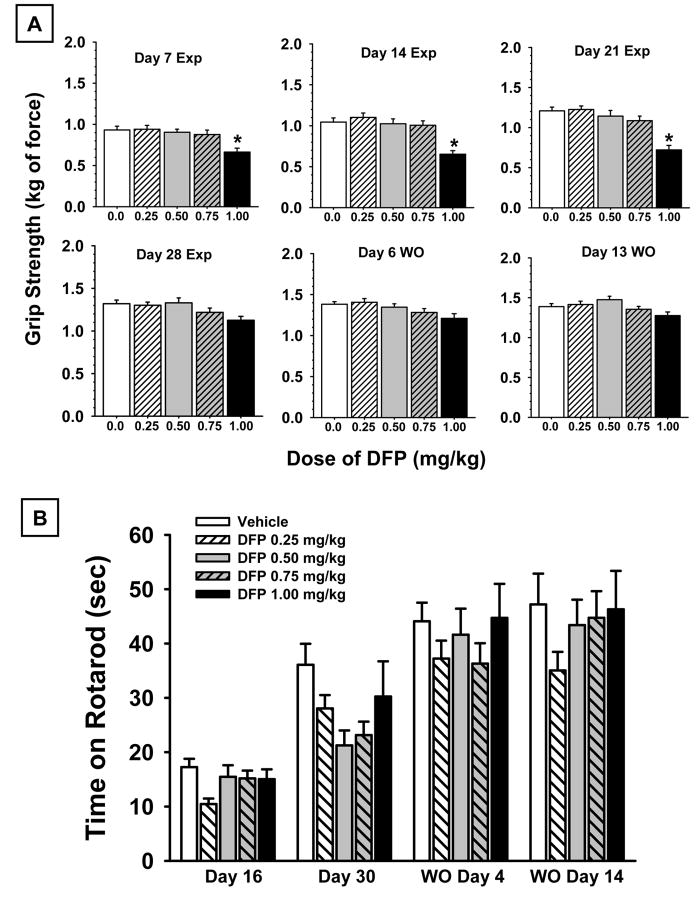
Grip Strength
The effects of DFP exposure on forelimb grip strength at various points during DFP exposure and during the drug free washout period are illustrated in Fig 3A. There was a statistically significant main effect of dose F4,92 = 7.9, p<0.001; session F5,460 = 142.85, p<0.001, as well as a significant dose × session interaction F20,460 = 2.23, p=0.002.. Post hoc evaluations indicated that grip strength increased in all groups over the course of repeated testing; and that the higher dose of DFP (1.0 mg/kg) was associated with impairments of grip strength (compared to vehicle controls) in the early sessions during the DFP exposure period. This effect diminished, however, in the later sessions (i.e. during drug exposure and during the drug-free washout session).
Fig 3.
(A) Forelimb grip strength measured in kg of force. Day 7, day 14, day 21, and day 28 of DFP exposure are depicted as well as day 6 and day 13 of a DFP-free washout period (WO). (B) Accelerating rotarod performance expressed as time maintained on a rotating bar that accelerated from 4 to 40 rpm over a 5-min period. Day 16, and day 30 of CPF exposure are depicted as well as day 4 and day 14 of a DFP-free washout period (WO). The bars in each figure represent the mean ± S.E.M. N=11-26.
Rotarod Performance
The effects of DFP exposure on the performance of the rotarod task at various points during exposure and during the DFP-free washout period are illustrated in Fig 3B. As in the case of grip strength, there was a statistically significant effect of the test session F3,276 = 81.85, p<0.001, indicating that rotarod performance improved in all groups over the course of repeated testing. However, there was no significant dose effect or dose × session interaction indicating that DFP did not significantly affect rotarod performance.
3.3 Water Maze Testing
Hidden Platform Test
Fig 4A illustrates the efficiency of each experimental group to locate a hidden platform in a water maze task on 6 consecutive days of testing, beginning on day 7 of the DFP-free washout period (i.e., after the 30 day regimen of DFP exposure). The acquisition curves for swim distance are depicted. Statistical comparisons of distances across the 4 groups revealed the following, main effect of dose, F4,92 = 0.97, p=0.43; test session, F5,460 = 48.45, p<0.001; dose × session interaction, F20,460 = 1.83, p<0.02. Similar results were evident when swim latencies were analyzed (data not shown). Thus, after exposure to vehicle or DFP, the rats learned to locate the hidden platform with progressively shorter swim distances (and latencies) across the 6 days of training. Post hoc analyses indicated that the 0.75 and 1.0 mg/kg doses of DFP were associated with significant (p<0.05) impairments in performance of the task (i.e., indicated by higher mean swim distances to locate the hidden platform) on days 1 and 5 of testing, respectively.
Swim Speeds
Fig 4B illustrates the swim speeds i.e., the distance swam (cm) divided by the latency to find the platform (sec) during water maze testing. Swim speeds ranged (on average) from approximately 23 and 26 cm/sec across the treatment groups for all 6 days of water maze testing and were not statistically different.
Probe Trials
Fig 4C illustrates the performance of probe trials in the water maze by the various treatment groups conducted on day 7 of water maze testing. Statistical comparisons of the percentage of time spent swimming in the previous target quadrant revealed a significant main effect for dose, F4,92 =3.09, p=0.02. Post hoc analysis indicated that the 1.0 mg/kg dose of DFP was associated with significant (p<0.05) impairments in performance.
Visible Platform Test
The average times required to reach a highly visible (reflective) platform in the water maze (data not shown) ranged between 11.8 and 15.9 seconds across all groups in the study and were not significantly different (i.e., all p values were >0.05), indicating that differences in performance of the previous hidden platform tests or probe trials were unlikely to be a result of gross impairments in visual acuity associated with DFP.
