Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) causes various signs of toxicity in early life stages of vertebrates through activation of the aryl hydrocarbon receptor (AHR). The AHR also plays important roles in normal development in mice, and AHR-/- mice show abnormal development of vascular structures in various blood vessels. Our previous studies revealed that Ahr type 2 (Ahr2) activation by TCDD and β-naphthoflavone (BNF) caused a significant decrease in blood flow in the dorsal midbrain of zebrafish embryos. Here we report effects of TCDD exposure on the morphology of some blood vessels in the head of developing zebrafish. TCDD caused concentration-dependent anatomical rearrangements in the shape of the prosencephalic artery in zebrafish larvae. In contrast, no major vascular defects were recognized in the trunk and tail regions following exposure to TCDD at least at the concentrations used. Essentially, the same observations were also confirmed in BNF-exposed larvae. Knock-down of either Ahr2 or Ahr nuclear translocator type 1 (Arnt1) by morpholino oligonucleotides (MOs) protected larvae against abnormal shape of the prosencephalic artery caused by TCDD and BNF. On the other hand, knock-down of Ahr2 or Arnt1 in vehicle-exposed zebrafish larvae had no clear effect on morphology of the prosencephalic artery or trunk vessels. Ascorbic acid, an antioxidant, protected against the TCDD-induced decrease in blood flow through the prosencephalic artery, but not the abnormal morphological changes in the shape of this artery. These results indicate that activation of Ahr2/Arnt1 pathway by TCDD and BNF affects the shape of certain blood vessels in the brain of developing zebrafish.
Keywords: TCDD, β-naphthoflavone, circulation failure, brain blood vessel, vascular development, zebrafish
1. Introduction
Fish embryos are among the most sensitive organisms to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) toxicity. Exposure of fish larvae to TCDD causes cardiovascular toxicities such as edema and circulation failure, craniofacial malformation, and growth retardation resulting in mortality (Walker and Peterson, 1994; Henry et al., 1997; Teraoka et al., 2002). Among these, the cardiovascular system is one of the most characteristic and important targets in developmental toxicity caused by TCDD and polycyclic aromatic hydrocarbons in various fish larvae (Walker and Peterson, 1994; Guiney et al., 1997; Cantrell et al., 1996; 1998; Wassenberg and Di Giulio, 2004). In zebrafish (Danio rerio), a model fish for environmental toxicology (Teraoka et al., 2003a; Hill et al., 2005), TCDD exposure disrupts heart development with a reduction in the number of cardiac myocytes and a reduction in cardiac output culminating in heart failure (Antkiewicz et al., 2005; 2006; Carney et al., 2006). The cardiovascular system is a common target of TCDD also in other vertebrates, including rodents and chick, which show edema, hemorrhage and heart malformation upon exposure (Cheung et al., 1981; Ishimura et al., 2009).
It is well established that TCDD binds the AHR, a ligand-activated basic-helix-loop-helix transcription factor, and the complex dimerizes with aryl hydrocarbon receptor nuclear translocator (ARNT) to induce the expression of a battery of genes (the AHR gene battery), including cytochrome P4501A (CYP1A) (Nebert et al., 2000). Studies in AHR knock-out mice (AHR-/- mice) have established that TCDD causes various developmental toxicities by way of AHR activation (Fernandez-Salguero et al., 1996; Mimura et al., 1997). Whereas mammalian species have a single AHR, there are multiple Ahr isoforms in teleosts, including zebrafish, which has Ahr1a, Ahr1b and Ahr2 (Hahn et al., 1997; Tanguay et al., 1999; 2000; Karchner et al., 2005). Knock-down studies with morpholino antisense oligonucleotides (MOs) indicate that pericardial edema as well as other endpoints of TCDD toxicity such as reduced peripheral blood flow are mediated by Ahr2 (Prasch et al., 2003; Teraoka et al., 2003b) and Arnt1 in zebrafish (Antkiewicz et al., 2006).
In addition to adaptive responses to environmental xenobiotics, AHR plays important roles in normal development in mice, and AHR-/- mice show abnormal development of some vascular structures. AHR-/- mice exhibit portocaval shunting of blood within the liver parenchyma (Lahvis et al., 2000; Walisser et al., 2005). In adult AHR-/- mice, more than half of the portal venous blood that flows to the liver bypasses the liver sinusoids (Lahvis et al., 2000), suggesting a role for AHR in vasculogenesis in rodents.
Previously, we reported that TCDD causes a transient decrease in blood flow in the mesencephalic vein in the head of the zebrafish embryo at about 50 hpf. This transient ischemic event precedes the occurrence of pericardial edema, and is a possible cause of TCDD-induced apoptosis in the dorsal midbrain (Dong et al., 2001; 2002). Using an Ahr2-MO knock-down approach, Dong et al. (2004) suggested that Ahr2 was involved in the mesencephalic vein transient ischemia response. Subsequently it was shown that this TCDD-induced response is also sensitive to antioxidants, inhibitors of CYP, inhibitors of cyclooxygenase 2 (COX2) and thromboxane receptor (TP) antagonists, suggesting the involvement of oxidative stress, CYP and the prostaglandin pathway (Dong et al., 2002; Teraoka et al., 2009).
During our investigation of mesencephalic circulation in zebrafish embryos, we discovered that some blood vessels in the head of the embryos showed a striking morphological variation when exposed to TCDD. In the present study we report that altered shape of certain brain blood vessels is caused by TCDD and BNF exposure in the zebrafish embryo, and this effect requires activation of Ahr2/Arnt1 signaling.
2. Materials and methods
2.1. Chemicals
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) was obtained from Cambridge Isotope Laboratories (98% purity: Andover, MA); β-naphthoflavone (BNF) was purchased from Sigma (St. Louis, MO). Other chemicals were obtained from Kanto Chemical (Japan).
