Abstract
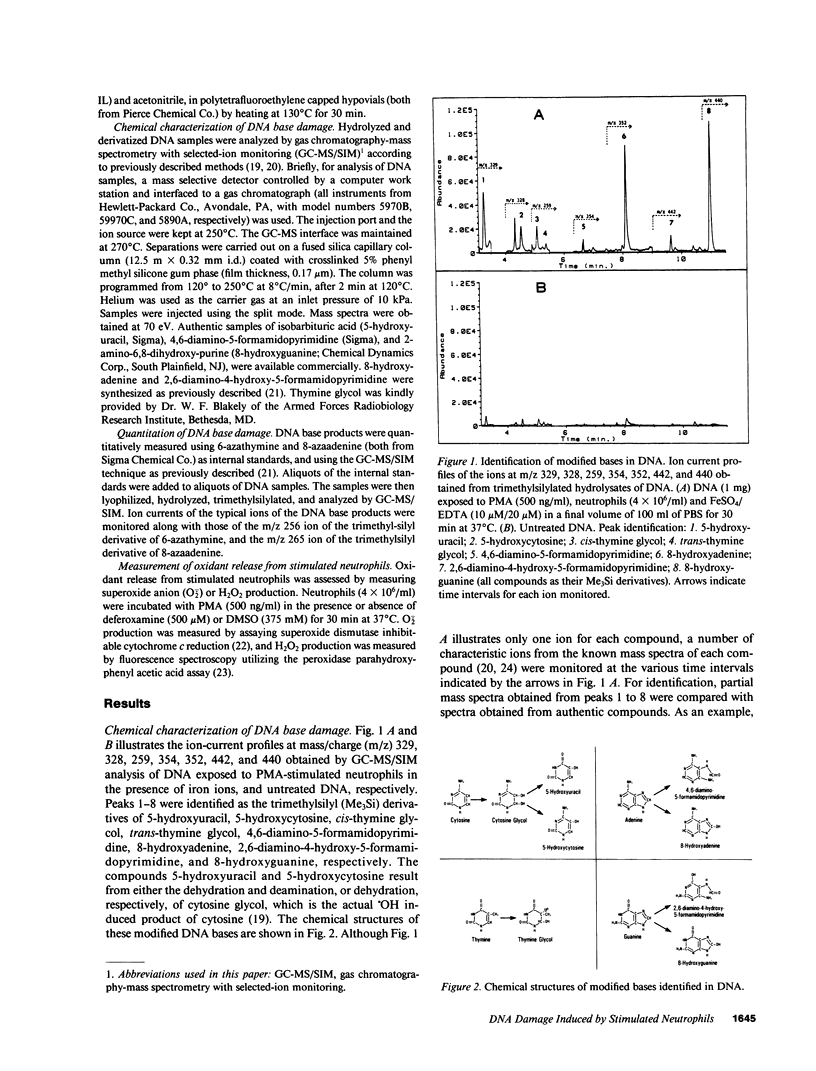
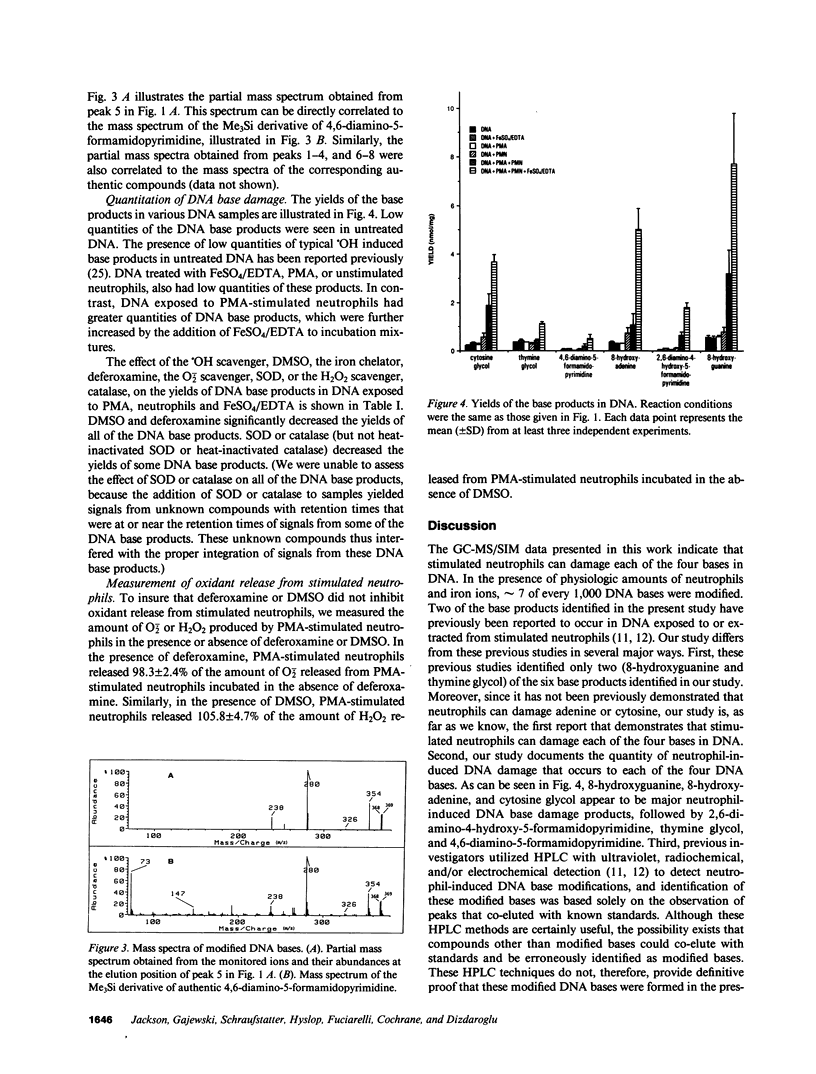
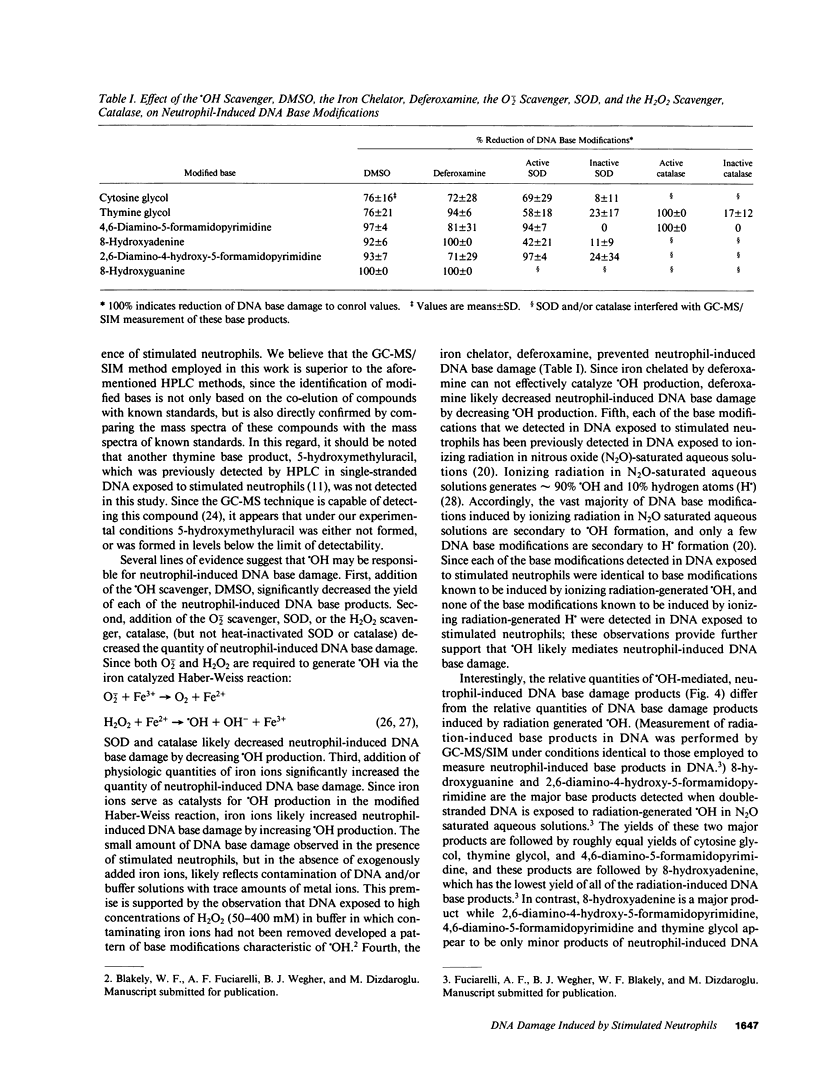
Leukocyte-induced DNA damage may partially account for the known association between chronic inflammation and malignancy. Since elucidation of the chemical nature of leukocyte-induced DNA damage may enhance our understanding of the mechanisms underlying leukocyte-induced DNA damage and the carcinogenesis associated with inflammation, the present study was undertaken to characterize the chemical modifications that occur in DNA exposed to stimulated human neutrophils. Calf thymus DNA was exposed to phorbol myristate acetate (PMA)-stimulated neutrophils in the presence or absence of exogenously added iron ions. DNA samples were subsequently hydrolyzed, derivatized and analyzed by gas chromatography-mass spectrometry with selected-ion monitoring. A variety of base modifications including cytosine glycol, thymine glycol, 4,6-diamino-5-formamidopyrimidine, 8-hydroxyadenine, 2,6-diamino-4-hydroxy-5-formamidopyrimidine, and 8-hydroxyguanine were identified. The yield of these various base products was increased by the addition of iron ions. Specifically, in the presence of physiologic quantities of iron ions, approximately 7 of every 1,000 DNA bases were modified. Addition of the superoxide anion scavenger, superoxide dismutase, the hydrogen peroxide scavenger, catalase, the hydroxyl scavenger, dimethylsulfoxide, or the iron chelator, deferoxamine, to DNA mixtures containing PMA, neutrophils, and iron ions, greatly decreased the yield of the damaged DNA base products. Our results indicate that stimulated human neutrophils can damage each of the four bases in DNA. It is likely that hydroxyl radical, generated via an iron catalyzed Haber-Weiss reaction, mediates neutrophil-induced DNA base damage, since: (a) the chemical structure of neutrophil-induced DNA base damage is consistent with a hydroxyl radical-mediated mechanism, (b) hydroxyl radical generated via ionizing radiation in aqueous solution produces DNA base modifications that are identical to neutrophil-induced DNA