CONSPECTUS
Conventional imaging methods, such as angiography, computed tomography, magnetic resonance imaging and radionuclide imaging, rely on contrast agents (iodine, gadolinium, radioisotopes) that are “always on”. While these agents have proven clinically useful, they are not sufficiently sensitive because of the inadequate target to background ratio. A unique aspect of optical imaging is that fluorescence probes can be designed to be activatable, i.e. only “turned on” under certain conditions. These probes can be designed to emit signal only after binding a target tissue, greatly increasing sensitivity and specificity in the detection of disease. There are two basic types of activatable fluorescence probes; 1) conventional enzymatically activatable probes, which exist in the quenched state until activated by enzymatic cleavage mostly outside of the cells, and 2) newly designed target-cell specific activatable probes, which are quenched until activated in targeted cells by endolysosomal processing that results when the probe binds specific cell-surface receptors and is subsequently internalized. Herein, we present a review of the rational design and in vivo applications of target-cell specific activatable probes. Designing these probes based on their photo-chemical (e.g. activation strategy), pharmacological (e.g. biodistribution), and biological (e.g. target specificity) properties has recently allowed the rational design and synthesis of target-cell specific activatable fluorescence imaging probes, which can be conjugated to a wide variety of targeting molecules. Several different photo-chemical mechanisms have been utilized, each of which offers a unique capability for probe design. These include: self-quenching, homo- and hetero-fluorescence resonance energy transfer (FRET), H-dimer formation and photon-induced electron transfer (PeT). In addition, the repertoire is further expanded by the option for reversibility or irreversibility of the signal emitted using the aforementioned mechanisms. Given the wide range of photochemical mechanisms and properties, target-cell specific activatable probes possess considerable flexibility and can be adapted to specific diagnostic needs. Herein, we summarize the chemical, pharmacological, and biological basis of target-cell specific activatable imaging probes and discuss methods to successfully design such target-cell specific activatable probes for in vivo cancer imaging.
1. Introduction
The contrast agents used in conventional imaging, such as CT, MRI, and angiography, continuously emit signal and hence are, “always on”.1 Even in the case of radionuclide imaging which can be specific for particular pathologic conditions, probes consist of a targeting moiety conjugated to an “always on” signaling payload.2–3 The fundamental disadvantage of “always on” probes is that they emit signals regardless of their proximity or interaction with target tissues or cells and as a result, there is considerable