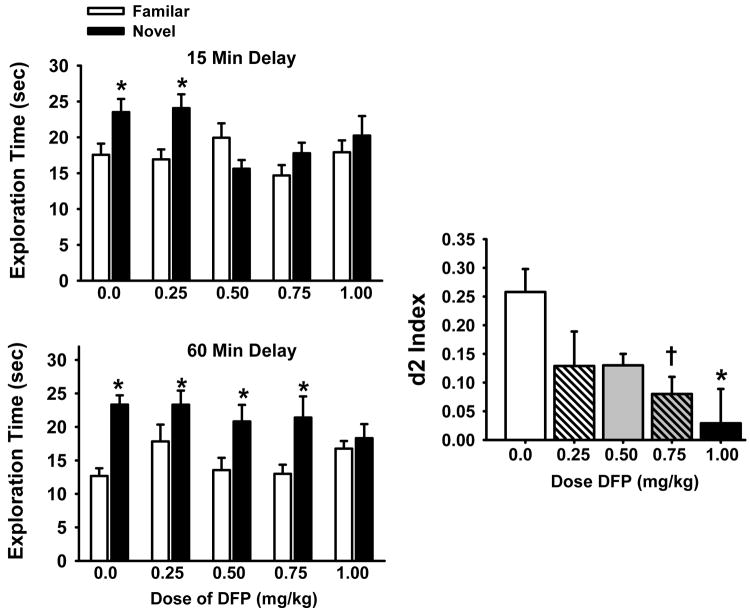
3.4 Spontaneous Novel Object Recognition Test (NOR)
Fig 5 illustrates the effects of prior DFP exposure on performance in the OR task. The most notable results were a significant main effect of dose F4,91 = 2.74, p=0.029 and object type, F1,262 = 29.01, p<0.0001. Post hoc analysis indicated a significant (p<0.05) preference for the novel object at both delay intervals under vehicle-control conditions (* in Fig 5). In the case of DFP-treated subjects, this preference was lost at the 0.5, 0.75 and 1.0 mg/kg doses at the 15 minute delay and at the 1.0 mg/kg dose at the 60 min delay (see Fig 5A). Average d2 indices (i.e., averaged across delay) were also calculated and are presented in Fig 5B. There was a significant main effect of dose in this analysis, F4,79 = 2.78, p=0.032. Post hoc analysis indicated that the 1.0 mg/kg dose of DFP was associated with a significant decrease in the d2 index (p<0.05) while the 0.75 mg/kg dose was associated with a clear trend (p<0.07) toward (significantly) impaired NOR performance.
Fig 5.
Effects of prior chronic exposure to DFP (i.e., testing on days 2-5 of a DFP-free washout) on the performance of a spontaneous novel object recognition task. The illustrations at the left indicate the preference for the novel object compared with the familiar object (*= p<0.05) at each of the 3 delays. Discrimination (d2) indices (i.e., averaged across delay) are presented at the right. d2 was calculated on each A/B trial and was defined as the difference in time spent exploring the novel and familiar objects divided by the total exploration time for both objects: d2 index = (novel-familiar)/(novel + familiar). Data are expressed as the mean ± S.E.M. N=11-26. * = significantly different from vehicle controls (p<0.05); † = p<0.07.
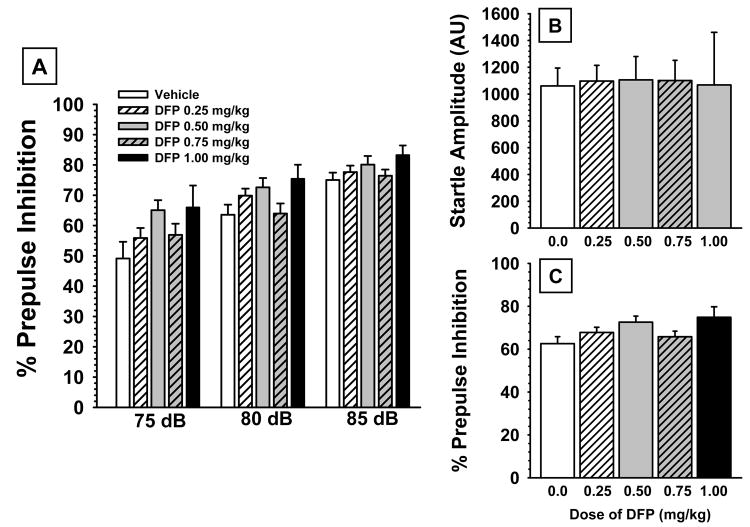
3.5 PPI Experiments
The effects of prior exposure to DFP on PPI testing assessed on day 12 of the drug-free washout are presented in Figs 6A-C. While there appeared to be a mild trend toward improved PPI (particularly at the 5.0 db prepulse level above background) associated with DFP, the effect was not statistically significant, main effect of dose, F4,92 = 2.23, p=0.07 (see Table 2). This non-significant trend was also apparent when the data were averaged across the prepulse levels (see Fig 6C). As expected, there was a highly significant difference in response to the different prepulse levels, F2,184 = 91.18, p<0.001; while the dose × prepulse level interaction was not significant. In addition, there were no significant effects of any of the doses of DFP on startle amplitude (Fig 6B).
Fig 6.
(A) Effects of prior chronic exposure to DFP (i.e., testing on day 13 of a drug free washout) on the percentage of prepulse inhibition (PPI) in rats for three prepulse intensities (5, 10, and 15 dB above background). (B) DFP effects on the mean startle amplitude to 120-dB, 20-ms noise burst. (C) DFP effects on the percentage of prepulse inhibition averaged across the three prepulse intensities. Bars represent mean ± S.E.M. for each treatment. N=11-26.
Table 2.