base modifications, (c) iron ions increase neutrophil-induced DNA base damage, and (d) iron chelators or scavengers of superoxide anion, hydrogen peroxide or hydroxyl radical decrease neutrophil-induced DNA base damage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander-Williams J. Inflammatory disease of the bowel: the risk of cancer. Dis Colon Rectum. 1976 Oct;19(7):579–581. doi: 10.1007/BF02590970. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Berkow R. L., Tzeng D. Y., Williams L. V., Baehner R. L. The comparative responses of human polymorphonuclear leukocytes obtained by counterflow centrifugal elutriation and Ficoll-Hypaque density centrifugation. I. Resting volume, stimulus-induced superoxide production, and primary and specific granule release. J Lab Clin Med. 1983 Nov;102(5):732–742. [PubMed] [Google Scholar]
- Birnboim H. C. DNA strand breakage in human leukocytes exposed to a tumor promoter, phorbol myristate acetate. Science. 1982 Mar 5;215(4537):1247–1249. doi: 10.1126/science.6276978. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Beardsley G. P. Functional effects of cis-thymine glycol lesions on DNA synthesis in vitro. Biochemistry. 1987 Aug 25;26(17):5398–5403. doi: 10.1021/bi00391a027. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M. Application of capillary gas chromatography-mass spectrometry to chemical characterization of radiation-induced base damage of DNA: implications for assessing DNA repair processes. Anal Biochem. 1985 Feb 1;144(2):593–603. doi: 10.1016/0003-2697(85)90158-7. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M., Bergtold D. S. Characterization of free radical-induced base damage in DNA at biologically relevant levels. Anal Biochem. 1986 Jul;156(1):182–188. doi: 10.1016/0003-2697(86)90171-5. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M. The use of capillary gas chromatography-mass spectrometry for identification of radiation-induced DNA base damage and DNA base-amino acid cross-links. J Chromatogr. 1984 Jul 6;295(1):103–121. doi: 10.1016/s0021-9673(01)87602-0. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Harris J., West M., Wong P. K. Formation of 8-hydroxydeoxyguanosine, hydroxyl free radical adduct of DNA in granulocytes exposed to the tumor promoter, tetradecanoylphorbolacetate. Biochem Biophys Res Commun. 1986 Jun 13;137(2):841–846. doi: 10.1016/0006-291x(86)91156-3. [DOI] [PubMed] [Google Scholar]