background signal to contend with. In order to design superior molecular imaging probes one seeks to either 1. maximize signal from the target, or 2. minimize signal from the background or 3 do both. Doing any of these leads to improved target-to-background ratio (TBR), which, in turn, improves sensitivity and specificity for detecting diseases with imaging. A long term approach to maximizing signal from the target is based on altering biodistribution and pharmacokinetics of the imaging probe.3 However, recent advances in activatable agents that emit signal after enzymatic digestion4–5, or acidification6–7, represent an exciting strategy for improving target to background ratios.
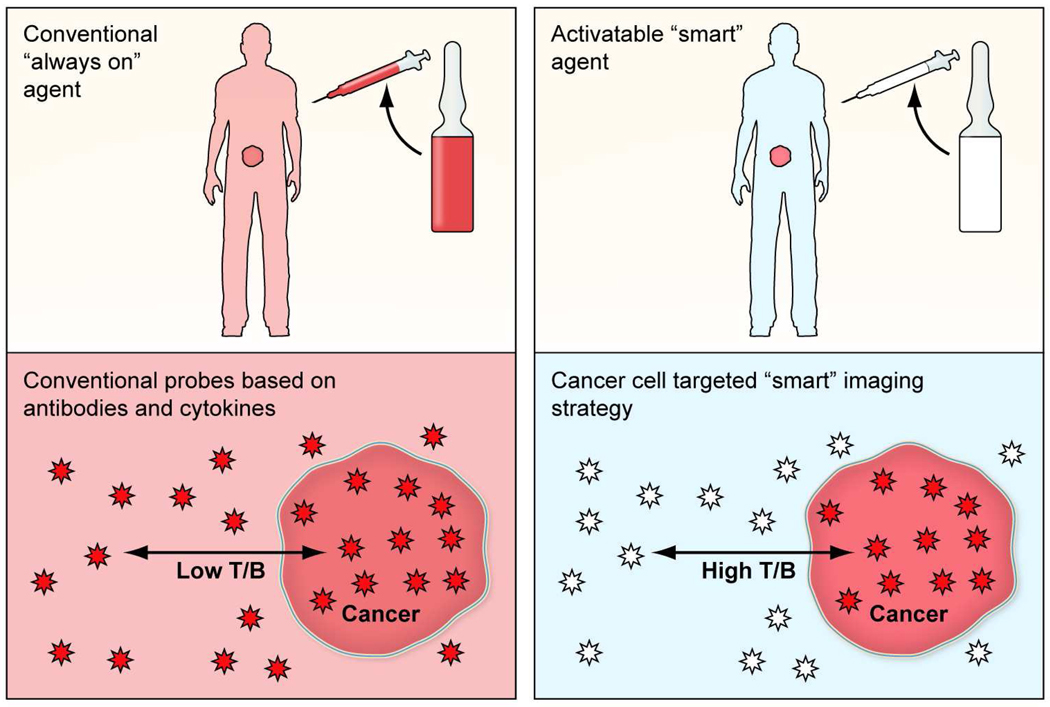
Optical imaging with fluorescent probes is a relatively new medical imaging technique. It has advantages of lower cost, portabililty, and real time capabilities. Of course, the limited depth of light penetration in tissue necessitates that fluorescence optical imaging be reserved for surfaces such as those encountered during a multitude of endoscopic or surgical procedures. A unique feature of fluorescence is that optical probes can be designed so that they do not emit light, if they are unbound, while only generate light upon internalization. This attribute of “turning on” only at the target, has been termed activatable or “smart”. Therefore, activatable agents show have low to no background signal and generate signal only after being bound to the specific molecular target.8–9 These activatable fluorescence probes maximize the target signal while minimizing the background signal, thereby resulting in higher target-to-background ratios than conventional “always on” imaging agents (Fig. 1).
FIGURE 1.
(a) A schema demonstrating targeted cancer imaging with a conventional “always on” probe (left) compared to a target-specific activatable probe (right). (b) A schema demonstrating target signal-to-background ratio of a conventional “always on” probe (left) is compared with a target-cell specific activatable probe (right).
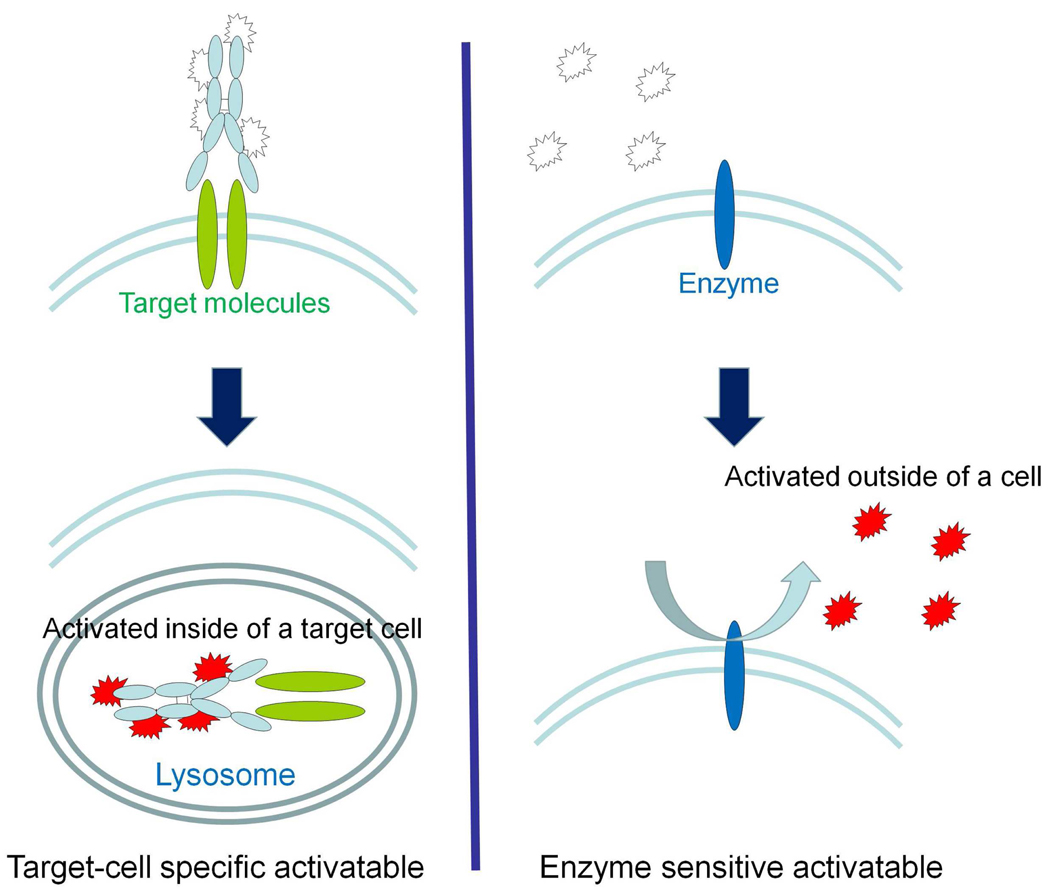
There are several ways to design activatable fluorescence probes. The typical method involves enzymatic activation of the probe by secreted extracellular or cell-surface enzymes. In this strategy, the probe is silent until it is activated by specific microenvironments. Enzymatic cleavage then leads to generation of fluorescence signal mostly in the extracellular space9–10. The second strategy involves target-cell specific activatable probes, which are quenched until activated within targeted cells by lysosomal processing of the probe. This occurs when the probe binds to the target cell-surface molecule, which leads to subsequent internalization of the probe and fluorescence activation through chemical processes of the fluorescent probe within the endolysosome. This strategy results in intracellular signal activationonly in the target cells (Fig. 2).11–12
FIGURE 2.