Protracted Effects of DFP on Choline Acetylcholinesterase (ChAT) Activity
| Treatment | Measurement Time Point | Brain Region | ||||
|---|---|---|---|---|---|---|
| BF | HIPP | PFC | CTX | STR | ||
| Vehicle | Last Injection Day | 12.37±0.78 | 4.42±0.24 | 3.83±0.33 | 3.01±0.15 | 8.94±0.42 |
| DFP 0.25 mg/kg | Last Injection Day | 9.33±1.50*** | 4.18±0.33 | 4.16±0.45 | 3.41±0.09 | 9.39±0.64 |
| DFP 0.50 mg/kg | Last Injection Day | 9.88±1.02* | 4.74±0.11 | 3.48±0.08 | 3.42±0.32 | 9.83±0.63 |
| DFP 0.75 mg/kg | Last Injection Day | 8.21±1.11*** | 4.69±0.35 | 3.82±0.37 | 2.94±0.20 | 8.34±0.39 |
| DFP 1.00 mg/kg | Last Injection Day | 6.67±1.35*** | 3.86±0.20 | 3.76±0.28 | 2.73±0.27 | 10.00±0.51 |
| Vehicle | Washout Day 7 | 9.52±0.60 | 4.37±0.15 | 2.75±0.22 | 3.70±0.19 | 6.48±0.44 |
| DFP 0.25 mg/kg | Washout Day 7 | 7.72±1.24§ | 4.29±0.13 | 2.32±0.16 | 3.12±0.17 | 6.59±0.44 |
| DFP 0.50 mg/kg | Washout Day 7 | 6.44±1.23** | 4.40±0.35 | 2.58±0.07 | 3.26±0.12 | 6.23±0.76 |
| DFP 0.75 mg/kg | Washout Day 7 | 8.06±0.94 | 4.64±0.21 | 2.21±0.12 | 3.03±0.09 | 6.41±0.65 |
| DFP 1.00 mg/kg | Washout Day 7 | 7.88±0.61§ | 4.85±0.19 | 2.25±0.10 | 3.07±0.17 | 7.37±0.46 |
| Vehicle | Washout Day 14 | 9.06±2.22 | 4.69±0.37 | 4.95±0.37 | 2.99±0.13 | 8.89±0.80 |
| DFP 0.25 mg/kg | Washout Day 14 | 8.04±0.60 | 4.57±0.72 | 3.78±0.16 | 2.76±0.26 | 9.26±1.02 |
| DFP 0.50 mg/kg | Washout Day 14 | 8.52±1.66 | 4.93±0.54 | 3.97±0.0.63 | 2.66±0.20 | 8.27±0.83 |
| DFP 0.75 mg/kg | Washout Day 14 | 8.50±2.33 | 4.74±0.65 | 3.63±0.29 | 3.39±0.33 | 8.01±0.93 |
| DFP 1.00 mg/kg | Washout Day 14 | 5.61±1.55** | 4.93±0.34 | 3.65±0.42 | 2.97±0.38 | 7.69±0.53 |
Choline acetylcholinesterase (ChAT) activity (expressed as pmoles/μg of protein/min) measured in 5 brain regions on the last day of a 30-day alternate injection regimen as well as in separate groups of animals at 7 and 14 days after the last DFP administration. Each value represents the mean ± S.E.M. derived from 5-6 rats.
p<0.001;
p<0.01;
p<0.05
p<0.1 with respect to vehicle control mean at the same measurement point.
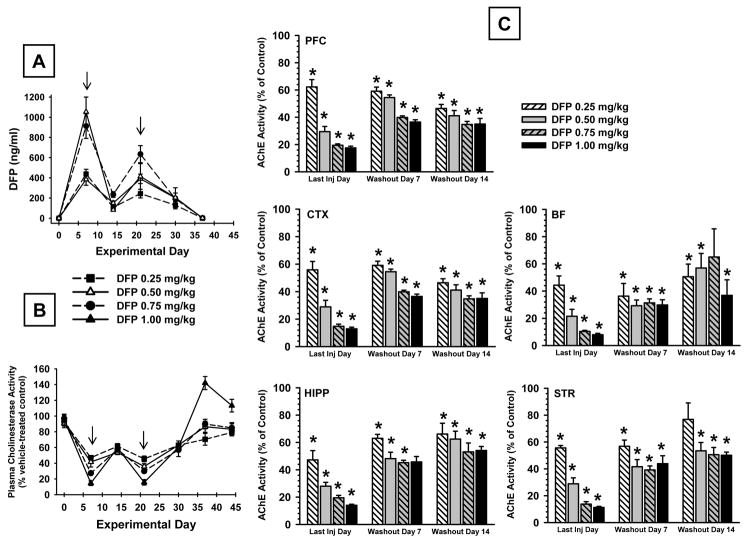
3.6 DFP Concentrations (Plasma and Brain)
Alternate day administration of DFP over 30 days produced a concentration-dependent increase in the plasma levels of DFP (Fig. 7A). Maximal plasma concentrations were detected when assessed on the day of injection (arrows in Fig 7A), and the concentrations were markedly diminished on alternate days (i.e., assessed the day after injection). After the final DFP injection on experimental day 30, plasma concentrations dropped sharply such that minimally detectable levels were present after the 2-week washout. Whole brain concentrations of DFP were measured on experimental day 30 (the last day of DFP exposure) and after a 7 day drug-free washout. DFP concentrations detected (ng/g) on experimental day 30 were as follows: 0.25 mg/kg = 3.11±2.08; 0.50 mg/ kg = 5.22±1.54; 0.75 mg/kg = 5.32±1.16; 1.00 mg/kg = 4.42±0.44. At the 7-day washout time point DFP concentrations were below the limit of quantitation in all dose groups.
Fig 7.
(A) Plasma concentrations of DFP during a treatment regimen in which DFP was administered s.c. on alternate days over a 30 day period followed by a 14 day wash-out period. The arrows indicate days during which DFP was administered prior to removing the blood sample for analysis. Each value represents the mean ± S.E.M. derived from 6 rats. (B). Plasma cholinesterase activity as a percent of control during a treatment regimen in which DFP was administered s.c. on alternate days over a 30 day period followed by a 14 day wash-out period. The arrows indicate days during which DFP was administered prior to removing the blood sample for analysis. Each value represents the mean ± S.E.M. derived from 5-6 rats. (C) Brain acetylcholinesterase activity as a percent of control measured in 5 brain regions on the last day of a 30-day alternate injection regimen as well as in separate groups of animals at 7 and 14 days after the last DFP administration. Each value represents the mean ± S.E.M. derived from 5-6 rats. *p<0.05 with respect to vehicle control mean.
3.7 Cholinesterase Activity (Plasma and Brain)
Alternate day administration of DFP produced a concentration-dependent decrease in plasma cholinesterase activity that correlated closely with the plasma levels of DFP achieved with each dose, dose effect, F4,23=9.57, p<0.001; day effect, F6,138=303.04, p<0.001; dose × day interaction, F24,138=29.71, p<0.001. Maximal plasma cholinesterase inhibition was detected when assessed on the day of DFP injection (arrows in Fig 7B), and inhibition was considerably lower on alternate days (i.e., assessed the day after injection). Enzyme activity was reduced to about 14% of control by the highest, 1.00 mg/kg DFP regimen at the 7 day time point and to about 20% of control at the 21 day time point. During the 2-week washout period, cholinesterase activity returned to baseline in all of the treatment groups, although, in the case of the highest dose of DFP (1.00 mg/kg), cholinesterase activity exceeded control levels significantly (e.g., p<0.001) on washout day 7.
