Abstract
Memories of learned associations between the rewarding properties of drugs and environmental cues contribute to craving and relapse in humans. The mesocorticolimbic dopamine (DA) system is involved in reward-related learning induced by drugs of abuse. DA D3 receptors are preferentially expressed in mesocorticolimbic DA projection areas. Genetic and pharmacological studies have shown that DA D3 receptors suppress locomotor-stimulant effects of cocaine and reinstatement of cocaine-seeking behaviors. Activation of the extracellular signal-regulated kinase (ERK) induced by acute cocaine administration is also inhibited by D3 receptors. How D3 receptors modulate cocaine-induced reward-related learning and associated changes in cell signaling in reward circuits in the brain, however, have not been fully investigated. In the present study, we show that D3 receptor mutant mice exhibit potentiated acquisition of conditioned place preference (CPP) at low doses of cocaine compared to wild-type mice. Activation of ERK and Ca2+/calmodulin-dependent protein kinase IIα (CaMKIIα), but not the c-Jun N-terminal kinase and p38, in the nucleus accumbens, amygdala and prefrontal cortex is also potentiated in D3 receptor mutant mice compared to that in wild-type mice following CPP expression. These results support a model in which D3 receptors modulate reward-related learning induced by low doses of cocaine by inhibiting activation of ERK and CaMKIIα in reward circuits in the brain.
Keywords: dopamine, D3 receptors, cocaine, reward learning, ERK, CaMKIIα
Drug addiction is a chronic brain disease and relapse to drug-seeking is the main obstacle to long-term treatment and cure for drug addiction in humans (Dackis and O'Brien, 2005). Memories of drug effects or learned associations between the rewarding properties of drugs and environmental cues contribute significantly to craving and relapse in humans (Hyman et al., 2006; Kauer and Malenka, 2007; Kalivas and O'Brien, 2008). The neurotransmitter dopamine (DA) is involved in reward-related learning (Schultz, 2002; Wise, 2008; Volkow et al., 2009). Drugs of abuse can pathologically change neuronal circuits in the mesocorticolimbic DA system, which projects from the ventral tegmental area to the nucleus accumbens (NAc), amygdala (AMG), prefrontal cortex (PFC) and other structures (Everitt and Robbins, 2005; Koob and Volkow, 2010). The basolateral AMG (BLA) mediates learning of conditioned associations between the rewarding effects of drugs of abuse and cues. The PFC contributes to decision-making and execution of goal-directed actions. The NAc modulates motivation for drug seeking by integrating information from the BLA and PFC and relaying it to motor output structures, and it mediates reinforcement. These different brain structures coordinate to modulate reward-related learning induced by drugs of abuse.
There are two families of DA receptors (Neve et al., 2004). The D1-like family includes D1 and D5 receptors and activation of these receptors leads to increased intracellular levels of cAMP. The D2-like family includes D2, D3 and D4 receptors and activation of these receptors is negatively linked to the cAMP production (Neve et al., 2004). D3 receptors are preferentially expressed in mesocorticolimbic DA projection areas (Le Foll et al., 2005; Heidbreder et al., 2005). We previously found that D3 receptor mutant mice exhibit potentiated acute locomotor activation compared to wild-type mice following injections of low (5 mg/kg) but not higher doses (10 and 20 mg/kg) of cocaine (Xu et al., 1997). We and others also demonstrated that a D3 receptor mutation or D3 receptor-selective agonists and antagonists can alter locomotor sensitization, discriminative stimulus and conditioned place preference (CPP) induced by stimulants (Xu et al., 1997; Carta et al., 2000; Karasinska et al., 2005; Richtand, 2006; Martelle et al., 2007; Chen et al., 2007; Beninger and Banasikowski, 2008; Chen and Xu, 2010). Self-administration of cocaine can be attenuated by the co-administration of D3 receptor-selective agonists (Neisewander et al., 2004; Martelle et al., 2007). Administration of a partial D3 receptor agonist or D3 receptor antagonists generally results in inhibition of cocaine seeking behavior (Pilla et al., 1999; Xi et al., 2006; Di Ciano, 2008; Peng et al., 2009). Taken together, these results suggest that D3 receptors contribute to the development and reinstatement of stimulant-induced behaviors.
The signaling pathways associated with DA receptors have been suggested to play a critical role in drug-induced neuroadaptations in the brain Hyman et al., 2006). D3 receptors regulate cocaine-induced cell signaling events. We previously found that activation of extracellular signal-regulated kinase (ERK) which is a member of the mitogen-activated protein kinase (MAPK) family by acute cocaine injections is inhibited by the activation of D3 receptors (Zhang et al., 2004; Chen and Xu, 2010). D3 receptors also inhibit N-methyl-D-aspartate (NMDA)-induced activation of Ca2+/calmodulin-dependent protein kinase IIα (CaMKIIα, Jiao et al., 2007). On the other hand, D3 receptors can be phosphorylated by CaMKIIα in an activity-dependent manner, resulting in the inhibition of D3 receptor function (Liu et al., 2009).