- Frenkel K., Chrzan K., Troll W., Teebor G. W., Steinberg J. J. Radiation-like modification of bases in DNA exposed to tumor promoter-activated polymorphonuclear leukocytes. Cancer Res. 1986 Nov;46(11):5533–5540. [PubMed] [Google Scholar]
- Fuciarelli A. F., Wegher B. J., Gajewski E., Dizdaroglu M., Blakely W. F. Quantitative measurement of radiation-induced base products in DNA using gas chromatography-mass spectrometry. Radiat Res. 1989 Aug;119(2):219–231. [PubMed] [Google Scholar]
- Guilbault G. G., Brignac P. J., Jr, Juneau M. New substrates for the fluorometric determination of oxidative enzymes. Anal Chem. 1968 Jul;40(8):1256–1263. doi: 10.1021/ac60264a027. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987 Nov;1(5):358–364. [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron chelates: is it a mechanism for hydroxyl radical production in biochemical systems? FEBS Lett. 1978 Aug 15;92(2):321–326. doi: 10.1016/0014-5793(78)80779-0. [DOI] [PubMed] [Google Scholar]
- Jackson J. H., Schraufstatter I. U., Hyslop P. A., Vosbeck K., Sauerheber R., Weitzman S. A., Cochrane C. G. Role of oxidants in DNA damage. Hydroxyl radical mediates the synergistic DNA damaging effects of asbestos and cigarette smoke. J Clin Invest. 1987 Oct;80(4):1090–1095. doi: 10.1172/JCI113165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Mori F., Kasai H., Inoue H., Iwai S., Miura K., Ohtsuka E., Nishimura S. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987 May 7;327(6117):77–79. doi: 10.1038/327077a0. [DOI] [PubMed] [Google Scholar]