Cholinesterase activity was also measured in five brain regions at 3 time points: the last day of DFP injection, washout day 7, and washout day 14 (Fig 7C). Statistical analyses revealed the following results, main effect for group, F14,45=86.1, p<0.001; brain region, F4,180=485.06, p<0.001; group × brain region interaction, F56,180=2.21, p<0.001. Thus, as indicated in Fig 7C, cholinesterase activity was inhibited (relative to vehicle controls) in all of the treatment groups in all of the brain regions analyzed. While there was some restoration in cholinesterase activity during the washout period in the DFP treated groups, activity remained inhibited (relative to controls) in all brain regions analyzed until the end of the study.
3.8 Cholinergic Proteins
Choline acetyltransferase (ChAT) activity was also measured in five brain regions at 3 time points (i.e., in different groups of rats): the last day of injection, washout day 7, and washout day 14 (see Table 2). Statistical analysis revealed the following results, main effect for group, F14,56=2.94, p<0.001; brain region, F4,166=178.9, p<0.0001; group × brain region interaction, F56,166=1.96, p<0.001. As expected, under vehicle control conditions, ChAT activity was highest in regions that are rich in cholinergic neurons (basal forebrain, striatum). Post hoc analysis revealed that the only brain region significantly affected by DFP exposure was the basal forebrain. In this case, ChAT activity was found to be significantly reduced by all of the doses of DFP when measurements were made on the last day of the 30-day exposure period. There were also significant (or nearly significant, p<0.1) DFP-related decreases in animals that were evaluated during the 7 day washout period. Finally, at the two week DFP-free washout time point there remained a significant decrease in ChAT activity in the basal forebrain in rats previously treated with the 1.00 mg/kg dose of DFP.
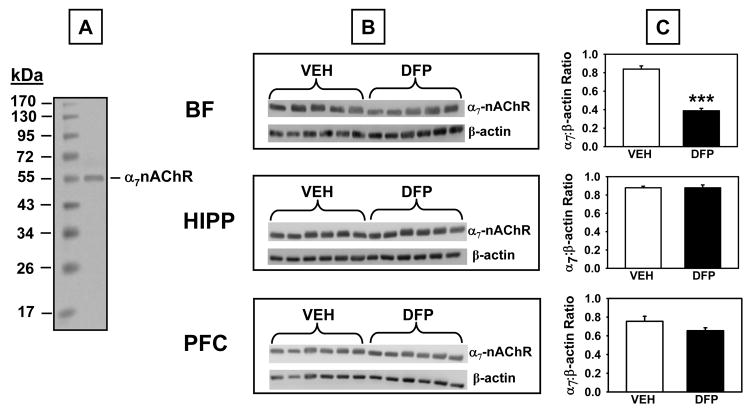
α7-nicotinic acetylcholine receptor (α7-nAChR) protein was also assessed in three brain regions (basal forebrain, hippocampus, and prefrontal cortex) in tissues harvested on experimental day 44 (last day of the two-week washout period) from animals exposed to the vehicle or 1.00 mg/kg DFP treatment regimen (see Fig 8). Western blot analysis using an antibody that recognizes the α7-nAChR (~56 kDa immunoreactive band) indicated that α7-nAChR protein was significantly decreased in the basal forebrain in DFP-treated rats compared to controls (t=6.10, p<0.001), but was not significantly affected in the hippocampus or prefrontal cortex.
Fig 8.
α7 nicotinic acetylcholine receptor (α7 nAChR) levels measured by Western Blot in brain lysates derived from rats that had completed the 1.00 mg/kg alternate day regimen of DFP plus the two-week washout period. A. Representative blot illustrating a molecular weight marker and α7 nAChR protein (~56 kDa). B. Blots illustrating α7 nAChR protein and β-actin (~38-40 kDa) in the same samples from basal forebrain (BF), hippocampus (HIPP), and prefrontal cortex (PFC). C. Data presented in the bar graphs were obtained from densitometry measurements of the bands for α7 nAChR and β-actin and the represent the mean ± SEM for the ratio. *** indicates significant difference compared to vehicle-treated control rats, p<0.001, two-tailed Student’s t-tests. N = 5-6.
3.9 NGF-Related Proteins
ProNGF and mNGF protein levels (Fig 9)
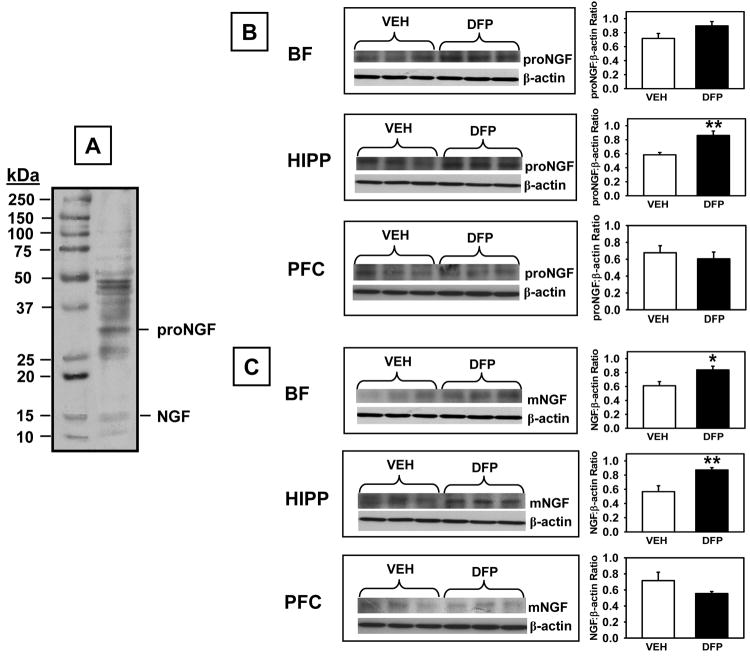
Fig 9.
