Abstract
BACKGROUND
The present study tested the hypothesis that inappropriate activation of the brain renin–angiotensin system (RAS) contributes to the pathogenesis of blood–brain barrier (BBB) disruption and cognitive impairment during development of salt-dependent hypertension. Effects of an angiotensin II (AngII) type-1 receptor blocker (ARB), at a dose that did not reduce blood pressure, were also examined.
METHODS
Dahl salt-sensitive (DSS) rats at 6 weeks of age were assigned to three groups: low-salt diet (DSS/L; 0.3% NaCl), high-salt diet (DSS/H; 8% NaCl), and high-salt diet treated with ARB, olmesartan at 1 mg/kg.
RESULTS
DSS/H rats exhibited hypertension, leakage from brain microvessels in the hippocampus, and impaired cognitive functions, which were associated with increased brain AngII levels, as well as decreased mRNA levels of tight junctions (TJs) and collagen-IV in the hippocampus. In DSS/H rats, olmesartan treatment, at a dose that did not alter blood pressure, restored the cognitive decline, and ameliorated leakage from brain microvessels. Olmesartan also decreased brain AngII levels and restored mRNA expression of TJs and collagen-IV in DSS/H rats.
CONCLUSIONS
These results suggest that during development of salt-dependent hypertension, activation of the brain RAS contributes to BBB disruption and cognitive impairment. Treatment with an ARB could elicit neuroprotective effects in cognitive disorders by preventing BBB permeability, which is independent of blood pressure changes.
Keywords: blood–brain barrier, blood pressure, cognitive impairment, hypertension, receptors, vascular cognitive impairment
Although increased blood–brain barrier (BBB) permeability is observed as a result of cerebral ischemia,1,2 disruption of the BBB has also been identified in other pathological conditions, such as multiple sclerosis.3 Furthermore, cerebrovascular abnormalities have been observed in cognitive disorders.4 BBB permeability increases with normal aging, and further increases in patients with vascular dementia,5 suggesting that BBB dysfunction is present from an early stage in patients exhibiting mild symptoms of cognitive impairment and minor cerebral microvascular diseases, such as lacunar stroke.
Accumulating evidence has suggested a potential relationship between hypertension and the risk of cognitive impairment.6–8 For instance, the Honolulu-Asia Aging Study and other clinical studies have shown that high midlife systolic blood pressure is a significant predictor of reduced cognitive function in later life, as well as a risk factor for vascular dementia.6–8 Additionally, a strong relationship between average dietary sodium intake and mortality due to stroke and cardiovascular diseases risk has been determined.9,10 In spontaneously hypertensive rats and stroke-prone spontaneously hypertensive rats, a disturbed fence function of tight junctions (TJs) has been demonstrated in BBB endothelial cells.11,12 However, it remains unclear whether BBB disruption and cognitive impairment occur during development of salt-dependent hypertension.
The brain renin–angiotensin system (RAS) plays an important role in the pathogenesis of cognitive impairment. Increased angiotensin II (AngII) levels result in impaired cognitive function in renin/angiotensinogen transgenic mice.13 Furthermore, treatment with an angiotensin receptor blocker (ARB) ameliorates the cognitive impairment in mice fed a high-salt and cholesterol diet, or type-2 diabetic mice.14,15 In addition, ARB treatment decreases BBB permeability in diabetic rats,16 which suggests that activation of the brain RAS is involved in the pathogenesis of cognitive impairment and BBB permeability in certain pathophysiological conditions. However, Hirawa et al.17 reported that long-term inhibition of RAS improves memory function in aged, low-salt treated, normotensive, Dahl salt-sensitive (DSS) rats. However, it has not been determined whether increased BBB permeability induced by inappropriate activation of brain RAS contributes to cognitive deficits observed during development of salt-dependent hypertension.
Therefore, the present study tested the hypothesis that augmentation of BBB permeability and cognitive impairment are associated with inappropriate activation of the brain RAS during development of salt-dependent hypertension. To test this, BBB permeability was analyzed, as well as TJ expression, cognitive function, and the effects of an ARB at a subpressor dose, in DSS hypertensive rats.
METHODS
Animals
All experimental procedures were performed according to guidelines for the care and use of animals established by the Kagawa University Medical School (Kagawa, Japan). Adult, male, 5-week-old, DSS rats (average weight 190 g) were purchased from Seak-Yoshitomi, Fukuoka, Japan. All animals were housed in a room with controlled lighting and temperature (25 °C). After a 1-week adjustment period, the 6-week-old rats were assigned to three groups: low-salt diet (DSS/L; 0.3% NaCl; Oriental Yeast, Osaka, Japan, n = 16), high-salt diet (DSS/H; 8% NaCl; Oriental Yeast, n = 16), and high-salt diet treated with the ARB, olmesartan (Daiichi-Sankyo, Tokyo, Japan, n = 16). The olmesartan dose was 1 mg/kg, once a day, by oral gavage for 4 weeks, which was determined on the basis of previous rat studies.18,19 Furthermore, our preliminary experiments showed that 1 mg olmesartan/kg body weight/day did not alter blood pressure in DSS/H rats (data not shown). Systolic blood pressure was monitored in conscious rats using tail-cuff plethysmography (BP-98A; Softron, Tokyo, Japan) each week. For BBB permeability detection, eight animals per group were anesthetized with pentobarbital sodium (50 mg/kg, intraperitoneal) and perfused with a fixative solution composed of 2.5% glutaraldehyde and 2% paraformaldehyde. In the remaining animals, brain tissues were collected following decapitation to measure AngII and gene expression, as well as for immunohistochemical analyses (n = 8 for each).