2.2. Zebrafish and TCDD treatment
Fertilized eggs were obtained from natural mating of adult zebrafish (long fin) in our laboratory according to the Zebrafish Book (Westerfield, 1993). Adult fish and developing fish were maintained at 28.5 °C with a lighting schedule of 14 hr light and 10 hr dark. Eggs were collected within 1 hr of spawning, rinsed, and placed into a clean polystyrene Petri dish (Asahi Techno, Japan). At 24 hr after spawning, fertilized embryos were exposed to either the TCDD vehicle, dimethyl sulfoxide (DMSO, 0.1%) or an apparent concentration of waterborne TCDD of 0.3 to 2.0 parts per billion (ppb) dissolved in 0.1% DMSO in 3 ml of Zebrafish Ringer solution (38.7 mM NaCl, 1.0 mM KCl, 1.7 mM HEPES-NaOH pH 7.2, 2.4 mM CaCl2) in 3 cm polystyrene Petri dishes until 48 hpf (hr post fertilization; n = 10 embryos/dish). After exposure, developing fish were maintained in Zebrafish Ringer solution until evaluation of vascular structure at 55 hpf. When the effect of antioxidant was investigated in some experiments, 10 mM ascorbic acid was included in the Zebrafish Ringer solution together with TCDD.
2.3. Knock-down with morpholino antisense oligonucleotides
Morpholino antisense oligonucleotides (MOs) against translation of Ahr2 (Ahr2-MO), and their respective negative controls having 4 different nucleotides (4mis-Ahr2-MO), were synthesized by Gene Tools (Philomath, OR), as described previously (Teraoka et al., 2003b). Translational inhibition type of MO against Arnt1 (Arnt1-MO) was used as previously reported (Prasch et al., 2006). Each morpholino was injected into the yolk of embryos at one to four cell stages with a fine glass needle connected to an automatic injector (IM-300: Narishige, Japan). 2 nL of 50 μM MOs in Ca2+-free Zebrafish Ringer solution were injected.
2.4. Evaluation of vascular morphology and blood flow
Using inverted microscopy (IX71, Olympus, Japan), blood flow in vessels in the head and other regions of zebrafish larvae were examined at 55 hpf by monitoring red blood cells passing through them. Some larvae were subjected to microangiography to visualize blood vessels under fluorescent microscopy (IX71, Olympus), just after injection of fluorescent polystyrene microspheres (FluoSpheres, Invitrogen, Carlsbad, CA) into the sinus venosus (Isogai et al., 2001). Blood flow in the prosencephalic artery was evaluated by time-lapse recording using a high-speed digital video camera (LRH1601BL, Digimo, Osaka, Japan), as originally described for measuring blood flow in the mesencephalic vein (Teraoka et al., 2002). Larvae were suspended in 200 μL of 3% methyl cellulose/Zebrafish Ringer solution in a hand-made plastic bath mounted on the stage of an inverted microscope (IMT-2: Olympus, Japan). Temperature of the suspension solution was maintained at 28.5°C with a PDMI-2 Micro-Incubator (Harvard Apparatus, Holliston, MA).
2.5. Statistics
Results are presented as mean ± SEM. Significance of mean differences between groups was determined by Tukey-Kramer's test (p < 0.05).
3. Results
3.1. Abnormal brain blood vessel morphology induced by TCDD exposure
We observed striking TCDD-induced alterations in the prosencephalic artery. At 55 hpf, two types of prosencephalic arteries were observed in control larvae; i.e., one with a typical arch (Typical; Fig. 1A, B) and the other with a meandering arch (Meandering; Fig. 1C, D). Prominent abnormal shapes of the prosencephalic artery found in TCDD-exposed larvae included a vessel with a split arch with both parts ending at the anterior cerebral vein (Split arch; Fig. 1E, F) and a vessel with a new branch ending at an unspecified blood vessel other than the anterior cerebral vein (New vessel; Fig. 1G, H). In addition, prosencephalic arteries with a small arch (Small arch) or that end at the prosencephalic artery on the opposite side of the head (Opposite end), were also observed in the TCDD-exposed group (Fig. 2).
Fig. 1.
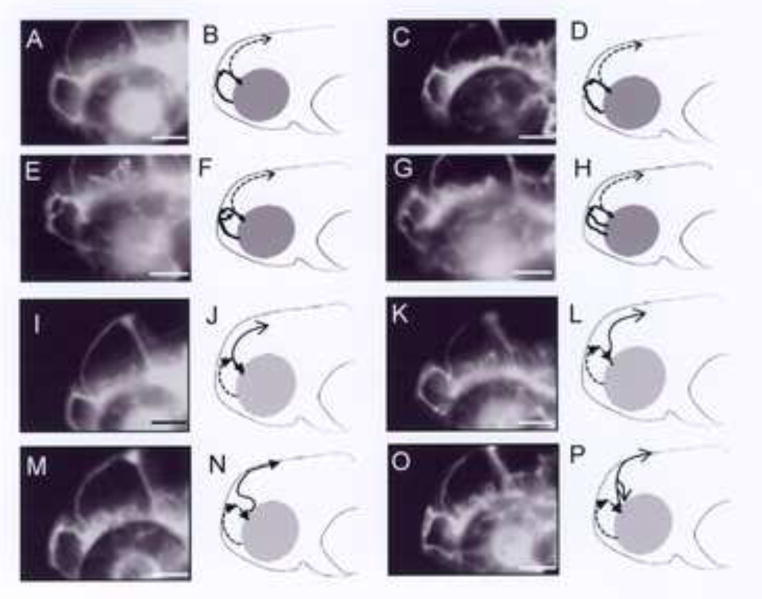
Malformation of blood vessel induced by TCDD in the brain of developing zebrafish. Embryos were exposed to vehicle or 1 ppb TCDD from 24 to 48 hpf Blood vessels, focusing on prosencephalic artery (A-H, bold line) and mesencephalic vein (I-P, bold line), were visualized by fluorescent beads injected into the sinus venosus at 55 hpf. Photographs and their schemes are presented as a set for each type of brain blood vessel. Dashed lines in panels B, D, F, H and in panels J, L, N, P indicate mesencephalic vein and prosencephalic artery, respectively. Panels A, B and I, J are control (vehicle) and the others are TCDD-treated larvae. A, B: Typical, C, D: Meandering, E, F: Split, G, H: New vessel for prosencephalic artery. I, J: Typical, K, L: Curved, M, N: Sigmoid, O, P: New vessel for mesencephalic vein. Detailed explanation is given in the text. Bars = 250 μm.
Fig. 2.