Although D3 receptors contribute to the development and reinstatement of cocaine-induced behaviors and related signaling events, how these receptors modulate cocaine-induced reward-related learning and associated changes in cell signaling in reward circuits in the brain have not been fully investigated. For example, we previous showed that a D3 receptor mutation in mice did not obviously affect the acquisition of CPP induced by relatively high doses (10 and 20 mg/kg) of cocaine (Chen and Xu, 2010). Whether these receptors inhibit reward-related learning induced by low does of cocaine remains unknown. Moreover, how D3 receptors regulate signaling involving ERK and other members of the MAPK family, c-Jun N-terminal kinase (JNK) and p38, as well as CaMKIIα in brain reward circuit is unclear. In the present study, we investigated these issues using D3 receptor mutant mice and the CPP paradigm. Our data support a model in which D3 receptors modulate reward-related learning induced by low doses (1 and 2.5 mg/kg) of cocaine by inhibiting activation of ERK and CaMKIIα in the NAc, AMG and PFC in the brain.
EXPERIMENTAL PROCEDURES
Mice
We previously generated DA D3 receptor mutant mice (Xu et al., 1997). Homozygous D3 receptor mutant mice and wild-type littermates were obtained by crossing D3 receptor heterozygous parents. The genotype of D3 receptor mutant and wild-type mice was determined by Southern blotting using a 3' gene-specific probe (Xu et al., 1997). The genetic background of all mice was initially 50% each of 129SvJ and C57BL/6J, and was subsequently bred with C57BL/6J mice for three additional generations. D3 receptor mutant and wild-type mice were group housed and were on a twelve hour light/dark cycle with food and water available ad libitum. Roughly equal numbers of male and female mice, 10 to 16 weeks old (mean age 12.2 weeks), were used in the current study. The temperature and humidity of the room were controlled. Animal use was in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animal and was approved by the University of Chicago Institutional Animal Care and Use Committee.
Drugs and antibodies
Cocaine hydrochloride was obtained from Sigma Chemical Co. (St. Louis, MO) and dissolved in sterile saline. All injections were administered intraperitoneally (i.p.) in a volume of 10 ml/kg body weight (Chen and Xu, 2010). Cocaine doses used in the current study were 1.0 and 2.5 mg/kg. Primary antibodies against phospho-ERK, phospho-JNK, phospho-p38, phospho-CaMKIIα, ERK, JNK, p38 and β-actin were purchased from Cell Signaling Technology (Beverly, MA). Primary antibodies for CaMKIIα and HRP-conjugated anti-rabbit or anti-goat second antibodies were purchased from Santa Cruz Technology (Santa Cruz, CA).
CPP
Eight three-chamber place preference apparati (MedAssociates, E. Fairfield, VT) were used in the present study (Zhang et al., 2006; Chen and Xu, 2010). The apparatus consisted of two large compartments (16.8×12.7×12.7 cm), and one small compartment (7.2×12.7×12.7 cm) which separated the large compartments. The two large compartments had different visual and tactile cues. One compartment was black with a stainless steel grid rod floor. The other compartment was white with a stainless steel mesh floor. The small compartment was gray with a smooth polyvinyl chloride floor. The apparatus had a clear Plexiglas top with a light on it.
We used a biased CPP procedure similar to that described before (Zhang et al., 2006; Chen and Xu, 2010). During the preconditioning phase (day 1–2, pre-test), mice were placed in the small compartment and were allowed to freely explore the 3 compartments for 20 minutes daily. The time spent in each compartment was recorded. Mice spending over 500 seconds in the small compartment or over 800 seconds in either large compartment were excluded. The next 12 days (day 3–14) were the conditioning and testing phase with one session per day. The drug-paired group received an i.p. cocaine injection and was confined in the white compartment for 30 minutes on day 3. On day 4, this group of mice received an i.p. saline injection and was confined in the black compartment for 30 minutes. As a control, the saline group received i.p. saline injections on days 3 and 4 and was confined in both compartments. On day 5 (test 1), mice were allowed to freely explore the three compartments for 20 minutes without injections, and the time spent in each compartment was recorded. Each mouse received three additional injections of cocaine plus saline and testing (tests 2–4) resulting in four conditioning sessions and four tests. All behavioral testing was performed during the light phase of the light/dark cycle (8 am–8 pm).
For the 1 mg/kg cocaine treatment, 18 wild-type mice and 16 receptor mutant mice were used; for the 2.5 mg/kg cocaine treatment, 20 each wild-type and D3 receptor mutant mice were used; for the saline treatment, 14 each mice were used. In general, 1–2 mice were excluded per group per treatment and a total of 9 out of 102 mice were excluded.
Protein extracts
Mice treated with cocaine at the 2.5 mg/kg dose and with saline were sacrificed by cervical dislocation immediately after the fourth test for CPP expression on day 14. Brains were rapidly removed, frozen on dry ice and stored at −80°C. Brains were sliced into 1 mm sections using the brain matrix as described (Chen and Xu, 2010). NAc, AMG and PFC were dissected according to a mouse brain atlas (Paxinos and Franklin, 1997). Tissues were homogenized in 300 μl ice-cold extraction buffer containing 50 mM Tris-HCl pH 7.5, 50 mM NaCl, 5 mM EDTA, 10 mM EGTA, 2 mM sodium pyrophosphate, 4 mM paranitrophenylphosphate, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 2 μg/ml aprotintin, 2 ug/ml leupeptin and 2 ug/ml pepstatin (Zhang et al., 2004). Homogenates were incubated on ice for 20 minutes and were centrifuged at 13,000 g for 20 minutes at 4°C (Chen and Xu, 2010). Supernatants were collected and protein concentrations were determined using a Bradford assay (Bio-Rad Laboratories, Hercules, CA).