Pro-NGF and mature NGF (mNGF) levels measured by Western Blot in brain lysates derived from rats that had completed the 1.00 mg/kg alternate day regimen of DFP plus the two-week washout period. A. Representative blot illustrating a molecular weight marker, proNGF (~32 kDa), and NGF (~14 kDa). B. Blots illustrating proNGF, NGF, and β-actin (~38-40 kDa) in the same samples from basal forebrain (BF), hippocampus (HIPP), and prefrontal cortex (PFC). C. Data presented in the bar graphs were obtained from densitometry measurements of the bands for proNGF, NGF, and β-actin and the represent the mean ± SEM for the ratio. * and ** indicates significant difference compared to vehicle-treated control rats, p<0.05, p<0.01 (respectively), two-tailed Student’s t-tests. N = 5-6.
Western blot analysis using an antibody that recognizes proNGF and mNGF (~32 kDa and ~14 kDa immunoreactive bands, respectively) indicated that proNGF levels (A) were increased in the hippocampus in DFP-treated rats compared to controls (t=3.62, p=0.009) with a trend toward an increase in the basal forebrain (t=1.92 p=0.09). Similarly, NGF levels (B) were increased in DFP-treated rats compared to controls in the hippocampus, (t=3.69, p=0.008) and basal forebrain (t=2.86, p=0.024). There were no statistically significant DFP-related effects on proNGF or NGF in the prefrontal cortex.
NGF receptors
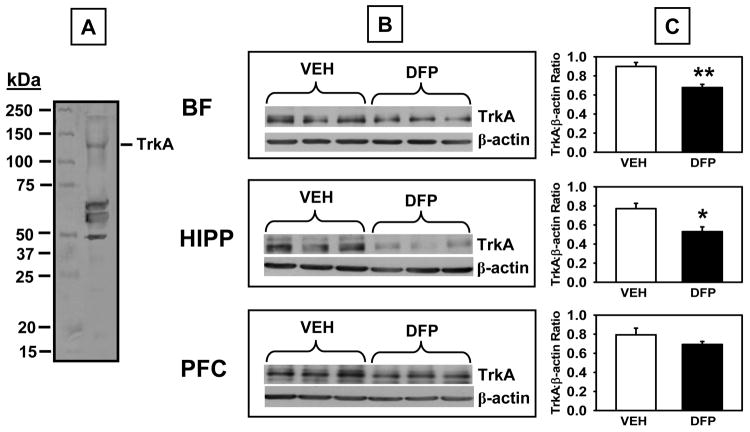
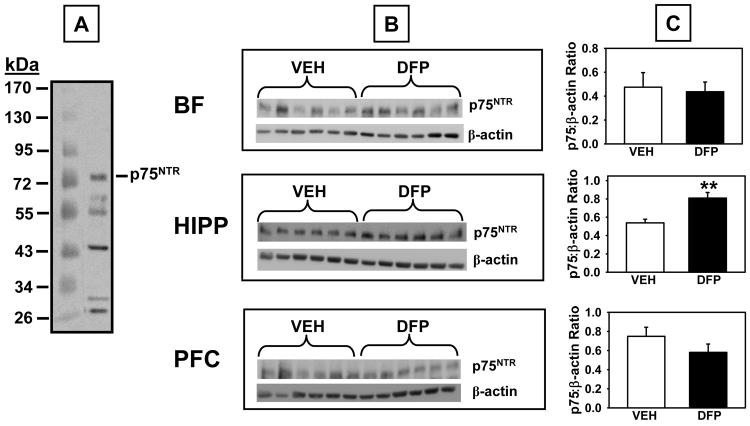
Western blot analysis using a specific antibody against TrkA (antibody from Santa Cruz Biotech, sc-118 detects TrkA at 140 kDa band) revealed significant DFP-related decreases in TrkA in the hippocampus (t=3.29, p=0.013) and basal forebrain, (t=4.17, p=0.006), but not the prefrontal cortex (Fig 10 top). Finally, significant increases in p75NTR protein (75 kDa band) levels were detected in the hippocampus (t=3.57, p=0.006), but not the basal forebrain or prefrontal cortex (Fig 11).
Fig 10.
(A) TrkA levels measured by Western Blot in brain lysates derived from rats that had completed the 1.00 mg/kg alternate day regimen of DFP plus the two-week washout period. A. Representative blot illustrating a molecular weight marker and TrkA (~140 kDa). B. Blots illustrating TrkA and β-actin (~38-40 kDa) in the same samples from basal forebrain (BF), hippocampus (HIPP), and prefrontal cortex (PFC). C. Data presented in the bar graphs were obtained from densitometry measurements of the bands for TrkA and β-actin and the represent the mean ± SEM for the ratio. * and ** indicates significant difference compared to vehicle-treated control rats, p<0.05, p<0.01 (respectively), two-tailed Student’s t-tests. N = 5-6.
Fig 11.
p75NTR levels measured by Western Blot in brain lysates derived from rats that had completed the 1.00 mg/kg alternate day regimen of DFP plus the two-week washout period. A. Representative blot illustrating a molecular weight marker and p75NTR (~75 kDa). B. Blots illustrating p75NTR protein and β-actin (~38-40 kDa) in the same samples from basal forebrain (BF), hippocampus (HIPP), and prefrontal cortex (PFC). C. Data presented in the bar graphs were obtained from densitometry measurements of the bands for p75NTR protein and β-actin and the represent the mean ± SEM for the ratio. ** indicates significant difference compared to vehicle-treated control rats, p<0.01, two-tailed Student’s t-tests. N = 5-6.
4. Discussion
The major findings of this rodent study can be summarized as follows: 1) repeated exposures to subthreshold doses of DFP (i.e., doses not associated with overt signs of cholinergic toxicity) were associated with protracted impairments in both acquisition and recall in a water maze spatial learning task as well as in a spontaneous novel object recognition (NOR) procedure, but not prepulse inhibition of the acoustic startle response; 2) DFP was associated with protracted (brain region-dependent) alterations in cholinergic marker proteins (ChAT and the α7-nAChR) as well as several NGF-related proteins in brain regions known to support information processing and cognitive function (e.g., basal forebrain, hippocampus), thus indicating a potential mechanism for the behavioral effects described above.