BBB permeability
As previously described,12 microvessel permeability was analyzed in the hippocampus and corpus callosum through the use of horseradish peroxidase. Following perfusion with 2.5% glutaraldehyde/2% paraformaldehyde, the brains were separated and maintained in another fixative solution composed of 1.25% glutaraldehyde/1% paraformaldehyde, for 24 h. For light microscope observation, sections were incubated in 0.01 mol/l acetate buffer (pH 3.3), followed by tetramethylbenzidine and hydrogen peroxide.
Passive avoidance test
To analyze cognitive functions, a shuttle avoidance cage (64 × 35 × 33 cm; Melquest, Toyama, Japan) and an isolation cabinet (72 × 67 × 63 cm; Melquest) were used, as previously described.20 Briefly, the rats were individually placed in a chamber and administered 20 inescapable, electric shocks (0.4 mA) for 5 s each. A tone signal was presented during the first 5 s of each trial. If there was no avoidance response within that period, the tone signal remained on and the shock was delivered through the grid floor. In the case of a no-escape response during this period, both tone and shock were automatically terminated. Cognitive function was determined by avoidance rate percentage.
Immunohistochemistry
Occludin colocalization with PECAM-1-positive endothelial cells was assessed in hippocampal microvessels. Brain tissue was immersed in optimal cutting temperature compound (Tissue-Tek; Sakura Finetek, Tokyo, Japan) and stored at −80 °C. The tissue was then cut into 10-μm thick frozen sections. Following immersion in methanol and phosphate- buffered saline wash steps, the sections were blocked with serum-free protein block (Dako Cytomation, Glostrup, Denmark) for 15 min. Subsequently, the primary antibodies were diluted with Canget signal immunostain (Toyobo, Tokyo, Japan) and incubated overnight at 4 °C. The antibodies included goat polyclonal anti-mouse platelet endothelial adhesion molecule (PECAM-1; 1:200; Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit anti-occludin (1:200; Santa Cruz Biotechnology). The sections were then incubated at room temperature for 1 h with fluorescein-conjugated secondary antibody in Canget signal immunostain (Toyobo; 1:800, donkey anti-goat and donkey anti-rabbit; Molecular Probes, Eugene, OR), mounted with Vectashield (Vector, Burlingame, CA), and examined under a fluorescence microscope (IX71SIF-2; Olympus, Tokyo, Japan).
Gene expression
Occludin, claudin-5, collagen-IV, and metalloproteinase (MMP)-2 and 9 were analyzed by laser-capture microdissection in the hippocampus and corpus callosum of frozen brain samples. First, tissue blocks were sectioned at 10-μm thickness using a cryostat microtome (Leica CM1950; Leica Microsystems, Tokyo, Japan). The sections were incubating in increasing concentrations of ethanol, followed by xylene. For RNA isolation from laser-capture microdissection samples, a Micro Scale RNA Isolation Kit was used (RNAqueous-Micro; Ambion, Austin, TX). All primers for real-time PCR are shown in Table 1.
Table 1.
Primers used for real-time Pcr
| Occludin | Claudin-5 | Collagen-IV | MMP-2 | MMP-9 | GAPDH | |
|---|---|---|---|---|---|---|
| F | 5′-ATGATGAACAG CCCCCTAATGT-3′ | 5′-TGTGAGGACT TGACCGACCTT-3′ | 5′-GAGGGTGCTG GACAAGCTCTT-3′ | 5′-GGTGGTGGTCA CAGCTATTTCTT-3′ | 5′-GGATCCCCC AACCTTTACCA-3′ | 5′-TGAACGGGA AGCTCACTGG-3′ |
| R | 5′-TGTCGACTCTT TCCGCATAGTC-3′ | 5′-CGGCAGTTTG GTGGCTACTT-3′ | 5′-TAAACGGACTG GGCTCGGAATTC-3′ | 5′-GCCAGTCCGAT TTGATGCTT-3′ | 5′-AAGGTCAGAAC CGACCCTACAA-3′ | 5′-TCCACCACCC TGTTGCTGTA-3′ |
Data were normalized against GAPDH expression and were expressed as fold-differences between control and treated groups.
MMP, metalloproteinase.