Western blotting
Changes in protein levels were analyzed by using western blotting as described (Chen and Xu, 2010). Equal amounts of protein (10–20 μg) were separated by 10% SDS-PAGE. Proteins were then transferred onto polyvinylidene difluoride membranes following electrophoresis for two hours. The membranes were blocked with 5% BSA for one hour at room temperature. Membranes were then incubated overnight at 4°C with different primary antibodies. After three washes with a 0.1% Tween 20 Tris-buffered saline (pH8.4), membranes were incubated with appropriate HRP-conjugated anti-rabbit or anti-goat secondary antibodies. Signals were detected by enhanced chemiluminescence. Primary antibodies against phospho-ERK, phospho-JNK, phospho-p38 and phospho-CaMKIIα were used at 1:2000 dilutions. The secondary antibodies were used at 1:5000 dilutions. The same membranes were stripped and incubated with antibodies against total ERK, JNK, p38 and CaMKIIα (1:2000; Chen and Xu, 2010). The membranes were stripped and re-probed with an anti-actin antibody (1:2000) to assure equal loading of the samples. All western blot analyses were performed a minimum of three times.
Quantification and data analysis
The behavioral data were analyzed as time spent on the saline-paired side subtracted from time spent on the drug-paired side and were presented as mean ± SEM (Chen and Xu, 2010). Two-way repeated measure ANOVA with test as within-subjects factor, and genotype and treatment as between-subjects factors were used, followed by one-way ANOVA test with post-hoc LSD. Paired-sample t-test was used for within-subjects comparisons.
The results for Western blotting were analyzed using densitometry. Ratios of phospho- to total ERK, JNK, p38 and CaMKIIα densities were calculated for each sample. Saline controls were set at 1. A one-way ANOVA with post-hoc LSD was used to analyze changes in phosphorylation. The statistically significant level was set at p<0.05.
RESULTS
DA D3 receptor mutant mice show potentiated CPP acquisition compared to wild-type mice at low doses of cocaine
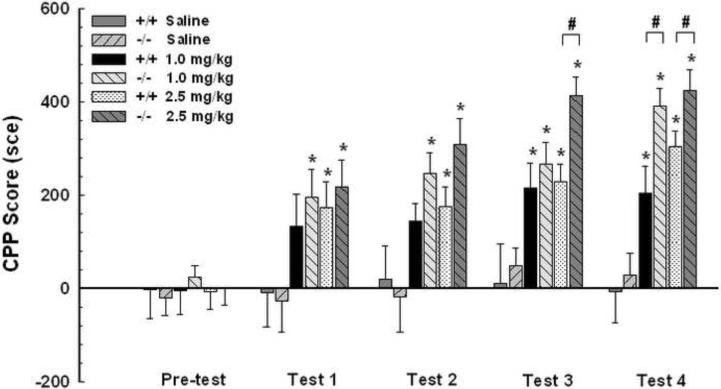
To determine the role of D3 receptors in reward-related learning, we used three groups of D3 receptor mutant and wild-type mice including two different doses of cocaine and saline (0, 1 and 2.5 mg/kg, n=12–19 mice per group) and performed a CPP study. There was a significant main effect of treatment [F (2, 92)=13.571, p<0.05] and genotype [F (1,92)=4.318, p<0.05] after conditioning (Fig. 1). Oneway ANOVA test showed that both D3 receptor mutant and wild-type mice developed CPP after conditioning [Fig. 1, F (5, 92)=14.597, p<0.05]. D3 receptor mutant and wild-type mice had developed CPP at the first [F (5, 92)=2.625, p<0.05] and third [F (5,92)=8.404, p<0.05] test sessions respectively at the 1 mg/kg dose of cocaine. At the 2.5 mg/kg dose of cocaine, both wild-type and D3 receptor mutant mice exhibited CPP at the first test session [F (5, 92)=2.625, p<0.05]. Significantly, D3 receptor mutant mice exhibited higher CPP acquisition than wild-type mice on test 4 at 1 mg/kg cocaine and both tests 3 and 4 at 2.5 mg/kg cocaine. Paired-sample t-tests indicated that D3 receptor mutant and wild-type mice do not show CPP acquisition after saline injections (Fig. 1, p>0.05). Together, these results suggest that, at very low doses of cocaine, D3 receptors inhibit acquisition of CPP induced by cocaine.
Fig. 1.
D3 receptor mutant mice show heightened CPP acquisition compared to wild-type mice. Three groups of D3 receptor mutant (−/−) and wild-type (+/+) mice received either cocaine (1 mg/kg, n=17 and 14 each; or 2.5 mg/kg, n=18 and 19 each) or saline (n=12 and 13 each) injections on alternative days, and were confined to specific compartments in a biased design. These mice were tested for place preference without injections. Both D3 receptor mutant and wild-type mice showed CPP acquisition induced by 1 and 2.5 mg/kg doses of cocaine but not by saline. Moreover, D3 receptor mutant mice exhibited higher CPP acquisition than wild-type mice at both doses of cocaine. Results represent mean ± SEM time spent on the drug-paired side minus that on the saline-paired side. *p<0.05 compared with the saline mouse group of the same genotype within the same test. #p<0.05 compared between two genotypes at the same cocaine dose and test.