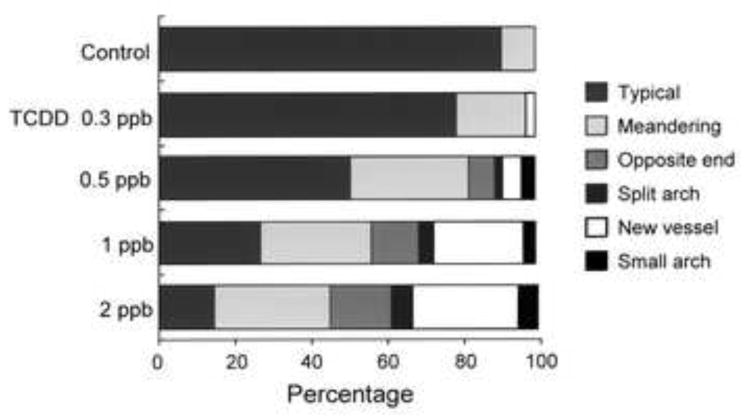
Percent incidence of different types of abnormalities in the prosencephalic artery caused by exposure to graded concentrations of TCDD. Embryos were exposed to vehicle or graded concentrations of TCDD (0.3, 0.5, 1 and 2 ppb) from 24 to 48 hpf. At 55 hpf, abnormally shaped prosencephalic arteries could be divided into 4 groups: vessels with split arch (Split arch), branch of new vessel (New vessel) as indicated in Fig. 1 and vessels, which ended at prosencephalic artery at other side (Opposite end) and vessels with small arch (Small arch). Meandering prosencephalic arteries as well as typical ones (Typical) were also observed in control larvae. Eighty one to one hundred and ninety three larvae were assessed at each concentration of TCDD.
At 55 hpf, control zebrafish had developed a fully arched mesencephalic vein (Fig. 1I, J), while some control zebrafish exhibited a mesencephalic vein with a curved arch (Curved; Fig. 1K, L). Variant shapes of mesencephalic veins were observed in zebrafish exposed to TCDD (1 ppb), including a vein with a sigmoid arch (Sigmoid) as compared to the curved arch in a normal mesencephalic vein (Fig. 1M, N), and a vein with a new branch ending at a different region (New vessel; Fig. 1O, P). Mesencephalic veins with these latter shapes were never seen in control larvae and are regarded as abnormal. The morphology of other vessels in the head was more complex and could not be rigorously assessed for effects of TCDD.
In contrast to vasculature in the head region, we did not detect any clear alterations in the arteries and veins in the trunk and tail region, including dorsal aorta, caudal artery and vein, intersegmental artery and vein and posterior cardinal vein, at least upon the exposure condition of TCDD used in this study. In a few cases, however, a relatively smaller arch in the posterior cardinal vein and some alterations, such as bifurcation with the same ending were observed in intersegmental vessels.
3.2. Concentration-dependent effects of TCDD on prosencephalic artery shape
In our initial experiments, the incidence of abnormal morphological changes caused by TCDD in the mesencephalic vein was relatively low and there was no clear TCDD concentration dependency. Thus we focused on the effects of exposure to graded concentrations of TCDD on the morphological changes observed in the prosencephalic artery.
Fig. 2 shows the percent incidence of each type of prosencephalic artery morphology observed in control and TCDD-treated larvae at 55 hpf (n = 81-193 for each concentration of TCDD). The incidence of each type of artery abnormality was increased by TCDD exposure in a concentration-dependent manner. Generally, there was no preferential increase among the four types of abnormal artery formed. On the other hand, Meandering prosencephalic artery, one of the normal types observed in control larvae, was increased in frequency, while there was a gradual decrease of frequency of the Typical artery shape caused by TCDD.
Typical and Meandering were regarded as normal, and Opposite end, Split arch, New vessel and Small arch caused by TCDD were counted as abnormal in Figs. 2 and 3. In assessing the occurrence of these abnormal prosencephalic arteries, we used 18-22 larvae for each treatment group to determine the percentage of malformation (Fig. 3). We repeated the experiment five to twenty one times, and the mean and SEM were calculated for statistical analysis. As shown in Fig. 3, TCDD at doses from 0.3 to 2 ppb resulted in a distinct concentration-dependent increase in abnormal prosencephalic artery shape in zebrafish larvae at 55 hpf.
Fig. 3.
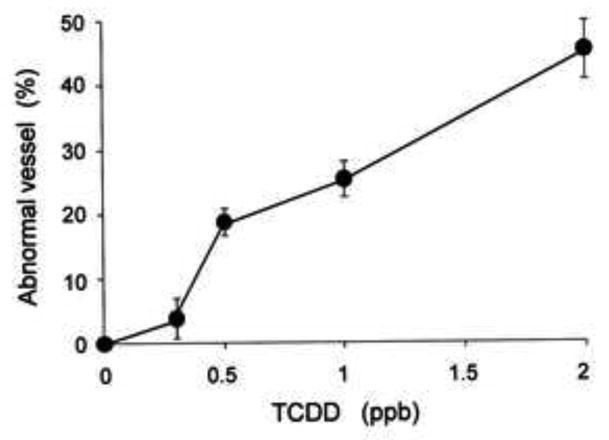
Concentration-dependent formation of abnormally shaped prosencephalic artery induced by TCDD in the zebrafish larva. Prosencephalic arteries with split arch, small arch, branch of new vessel and the end at the opposite side were defined as abnormal vessels (Abnormal vessel), since those were never observed in vehicle-treated control larvae. Abnormal vessels were counted in 18-22 larvae exposed to graded concentrations of TCDD (0.3, 0.5, 1 and 2 ppb) for each group. Average of the five to twenty one groups was presented ± SEM (n = 21 for control, n = 5 for 0.3 and 2 ppb TCDD, n = 12 for 0.5 ppb TCDD, n = 16 for 1 ppb TCDD).