In behavioral experiments, the water maze procedure was utilized since it is a visuospatial learning task that is sensitive cholinergic alterations (e.g., McNamara and Skelton, 1993), and importantly, deficits in visuospatial processing have been identified as one of the negative outcomes in patients previously exposed to OPs for chronic periods (Rolda′n-Tapia et al., 2005). In the present study, rats previously exposed to the 1.0 mg/kg dose of DFP were impaired in the hidden platform task as well as in probe trials indicating protracted (DFP-related) disruptions in learning (acquisition) as well as retention. Prior DFP exposure (particularly at the higher doses we evaluated) also resulted in impairments in the NOR task. This procedure (a test of recognition memory for rodents) was used to complement the water maze studies since it is a non-spatial, memory-related task (see Ennaceur and Delacour 1988) that has also been shown to be sensitive to cholinergic alterations (Bartolini et al., 1996).
Prepulse inhibition (PPI) experiments were conducted due to our previous findings of impairments in rats administered the OP insecticide, chlorpyrifos (Terry et al., 2007b) and earlier reports of sensorimotor abnormalities (see Kamel and Hoppin, 2004) and attentional impairments in humans previously exposed to OPs. PPI, defined as the reduction in startle response produced by a low-intensity stimulus presented before a high-intensity, startle-producing stimulus has been widely used as a neurophysiological measure of the early pre-attentive stages of information processing (Braff and Geyer, 1990). Further, PPI has also been shown in animals to be sensitive to cholinergic manipulations (Hohnadel et al., 2007). Surprisingly, in the present study, there were no impairments in PPI associated with any dose of DFP and in fact, there was a trend toward improvement (overall main effect of dose, p<0.07). This observation is interesting given our previous findings that reversible acetylcholinesterase inhibitors (i.e., galantamine and donepezil) can improve PPI in pharmacological impairment models. This finding also suggests that insecticide OPs and alkylphosphate OPs may differ in their effects on sensorimotor gating.
Additional behavioral testing revealed modest DFP-related decreases in open field locomotor activity and grip strength and rats treated with the higher doses of DFP gained slightly less weight over the course of the 30-day exposure period. Taken together, these findings raise the question of whether some type of psychomotor impairment or general compromise in health might have contributed to poor performance in the memory-related behavioral tasks described above. It should be noted, however, that performance of the rotarod task (which requires fine motor control and balance as well as motor learning) was not significantly impaired and grip strength (which was impaired in the early test sessions during the DFP exposure period) was not impaired during the DFP-free washout period when the memory-related tasks were performed. Furthermore, there were no significant DFP-related effects on swim speed or visible platform tests in the water maze, or on the total time spent with the test objects in the NOR task. Finally, the dose of DFP (1.0 mg/kg) associated with the most significant effects on memory-related task performance was, in fact, associated with similar or less impact on weight gain than lower doses (i.e., specifically the 0.5 and 0.75 mg/kg doses). Collectively, the observations described here argue against the premise that gross drug effects on motor function, visual acuity, exploratory activity, or general health status could explain the deficits in the memory-related behavioral tasks.
The activity of cholinesterase (ChE), a known target of OPs including DFP, was assessed in both plasma and brain at various times throughout the study. As indicated in the results, ChE activity was decreased significantly by all of the doses of DFP evaluated, and while inhibition abated to control levels (or even above control levels with the highest dose of DFP) in plasma, during the washout period, activity remained depressed in all of the brain regions evaluated even up to the fourteenth day. Thus, such residual impairments on ChE activity cannot be ruled out as a contributing factor to the behavioral impairments. It is also important to note that the highest dose of DFP evaluated (1.0 mg/kg) was associated with a 90% inhibition of cholinesterase by day 6 of treatment, a level of inhibition that would normally be expected to produce overt cholinergic signs. The lack of acute signs of toxicity in this study is suggestive of some type of adaptive mechanism or tolerance that protected the animals against the more severe toxic effects of DFP after repeated exposure. Behavioral tolerance to repeated exposures to DFP (and other OPs) have been described previously (Bushnell et al., 1991), although most such reports describe an attenuation of overt signs of toxicity with repeated exposure.
Subsequent neurochemical studies were designed to determine whether the protracted behavioral deficits described above could be related to alterations in proteins that have important roles in (or influence on) the brain cholinergic pathways best documented play important roles in cognition (i.e., those originating from the basal forebrain and projecting to cortical and hippocampal areas, see review, Bartus 2000). Interestingly, activity of the cholinergic enzyme, ChAT was selectively decreased in the basal forebrain by exposure to DFP, and this effect persisted throughout the washout period in animals previously exposed to the highest dose of DFP. The levels of α7-nAChR protein were also selectively diminished in the basal forebrain by prior exposure to DFP. This observation of brain-region specific cholinergic alterations is somewhat perplexing, although damage to the basal forebrain cholinergic system (often correlated with cognitive deficits) has been observed in association with brain insults from a variety of sources (e.g., Alzheimer’s disease, traumatic head injury, ischemia). It has been suggested that cholinergic neurons may be particularly vulnerable to brain insults due to their unique metabolic demands (i.e., they use choline for the synthesis of acetylcholine as well as for neuronal cell membrane synthesis, see Wurtman, 1992). Thus, when cholinergic neurons are subjected to excitotoxicity (such as associated with exposure to amyloid peptides, ischemia, or head injury, etc.) and choline levels are depleted, the neurons may use the choline bound in the cell membrane to create acetylcholine, potentially leading to cholinergic cell damage and/or loss (see Salmond et al., 2005). It is interesting to speculate that a similar process could occur as a result of elevations in cholinergic activity as a result of OP exposure.