AngII measurements
After brain tissues were quickly removed, part of the cerebral cortex and hippocampus were immediately separated and frozen. For plasma sample collection, a proteolytic enzyme inhibitor cocktail (EDTA-2Na 500 mmol/l; Captopril 10 nmol/l; Pepstatin 1 mmol/l + 1,10-phenanthroline 125 mmol/l) was added to each sample. Brain and plasma AngII were measured using a previously described radioimmunoassay method.21,22
Statistical analyses
All values were expressed as mean ± s.d. Differences in blood pressure and passive avoidance test results among groups were determined using repeated measurements of analysis of variance. Differences in AngII protein concentrations and mRNA levels were examined using one-way analysis of variance. When overall analysis of variance P values were <0.01, Bonferroni’s correction for multiple comparisons was used to assess individual group differences.
RESULTS
Systolic blood pressure and body weight
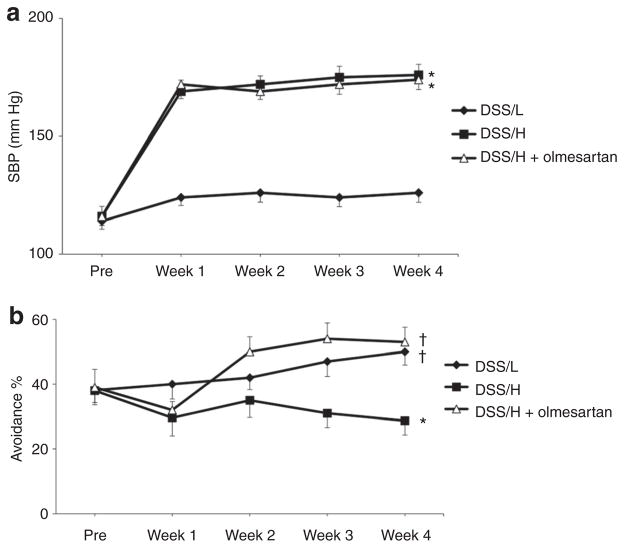
Low-salt diet treatment for 4 weeks did not alter systolic blood pressure in the DSS rats (123 ± 4 mm Hg, Figure 1a), whereas DSS/H rats exhibited increased systolic blood pressure (176 ± 5 mm Hg) at 4 weeks. Olmesartan did not alter systolic blood pressure in DSS/H rats (174 ± 4 mm Hg) at 4 weeks. Changes in body weight throughout the experimental period were similar between groups (data not shown).
Figure 1.
Blood pressure and cognitive functions. (a) Systolic blood pressure. High-salt diet intake elevates systolic blood pressure in DSS/H rats. Treatment with olmesartan (1 mg/kg body weight/day) does not significantly decrease systolic blood pressure in DSS/H rats. *P < 0.01 vs. DSS/L. (b) Cognitive functions. DSS/L rats exhibit gradual improvement in cognitive functions, with regard to percentages in avoidance tests, which were assessed once per week for 4 weeks. In contrast, at week 4, DSS/H rats exhibit learning and memory deficits. Olmesartan treatment restores cognitive decline and improves cognitive ability to levels similar to the DSS/L rats. *P < 0.01 vs. DSS/L. †P < 0.01: vs. DSS/H. DSS/H, Dahl salt-sensitive/high-salt diet; DSS/L, Dahl salt-sensitive/low-salt diet; SBP, systolic blood pressure.
Cognitive functions
Figure 1b shows cognitive function as determined by avoidance rate percentage. DSS/L rats exhibited gradual improvement in cognitive functions, whereas cognitive functions in the DSS/H rats were significantly decreased. Olmesartan treatment significantly ameliorated the cognitive deficits in the DSS/H rats.
BBB microvessel leakage
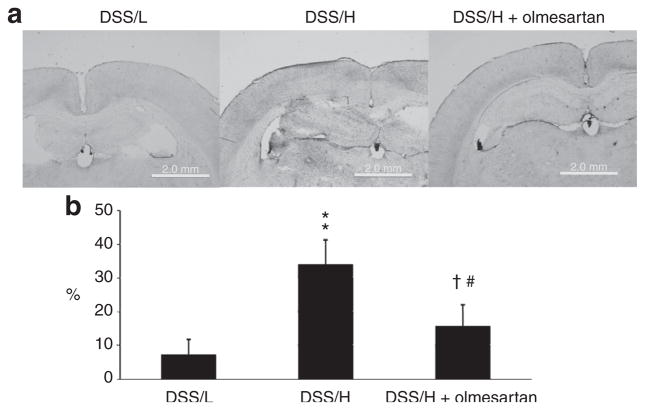
As previously mentioned, BBB permeability was evaluated by measuring percentage leakage of horseradish peroxidase-tetramethylbenzidine. 12 DSS/L rats exhibited very little leakage (7 ± 1%) from microvessels in the hippocampus and corpus callosum (Figure 2). In contrast, substantial leakage took place in the ventral hippocampus and corpus callosum of DSS/H rats (34 ± 3%). Olmesartan markedly reduced BBB microvessels permeability in DSS/H rats (16 ± 7%).
Figure 2.