A schema for the activation process involved with a target-cancer cell specific activatable molecular probe based on an anti growth factor receptor monoclonal antibody conjugated with an activatable fluorophore. Note that the agent is non-fluorescent in the unbound state and becomes irreversibly activated within the target cell.
There are advantages and disadvantages to both methods. In enzymatic activation, a single target enzyme can activate many fluorescence molecules, thus amplifying the signal from the target tissue. However, a disadvantage of enzymatic activation is that the activation occurs in the extracellular space. Therefore, after activation the probe can diffuse away from the target and contribute to background signal. A few of probes can be reacted with enzymes in the lysosome, however, the reaction mostly happened in the macrophage because of no cell selectivity on the probe. Further, none of the currently utilized enzymes for fluorescence activation are specific for carcinogenesis but are instead, released secondary to host-cancer interactions thereby decreasing specificity. In contrast, probes that are activated by endolysosomal processing, are highly specific and generally remain localized to the target as activation relies upon the probe binding specific cell-surface receptors. Signal amplification can be achieved by conjugating multiple fluorophores to a single targeting moiety, such as a monoclonal antibody13–14. However, there is a limited number of fluorophores (up to around 10) that can be conjugated to a single molecule without compromising binding specificity, making amplification less potent that that achieved by extracellular enzymatic activation. Due to the highly sensitive (pM range) nature of fluorescence imaging, molecularly targeted cell-specific probes can permit early in vivo cancer detection of extremely small lesions. Moreover, the multitude of cell surface molecules, such as overexpressed growth factor receptors, which are directly related to carcinogenesis, provide numerous targets that are highly specific for cancer. With this in mind, molecularly targeted activation may yield better specificity for detecting cancer than enzymatic activation. In this review article, we focus on the chemical and biological strategies involved in designing target-cell specific activatable fluorescence imaging probes for in vivo cancer diagnosis.
2. General strategy for designing target-cell specific activatable fluorescence imaging probes
Targeted molecular imaging probes consist of three basic parts; a targeting moiety for specificity, a carrier to optimize pharmacokinetics, and an activatable fluorophore for signaling. These three components must be integrated in such a way that the biologic specificity, pharmacokinetics and photochemistry, of the probe work in concert to achieve the desired application of the target-cell specific molecular probe.
2.1 Achieving Biological Specificity
In order to design a target-specific probe, ligands should bind with specificity to the target but not the background tissues. Among the biological targeting molecules, monoclonal antibodies and receptor ligands generally have the highest specificity for their respective antigens or receptors. In addition, large proportions of these ligands are internalized after binding to their targets and then undergo lysosomal processing. Within the lysosome, the antigen-antibody or ligand-receptor pairs are catabolized under unique conditions including low pH, high protease activity, and oxidation, which can separately or collectively serve as a chemical switch to “turn on” the activatable fluorophore. For these reasons, we commonly rely on these cell surface proteins to develop targeting probes (Fig. 3).
FIGURE 3.
A schematic explanation of the function of a target-cancer cell specific activatable probe (left) versus an enzyme activatable probe (right). The signal activation of target-cell specific activatable probes occurs intracellularly, whereas enzyme activation typically occurs in the extracellular environment permitting diffusion away from the target cell.