We subsequently evaluated residual effects of DFP on a series of NGF-related proteins (including receptors) that are involved the maintenance and support of adult basal forebrain cholinergic neurons in mammalians. Under normal conditions, mNGF binding to its high affinity receptor, TrkA, activates pathways that enhance cholinergic neuron survival (reviewed, Counts and Mufson 2005). Conversely, proNGF, the uncleaved precursor form of NGF, binds to the p75NTR receptor with higher affinity than mNGF and it is more selective for the p75NTR receptor relative to TrkA (Lee et al., 2001). Notably, the p75NTR receptor is well-known for its role in mediating neuronal cell death (reviewed Underwood and Coulson, 2008). The brain regions analyzed (basal forebrain hippocampus, prefrontal cortex) were selected based on their well-established functional roles in human cognition and performance of the specific rodent behavioral tasks we found to be adversely affected by DFP exposure (i.e. the water maze and NOR, see McNamara and Skelton, 1993; McDonald and White, 1995; Myhrer, 1988; Rampon et al., 2000; Broadbent et al., 2004 DeVito and Eichenbaum, 2009). We detected DFP-related elevations in neurotrophin proteins that have been associated with apoptosis and neuronal death (i.e., proNGF and p75NTR) in the hippocampus, observations that are interesting in light of the deficits in performance of hippocampus dependent memory tasks (water maze, NOR) described above. Furthermore, TrkA protein, a neurotrophin receptor normally associated with neuronal survival and plasticity was diminished in the basal forebrain and hippocampus. Surprisingly, mNGF concentrations (similar to proNGF) were elevated in the basal forebrain and hippocampus. This observation is somewhat difficult to interpret given the common literature descriptions of proNGF and mNGF as having opposing roles (apoptotic versus neurotrophic, respectively). It should be noted, however, that both apoptotic and neurotrophic actions have been attributed to proNGF (e.g., Lee et al., 2001; Buttigieg et al., 2007) and other factors such as the p75NTR/TrkA ratio may be the determining factor as to whether a cell undergoes apoptosis or survives in the presence of neurotrophins (Yoon et al., 1998).
The contribution of residual levels of DFP to the protracted effects on neurotrophin receptors and cholinergic proteins appears minimal since the concentrations of DFP were undetectable at the time of the neurochemical assessments. However, (as noted above) residual cholinesterase inhibition was detected (in all of the brain regions analyzed). It seems unlikely, however, that the selective alterations of ChAT, α7-nAChRs, and NGF-related proteins (in specific brain regions known to influence cognitive processes, see above) would be explained by generalized cholinesterase inhibition.
From a translational perspective there are several points that should be considered. As noted above, the intermittent (subthreshold) dosing regimen was used in this study to provide a model for the types of environmental exposures that might be experienced by individuals who live in and around areas where OP nerve agents have been released. While nerve agent OPs are generally believed to rapidly degrade after release, it is not widely appreciated that this degradation depends on the specific type of OP, the environmental conditions, and the type of contact surface. Sarin, tabun, and soman evaporate relatively quickly and (depending on the weather conditions) are generally thought to persist no more than a few hours to a few days. However, chemical analyses of clothing, grave debris, soil, and munitions fragments collected from Birjinni four years after the Iraqi attack revealed hydrolysis products of sarin as well as traces of the intact compound (Black et al., 1994). It thus appears that sarin can remain as a potential threat (considering its extreme potency) for extended periods. In addition, VX is much more persistent in the environment due to its lower volatility and higher stability on a wide variety of surfaces (Gura et al., 2006). In fact, VX has a remarkably high stability in soil at low temperatures as exemplified in a study by Groenewold et al., 2002, where it was stable at 4°C for six months. Gura and colleagues reported that about 2% of VX applied to asphalt could be recovered after 425 days (obviously a concern given its estimated human LD50 of 10 μg/kg).
In conclusion, the results of this prospective animal study thus provide evidence to support two novel hypotheses: 1) that intermittent, subthreshold exposures alkyl OPs can lead to protracted deficits in specific domains of cognition (i.e., spatial learning and recall as well as recognition memory) and 2) that such cognitive deficits may be related to persistent functional changes in brain neurotrophin and cholinergic pathways.
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences (ES012241 to AVT). Salary support (JJB) also was contributed through a Merit Review Award from the Veterans Administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnaby F. The nuclear arsenals and nuclear disarmament. Med Confl Surviv. 1998;14(4):314–20. doi: 10.1080/13623699808409411. [DOI] [PubMed] [Google Scholar]
- Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- Bartolini L, Casamenti F, Pepeu G. Aniracetam restores object recognition impaired by age, scopolamine, and nucleus basalis lesions. Pharmacol Biochem Behav. 1996;53:277–283. doi: 10.1016/0091-3057(95)02021-7. [DOI] [PubMed] [Google Scholar]
- Black RM, Clarke RJ, Read RW, Reid MT. Application of gas chromatography-mass spectrometry and gas chromatography-tandem mass spectrometry to the analysis of chemical warfare samples, found to contain certain residues of the nerve agent sarin, sulphur mustard and their degradation products. J Chromatogr A. 1994;662(2):301–21. doi: 10.1016/0021-9673(94)80518-0. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia: human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell PJ, Padilla SS, Ward T, Pope CN, Olszyk VB. Behavioral and neurochemical changes in rats dosed repeatedly with diisopropylfluorophosphate. J Pharmacol Exp Ther. 1991;256:741–750. [PubMed] [Google Scholar]
- Buttigieg H, Kawaja MD, Fahnestock M. Neurotrophic activity of proNGF in vivo. Exp Neurol. 2007 Apr;204(2):832–5. doi: 10.1016/j.expneurol.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Counts SE, Mufson EJ. The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J Neuropathol Exp Neurol. 2005;64:263–272. doi: 10.1093/jnen/64.4.263. [DOI] [PubMed] [Google Scholar]