BBB permeability. (a) DSS/H rats exhibit increased microvessels permeability in the hippocampus and corpus callosum compared with DSS/L rats, as indicated by HRP-TMB reaction (violet stain). Olmesartan treatment reduces microvessels permeability in the hippocampus and corpus callosum of DSS/H rats. (b) Quantitative analysis of BBB permeability in terms of the percentage stained area of hippocampus and corpus callosum. *P < 0.01 vs. DSS/L. †P < 0.01 vs. DSS/H. BBB, blood–brain barrier; DSS/H, Dahl salt-sensitive/high-salt diet; DSS/L, Dahl salt-sensitive/low-salt diet; HRP, horseradish peroxidase; TMB, tetramethylbenzidine.
Gene expression in the hippocampus and corpus callosum
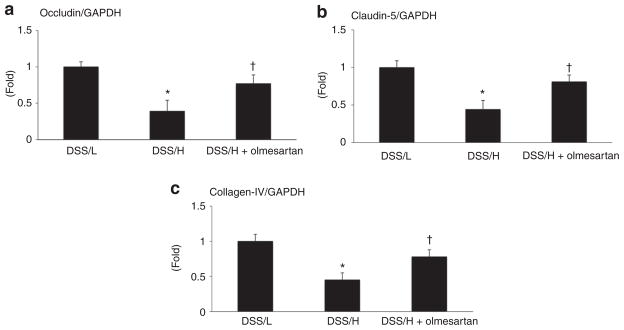
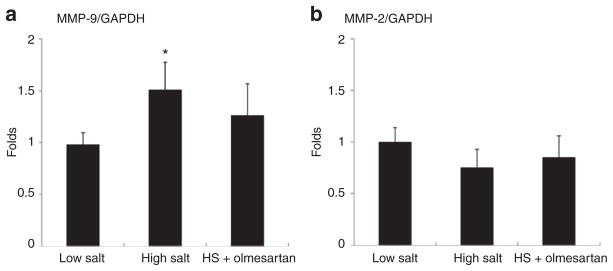
mRNA expressions of occludin, claudin-5, and collagen-IV in the hippocampus and corpus callosum were less in the DSS/H rats compared with the DSS/L rats. In addition, DSS/H rats showed to increase the genes expression of MMP-9. Olmesartan treatment restored expression of genes encoding occludin, claudin-5, collagen-IV, and MMP-9 in DSS/H rats. MMP-2 mRNA expression remained similar between groups (Figures 3 and 4).
Figure 3.
mRNA expression of TJ genes and collagen-IV. Occludin, claudin-5, and collagen-IV mRNA levels in the hippocampus are less in DSS/H rats than in DSS/L rats. High-salt diet decreases mRNA levels of occludin and claudin-5, as well as collagen-IV, compared with levels in the DSS/L rats. Olmesartan treatment in DSS/H rats restores expression of genes encoding TJ proteins and collagen-IV. *P < 0.01 vs. DSS/L. †P < 0.01 vs. DSS/H. DSS/H, Dahl salt-sensitive/high-salt diet; DSS/L, Dahl salt-sensitive/low-salt diet; TJ, tight junction.
Figure 4.
mRNA expression of MMP-2 and MMP-9. (a) MMP-9 gene expression is increased in the hippocampus of DSS/H rats compared with control rats. In addition, there are no differences between the DSS/H+ olmesartan and DSS/L groups. (b) No changes are observed in MMP-2 gene expression between the groups. *P < 0.01 vs. DSS/L. DSS/H, Dahl salt-sensitive/high-salt diet; DSS/L, Dahl salt-sensitive/low-salt diet; HS, high salt; MMP, metalloproteinase.
Immunohistochemistry
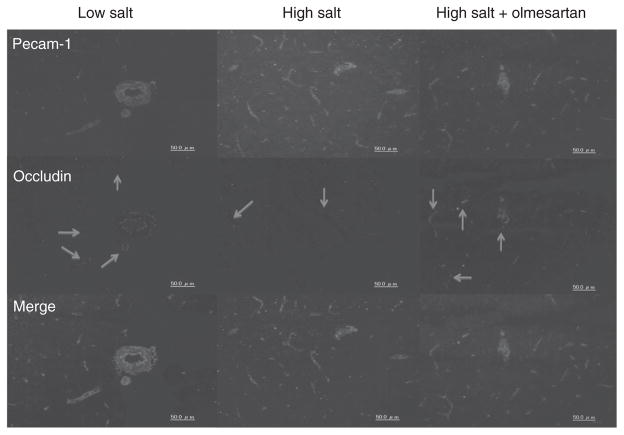
To determine TJ expression, occludin colocalization in PECAM-1-positive endothelial cells was analyzed in microvessels of the hippocampus and corpus callosum by immunohistochemistry. PECAM-1-positive endothelial cells were present in all groups (Figure 5). Occludin expression was observed around microvessels of the hippocampus and corpus callosum in the DSS/L rats. However, very low occludin expression was observed in hippocampal microvessels of the DSS/H rats, but olmesartan treatment significantly restored expression.
Figure 5.