- Lewis J. G., Adams D. O. Induction of 5,6-ring-saturated thymine bases in NIH-3T3 cells by phorbol ester-stimulated macrophages: role of reactive oxygen intermediates. Cancer Res. 1985 Mar;45(3):1270–1275. [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hinshaw D. B., Hyslop P. A., Spragg R. G., Cochrane C. G. Oxidant injury of cells. DNA strand-breaks activate polyadenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J Clin Invest. 1986 Apr;77(4):1312–1320. doi: 10.1172/JCI112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hyslop P. A., Hinshaw D. B., Spragg R. G., Sklar L. A., Cochrane C. G. Hydrogen peroxide-induced injury of cells and its prevention by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4908–4912. doi: 10.1073/pnas.83.13.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstätter I., Hyslop P. A., Jackson J. H., Cochrane C. G. Oxidant-induced DNA damage of target cells. J Clin Invest. 1988 Sep;82(3):1040–1050. doi: 10.1172/JCI113660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirnamé-Moré L., Rossman T. G., Troll W., Teebor G. W., Frenkel K. Genetic effects of 5-hydroxymethyl-2'-deoxyuridine, a product of ionizing radiation. Mutat Res. 1987 Jun;178(2):177–186. doi: 10.1016/0027-5107(87)90267-3. [DOI] [PubMed] [Google Scholar]
- Wallace S. S. The biological consequences of oxidized DNA bases. Br J Cancer Suppl. 1987 Jun;8:118–128. [PMC free article] [PubMed] [Google Scholar]
- Weitberg A. B., Weitzman S. A., Destrempes M., Latt S. A., Stossel T. P. Stimulated human phagocytes produce cytogenetic changes in cultured mammalian cells. N Engl J Med. 1983 Jan 6;308(1):26–30. doi: 10.1056/NEJM198301063080107. [DOI] [PubMed] [Google Scholar]
- Weitzman S. A., Stossel T. P. Effects of oxygen radical scavengers and antioxidants on phagocyte-induced mutagenesis. J Immunol. 1982 Jun;128(6):2770–2772. [PubMed] [Google Scholar]
- Weitzman S. A., Weitberg A. B., Clark E. P., Stossel T. P. Phagocytes as carcinogens: malignant transformation produced by human neutrophils. Science. 1985 Mar 8;227(4691):1231–1233. doi: 10.1126/science.3975611. [DOI] [PubMed] [Google Scholar]
- de Mello Filho A. C., Meneghini R. Protection of mammalian cells by o-phenanthroline from lethal and DNA-damaging effects produced by active oxygen species. Biochim Biophys Acta. 1985 Oct 30;847(1):82–89. doi: 10.1016/0167-4889(85)90156-9. [DOI] [PubMed] [Google Scholar]