2.2 Pharmacological strategy
The pharmacokinetic strategy of target-specific activatable probes is very different from that of conventional “always on” imaging probes.3 Since target-specific activatable probes do not emit signal before hitting the target molecules, unbound probes do not yield signal. Therefore, there is less background signal to compromise sensitivity and specificity, yielding an absolute increase in target-to-background ratios as the signal from the unbound agent is negligible.15 Thus, activatable probes can have longer clearance times without compromising imaging, which allows a greater amount of injected reagent to accumulate in target tissue/cells. With “always on” probes, there is a premium on optimizing pharmacokinetics to remove unbound probe as quickly as possible to improve the target-to-background ratio. However, this is not always possible. For instance, monoclonal IgGs labeled with “always on” signaling payloads, exhibit prolonged clearance, which leads to high background signal and low target-to-background ratios.3 Yet, when the same monoclonal IgG is labeled with an activatable signaling payload, high target-to-background ratios can be achieved despite the presence of unbound probe remaining in circulation.14,16 (Fig. 1b)
2.3 Photo-chemical strategy
In order to permit imaging with high target-to-background, the activation ratio (post-activation signal/ pre-activation signal) must be high. In effect this means that the signal intensity of the quenched (or off) state of the fluorophore should approach zero. Therefore, optimal dequenchable quenching of the photo-chemical reaction including H- or J-type dimer formation, homo- or hetro- Förster resonance energy transfer, and photon induced electron transfer (See section 3), is required in the non-activated state. However, in order to produce an image, the signal generated by activation must have sufficient quantum yields to be detected by a camera. Therefore, an ideal probe yields little or no signal in the unbound state, but becomes brightly fluorescent in the activated state after binding and internalization.
3. Photo-chemical basis for the fluorescence signal quenching and activation
Several different photo-chemical mechanisms for fluorescence signal quenching and activation have recently been discovered. The best known mechanism is Förster (fluorescence) resonance energy transfer (FRET), wherein energy from one fluorophore is transferred to another molecule, when the two molecules are in close (<10nm) proximity. The FRET pair can consist of two fluorophores (self-quenching) or a fluorophore and a quencher molecule. The FRET or H-dimer formation effect is based on inter-fluorophore processing between two fluorophore molecules in proximity of one another. Another mechanism of activation, which has been more recently discovered, is photon-induced electron transfer (PeT), which operates within a single fluorophore molecule and does not require the presence of a second fluorophore. Both of these mechanisms can then be utilized in the design of target-cell specific activatable probes.
3.1 Self-quenching (Homo-FRET)
Self-quenching or Homo-FRET occurs, when excited fluorophores of the same type absorb energy from each other that otherwise would have led to an emitted photon, thus diminishing the fluorescence of the entire compound. This design is commonly employed because of its simplicity. For instance, this mechanism has been employed for producing enzyme activatable imaging probes activated by the cathepsin and matrix metalloproteinase family of enzymes found in cancers and inflammation.10 However, Homo-FRET quenching can only take place when multiple fluorophores are within proximity of one another. Homo-FRET quenching can be validated by the absorbance spectra of the quenched conjugates.17 While Homo-FRET can produce high post-activation signal from the multiple fluorophores involved, there is often considerable pre-activation signal reducing the activation ratio (Fig. 4a).11,13–14
FIGURE 4.
The schematic explanation for five available photo-chemical activation strategies; (a) self-quenching (Homo-FRET), (b) quencher-fluorophore combination, (c) auto-quenching, (d) H-type dimer formation, (e) photon induced electron transfer (PeT), and (f) a dual functional activatable fluorophore based on the combination of H-type dimer formation and PeT.
3.2 Quencher-fluorophore combination
While self-quenching fluorophores have thus far been limited by background production of fluorescence in the pre-activation quenched state, quencher-fluorophore pairs produce little pre-activation state fluorescence. Chemical quencher-fluorophore combinations are commonly utilized in the design of small molecular activatable probes. Quencher-fluorophore combinations can achieve higher activation ratios due to low pre-activation signal in the quenched state compared to self-quenching activatable probes (Fig. 4b).18 However, this strategy is limited by the intensity of the post-activation signal due to the smaller total number of fluorophores in the probe (compared to the self-quenching strategy, where there are numerous fluorophores available). Furthermore, there are only a limited number of biocompatible quencher-fluorophore pairs with sufficiently high activation ratios.19
3.3 Auto-quenching
Some fluorochromes can spontaneously induce a low signal quenched state, when conjugated with proteins, likely due to interactions between fluorophores and aromatic rings on the side chain of amino acids, such as tryptophan or phenylalanine. A good example of this is indocyanine green (ICG)-like dye, which can be fully quenched when covalently conjugated with humanized monoclonal antibodies via a side chain of lysine, even at low conjugation ratios (Fig. 4c). The activation ratio of ICG-conjugated antibodies can be as high as 50, an extremely high value among the activatable near infrared probes with emission wavelengths over 800 nm.16 Moreover, ICG has a long proven record of safety in humans.