3.3. Effects of BNF on brain blood vessel morphology
The effects of β-naphthoflavone (BNF), a non-halogenated AHR agonist, on vascular formation were studied, to determine whether another type of AHR agonist might elicit effects similar to TCDD. As shown in Table 1, BNF (0.1-1.0 μM) caused the same types of malformations in the prosencephalic artery that were caused by TCDD. Similar to TCDD, BNF caused concentration-dependent increases in abnormally shaped prosencephalic arteries on the whole (Table 2). BNF also induced abnormalities in the mesencephalic vein, producing new vessel and sigmoid arch deformities, in addition to absence of blood flow through the vein. Alterations in the morphology of trunk and tail blood vessels were not observed in BNF-exposed larvae. Thus, BNF caused qualitatively similar malformation responses to TCDD in the mesencephalic vein and prosencephalic artery.
Table 1.
Incidence of morphological changes in the prosencephalic artery in developing zebrafish exposed to graded concentrations of β-naphthoflavone. Embryos were exposed to various concentrations of β-naphthoflavone (BNF) or vehicle from 24 to 48 hpf Prosencephalic arteries in control and BNF-treated larvae were classified into 6 types at 55 hpf. These are typical and meandering arteries also observed in vehicle control larvae (Typical, Meandering). The rest are abnormally shaped arteries never observed in vehicle control larvae, including arteries with the opposite end (Opposite end), arteries with split arch (Split arch), arteries with newly formed vessel (New vessel) and arteries with small arch (Small arch). The percent incidence of these types was calculated for 48-95 larvae per treatment, as indicated by numericals in parenthesis.
| Classification of observed arteries | ||||||||
|---|---|---|---|---|---|---|---|---|
| Typical | Meandering | Opposite end | Split arch | New vessel | Small arch | |||
| Control | (48) | 97.9% | 2.1% | 0.0% | 0.0% | 0.0% | 0.0% | |
| β-naphthoflavone | 0.1 μM | (74) | 82.4% | 8.1% | 2.7% | 2.7% | 2.7% | 1.4% |
| 0.3 μM | (95) | 66.3% | 17.9% | 3.2% | 6.3% | 5.3% | 1.1% | |
| 0.5 μM | (94) | 60.6% | 17.0% | 6.4% | 3.2% | 12.8% | 0.0% | |
| 1.0 μM | (93) | 54.8% | 16.1% | 9.7% | 40.8% | 8.6% | 0.0% | |
Table 2.
Abnormal prosencephalic arteries in developing zebrafish exposed to graded concentrations of β-naphthoflavone. Embryos were exposed to various concentrations of β-naphthoflavone (BNF) or vehicle to evaluate the shape of the prosencephalic artery at 55 hpf Arteries with opposite end (Opposite end), split arch (Split arch), new vessel formation (New vessel) and small arch (Small arch) were counted as abnormal, as these were never recognized in vehicle control larvae (Fig. 1). Ten to twenty larvae were used for each group and the experiment was repeated 5 - 11 times (n = 5 or11). Results are expressed as mean ± SEM.
| Concentration of β-naphthoflavone | |||||
|---|---|---|---|---|---|
| Control | 0.1 μM | 0.3 μM | 0.5 μM | 1 μM | |
| Abnormal vessel | 0.0± 0.0 % (6) | 6.8±4.3% (5) | 12.6±2.2%(6) | 27.9±3.7 % (11) | 19.4±4.5 % (6) |
3.4. Effects of morpholino knock-down of Ahr2 and Arnt1
Knock-down of Ahr2 translation with antisense morpholino oligonucleotides was carried out to address the possibility that Ahr2 may have a role in normal formation of blood vessels in the brain, as well as in producing abnormalities in shape of the brain vasculature induced by TCDD and BNF. First, the effect of Ahr2 knock-down (Ahr2-KD) in vehicle-exposed larvae was studied. We examined 203 control larvae injected with Ahr2-MO at a concentration that strongly blocked the circulation failure in TCDD-exposed larvae (Prasch et al., 2003; Dong et al., 2004). Effectiveness of Ahr2-MO was also confirmed by rescue of pericardial edema and retardation of jaw growth caused by TCDD, as well as blocking TCDD induction of Cyp1a gene, in this study (data not shown). Alterations were rarely observed in these vehicle-exposed larvae in morphology of the prosencephalic artery, mesencephalic vein or other vessels in the brain, trunk and tail. A few changes in mesencephalic veins and prosencephalic arteries were seen in Ahr2-KD larvae (Ahr2 morphants), i.e., there were 3 larvae with a loss of blood flow in the mesencephalic vein and 2 larvae having either a new vessel or a split arch in the prosencephalic artery. A similar very low incidence of malformation was also observed in larvae injected with the negative Ahr2-MO homologs (4mis-Ahr2-MO, 4mis-MO) in a similar ratio (1/178 and 2/178 for mesencephalic vein and prosencephalic artery, respectively), suggesting a non-specific effect of these MO molecules, rather than an effect due to knock-down of Ahr2.
Fig. 4 indicates the effects of Ahr2-KD on altered shape of the prosencephalic artery by TCDD. Ahr2-KD markedly inhibited the abnormal shapes of this artery that were caused by both concentrations of TCDD (0.5 and 1 ppb). On the other hand, treatment with the 4mis-Ahr2-MO did not alter the TCDD response significantly. Similarly, the BNF-induced formation of abnormalities in the prosencephalic artery was effectively blocked by Ahr2-KD, but not by the negative control MO homologs (Table 3). Ahr2-KD was also effective in completely blocking all shape malformations caused by TCDD in the mesencephalic vein (1 ppb TCDD 13.9 ± 4.6%, Ahr2-KD + 1 ppb TCDD 0%).
Fig. 4.

Involvement of Ahr2 in TCDD-induced abnormal vessel formation in prosencephalic artery. After injection of either a morpholino antisense oligonucleotide against Ahr2 (Ahr2-MO) or its negative homolog (4mis-Ahr2-MO or 4mis-MO), embryos were exposed to vehicle (Control) and 0.5 ppb or 1 ppb TCDD from 24-48 hpf. At 55 hpf, the number of abnormal vessels was determined in 17-24 larvae as a group for each treatment. Average of the five groups was presented ± SEM (n = 5). * P < 0.05, compared to respective controls (TCDD 0.5 ppb and 1 ppb).
Table 3.