Potentiated activation of ERK, but not JNK and p38, in the NAc, AMG and PFC in D3 receptor mutant mice following CPP expression
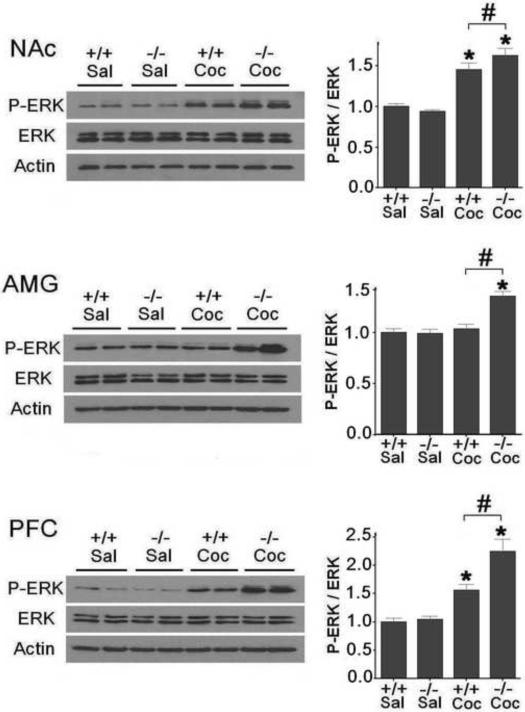
We systematically investigated the status of all members of the MAPK family, ERK, JNK and p38 in different brain regions in D3 receptor mutant and wild-type mice immediately following CPP expression tests. Western blot analyses indicated that ERK was activated in the NAc, AMG and PFC in D3 receptor mutant mice compared to saline-treated control group [Fig. 2, NAc: F (3, 27)=32.478, p<0.05; AMG: F (3, 27)=17.728, p<0.05; PFC: F (3, 27)=22.127, p<0.05]. In wild-type mice, ERK was activated in the NAc and PFC as compared to the saline-treated group [Fig. 2, NAc: p<0.05; PFC: p<0.05], but not in the AMG (p>0.05). Notably, phospho-ERK levels are higher in all three brain regions in D3 receptor mutant mice than those in wild-type mice following CPP expression (Fig. 2, p<0.05). Levels of phospho-ERK and total ERK were similar in each of the three brain regions in D3 receptor mutant and wild-type mice following saline treatment (Fig 2).
Fig. 2.
Potentiated ERK activation in the NAc, AMG and PFC in D3 receptor mutant mice compared to that in wild-type mice following CPP expression. Mice were given cocaine (Coc, 2.5 mg/kg) or saline (Sal) injections and were sacrificed at the end of the fourth CPP test. Western blotting was performed using brain samples from D3 receptor mutant (−/−) and wild-type (+/+) mice (n=7 mice each). Ratios of phospho-ERK (P-ERK) relative to total ERK protein levels in the NAc, AMG and PFC were analyzed. Data were expressed as mean± SEM relative to saline controls that were set as 1. Actin levels were used as a loading control. *p<0.05 compared with the saline control mouse group of the same genotype. #p<0.05 compared between the two genotypes.
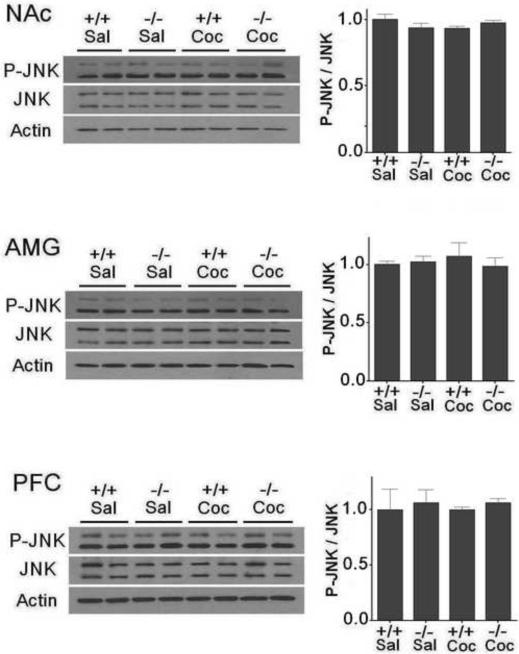
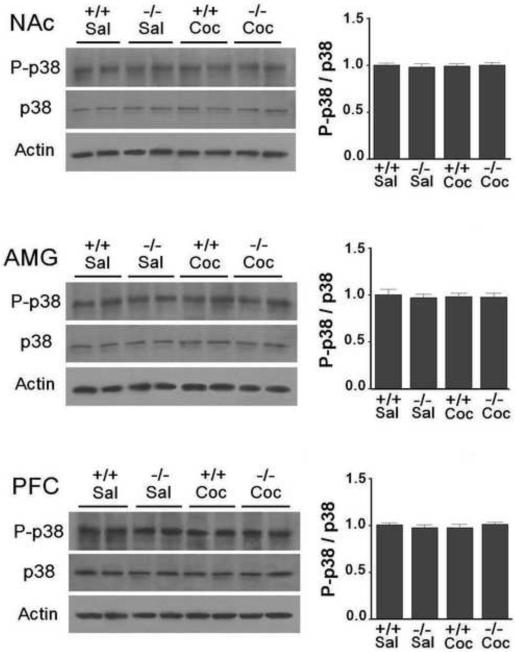
There were no significant changes in phospho-JNK (Fig. 3) and phospho-p38 (Fig. 4) levels in any of the three brain regions in D3 receptor mutant and wild-type mice following cocaine treatment compared to that following saline treatment (p>0.05). Moreover, levels of phospho-JNK and total JNK as well as phospho-p38 and total p38 were similar in each of the brain regions in wild-type and D3 receptor mutant mice (Figs 3 and 4).