3.4 H-dimer formation
Xanthene derivatives are known to form homo-dimers at high concentrations (~mM) in aqueous solutions. This homo-dimer formation induces short (H-dimer) or long (J-dimer) shifts of absorbance spectra, which completely quench the emission fluorescence signal.20–21 When covalently conjugated with long proteins, two fluorophores in close (< 0.5nm) proximity can maintain the homo H-dimer formation at a lower concentration (<nM) and yet easily dissociate to dequench after conformational change or unfolding of the protein (Fig. 4d). Therefore, H-dimer formation represents an alternative mechanism for designing activatable imaging probes with high activation ratios.22 Unfortunately, only a few fluorochromes form H-dimers under random protein conjugation reactions. Therefore, even though the fluorochromes forming H-dimers are completely quenched, the remaining fluorochromes in the probe may contribute to pre-activation light emission, thus lowering the overall activation ratio of the macromolecular probe.
3.5 Photon-induced electron transfer
Another mechanism for developing activatable fluorescence imaging probes is “photon-induced electron transfer” (PeT). PeT is a widely accepted mechanism for fluorescence quenching, in which electron transfer from the PeT donor to the excited fluorophore diminishes the fluorescence signal. When the PeT donor is cleaved from the fluorophore or inactivated, full activation is achieved. Recently, it was reported that the fluorescence of the commonly utilized fluorophore, fluorescein and its derivatives, could, in part, be controlled by the PeT mechanism, i.e. the fluorescence properties of fluorescein derivatives could be modulated by intra-molecular PeT. However, the PeT mechanism was thought to be limited to lower wavelength fluorophores and was not thought to be possible in longer-wavelength fluorophores. Indeed, almost all the reports of PeT activation strategies utilize UV-excitable fluorophores including anthracene. However, Urano et al. recently found that when the fluorescein structure was deconstructed into two parts, i.e., the benzoic acid moiety as the PeT donor and the xanthene ring as the fluorophore, only small reductions in emission were observed with fluorescein and its derivatives. Yet, when the HOMO energy of the benzoic acid moiety was higher than a certain threshold, PeT occurred much more efficiently, resulting greatly reduced fluorescence.23 On the other hand, when the HOMO energy is lower than this threshold, as is the case for generic fluorescein, the PeT mechanism is less efficient and the molecule becomes highly fluorescent. This strategy has not only been used with fluorescein but also with a wide range of longer-wavelength families of fluorophores, such as BODIPYs, rhodamines, and even cyanines (Fig. 4e). Utilizing the PeT mechanism, fluorochromes can be quenched and activated with extremely high activation ratios, as high as 750.12 However, the conjugation reactions required for attaching targeting ligands to the PeT fluorophore may affect the HOMO energy state, resulting in a decrease in the effective activation ratio of the entire probe.
3.6 Combination of different mechanisms
PeT activation theory relies on intra-fluorophore electron donation, whereas the other activation strategies are based on inter-fluorophore interactions. Therefore, by employing PeT in combination with inter-fluorophore strategies, synergic effects may further improve the activation ratio. Ogawa et al. simultaneously employed both PeT and H-dimer formation mechanisms to synthesize an activatable fluorescence probe with higher activation ratios than were achievable with either PeT or H-dimer formation alone. This hybrid agent was able to depict target cancer tissues in vivo with high tumor-to-background ratios during fluorescence endoscopy (Fig. 4f).24
4. In vivo imaging applications of target-cell specific activatable probes
Signal activation is irreversible in most activatable probes; once activated these probes will continue to emit fluorescence signal until the fluorophore is physically destroyed or biologically catabolized. Therefore, the signal strength of an irreversible activatable probe is related to the cumulative number of activated molecules in target cells.11,13–14,16,22 In contrast, signal activation in some activatable probes is reversible, i.e. the probe deactivates in response to environmental changes such as pH. Therefore, the signal strength of the reversible activatable probes reflects the number of activated probe molecules at a given moment whereas the signal derived from irreversible activatable probes is cumulative over time.12 For this reason, most irreversible activatable probes yield stronger signal and are more sensitive than reversible activatable probes, however, only reversible activatable probes have the potential for real-time monitoring of in vivo events.