Immunostaining of microvessels. PECAM-1-positive endothelial cells are observed in all groups. Occludin is expressed in microvessels of the hippocampus and corpus callosum in DSS/L rats. However, corresponding levels are low in DSS/H rats. Olmesartan treatment restores occludin expression in microvessels of the hippocampus and corpus callosum in DSS/H rats. DSS/H, Dahl salt-sensitive/high-salt diet; DSS/L, Dahl salt-sensitive/low-salt diet.
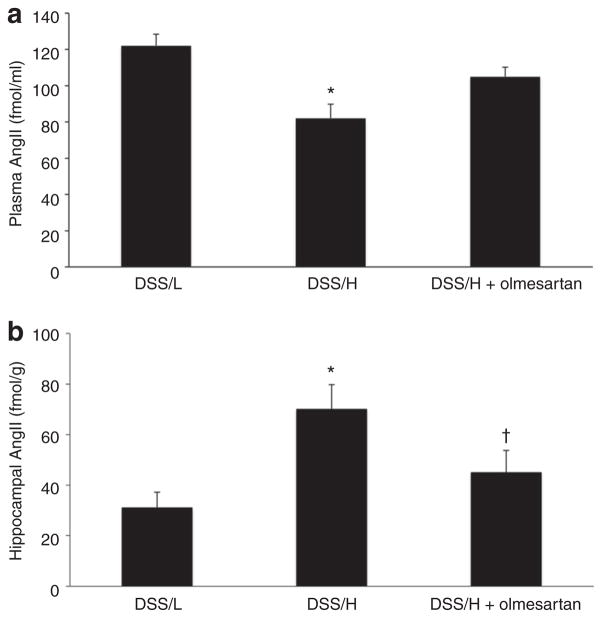
Plasma and brain AngII levels
Plasma AngII levels were decreased in DSS/H rats, compared with DSS/L rats (Figure 6a). In contrast, DSS/H rats exhibited approximately twofold greater AngII levels in the hippocampus, compared with DSS/L rats (Figure 6b). However, cerebral cortical AngII levels were similar between DSS/L and DSS/H rats (data not shown). In the DSS/H rats, olmesartan treatment significantly decreased hippocampal AngII concentrations to levels similar to the DSS/L rats (Figure 6b). Treatment with olmesartan tended to increase plasma AngII levels, but these changes were not statistically significant (Figure 6a).
Figure 6.
Plasma and brain AngII levels. (a) Plasma AngII expression is decreased in DSS/H rats compared with DSS/L rats. *P < 0.01 vs. DSS/L. (b) High-salt diet increases AngII levels in the hippocampus of DSS/H rats. Olmesartan treatment decreases AngII levels in the hippocampus of DSS/H rats. *P < 0.01 vs. DSS/L. †P < 0.01 vs. DSS/H. AngII, angiotensin II; DSS/H, Dahl salt-sensitive/high-salt diet; DSS/L, Dahl salt-sensitive/low-salt diet.
DISCUSSION
Small-vessel disease is a major risk factor for vascular dementia,23,24 and brain vessel remodeling is a key contributor to cerebrovascular dysfunction in several diseases.25 The present study demonstrated, for the first time, that cognitive impairment is associated with increased hippocampal AngII levels and BBB permeability during hypertension development in DSS/H rats. Furthermore, treatment with the ARB, olmesartan, at a dose that did not alter blood pressure, markedly restored BBB disruption and reduced cognitive decline in DSS/H hypertensive rats. These data were consistent with the hypothesis that inappropriate activation of the brain RAS contributes to the pathogenesis of BBB injury and cognitive impairment during the development of salt-dependent hypertension. ARB treatment could elicit beneficial effects for cognitive impairment by protecting against BBB injury in patients with salt-dependent hypertension, independently of blood pressure changes.
The present study showed that RAS inhibition with an ARB protected cognitive deficits in DSS/H hypertensive rats in a blood pressure-independent manner. These results were consistent with previous studies showing that long-term treatment with an ARB or angiotensin-converting enzyme inhibitor ameliorated impaired cognitive function in aged, low salt-treated, normotensive, DSS rats.17 Additionally, a series of studies reported disruption of passive avoidance retention by AngII infusion in the central nervous system.26,27 Interestingly, AngII administration to the hippocampal area resulted in induction of long-term potentiation in the hippocampus. 28 The present study showed that cognitive impairment was associated with increased hippocampal AngII levels in DSS/H hypertensive rats. These data were consistent with the hypothesis that augmentation of brain RAS activity contributes to the progression of cognitive decline.13–17,28 It should be noted that AngII levels were measured using the direct radioimmunoassay method,21,22 without separation of Ang peptides by high-performance liquid chromatography. Therefore, AngII values should represent actual concentrations of AngII + AngIII.