Fig. 3.
JNK is not significantly activated in the NAc, AMG and PFC in D3 receptor mutant and wild-type mice following CPP expression. Western blotting for JNK was performed using brain samples from D3 receptor mutant (−/−) and wild-type (+/+) mice (n=7 mice each) after the last CPP expression test as before. The cocaine dose used for CPP induction was 2.5 mg/kg. Ratios of phospho-JNK (P-JNK) over total JNK protein levels in the NAc, AMG and PFC were analyzed. Data represent mean± SEM relative to saline (Sal) controls and were set as 1. Actin levels were used as a loading control.
Fig. 4.
p38 is not obviously activated in the NAc, AMG and PFC in D3 receptor mutant and wild-type mice following CPP expression. Western blotting for p38 was performed using brain samples from D3 receptor mutant (−/−) and wild-type (+/+) mice (n=7 mice each) after the last CPP expression test as before. The cocaine dose used for CPP induction was 2.5 mg/kg. Ratios of phospho-p38 (P-p38) over total p38 protein levels in the NAc, AMG and PFC were analyzed. Data represent mean± SEM relative to saline (Sal) controls and were set as 1. Actin levels were used as a loading control.
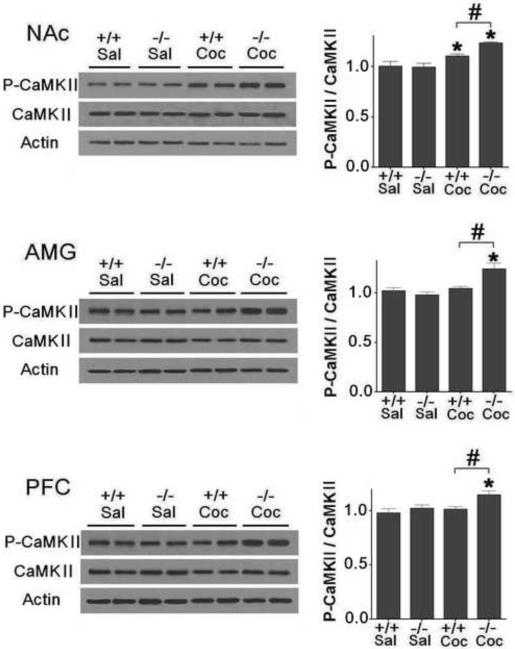
Heightened CaMKIIα activation in D3 receptor mutant mice following CPP expression
We next investigated whether there is a corresponding difference in CaMKIIα activation in the three brain regions between D3 receptor mutant and wild-type mice following CPP expression. We sacrificed D3 receptor mutant and wild-type mice (n=7 each) immediately after the fourth CPP expression test. Western blot experiments indicated that, compared to saline groups, CaMKIIα was activated in the NAc, AMG and PFC in D3 receptor mutant mice, and in the NAc in wild-type mice [Fig. 5, NAc: F (3,27)=13.346, p<0.05; AMG: F (3,27)= 9.669, p<0.05; PFC: F (3,27)=5.066, p<0.05], but not in the AMG and PFC in wild-type mice (p>0.05). Moreover, phospho-CaMKIIα levels are higher in the NAc, AMG and PFC in D3 receptor mutant mice than those in wild-type mice (Fig. 5, p<0.05). Levels of phospho-CaMKIIα and total CaMKIIα were similar in all brain regions in D3 receptor mutant and wild-type mice (Fig. 5).
Fig. 5.
Higher CaMKIIα activation in the NAc, AMG and PFC in D3 receptor mutant than wild-type mice following CPP expression. Mice were given cocaine (Coc, 2.5 mg/kg) or saline (Sal) and were sacrificed immediately after the last CPP test. Western blotting was performed using brain samples from D3 receptor mutant (−/−) and wild-type (+/+) mice (n=7 mice each). Ratios of phospho-CaMKIIα (P-CaMKIIα) relative to total CaMKIIα protein levels in the NAc, AMG and PFC were analyzed. Data were expressed as mean± SEM relative to saline controls that were set as 1. Actin levels were used as a loading control. *p<0.05 compared with the saline control mouse group of the same genotype. #p<0.05 compared between two genotypes.
DISCUSSION
We previously found that a D3 receptor mutation in mice did not obviously affect the acquisition of CPP induced by relatively high doses of cocaine (Chen and Xu, 2010). In the current study, we investigated how D3 receptors modulate CPP induction at low doses of cocaine and the associated changes in signaling events mediated by MAPK and CaMKIIα in several brain areas. We found that D3 receptor mutant mice exhibit potentiated CPP acquisition at low doses of cocaine compared to wild-type mice. Moreover, activation of ERK, but not JNK and p38, as well as CaMKIIα in brain reward circuits is also potentiated in D3 receptor mutant mice compared to that in wild-type mice after CPP expression. These data suggest a model in which D3 receptors contribute to reward-related learning induced by low doses of cocaine by inhibiting activation of ERK and CaMKIIα in the NAc, AMG and PFC.