Effects of gene knock-down of Ahr2 and Arnt1 on percent incidence of abnormal prosencephalic artery shape caused by TCDD and BNF. Embryos were injected with morpholino antisense oligos against aryl hydrocarbon receptor 2 (Ahr2), the negative homologue (4mis-Ahr2-MO) and Arnt1 (Arnt1-MO) at one to four cell stages. After these treatments, the embryos were exposed to 1 ppb TCDD (TCDD), 0.5 μM β-naphthoflavone (BNF) or vehicle (DMSO: Control), from 24-48 hpf. At 55 hpf, the number of abnormal vessels was determined in 17-30 larvae as a group for each treatment to express as percentage. Mean of 4-7 groups is presented ± SEM (n = 4-7).
| Control | TCDD | Control | BNF | |
|---|---|---|---|---|
| No treatment | 0.0 ± 0.0% (5) | 23.3 ± 6.3% (5) | 0.0 ± 0.0% (7) | 24.9± 5.2% (7) |
| 4mis-Ahr2-MO | 0.0 ± 0.0% (5) | 33.7 ± 7.5% (5) | 1.4 ± 1.5% (6) | 27.5 ± 3.8% (6) |
| Ahr2-MO | 0.0 ± 0.0% (5) | 1.1 ± 1.2% (5)a | 3.1 ± 1.6% (7) | 2.9 ± 2.0% (7)a |
| No treatment | 0.0 ± 0.0% (4) | 37.2 ± 5.0% (4) | 0.0 ± 0.0% (4) | 33.3 ± 4.9% (4) |
| Arnt1-MO | 3.7 ± 4.1% (4) | 11.4 ± 2.9% (4)a | 1.3 ± 1.4% (4) | 11.5 ± 4.7% (4)a |
(p < 0.05)
As shown in Table 3, morpholino knock-down of Arnt1 expression (Arnt1-KD) significantly inhibited the prosencephalic artery malformations induced by both TCDD (1 ppb) and BNF (0.5 μM). Prosencephalic artery morphology was rarely affected by Arnt1-KD alone, although the incidence of the abnormal prosencephalic artery in the Arnt1-KD morphants (8/159) was slightly higher than that seen in the Ahr2-KD morphants described above.
3.5. Effects of antioxidants on TCDD-induced increases in prosencephalic artery malformations and decreases in blood flow
Previously we reported that some antioxidants protected against TCDD-induced decreases in blood flow through the mesencephalic vein of developing zebrafish (Dong et al., 2002). In the present study we investigated the possible involvement of oxidative stress in the TCDD-evoked abnormal prosencephalic artery formation. As shown in Fig. 5A, TCDD (1 ppb) decreased blood flow through the prosencephalic artery at 55 hpf well before erythropoiesis is reduced (Belair et al., 2001). Exposure to ascorbic acid (10 mM) protected against this effect of TCDD by restoring blood flow to almost the control level. On the other hand, the same application of ascorbic acid did not affect the abnormal shapes of the prosencephalic artery caused by TCDD (Fig. 5B).
Fig. 5.
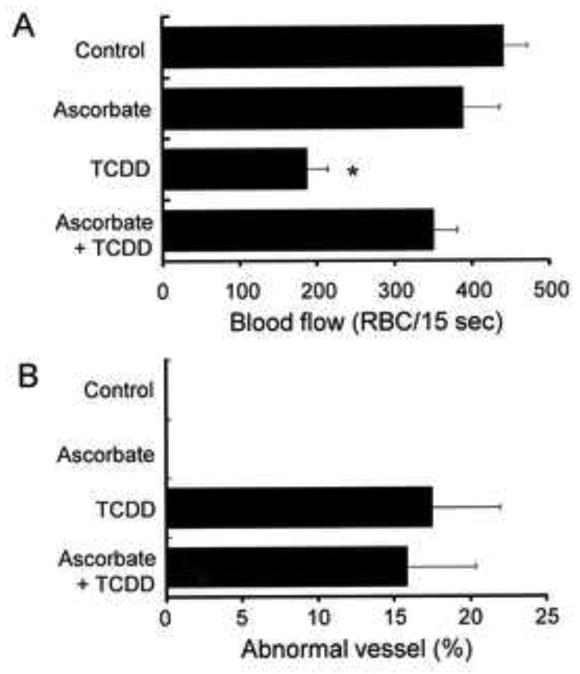
Differential effect of antioxidant treatment on the reduced blood flow and abnormal vessel formation in the prosencephalic artery of TCDD exposed zebrafish. Embryos were exposed to vehicle, ascorbate (10 mM), TCDD (1 ppb) or their combination (Ascorbate + TCDD) from 24-48 hpf. At 55 hpf, the number of red blood cells passing through the prosencephalic artery per 15 sec (RBC/15 sec) was determined as an index of blood flow (A), in addition to the percent incidence of abnormal vessel formation in prosencephalic artery (B). A: Fifteen to nineteen larvae were evaluated for each treatment. B: Seventeen to twenty one larvae were observed for each group and 4 groups were used for each treatment (n = 4). Results are expressed as mean ± SEM. * P < 0.05, compared to control.
4. Discussion
TCDD and BNF exposure affected the shape of two blood vessels in the head of developing zebrafish, the mesencephalic vein and the prosencephalic artery. Vascular effects of TCDD have been seen in other blood vessels of fish. For example, delayed regression of the common cardinal vein was reported during early development in zebrafish (Bello et al., 2004) and red seabream (Yamauchi et al., 2006). However, a TCDD-induced change in the shape of a vein and artery in the zebrafish larval brain, in some cases including neovascularization, is a new endpoint of TCDD developmental toxicity involving the vasculature. This effect of TCDD exposure was essentially absent in Ahr2 morphants and greatly reduced in Arnt1 morphants, suggesting that hyperactivation of Ahr2/Arnt1 signaling is involved, similar to other TCDD-induced responses in zebrafish (Antkiewicz et al., 2006). Our finding that TCDD caused neovascularization (new branches) as one type of malformation in the mesencephalic vein and prosencephalic artery in zebrafish larvae is particularly significant. It has also been reported that TCDD treatment exacerbates photocoagulation-induced choroidal neovascularization in mice (Takeuchi et al., 2009), induces remodeling of the placental vascular network in rats (Ishimura et al., 2009) and causes aortic arch anomalies in the chick embryo (Cheung et al., 1981). Thus, hyperactivation of AHR/ARNT signaling by TCDD has modulatory effects on vascularization in a variety of vertebrates.