The present study also demonstrated that cognitive deficits were associated with augmented BBB permeability in the DSS rats. The BBB neurovascular unit acts as an obstacle for substance delivery to the central nervous system. In particular, under physiological conditions, TJs and collagen-IV are key components in the regulation of BBB function.2 Impaired BBB function has been shown to contribute to the pathophysiology of several brain diseases, including cognitive disorders.29 In the present study, cognitive deficits and increased BBB permeability were accompanied by reduced expression of TJs and collagen-IV and increased MMP-9 gene expression in the hippocampus and corpus callosum of DSS/H hypertensive rats. Furthermore, ARB treatment attenuated BBB injury and the reduction in TJ and collagen-IV expression in the DSS/H rats. Collectively, these results were consistent with the hypothesis that AngII-induced reductions in neurovascular unit components of the BBB, such as TJs and collagen-IV, contribute to altered BBB permeability and associated cognitive impairments.
Although the precise mechanisms responsible for the deleterious effect of AngII on BBB injury are not clear, one possible mechanism is that AngII stimulates the production of proinflammatory cytokines and activates MMPs, which further mediate TJ disruption and BBB permeability and ultimately lead to cognitive dysfunction.30–32 In aged, low-salt-treated, normotensive, DSS rats, improved cognitive function mediated by RAS inhibitors was associated with preservation of neuronal capillary densities in the hippocampal area.17 Therefore, it is possible that some of the neuroprotective effects of ARBs on BBB permeability are accompanied by restoration of TJ and collagen-IV expression in the BBB. In the present study, MMP-9 expression was significantly increased in hippocampal tissues of DSS/H rats. Although in situ zymography was not performed to measure MMP activity due to the small amount of tissue, these results suggest a potential role for MMP-9 in BBB injury in DSS rats. Olmesartan treatment also resulted in significantly decreased hippocampal MMP-9 expression in the DSS/H rats. Previous studies have shown that cognitive deficits and BBB permeability are associated with increased oxidative stress.13,33 Therefore, it can be speculated that ARB effects on MMP-9 expression are mediated by their antioxidative stress effects. However, further studies are needed to address these issues.
The present study showed that a high-salt diet inappropriately increased AngII contents in the hippocampus of DSS rats, whereas plasma AngII levels were significantly decreased. These results were consistent with previous studies showing that a high-salt diet increased AngII levels and AT1 receptor density in tissues.34,35 Systemic olmesartan treatment decreased AngII levels in hippocampal tissues of DSS/H rats, suggesting that at least some neuroprotective effects of ARB could be explained by reduced brain AngII levels. However, the precise mechanisms responsible for high salt-induced activation of the brain RAS, as well as effects of olmesartan on brain AngII levels, remain poorly understood. Nevertheless, we recently demonstrated that systemic treatment for 2 weeks with the ARB candesartan decreases brain AngII levels, as well as angiotensin-converting enzyme and angiotensinogen gene expression, in AngII-infused hypertensive rats, although candesartan concentrations in brain tissues were below detectable limits.36 In contrast, other ARBs, such as losartan, partially penetrate the BBB and selectively inhibit central AT1 receptors.37 These results suggest that ARBs penetrate the BBB at very low concentrations, but these levels could be sufficient to regulate the brain RAS.
In conclusion, the present study supported the hypothesis that RAS activation in the hippocampus plays a key role in the pathogenesis of reduced neurovascular TJs and BBB disruption, which leads to cognitive deficits in DSS hypertensive rats. Furthermore, in the DSS/H hypertensive rats, RAS inhibition attenuated these changes without altering blood pressure. These data supported the recently introduced clinical concept that the neurovascular unit is an important therapeutic target for brain protection.38 However, the precise mechanisms underlying the neurovascular protective effects of RAS inhibitors, as well as the pathophysiological relevance in preventing cognitive decline, need to be further investigated to better understand the role of local RAS in the pathogenesis of cognitive disorders. Furthermore, DSS rats do not emulate exactly the pathophysiology of salt-sensitive hypertensive patients. Therefore, future clinical studies will be needed.