Activation of D3 receptors inhibits cocaine-induced reward-related learning
DA is involved in reward-related learning (Schultz, 2002; Wise, 2008; Volkow et al., 2009). We previously found that D1 receptor mutant mice do not acquire CPP over a wide dose range of cocaine (2.5–20 mg/kg, Chen and Xu, 2010). This result is similar to those from pharmacological studies demonstrating that D1 receptor antagonists block cocaine-induced CPP (Tzschentke, 1998). This finding is also in agreement with those showing that D1 receptors are required for mice to learn to self administer cocaine (Caine et al., 2007). These results suggest an important role for D1 receptors in the acquisition of reward-related learning induced by cocaine. Interestingly, evidence suggests that D3 receptors play a role in cocaine-induced behavior that is opposite of D1 receptors. For example, D3 receptor mutant mice exhibit potentiated acute locomotor activation compared to wild-type mice at the 5 mg/kg dose of cocaine (Xu et al., 1997) whereas D1 receptor mutant mice fail to exhibit locomotor activation following cocaine injections over a wide dose range (Xu et al., 1994a; 1994b; 2000). D3 receptor mutant mice also exhibit opposite signaling patterns compared to those in D1 receptor mutant mice following cocaine administration (Zhang et al., 2004; Jiao et al., 2007). Whereas D1 receptor mutant mice fail to acquire CPP, D3 receptor mutant mice exhibit potentiated CPP induced by low but not high does of amphetamine or methamphetamine (Xu et al., 1997; Chen et al., 2007). At higher doses of cocaine (10 and 20 mg/kg), a D3 receptor mutation in mice does not obviously affect the acquisition of CPP (Chen and Xu, 2010). In the current study, we found that D3 receptor mutant mice exhibit potentiated CPP acquisition compared to wild-type mice at low doses of cocaine (1 and 2.5 mg/kg, Fig. 1). These findings suggest that, in contrast to D1 receptors, D3 receptors inhibit reward-related learning induced by low doses of cocaine.
D3 receptors inhibit ERK and CaMKIIα activation in brain reward circuits during acquisition of reward learning
The NAc, BLA and PFC are components of the circuitry that process learned associations between the rewarding properties of drugs and cues (Kalivas and McFarland, 2003; Di Ciano and Everitt, 2004; Weiss, 2005; Everitt and Robbins, 2005; Feltenstein and See 2008; Koob and Volkow, 2010). Conditioned cues can activate these brain regions (Neisewander et al., 2000; Ito et al, 2000; Volkow et al., 2004; 2005). In the context of CPP, the excitatory drive from the BLA to the NAc is enhanced during cocaine seeking (Miller and Marshall, 2005b). The NAc is involved in the acquisition, retrieval and reconsolidation of cocaine-paired contextual memory (Miller and Marshall, 2005a). Drug-related cues can increase DA levels (Volkow et al., 2008; Koob and Volkow, 2010). These results imply the importance of DA receptors and associated signaling mechanisms in these brain regions in establishing memories for drug-associated cues. We thus investigated signaling events in these brain regions following CPP expression.
ERK activation is oppositely regulated by D1 and D3 receptors following exposure to cocaine (Zhang et al., 2004; Chen and Xu, 2010). Inhibiting the ERK signaling pathway or an ERK mutation can attenuate cocaine-induced behavioral responses (Pierce et al., 1999; Valjent et al., 2000; 2006; Ferguson et al., 2006). The NAc, BLA and PFC express D3 receptors (Neve et al., 2004). Thus we studied D3 receptor-mediated ERK activation in these brain regions following CPP acquisition. ERK is activated in the NAc, AMG and PFC in D3 receptor mutant mice and in the NAc and PFC but not in AMG in wild-type mice following CPP acquisition (Fig. 2). These results imply that ERK activation in the NAc and PFC accompanies CPP expression induced by low doses of cocaine. Exposure to drug-associated cues increased ERK activation in the central AMG after 30 days, but not 1 day of withdrawal (Lu et al., 2005), implying that the rise in phospho-ERK levels in the AMG depends on length of withdrawal (Chen and Xu, 2010). It is possible that, in the absence of D3 receptor, the withdrawal time necessary for ERK activation in the AMG is shortened following CPP acquisition. No obvious changes in phospho-JNK and p38 levels were found in the NAc, AMG and PFC following CPP acquisition (Figs. 3 and 4). This finding is similar to those using higher doses of cocaine (Chen and Xu, 2010) or methamphetamine (Mizoguchi et al., 2004). These results suggest that activation of the ERK signaling pathway in the NAc, AMG and PFC, but not the JNK and p38 signaling pathways, via D3 receptors contributes to cocaine-induced reward learning as measured by the CPP paradigm.