4.1 Irreversible activatable probes
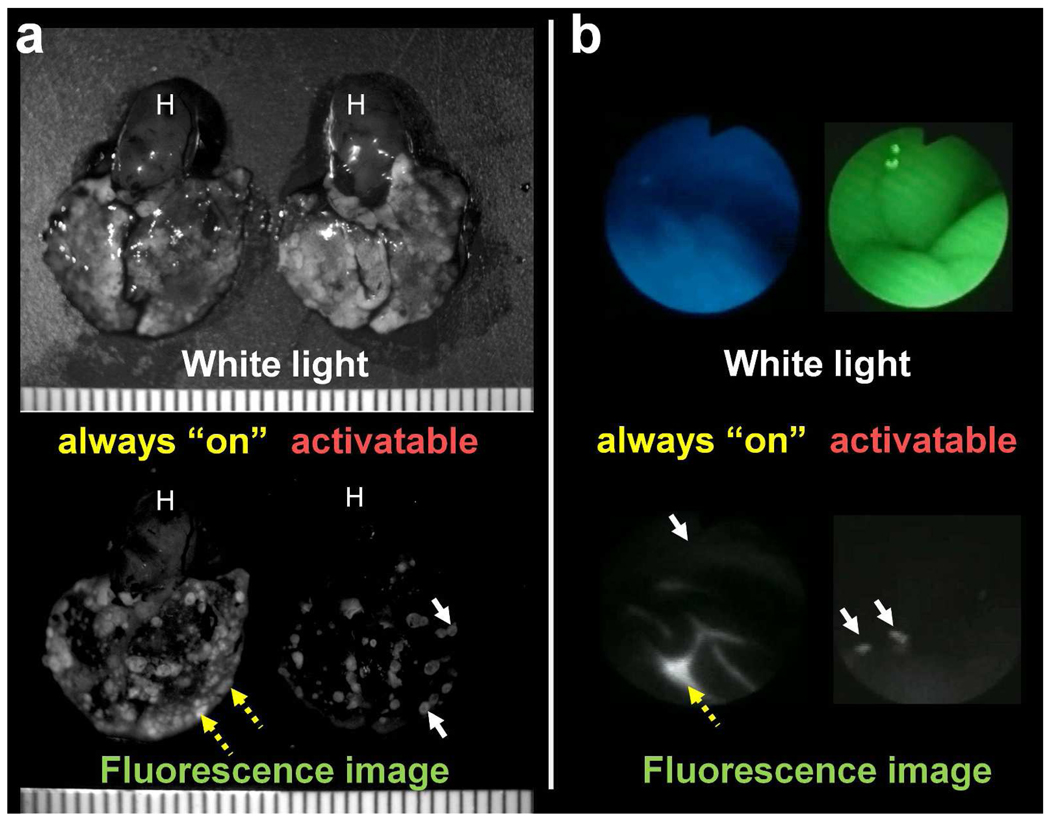
Target-cell specific irreversible activatable probes are ideal for highly specific and sensitive cancer detection. The combination of highly specific targeting (e.g. monoclonal antibodies), high activation ratios, and accumulation of the probe in target cells over time enables the detection of cancer nodules as small as ~100 µm in diameter with high (>95%) specificity (Fig. 5a).11,14,16 Since unbound reagent does not contribute to the image, clear images of tiny deposits of cancer can be achieved rapidly with either intravenous or intraperitoneal administration. Moreover, unlike “always on” agents, there is no need to wait for clearance of the injected agent to reduce the background signal, but only need to wait for activation. The rapidity, with which images are formed with this strategy, is ideal for numerous real-time clinical applications, including surgical or endoscopic procedures (Fig. 5b).22
FIGURE 5.
Comparison of in vivo imaging utilizing an “always on” probe (right) and a cancer cell-specific activatable probe (left). (a) A mouse model of metastatic HER2-positive lung cancer imaged 1 day after intravenous injection of “always on” (left) and activatable (right) fluorescence-labeled trastuzumab (monoclonal antibody against the HER2 receptor). The target-cell specific activatable probe (right) exclusively images targeted lung metastasis (white arrow) without visible background signal remaining unbound reagent within the blood pool. In contrast, the “always on” probe images lung cancer metastases, yet considerable background signal results from unbound agent within the blood vessels (yellow dashed arrow) and heart (H).
