Abstract
Inflammatory bowel diseases (IBDs) are complex and chronic disabling conditions resulting from a dysregulated dialogue between intestinal microbiota and components of both the innate and adaptive immune systems. Cytokines are essential mediators between activated immune and non-immune cells, including epithelial and mesenchymal cells. They are immunomodulatory peptides released by numerous cells and these have significant effects on immune function leading to the differentiation and survival of T cells. The physiology of IBD is becoming a very attractive field of research for development of new therapeutic agents. These include cytokines involved in intestinal immune inflammation. This review will focus on mechanisms of action of cytokines involved in IBD and new therapeutic opportunities for these diseases.
Keywords: Inflammatory bowel disease, Ulcerative colitis, Crohn’s disease, Cytokine, Pathophysiology, Biological therapy
INTRODUCTION
Ulcerative colitis (UC) and Crohn’s disease (CD) are immune-mediated disorders of the intestine[1]. Accumulating data suggests that inflammatory bowel disease (IBD) results from an inappropriate inflammatory response to intestinal microbes in a genetically susceptible host[2]. Emerging evidence suggests that disease development implicates a dysregulated dialogue between the intestinal flora and components of both the innate and adaptive immune systems[3,4].
Active IBD is defined as an infiltration of the lamina propria by innate immune cells [neutrophils, macrophages, dendritic and natural killer (NK) T cells] and adaptive immune cells (B and T cells). Increased numbers and activation of these cells in the intestinal mucosa enhance local levels of tumor necrosis factor-α (TNF-α) and several proinflammatory interleukins (IL)[2,5]. Cytokines are essential mediators of the interaction between activated immune cells and non-immune cells, including epithelial and mesenchymal cells[6,7].
Recent advances in the study of the regulation of key cytokines during major forms of IBD promise the development of more effective mechanism-based therapies[8]. Given that many of these involve regulation of dynamic biological processes, it is likely that the most effective agents will fall within the broad rubric of biologic therapy. The prototypic example of the ability of a biologic agent to effectively change the therapeutic landscape is provided by anti-TNF-α, first demonstrated through clinical validation of the prototypic agent infliximab[8]. The advent of anti-TNF-α agents has changed the way of treating IBD refractory to standard medications[3,9].
Advances in the understanding of IBD pathophysiology have become a very active area for the development of novel therapeutic agents. New targets for biologics include cytokines involved in intestinal immune inflammation that have led to new therapeutic opportunities[10,11]. Although IBD etiology is unknown, some molecules which are involved in the physiopathology have been identified and can be targeted by biological therapies[12]. This review will focus on cytokines involved in the dysregulated inflammatory response in IBD and targeted by biological therapies.
CYTOKINE NETWORK AND IMMUNITY
Cytokines (from greek cyto: cell; kinos: movement) are substances that are secreted by specific cells of the immune system and carry signals locally between cells, with extensive use in cellular communication. The term “cytokine” encompasses a large and diverse family of polypeptide regulators that are produced widely throughout the body by cells of diverse embryological origin. Basically, the term “cytokine” has been used to refer to the immunomodulating agent. Interferon was the first cytokine to be described in 1957[13]. The clinical efficacy of targeting TNF-α indicates that cytokines are potential therapeutic targets in IBD[6].
Cytokines have profound effects on immune functions[14]. Beyond the classical T helper Th1/Th2 paradigm indicating predominant Th1-mediated responses dominated by the production of interferon-γ (IFN-γ) in CD and an exaggerated Th2-like inflammation in UC characterized by an increased production of IL-13[2,15], there has been a surge of information with regard to the role of innate immunity in IBD pathogenesis. Thus new data on adaptive immunity are emerging, indicating that: (1) the mucosal Th1 and Th2 responses of CD and UC may be actually secondary to defects of the innate immune response; (2) the dysfunction of regulatory T cells may be contributing to mucosal immune abnormalities; and (3) the newly described Th17 cells are also prominently involved in the gut inflammatory response in both forms of IBD[5,15].
The differentiation and survival of T cells depend on the relative amount of key regulatory cytokines produced mainly by macrophages and dendritic cells[12]. In the presence of IL-12 and IFN-γ, naive CD4+ T cells adopt a Th1 phenotype which then activate macrophages that release IL-1, IL-6 and TNF-α. Thus this creates a positive feedback loop[3,6,12]. In the presence of IL-4, naive CD4-T cells adopt a Th2 phenotype[12,16]. The Th17 development is triggered by both IL-6, IL-21, IL-23 and transforming growth factor-β (TGF-β), leading to secretion of the IL-17 cytokine family and IL-22[6]. Although the function of Th17 cells is not clearly known, there is probably an important part of this T cell population which expresses IL-23 receptors. This has been recently demonstrated as an IBD susceptibility gene in genome-wide association studies.