Protein kinase A (PKA)-regulated CaMKIIα activation contributes to neuronal plasticity (Wayman et al., 2008). We previously found that D1 and D3 receptors can mediate and inhibit NMDA-induced CaMKIIα activation presumably by oppositely regulating PKA activity (Jiao et al., 2007). Consistent with this finding, we found potentiated CaMKIIα activation in the NAc, AMG and PFC in D3 receptor mutant mice compared to that in wild-type mice following CPP acquisition in the current study (Fig. 5). These results suggest that, in the absence of D3 receptors, there is potentiated CaMKIIα activation compared to that in wild-type mice, likely due to activation of NMDA receptors (Jiao et al., 2007; Wayman et al., 2008). Moreover, CaMKIIα activation in the NAc accompanies CPP expression induced by low doses of cocaine. Furthermore, requirement for CaMKIIα and ERK activation in different brain regions is not identical for CPP expression at low doses of cocaine.
A model for D3 receptor function in reward learning
D3 receptors have higher affinities for DA than other DA receptors, including D1 receptors (Sokoloff et al., 1992). It has been suggested that D2 family receptors like D3 receptors can respond to tonic DA release while D1 receptors are activated when there is phasic DA release (Goto et al., 2007; Grace et al., 2007). A large percentage of D3 receptor-bearing neurons co-express D1 receptors, especially in the NAc (Surmeier et al., 1996; Schwartz et al., 1998). D3 receptors are activated by basal levels of DA to inhibit adenylyl cyclase and related signaling including ERK and CaMKIIα. When DA release is enhanced and DA levels increase, CaMKIIα phosphorylates D3 receptors at the serine 229 site in the third intracellular loop (Liu et al., 2009). Consequently, the inhibitory tone of D3 receptors on cocaine-induced behavior and related signaling is apparently removed. This allows other DA receptors including D1 receptors to fully mediate behavioral activation and related signaling induced by cocaine. Our current results and those from previous studies support the above model in which D3 receptors contribute to reward-related learning induced by low doses of cocaine by regulating activation of ERK and CaMKIIα in specific areas of the brain.
Research Highlightsa.
D3 receptors inhibit reward-related learning induced by cocaine
D3 receptors also inhibit cocaine-induced activation of ERK and CaMKIIα in brain reward circuits
D3 receptors modulate reward learning by regulating specific signaling events
ACKNOWLEDGEMENTS
We thank Dr. H. Jiao for general help, and members of our laboratory for discussions, and Drs. D.A. Hall and Y. Yan for critically reading the manuscript. H.K. and W.K. were supported, in part, by the China Scholarship Council. M.X. was supported by grants from NIDA (DA17323 and DA025088).
ABBREVIATIONS
- (AMG)
amygdala
- (CaMKII)
Ca2+/calmodulin-dependent protein kinase II
- (CPP)
conditioned place preference
- (JNK)
c-Jun N-terminal kinase
- (DA)
dopamine
- (ERK)
extracellular signal-regulated kinase
- (MAPK)
mitogen-activated protein kinase
- (NMDA)
N-methyl-D-aspartate
- (NAc)
nucleus accumbens
- (PFC)
prefrontal cortex
- (PKA)
protein kinase A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Beninger RJ, Banasikowski TJ. Dopaminergic mechanism of reward-related incentive learning: focus on the dopamine D(3) receptor. Neurotox. Res. 2008;14:57–70. doi: 10.1007/BF03033575. [DOI] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, Tonegawa S, Zhang J, Xu M. Lack of cocaine self-administration in dopamine D1 receptor knockout mice. J. Neurosci. 2007;27:13140–13150. doi: 10.1523/JNEUROSCI.2284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta AR, Gerfen CR, Steiner H. Cocaine effects on gene regulation in the striatum and behavior: increased sensitivity in D3 dopamine receptor-deficient mice. Neuroreport. 2000;11:2395–2399. doi: 10.1097/00001756-200008030-00012. [DOI] [PubMed] [Google Scholar]
- Chen LP, Xu M. Dopamine D1 and D3 receptors are differentially involved in cue-elicited cocaine seeking. J. Neurochem. 2010;114:530–541. doi: 10.1111/j.1471-4159.2010.06775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PC, Lao CL, Chen JC. Dual alteration of limbic dopamine D1 receptor-mediated signaling and the Akt/GSK3 pathway in dopamine D3 receptor mutant during the development of methamphetamine sensitization. J. Neurochem. 2007;100:225–241. doi: 10.1111/j.1471-4159.2006.04203.x. [DOI] [PubMed] [Google Scholar]
- Dackis C, O'Brien C. Neurobiology of addiction: treatment and public policy ramifications. Nat. Neurosci. 2005;8:1431–1436. doi: 10.1038/nn1105-1431. [DOI] [PubMed] [Google Scholar]
- Di Ciano P. Drug seeking under a second-order schedule of reinforcement depends on dopamine D3 receptors in the basolateral amygdala. Behav. Neurosci. 2008;122:129–139. doi: 10.1037/0735-7044.122.1.129. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J. Neurosci. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1488. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br. J. Phar. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. Knockout of ERK1 enhances cocaine evoked immediate early gene expression and behavioral plasticity. Neuropsychopharm. 2006;31:2660–2668. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- Goto Y, Otani S, Grace AA. The yin and yang of dopamine release: a new perspective. Neuropharm. 2007;53:583–587. doi: 10.1016/j.neuropharm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR., Jr The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res. Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu. Rev. Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J. Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao H, Zhang L, Gao F, Lou D, Zhang J, Xu M. Dopamine D1 and D3 receptors oppositely regulate NMDA- and cocaine-induced MARK signaling via NMDA receptor phosphorylation. J. Neurochem. 2007;103:840–848. doi: 10.1111/j.1471-4159.2007.04840.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharm. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharm. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Karasinska JM, George SR, Cheng R, O'Dowd BF. Deletion of dopamine D1 and D3 receptors differentially affects spontaneous behaviour and cocaine-induced locomotor activity, reward and CREB phosphorylation. Eur. J. Neurosci. 2005;22:1741–1750. doi: 10.1111/j.1460-9568.2005.04353.x. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat. Rev. Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharm. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, Sokoloff P. The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharm. 2005;49:525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Liu X, Mao L, Zhang G, Papasian CJ, Fibuch EE, Lan H, Zhou HF, Xu M, Wang JQ. Activity-dependent modulation of limbic dopamine D3 receptors by CaMKII. Neuron. 2009;61:425–438. doi: 10.1016/j.neuron.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat. Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Martelle JL, Claytor R, Ross JT, Reboussin BA, Newman AH, Nader MA. Effects of two novel D3-selective compounds, NGB 2904 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide] and CJB 090 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide], on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J. Phar. Exp. Ther. 2007;321:573–582. doi: 10.1124/jpet.106.113571. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005a;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered Fos expression in neural pathways underlying cue-elicited drug seeking in the rat. E. J. Neurosci. 2005b;21:1385–1393. doi: 10.1111/j.1460-9568.2005.03974.x. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Mizuno M, Mizuno T, Nitta A, Noda Y, Nabeshima T. Regulations of methamphetamine reward by extracellular signal-regulated kinase 1/2/ets-like gene-1 signaling pathway via the activation of dopamine receptors. Mol. Pharmacol. 2004;65:1293–1301. doi: 10.1124/mol.65.5.1293. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J. Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Fuchs RA, Tran-Nguyen LT, Weber SM, Coffey GP, Joyce JN. Increases in dopamine D3 receptor binding in rats receiving a cocaine challenge at various time points after cocaine self-administration: implications for cocaine-seeking behavior. Neuropsychopharm. 2004;29:1479–1487. doi: 10.1038/sj.npp.1300456. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J. Recept. Signal Transduct. Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Peng XQ, Ashby CR, Jr, Spiller K, Li X, Li J, Thomasson N, Millan M, Mocaër E, Muńoz C, Gardner EL, Xi ZX. The preferential dopamine D3 receptor antagonist S33138 inhibits cocaine reward and cocaine-triggered relapse to drug-seeking behavior in rats. Neuropharm. 2009;56:752–760. doi: 10.1016/j.neuropharm.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Pierce-Bancroft AF, Prasad BM. Neurotrophin-3 contributes to the initiation of behavioral sensitization to cocaine by activating the Ras/Mitogen-activated protein kinase signal transduction cascade. J. Neurosci. 1999;19:8685–8695. doi: 10.1523/JNEUROSCI.19-19-08685.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz J-C, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Richtand NM. Behavioral sensitization, alternative splicing, and D3 dopamine receptor-mediated inhibitory function. Neuropsychopharm. 2006;31:2368–2375. doi: 10.1038/sj.npp.1301163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Diaz J, Bordet R, Griffon N, Perachon S, Pilon C, Ridray S, Sokoloff P. Functional implications of multiple dopamine receptor subtypes: the D1/D3 receptor coexistence. Brain Res. Brain Res. Rev. 1998;26:236–242. doi: 10.1016/s0165-0173(97)00046-5. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Martres MP, Giros B, Bouthenet ML, Schwartz JC. The third dopamine receptor (D3) as a novel target for antipsychotics. Biochem. Pharmacol. 1992;43:659–666. doi: 10.1016/0006-2952(92)90227-a. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J. Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog. Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J. Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corbillé AG, Bertran-Gonzalez J, Hervé D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc. Natl. Acad. Sci. USA. 2006;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharm. 2004;47:3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J. Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine's role in drug abuse and addiction. Neuropharm. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Lee YS, Tokumitsu H, Silva AJ, Soderling TR. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59:914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr. Opin. Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox. Res. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, Gitajn L, Gardner EL. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine's rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharm. 2006;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- Xu M, Moratalla R, Gold LH, Hiro N, Koob GF, Graybiel AM, Tonegawa S. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell. 1994a;79:729–742. doi: 10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]
- Xu M, Hu XT, Cooper DC, Moratalla R, Graybiel AM, White FJ, Tonegawa S. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell. 1994b;79:945–955. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT, White NM, Graybiel AM, White FJ, Tonegawa S. Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron. 1997;19:837–848. doi: 10.1016/s0896-6273(00)80965-4. [DOI] [PubMed] [Google Scholar]
- Xu M, Guo Y, Vorhees CV, Zhang J. Behavioral responses to cocaine and amphetamine in D1 dopamine receptor mutant mice. Brain Res. 2000;852:198–207. doi: 10.1016/s0006-8993(99)02258-1. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Zhang J, Xu M. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J. Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang L, Jiao H, Zhang Q, Zhang D, Lou D, Katz JL, Xu M. c-fos Facilitates acquisition and extinction of cocaine-induced persistent change. J. Neurosci. 2006;26:13287–13296. doi: 10.1523/JNEUROSCI.3795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]