A role for AHR in normal mammalian vascular formation has been inferred from studies using AHR-/- mice. In our study of vehicle-exposed zebrafish, however, Ahr2-KD alone had no major impact on development of the vasculature throughout the body. However, knockdown of Ahr2 did effectively block both the TCDD-induced decrease in blood flow (Dong et al., 2002) and increase in malformations in the prosencephalic artery, as well as the mesencephalic vein presented here. Since we used a transient knock-down technique with an Ahr2-MO, the small amount of Ahr2 protein remaining might still be sufficient for normal vascular formation to occur. On the other hand, Meandering prosencephalic artery, which was increased by TCDD in an Ahr2-dependent manner, was also observed in vehicle control larvae. Thus, further study, with zebrafish in which Ahr2 protein is completely absent, will be required to determine if Ahr2 has any role in normal vasculogenesis in zebrafish, like AHR has in mice (Lahvis et al., 2000). Zebrafish have two Ahr1 genes, ahr1a and ahr1b (Karchner et al., 2005) in addition to ahr2. The normal physiological functions of these three Ahrs are largely unknown, at least at the in vivo level, compared to in vitro experiments (Karchner et al., 2005). Ahr1a might be involved in Cyp1a induction by some polycyclic aromatic hydrocarbons in zebrafish larvae (Incardona et al., 2006). However, at all stages of vascular development it is possible that one or more of the Ahrs in zebrafish could be involved. Continued research is needed (1) to identify those processes in vasculogenesis and vascular remodeling that are dependent on particular forms of the Ahr in zebrafish, and (2) to determine to what extent AHR-mediated effects on vascular development in zebrafish and mice are similar. Ahr2, Ahr1a and Ahr1b knock-out zebrafish, prepared by the TILLING (Targeting Induced Local Lesions In Genomes) method (Wienholds et al., 2003), would be helpful in addressing these needs.
We have shown that malformation of the vasculature caused by Ahr2 activation is clearly present in certain blood vessels in the brain. Whether TCDD effects on malformation of blood vessels in zebrafish larvae are restricted to the head will need to be rigorously assessed, given the complexity of the vascular system throughout the body. In addition, it is recognized that although changes in vasculature shape were observed only in the prosencephalic artery and mesencephalic vein, other vessels in the head and brain might also be affected. Identifying additional malformed vessels was beyond the scope of this study. In general, mechanisms of blood vessel formation have been extensively studied almost exclusively in the trunk (Baldessari and Mione, 2008), but seldom in the head and we found no malformed blood vessels in the trunk. There are potentially two explanations for the lack of prominent defects in trunk vessels. First, the major vessels in the trunk region are set up before TCDD exposure began at 24 hpf, so it may be that a critical window for affecting development of the trunk vessels had past (Isogai et al., 2001). Alternatively, because cranial vessel patterning is intertwined with neural development, there may be other mechanisms involved in the development of certain brain blood vessels that is not present in the trunk, such as the interaction between vessels and neural tissue. This is not surprising, since it is generally accepted that molecular, structural and functional specializations are different among various vascular beds. For example, the zebrafish bubblehead (bbh) mutant exhibits hydrocephalus and severe cranial hemorrhage during early embryogenesis, whereas blood vessels in other regions of the embryo appear intact (Liu et al., 2007). Understanding differences in the response of zebrafish larval blood vessels to TCDD is an important first step in identifying the genes and signaling pathways that are being disrupted by hyperactivation of Ahr2/Arnt1 signaling leading to these blood vessel-specific malformations.
The downstream mechanism by which TCDD and BNF elicit these effects is unclear. The production of oxidative stress by TCDD has been extensively studied in various systems (Reichard et al., 2006; Goldstone and Stegeman, 2006). In developing zebrafish and medaka, antioxidants blocked edema and apoptosis in blood vessels caused by TCDD or 3,3′,4,4′,5-pentachlorobiphenyl exposure (Cantrell et al., 1996; Na et al., 2009). Previously, we reported that mesencephalic vein circulation failure caused by TCDD in zebrafish larvae could be inhibited by the antioxidants ascorbic acid and N-acetylcysteine (Dong et al., 2002). In the present study we confirmed that blood flow through the prosencephalic artery also was reduced by TCDD, and that this inhibitory effect of dioxin was protected against by ascorbic acid treatment. In contrast, ascorbic acid was without effect on TCDD-evoked deformities of the prosencephalic artery. Hemodynamic changes play an important role in blood vessel formation and changes in blood flow can lead to severe vascular distortion (Yashiro et al., 2007). The ascorbic acid protection against the TCDD-induced decrease in blood flow in these particular blood vessels but not the various types of malformations that TCDD caused in the same blood vessels, suggests it is unlikely that TCDD-induced circulation failure is the cause of the cephalic vessel anomalies in TCDD-exposed larvae in the present study.
Hypoxia-inducible factor 1α (HIF1α) is important in vasculogenesis and competition of AHR and HIF1α for ARNT might be a mechanism by which TCDD exposure could affect blood vessel formation (Ema et al., 1997). However, some of our results show that TCDD induced new vessel formation rather than regression. This cannot be explained by AHR and HIF1α competing for ARNT. Furthermore, TCDD-induced vascular malformations in the prosencephalic artery were greatly reduced in Arnt1 morphants, similar to other endpoints of TCDD toxicity (Prasch et al., 2006).
Reductions in peripheral blood flow were not protected in Arnt2-/- null mutant zebrafish (Prasch et al., 2004) and it seems likely that this will also be the case for prosencephalic artery malformations caused by TCDD. However, blood vessel deformities caused by TCDD exposure have yet to be examined in Arnt2 null larvae. The possible role of Arnt2 in the production of TCDD-induced blood vessel malformations will require further study. It was recently reported that TCDD increased photocoagulation-induced choroidal neovascularization in association with enhanced VEGF mRNA expression in mice (Takeuchi et al., 2009). On the other hand, TCDD has been reported to inhibit VEGF expression in other studies (Ishimura et al., 2009). Obviously further research is needed to elucidate the mechanism of these brain vessel malformations in zebrafish larvae caused by TCDD and BNF.