(b) A mouse model of ovarian cancer with disseminated peritoneal metastases imaged using a fluorescence endoscopy system, 2 hours after intraperitoneal injection of “always on” (left) or activatable (right) fluorescence-labeled D-galactose lectin binding probe. The target-cell specific activatable probe (right) only shows targeted peritoneal tumors (white arrow) without background signal from unbound reagent. In contrast, the “always on” probe demonstrates higher signal from unbound reagent (yellow dashed arrow) than that from targeted tumors (white arrow).
4.2 Reversible activatable probes
In addition to target-specific detection, reversibly activatable probes provide the opportunity to monitor the response of cancer cells to therapy in real time. For instance, it is established that lysosomal pH is maintained by an ATP-dependent proton pump. As cells become damaged or die as a result of effective therapy, the activity of ATP decreases, thus, reducing the acidification of the lysosome. The activatable pH-sensitive imaging probes within the lysosome then lose fluorescence signal, indicating cellular dysfunction or death. Loss of fluorescence then serves as a surrogate marker for successful anti-cancer therapy. Reversible activatable probes may thereby permit effective real-time monitoring of viable cancer cell burden (Fig 6).12
FIGURE 6.
Fluorescence imaging depicting real-time therapeutic effects in a peritoneal ovarian cancer model using a reversible pH-sensitive targeted activatable probe. The fluorescence signal derived from the activatable probe decreased with time indicating successful treatment.
5. Concluding remarks
Herein, we discuss the chemical, pharmacological, and biological strategies for designing target-cell specific activatable fluorescence imaging probes. We also present a discussion regarding their in vivo application for cancer imaging. Integration of these multi-disciplinary approaches enables the rational design and synthesis of target-cell specific activatable fluorescence imaging probes, which target a variety of different molecules (Fig. 7). Such activatable fluorescence probes have demonstrated the potential to depict tiny cancer nodules with high sensitivity and specificity, while exhibiting minimal background signal. Successful translation of target-cell specific activatable imaging to surgical and endoscopic procedures has promise for improving the diagnosis and treatment of cancer.
FIGURE 7.
A summary of the potential designs for target-cancer cell specific activatable fluorescence probes.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Biographies
Hisataka Kobayashi is the Chief scientist in the Molecular Imaging Program at the National Cancer Institute of the National Institutes of Health in Bethesda, MD. Dr. Kobayashi is awarded MD and PhD (Immunology/Medicine) from the Kyoto University in Japan. He joined as a post doctal fellow in the Nuclear Medicine Department at the Clinical Center of the National Institutes of Health in 1995 and moved to the current position in the Molecular Imaging Program at NCI in 2004. His interest is in developing the novel molecular imaging agents and technologies especially for targeting cancers.
Peter L. Choyke is the Director of the Molecular Imaging Program at the Center for Cancer Research of the National Cancer Institute in Bethesda, Maryland. Dr. Choyke is a graduate of Jefferson Medical School and received training in Diagnostic Radiology at Yale University and the University of Pennsylvania. He joined the Diagnostic Radiology Department at the Clinical Center of the National Institutes of Health in 1988 and formed the Molecular Imaging Program at NCI in 2004. His interest is in accelerating the treatment of cancer by using novel molecular imaging agents which target specific features of cancers.