Acknowledgments
This study was supported by Grants in Aid for Scientific Research from Ministry of Education, Culture, Sports & Technology of Japan (18590945 and 20590253), and by grants from the Kagawa University Project Research Fund 2009. We would like to express our gratitude to Mr Hua Fu (Kagawa University Medical School, Kagawa, Japan) for his technical assistance.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysiology of ischemic stroke. Thromb Res. 2000;98:73–81. doi: 10.1016/s0049-3848(00)00218-8. [DOI] [PubMed] [Google Scholar]
- 2.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 3.Floris S, Blezer EL, Schreibelt G, Döpp E, van der Pol SM, Schadee-Eestermans IL, Nicolay K, Dijkstra CD, de Vries HE. Blood-brain barrier permeability and monocyte infiltration in experimental allergic encephalomyelitis: a quantitative MRI study. Brain. 2004;127:616–627. doi: 10.1093/brain/awh068. [DOI] [PubMed] [Google Scholar]
- 4.Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34:806–812. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- 5.Popescu BO, Toescu EC, Popescu LM, Bajenaru O, Muresanu DF, Schultzberg M, Bogdanovic N. Blood-brain barrier alterations in ageing and dementia. J Neurol Sci. 2009;283:99–106. doi: 10.1016/j.jns.2009.02.321. [DOI] [PubMed] [Google Scholar]
- 6.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- 7.Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, Persson G, Odén A, Svanborg A. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 8.Stewart R, Xue QL, Masaki K, Petrovitch H, Ross GW, White LR, Launer LJ. Change in blood pressure and incident dementia: a 32-year prospective study. Hypertension. 2009;54:233–240. doi: 10.1161/HYPERTENSIONAHA.109.128744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He J, Ogden LG, Vupputuri S, Bazzano LA, Loria C, Whelton PK. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. JAMA. 1999;282:2027–2034. doi: 10.1001/jama.282.21.2027. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki S, Zhang XH, Kesteloot H. Dietary sodium, potassium, saturated fat, alcohol, and stroke mortality. Stroke. 1995;26:783–789. doi: 10.1161/01.str.26.5.783. [DOI] [PubMed] [Google Scholar]
- 11.Lippoldt A, Kniesel U, Liebner S, Kalbacher H, Kirsch T, Wolburg H, Haller H. Structural alterations of tight junctions are associated with loss of polarity in stroke-prone spontaneously hypertensive rat blood-brain barrier endothelial cells. Brain Res. 2000;885:251–261. doi: 10.1016/s0006-8993(00)02954-1. [DOI] [PubMed] [Google Scholar]
- 12.Ueno M, Sakamoto H, Tomimoto H, Akiguchi I, Onodera M, Huang CL, Kanenishi K. Blood-brain barrier is impaired in the hippocampus of young adult spontaneously hypertensive rats. Acta Neuropathol. 2004;107:532–538. doi: 10.1007/s00401-004-0845-z. [DOI] [PubMed] [Google Scholar]
- 13.Inaba S, Iwai M, Furuno M, Tomono Y, Kanno H, Senba I, Okayama H, Mogi M, Higaki J, Horiuchi M. Continuous activation of renin-angiotensin system impairs cognitive function in renin/angiotensinogen transgenic mice. Hypertension. 2009;53:356–362. doi: 10.1161/HYPERTENSIONAHA.108.123612. [DOI] [PubMed] [Google Scholar]
- 14.Mogi M, Tsukuda K, Li JM, Iwanami J, Min LJ, Sakata A, Fujita T, Iwai M, Horiuchi M. Inhibition of cognitive decline in mice fed a high-salt and cholesterol diet by the angiotensin receptor blocker, olmesartan. Neuropharmacology. 2007;53:899–905. doi: 10.1016/j.neuropharm.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Tsukuda K, Mogi M, Li JM, Iwanami J, Min LJ, Sakata A, Fujita T, Iwai M, Horiuchi M. Amelioration of cognitive impairment in the type-2 diabetic mouse by the angiotensin II type-1 receptor blocker candesartan. Hypertension. 2007;50:1099–1105. doi: 10.1161/HYPERTENSIONAHA.107.099374. [DOI] [PubMed] [Google Scholar]
- 16.Awad AS. Role of AT1 receptors in permeability of the blood-brain barrier in diabetic hypertensive rats. Vascul Pharmacol. 2006;45:141–147. doi: 10.1016/j.vph.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Hirawa N, Uehara Y, Kawabata Y, Numabe A, Gomi T, Ikeda T, Suzuki T, Goto A, Toyo-oka T, Omata M. Long-term inhibition of renin-angiotensin system sustains memory function in aged Dahl rats. Hypertension. 1999;34:496–502. doi: 10.1161/01.hyp.34.3.496. [DOI] [PubMed] [Google Scholar]
- 18.Porteri E, Rodella L, Rizzoni D, Rezzani R, Paiardi S, Sleiman I, De Ciuceis C, Boari GE, Castellano M, Bianchi R, Agabiti-Rosei E. Effects of olmesartan and enalapril at low or high doses on cardiac, renal and vascular interstitial matrix in spontaneously hypertensive rats. Blood Press. 2005;14:184–192. doi: 10.1080/08037050510034211. [DOI] [PubMed] [Google Scholar]