In summary, activation of Ahr2/Arnt1 signaling by TCDD and BNF caused dose-dependent increases in blood vessel malformation in the prosencephalic artery and mesencephalic vein in early life stages of zebrafish. These alterations in the shape of a specific artery and vein in the brain is novel and might serve as an early, sensitive biomarker for Ahr2-mediated developmental toxicity in zebrafish. It will be important to determine if similar early defects in brain vasculature occur in other species as well.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science (H.T.), Akiyama Foundation (H.T.), The Promotion and Mutual Aid Corporation for Private Schools of Japan (H.T.) and in part by NIH grant R01ES015912 (J.J.S.) The award of the Postdoctoral Fellowship for Researchers from the Japan Society for the Promotion of Science to A.K. (no. 4313) is acknowledged.
Footnotes
Zebrafish gene nomenclature is followed by http://zfin.org/zf_info/nomen.html and genes and proteins are described in lower case italics and in normal script with the first letter capitalized. All capitalized letters for proteins are used for general meaning or in rodents.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol Sci. 2005;84:368–377. doi: 10.1093/toxsci/kfi073. [DOI] [PubMed] [Google Scholar]
- Antkiewicz DS, Peterson RE, Heideman W. Blocking expression of AHR2 and ARNT1 in zebrafish larvae protects against cardiac toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2006;94:175–182. doi: 10.1093/toxsci/kfl093. [DOI] [PubMed] [Google Scholar]
- Baldessari D, Mione M. How to create the vascular tree? (Latest) help from the zebrafish. Pharmacol Ther. 2008;118:206–230. doi: 10.1016/j.pharmthera.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Belair CD, Peterson RE, Heideman W. Disruption of erythropoiesis by dioxin in the zebrafish. Dev Dyn. 2001;222:581–594. doi: 10.1002/dvdy.1213. [DOI] [PubMed] [Google Scholar]
- Bello SM, Heideman W, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits regression of the common cardinal vein in developing zebrafish. Toxicol Sci. 2004;78:258–266. doi: 10.1093/toxsci/kfh065. [DOI] [PubMed] [Google Scholar]
- Cantrell SM, Joy-Schlezinger J, Stegeman JJ, Tillitt DE, Hannink M. Correlation of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced apoptotic cell death in the embryonic vasculature with embryotoxicity. Toxicol Appl Pharmacol. 1998;148:24–34. doi: 10.1006/taap.1997.8309. [DOI] [PubMed] [Google Scholar]
- Cantrell SM, Lutz LH, Tillitt DE, Hannink M. Embryotoxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): the embryonic vasculature is a physiological target for TCDD-induced DNA damage and apoptotic cell death in Medaka (Orizias latipes) Toxicol Appl Pharmacol. 1996;141:23–34. doi: 10.1006/taap.1996.0256. [DOI] [PubMed] [Google Scholar]
- Carney SA, Chen J, Burns CG, Xiong KM, Peterson RE, Heideman W. AHR activation produces heart-specific transcriptional and toxic responses in developing zebrafish. Molec Pharmacol. 2006;70:1–13. doi: 10.1124/mol.106.025304. [DOI] [PubMed] [Google Scholar]
- Cheung MO, Gilbert EF, Peterson RE. Cardiovascular teratogenicity of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin in the chick embryo. Toxicol Appl Pharmacol. 1981;61:197–204. doi: 10.1016/0041-008x(81)90409-9. [DOI] [PubMed] [Google Scholar]
- Dong W, Teraoka H, Kondo S, Hiraga T. 2,3,7,8-Tetrachlorodibenzo-p-dioxin induces apoptosis in the dorsal midbrain of zebrafish embryos by activation of aryl hydrocarbon receptor. Neurosci Lett. 2001;303:169–172. doi: 10.1016/s0304-3940(01)01743-8. [DOI] [PubMed] [Google Scholar]
- Dong W, Teraoka H, Tsujimoto Y, Stegeman JJ, Hiraga T. Role of aryl hydrocarbon receptor in mesencephalic circulation failure and apoptosis in zebrafish embryos exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2004;77:109–116. doi: 10.1093/toxsci/kfh023. [DOI] [PubMed] [Google Scholar]
- Dong W, Teraoka H, Yamazaki K, Tsukiyama S, Imani S, Imagawa T, Stegeman JJ, Peterson RE, Hiraga T. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: local circulation failure in the dorsal midbrain is associated with increased apoptosis. Toxicol Sci. 2002;69:191–201. doi: 10.1093/toxsci/69.1.191. [DOI] [PubMed] [Google Scholar]
- Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140:173–179. doi: 10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- Goldstone HM, Stegeman JJ. Molecular mechanisms of 2,3,7,8-tetrachlorodibenzo-p-dioxin cardiovascular embryotoxicity. Drug Metab Rev. 2006;38:261–289. doi: 10.1080/03602530600570099. [DOI] [PubMed] [Google Scholar]
- Guiney PD, Smolowitz RM, Peterson RE, Stegeman JJ. Correlation of 2,3,7,8-tetrachlorodibenzo-p-dioxin induction of cytochrome P4501A in vascular endothelium with toxicity in early life stages of lake trout. Toxicol Appl Pharmacol. 1997;143:256–273. doi: 10.1006/taap.1996.8051. [DOI] [PubMed] [Google Scholar]
- Hahn ME, Karchner SI, Shapiro MA, Perera SA. Molecular evolution of two vertebrate aryl hydrocarbon (dioxin) receptors (AHR1 and AHR2) and the PAS family. Proc Natl Acad Sci USA. 1997;94:13743–13748. doi: 10.1073/pnas.94.25.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry TR, Spitsbergen JM, Hornung MW, Abnet CC, Peterson RE. Early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio) Toxicol Appl Pharmacol. 1997;142:56–68. doi: 10.1006/taap.1996.8024. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Day HL, Collier TK, Scholz NL. Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol Appl Pharmacol. 2006;217:308–321. doi: 10.1016/j.taap.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Ishimura R, Kawakami T, Ohsako S, Tohyama C. Dioxin-induced toxicity on vascular remodeling of the placenta. Biochem Pharmacol. 2009;77:660–669. doi: 10.1016/j.bcp.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Isogai S, Horiguchi M, Weinstein BM. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev Biol. 2001;230:278–301. doi: 10.1006/dbio.2000.9995. [DOI] [PubMed] [Google Scholar]