REFERENCES
- 1.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alford R, Ogawa M, Choyke PL, Kobayashi H. Molecular probes for the in vivo imaging of cancer. Mol Biosyst. 2009;5:1279–1291. doi: 10.1039/b911307j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23:1137–1146. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 4.Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. In vivo visualization of gene expression using magnetic resonance imaging. Nat Biotechnol. 2000;18:321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 5.Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 6.Aime S, Delli Castelli D, Terreno E. Novel pH-reporter MRI contrast agents. Angew Chem Int Ed Engl. 2002;41:4334–4336. doi: 10.1002/1521-3773(20021115)41:22<4334::AID-ANIE4334>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S, Wu K, Sherry AD. A Novel pH-Sensitive MRI Contrast Agent. Angew Chem Int Ed Engl. 1999;38:3192–3194. [PubMed] [Google Scholar]
- 8.Kobayashi H, Ogawa M, Alford R, Choyke PL, Urano Y. New Strategies for Fluorescent Probe Design in Medical Diagnostic Imaging. Chem Rev. 2009 doi: 10.1021/cr900263j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahmood U, Tung CH, Bogdanov A, Jr, Weissleder R. Near-infrared optical imaging of protease activity for tumor detection. Radiology. 1999;213:866–870. doi: 10.1148/radiology.213.3.r99dc14866. [DOI] [PubMed] [Google Scholar]
- 10.Tung CH, Bredow S, Mahmood U, Weissleder R. Preparation of a cathepsin D sensitive near-infrared fluorescence probe for imaging. Bioconjug Chem. 1999;10:892–896. doi: 10.1021/bc990052h. [DOI] [PubMed] [Google Scholar]
- 11.Hama Y, Urano Y, Koyama Y, Kamiya M, Bernardo M, Paik RS, Shin IS, Paik CH, Choyke PL, Kobayashi H. A target cell-specific activatable fluorescence probe for in vivo molecular imaging of cancer based on a self-quenched avidin-rhodamine conjugate. Cancer Res. 2007;67:2791–2799. doi: 10.1158/0008-5472.CAN-06-3315. [DOI] [PubMed] [Google Scholar]
- 12.Urano Y, Asanuma D, Hama Y, Koyama Y, Barrett T, Kamiya M, Nagano T, Watanabe T, Hasegawa A, Choyke PL, Kobayashi H. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat Med. 2009;15:104–109. doi: 10.1038/nm.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hama Y, Urano Y, Koyama Y, Gunn AJ, Choyke PL, Kobayashi H. A self-quenched galactosamine-serum albumin-rhodamineX conjugate: a "smart" fluorescent molecular imaging probe synthesized with clinically applicable material for detecting peritoneal ovarian cancer metastases. Clin Cancer Res. 2007;13:6335–6343. doi: 10.1158/1078-0432.CCR-07-1004. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa M, Regino CA, Choyke PL, Kobayashi H. In vivo target-specific activatable near-infrared optical labeling of humanized monoclonal antibodies. Mol Cancer Ther. 2009;8:232–239. doi: 10.1158/1535-7163.MCT-08-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19:316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa M, Kosaka N, Choyke PL, Kobayashi H. In vivo molecular imaging of cancer with a quenching near-infrared fluorescent probe using conjugates of monoclonal antibodies and indocyanine green. Cancer Res. 2009;69:1268–1272. doi: 10.1158/0008-5472.CAN-08-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogawa M, Kosaka N, Urano Y, Choyke PL, Kobayashi H. Activatable optical imaging probes with various fluorophore-quencher combinations. SPIE Proceedings. 2009;7190:6449–6418. [Google Scholar]
- 18.Ogawa M, Kosaka N, Longmire MR, Urano Y, Choyke PL, Kobayashi H. Fluorophore-quencher based activatable targeted optical probes for detecting in vivo cancer metastases. Mol Pharm. 2009;6:386–395. doi: 10.1021/mp800115t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogawa M, Kosaka N, Urano Y, Choyke PL, Kobayashi H. Activatable optical imaging probes with various fluorophore-quencher combinations. Proceeding of SPIE. 2009;7190:71900Z. [Google Scholar]
- 20.Packard BZ, Komoriya A, Toptygin DD, Ludwig B. Structural Characteristics of Fluorophores That Form Intramolecular H-Type Dimers in a Protease Substrate. J Phys Chem B. 1997;101:5070–5074. [Google Scholar]
- 21.Halterman RL, Moore JL, Mannel LM. Disrupting aggregation of tethered rhodamine B dyads through inclusion in cucurbit[7]uril. J Org Chem. 2008;73:3266–3269. doi: 10.1021/jo7026432. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa M, Kosaka N, Choyke PL, Kobayashi H. H-type dimer formation of fluorophores: a mechanism for activatable, in vivo optical molecular imaging. ACS Chem Biol. 2009;4:535–546. doi: 10.1021/cb900089j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urano Y, Kamiya M, Kanda K, Ueno T, Hirose K, Nagano T. Evolution of fluorescein as a platform for finely tunable fluorescence probes. J Am Chem Soc. 2005;127:4888–4894. doi: 10.1021/ja043919h. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa M, Kosaka N, Choyke PL, Kobayashi H. High Sensitivity Detection of Cancer in vivo using a Dual-Controlled Activation Fluorescent Imaging Probe Based on H-dimer Formation and pH Activation. Mol Biosyst. 2010 doi: 10.1039/b917876g. DOI: 10.1039/b917876g, (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]