- 19.Takeda S, Sato N, Takeuchi D, Kurinami H, Shinohara M, Niisato K, Kano M, Ogihara T, Rakugi H, Morishita R. Angiotensin receptor blocker prevented beta-amyloid-induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension. 2009;54:1345–1352. doi: 10.1161/HYPERTENSIONAHA.109.138586. [DOI] [PubMed] [Google Scholar]
- 20.Mogi M, Li JM, Iwanami J, Min LJ, Tsukuda K, Iwai M, Horiuchi M. Angiotensin II type-2 receptor stimulation prevents neural damage by transcriptional activation of methyl methanesulfonate sensitive 2. Hypertension. 2006;48:141–148. doi: 10.1161/01.HYP.0000229648.67883.f9. [DOI] [PubMed] [Google Scholar]
- 21.Fox J, Guan S, Hymel AA, Navar LG. Dietary Na and ACE inhibition effects on renal tissue angiotensin I and II and ACE activity in rats. Am J Physiol. 1992;262:F902–F909. doi: 10.1152/ajprenal.1992.262.5.F902. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension. 2002;39:129–134. doi: 10.1161/hy0102.100536. [DOI] [PubMed] [Google Scholar]
- 23.de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47:145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- 24.van der Flier WM, van Straaten EC, Barkhof F, Verdelho A, Madureira S, Pantoni L, Inzitari D, Erkinjuntti T, Crisby M, Waldemar G, Schmidt R, Fazekas F, Scheltens P. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke. 2005;36:2116–2120. doi: 10.1161/01.STR.0000179092.59909.42. [DOI] [PubMed] [Google Scholar]
- 25.Tong XK, Nicolakakis N, Kocharyan A, Hamel E. Vascular remodeling versus amyloid beta-induced oxidative stress in the cerebrovascular dysfunctions associated with Alzheimer’s disease. J Neurosci. 2005;25:11165–11174. doi: 10.1523/JNEUROSCI.4031-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Köller M, Krause HP, Hoffmeister F, Ganten D. Endogenous brain angiotensin II disrupts passive avoidance behavior in rats. Neurosci Lett. 1979;14:71–75. doi: 10.1016/0304-3940(79)95346-1. [DOI] [PubMed] [Google Scholar]
- 27.Morgan JM, Routtenberg A. Angiotensin injected into the neostriatum after learning disrupts retention performance. Science. 1977;196:87–89. doi: 10.1126/science.402696. [DOI] [PubMed] [Google Scholar]
- 28.Wayner MJ, Armstrong DL, Polan-Curtain JL, Denny JB. Ethanol and diazepam inhibition of hippocampal LTP is mediated by angiotensin II and AT1 receptors. Peptides. 1993;14:441–444. doi: 10.1016/0196-9781(93)90129-5. [DOI] [PubMed] [Google Scholar]
- 29.Popescu BO, Toescu EC, Popescu LM, Bajenaru O, Muresanu DF, Schultzberg M, Bogdanovic N. Blood-brain barrier alterations in ageing and dementia. J Neurol Sci. 2009;283:99–106. doi: 10.1016/j.jns.2009.02.321. [DOI] [PubMed] [Google Scholar]
- 30.Fleegal-DeMotta MA, Doghu S, Banks WA. Angiotensin II modulates BBB permeability via activation of the AT(1) receptor in brain endothelial cells. J Cereb Blood Flow Metab. 2009;29:640–647. doi: 10.1038/jcbfm.2008.158. [DOI] [PubMed] [Google Scholar]
- 31.Guo RW, Yang LX, Wang H, Liu B, Wang L. Angiotensin II induces matrix metalloproteinase-9 expression via a nuclear factor-kappaB-dependent pathway in vascular smooth muscle cells. Regul Pept. 2008;147:37–44. doi: 10.1016/j.regpep.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Smith C, Howlett C, Stanimirovic D. Inflammatory activation of human brain endothelial cells by hypoxic astrocytes in vitro is mediated by IL-1beta. J Cereb Blood Flow Metab. 2000;20:967–978. doi: 10.1097/00004647-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Lochhead JJ, McCaffrey G, Quigley CE, Finch J, DeMarco KM, Nametz N, Davis TP. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2010;30:1625–1636. doi: 10.1038/jcbfm.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang JM, Veerasingham SJ, Tan J, Leenen FH. Effects of high salt intake on brain AT1 receptor densities in Dahl rats. Am J Physiol Heart Circ Physiol. 2003;285:H1949–H1955. doi: 10.1152/ajpheart.00744.2002. [DOI] [PubMed] [Google Scholar]
- 35.Tojo A, Onozato ML, Kobayashi N, Goto A, Matsuoka H, Fujita T. Antioxidative effect of p38 mitogen-activated protein kinase inhibitor in the kidney of hypertensive rat. J Hypertens. 2005;23:165–174. doi: 10.1097/00004872-200501000-00027. [DOI] [PubMed] [Google Scholar]
- 36.Pelisch N, Hosomi N, Ueno M, Masugata H, Murao K, Hitomi H, Nakano D, Kobori H, Nishiyama A, Kohno M. Systemic candesartan reduces brain angiotensin II via downregulation of brain renin-angiotensin system. Hypertens Res. 2010;33:161–164. doi: 10.1038/hr.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhuo J, Song K, Abdelrahman A, Mendelsohn FA. Blockade by intravenous losartan of AT1 angiotensin II receptors in rat brain, kidney and adrenals demonstrated by in vitro autoradiography. Clin Exp Pharmacol Physiol. 1994;21:557–567. doi: 10.1111/j.1440-1681.1994.tb02555.x. [DOI] [PubMed] [Google Scholar]
- 38.Neuwelt EA. Mechanisms of disease: the blood-brain barrier. Neurosurgery. 2004;54:131–140. doi: 10.1227/01.neu.0000097715.11966.8e. discussion 141. [DOI] [PubMed] [Google Scholar]