- Karchner SI, Franks DG, Hahn ME. AHR1B, a new functional aryl hydrocarbon receptor in zebrafish: tandem arrangement of ahr1b and ahr2 genes. Biochem J. 2005;392:153–161. doi: 10.1042/BJ20050713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahvis GP, Lindell SL, Thomas RS, McCuskey RS, Murphy C, Glover E, Bentz M, Southard J, Bradfield CA. Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc Natl Acad Sci USA. 2000;97:10442–10447. doi: 10.1073/pnas.190256997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Fraser SD, Faloon PW, Rollins EL, Vom Berg J, Starovic-Subota O, Laliberte AL, Chen JN, Serluca FC, Childs SJ. A betaPix Pak2a signaling pathway regulates cerebral vascular stability in zebrafish. Proc Natl Acad Sci USA. 2007;104:13990–13995. doi: 10.1073/pnas.0700825104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K, Ema M, Sogawa K, Yasuda M, Katsuki M, Fujii-Kuriyama Y. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 1997;2:645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- Na YR, Seok SH, Baek MW, Lee HY, Kim DJ, Park SH, Lee HK, Park JH. Protective effects of vitamin E against 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) induced toxicity in zebrafish embryos. Ecotoxicol Environ Safety. 2009;72:714–719. doi: 10.1016/j.ecoenv.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol. 2000;59:65–85. doi: 10.1016/s0006-2952(99)00310-x. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Heideman W, Peterson RE. ARNT2 is not required for TCDD developmental toxicity in zebrafish. Toxicol Sci. 2004;82:250–258. doi: 10.1093/toxsci/kfh235. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Tanguay RL, Mehta V, Heideman W, Peterson RE. Identification of zebrafish ARNT1 homologs: 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity in the developing zebrafish requires ARNT1. Mol Pharmacol. 2006;69:776–787. doi: 10.1124/mol.105.016873. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Teraoka H, Carney SA, Dong W, Hiraga T, Stegeman JJ, Heideman W, Peterson RE. Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol Sci. 2003;76:138–150. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- Reichard JF, Dalton TP, Shertzer HG, Puga A. Induction of oxidative stress responses by dioxin and other ligands of the aryl hydrocarbon receptor. Dose Response. 2006;3:306–331. doi: 10.2203/dose-response.003.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A, Takeuchi M, Oikawa K, Sonoda KH, Usui Y, Okunuki Y, Takeda A, Oshima Y, Yoshida K, Usui M, Goto H, Kuroda M. Effects of dioxin on vascular endothelial growth factor (VEGF) production in the retina associated with choroidal neovascularization. Invest Ophthalmol Vis Sci. 2009;50:3410–3416. doi: 10.1167/iovs.08-2299. [DOI] [PubMed] [Google Scholar]
- Tanguay RL, Heideman W, Peterson RE. Cloning and characterization of the zebrafish (Danio rerio) aryl hydrocarbon receptor. Biochim Biophys Acta. 1999;1444:35–48. doi: 10.1016/s0167-4781(98)00252-8. [DOI] [PubMed] [Google Scholar]
- Tanguay RL, Andreasen EA, Heideman W, Peterson RE. Identification and expression of alternatively spliced aryl hydrocarbon nuclear translocator 2 (ARNT2) cDNAs from zebrafish with distinct functions. Biochim Biophys Acta. 2000;1494:117–128. doi: 10.1016/s0167-4781(00)00225-6. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Hiraga T. Zebrafish as a novel experimental model for developmental toxicology. Congenit Anom. 2003a;43:123–132. doi: 10.1111/j.1741-4520.2003.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Teraoka T, Dong W, Ogawa S, Tsukiyama S, Okuhara Y, Niiyama M, Ueno N, Peterson RE, Hiraga T. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: Altered regional blood flow and impaired lower jaw development. Toxicol Sci. 2002;65:192–199. doi: 10.1093/toxsci/65.2.192. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Tsujimoto Y, Iwasa H, Endoh D, Ueno N, Stegeman JJ, Peterson RE, Hiraga T. Induction of cytochrome P450 1A is required for circulation failure and edema by 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish. Biochem Biophys Res Commun. 2003b;304:223–228. doi: 10.1016/s0006-291x(03)00576-x. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Kubota A, Dong W, Kawai Y, Yamazaki K, Mori C, Harada Y, Peterson RE, Hiraga T. Role of the cyclooxygenase 2-thromboxane pathway in 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced decrease in mesencephalic vein blood flow in the zebrafish embryo. Toxicol Appl Pharmacol. 2009;234:33–40. doi: 10.1016/j.taap.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Walisser JA, Glover E, Pande K, Liss AL, Bradfield CA. Aryl hydrocarbon receptor-dependent liver development and hepatotoxicity are mediated by different cell types. Proc Natl Acad Sci USA. 2005;102:17858–17863. doi: 10.1073/pnas.0504757102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MK, Peterson RE. Aquatic toxicity of dioxins and related chemicals. In: Schecter A, editor. Dioxins and Health. Plenum; New York: 1994. pp. 347–387. [Google Scholar]
- Wassenberg DM, Di Giulio RT. Teratogenesis in Fundulus heteroclitus embryos exposed to a creosote-contaminated sediment extract and CYP1A inhibitors. Mar Environ Res. 2004;58:163–168. doi: 10.1016/j.marenvres.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Wienholds E, van Eeden F, Kosters M, Mudde J, Plasterk RH, Cuppen E. Efficient target-selected mutagenesis in zebrafish. Genome Res. 2003;13:2700–2707. doi: 10.1101/gr.1725103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. University of Oregon Press; Eugene: 1993. [Google Scholar]
- Yamauchi M, Kim EY, Iwata H, Shima Y, Tanabe S. Toxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in developing red seabream (Pagrus major) embryo: an association of morphological deformities with AHR1, AHR2 and CYP1A expressions. Aquat Toxicol. 2006;80:166–179. doi: 10.1016/j.aquatox.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Shiratori H, Hamada H. Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature. 2007;450:285–288. doi: 10.1038/nature06254. [DOI] [PubMed] [Google Scholar]