- DeVito LM, Eichenbaum H. Distinct contributions of the hippocampus and medial prefrontal cortex to the ‘what-where-when’ components of episodic-like memory in mice. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2009.09.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Radiochemical micro assays for the determination of choline acetyltransferase and acetylcholinesterase activities. Biochem J. 1969;115:465–472. doi: 10.1042/bj1150465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart DA, Middlemore M-L, Terry AV., Jr ELISA methods to measure cholinergic markers and nerve growth factor receptors in cortex, hippocampus, prefrontal cortex, and basal forebrain from rat brain. J Neurosci Meth. 2006;150:159–173. doi: 10.1016/j.jneumeth.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Gearhart DA, Sickles DW, Buccafusco JJ, Prendergast MA, Terry AV., Jr Chlorpyrifos, chlorpyrifos-oxon, and diisopropylfluorophosphate inhibit kinesin-dependent microtubule motility. Toxicol Appl Pharmacol. 2007;218:20–29. doi: 10.1016/j.taap.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Groenewold GS, Williams JM, Appelhans AD, Gresham GL, Olson JE, Jeffery MT, Rowland B. Hydrolysis of VX on concrete: rate of degradation by direct surface interrogation using an ion trap secondary ion mass spectrometer. Environ Sci Technol. 2002;36(22):4790–4. doi: 10.1021/es025754n. [DOI] [PubMed] [Google Scholar]
- Gura S, Tzanani N, Hershkovitz M, Barak R, Dagan S. Fate of the chemical warfare agent VX in asphalt: a novel approach for the quantitation of VX in organic surfaces. Arch Environ Contam Toxicol. 2006;51(1):1–10. doi: 10.1007/s00244-005-2116-y. [DOI] [PubMed] [Google Scholar]
- Heaton KJ, Palumbo CL, Proctor SP, Killiany RJ, Yurgelun-Todd DA, White RF. Quantitative magnetic resonance brain imaging in US army veterans of the 1991 Gulf War potentially exposed to sarin and cyclosarin. Neurotoxicology. 2007 Jul;28(4):761. doi: 10.1016/j.neuro.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Hobbiger F. Pharmacology of anticholinesterase drugs. In: Zaimis E, editor. Neuromuscular junction Handbook of experimental pharmacology. Berlin: Springer Verlag; 1972. pp. 487–581. [Google Scholar]
- Hohnadel E, Bouchard K, Terry Galantamine and donepezil attenuate pharmacologically induced deficits in prepulse inhibition in rats. Neuropharmacology. 2007;52:542–551. doi: 10.1016/j.neuropharm.2006.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Macilwain C. Study proves Iraq used nerve gas. Nature. 1993;363:3. doi: 10.1038/363003b0. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Hippocampal and nonhippocampal contributions to place learning in rats. Behav Neurosci. 1995;109:579–593. doi: 10.1037//0735-7044.109.4.579. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res Rev. 1993;18:33–49. doi: 10.1016/0165-0173(93)90006-l. [DOI] [PubMed] [Google Scholar]
- Myhrer T. Exploratory behavior and reaction to novelty in rats with hippocampal perforant path systems disrupted. Behav Neurosci. 1988;102:356–362. doi: 10.1037//0735-7044.102.3.356. [DOI] [PubMed] [Google Scholar]
- Kamel F, Hoppin JA. Association of pesticide exposure with neurologic dysfunction and disease. Environ Health Perspect. 2004;112:950–958. doi: 10.1289/ehp.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast MA, Terry AV, Buccafusco JJ. Effects of chronic, low-level organophosphate exposure on delayed recall, discrimination, and spatial learning in monkeys and rats. Neurotox and Terat. 1998;20(2):115–122. doi: 10.1016/s0892-0362(97)00098-6. [DOI] [PubMed] [Google Scholar]
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- Ray DE, Richards PG. The potential for toxic effects of chronic, low-dose exposure to organophosphates. Toxicol Lett. 2001;120:343–51. doi: 10.1016/s0378-4274(01)00266-1. [DOI] [PubMed] [Google Scholar]
- Roldan-Tapia L, Parron T, Sanchez-Santed F. Neuropsychological effects of long-term exposure to organophosphate pesticides. Neurotoxicol Teratol. 2005;27(2):259–266. doi: 10.1016/j.ntt.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Rusyniak DE, Nanagas KA. Organophosphate poisoning. Semin Neurol. 2004;24:197–204. doi: 10.1055/s-2004-830907. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Chatfield DA, Menon DK, Pickard JD, Sahakian BJ. Cognitive sequelae of head injury: involvement of basal forebrain and associated structures. Brain. 2005 Jan;128(Pt 1):189–200. doi: 10.1093/brain/awh352. [DOI] [PubMed] [Google Scholar]
- Saunders BC. Some aspects of the chemistry and toxic action of organic compounds containing phosphorus and fluorine. Cambridge: University Press; 1957. p. 42. [Google Scholar]
- Sungurtekin H, Gurses E, Balci C. Evaluation of several clinical scoring tools in organophosphate poisoned patients. Clin Toxicol (Phila) 2006;44:121–126. doi: 10.1080/15563650500514350. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Stone JD, Buccafusco JJ, Sickles DW, Sood A, Prendergast MA. Repeated exposures to subthreshold doses of chlorpyrifos in rats: hippocampal damage, impaired axonal transport, and deficits in spatial learning. J Pharmacol Exp Ther. 2003;305:375–384. doi: 10.1124/jpet.102.041897. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Parikh V, Gearhart DA, Pillai A, Hohnadel E, Warner S, Nasrallah HA, Mahadik SP. Time-dependent effects of haloperidol and ziprasidone on nerve growth factor, cholinergic neurons, and spatial learning in rats. J Pharmacol Exp Ther. 2006;318:709–724. doi: 10.1124/jpet.105.099218. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Gearhart DA, Beck WD, Truan JN, Middlemore ML, Williamson LN, Bartlett MG, Prendergast MA, Sickles DW, Buccafusco JJ. Chronic, Intermittent Exposure to Chlorpyrifos in Rats: Protracted Effects on Axonal Transport, Neurotrophin Receptors, Cholinergic Markers, and Information Processing. J Pharmacol Exper Ther. 2007a;322:1117–1128. doi: 10.1124/jpet.107.125625. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Gearhart DA, Warner SE, Zhang G, Bartlett MG, Middlemore ML, Beck WD, Jr, Mahadik SP, Waller JL. Oral haloperidol or risperidone treatment in rats: Temporal effects on nerve growth factor receptors, cholinergic neurons, and memory performance. Neuroscience. 2007b;146:1316–1332. doi: 10.1016/j.neuroscience.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa N, Morita H, Nakajima T. Sarin experiences in Japan: acute toxicity and long-term effects. J Neurol Sci. 2006;249:76–85. doi: 10.1016/j.jns.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Xu M, Terry AV, Jr, Bartlett MG. Determination of diisopropylfluorophosphate in rat plasma and brain tissue by headspace solid-phase microextraction gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:3069–3075. doi: 10.1002/rcm.3706. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ. Choline metabolism as a basis for the selective vulnerability of cholinergic neurons. Trends Neurosci. 1992 Apr;15(4):117–22. doi: 10.1016/0166-2236(92)90351-8. [DOI] [PubMed] [Google Scholar]
- Yoon SO, Casaccia-Bonnefil P, Carter B, Chao Mv. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neurosci. 1998 May 1;18(9):3273–81. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]