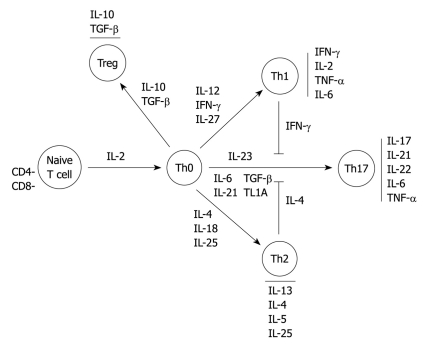
In contrast, TGF-β and IL-10 modulate differentiation of naïve T cells to T regulatory cell subgroups leading to high amounts of IL-10 and TGF-β, and are able to suppress bystander T cell activation. This could be defective in IBD[17-20]. There is a complex network between these different cell populations in the case of inflammation as, for example, in the negative crossregulation of the differentiation of Th17 cells by Th2 cells (IL-4, IL-27) and Th1 cells (IFN-γ) (Figure 1)[21].
Figure 1.
Overview of T cell differentiation and interleukin pathways. IL: Interleukin; IFN: Interferon; TGF: Transforming growth factor; TNF: Tumor necrosis factor; TL1A: TNF-like factor 1A.
PROINFLAMMATORY CYTOKINES
TNF family: TNF-α and TNF-like factor 1A
Mechanisms of action: TNF-α is a major mediator of inflammation in the gut[22-25]. It is synthesized by several cells including intestinal epithelial cells but predominantly by cells of the monocyte line and T lymphocytes[14]. TNF-α is a homotrimeric protein that mediates its diverse biologic effects through 2 distinct receptors known as TNF-α receptor type I expressed on all nucleated cells and TNF-α receptor type II restricted to cells of hematopoietic lineage[26]. Through the activation of nuclear factor-κB (NF-κB), TNF-α induces the expression of various genes such as urokinase plasminogen activator, cyclooxygenase II (COX II) and vascular endothelial growth factor (VEGF)[26]. By this method, TNF-α has multiple biological effects such as increasing leukocyte recruitment (induction of leukocyte adhesion molecules)[27,28], modulation of nitric oxide (NO) production (increasing the vascular permeability)[29,30], induction of secretion of proinflammatory cytokines[31], and the proliferation and differentiation of immune cells[26]. TNFSF15 encodes TNF-like factor 1A (TL1A), which is a TNF-like molecule that mediates co-stimulation of Th1 and Th17 cells. It is required for optimal differentiation of Th17 cells[21,32]. Variants in the TNFSF15 gene contribute to overall CD susceptibility[33,34] and an increased production of TL1A has been observed in CD[35]. Interestingly, in mice, colitis was prevented and attenuated by an anti-TL1A antibody[36].
Results of clinical trials (Table 1): Three anti-TNF agents, namely infliximab, adalimumab and certolizumab pegol have been approved by the US Food and Drug Administration for the treatment of luminal CD. In Europe, certolizumab has not yet received approval for IBD. Infliximab has also been approved for fistulizing CD and UC. In luminal CD, infliximab was effective in inducing clinical remission in 33% of patients compared with only 4% of a placebo group at week 4 (P = 0.005)[37], and in maintaining clinical remission (45% in the infliximab group vs 21% in the placebo group, P < 0.005). Adalimumab was also significantly more effective than placebo in inducing clinical remission (36% vs 12%, P < 0.001)[38], and more effective than placebo in maintaining clinical remission at week 56 (36% vs 16%). Infliximab and adalimumab have also been shown to be more effective than placebo in maintaining steroid-free remission at 1 year[39,40]. Regarding certolizumab pegol, results from large randomized, placebo-controlled trials are more controversial, with no improvement at week 6 and different long-term response rates between trials[41,42]. In fistulizing CD, 55% of the patients who received 5 mg/kg infliximab had complete fistula closure, as compared with only 13% of the patients assigned to placebo (P = 0.001)[43]. In UC, 2 large randomized, placebo-controlled studies, namely the ACT 1 and ACT 2 trials, evaluated the efficacy of infliximab for induction and maintenance therapy in UC[44]. In both trials, at week 8, nearly two-thirds of patients in the group receiving 5 mg of infliximab had had a clinical response, as compared with one-third of patients in the placebo group (P < 0.001).
Table 1.
Clinical efficacy and marketing approval for anti-tumor necrosis factor-α agents
| Drug name |
Efficacy (% of induction of remission/% sustained remission) |
Approved (FDA/Europe) |
||||
| Luminal CD | Fistulizing CD | UC | Luminal CD | Fistulizing CD | UC | |
| Infliximab (Remicade®) | 33/45 | 55/36 | 38.8/23.1 | Yes/Yes | Yes/Yes | Yes/Yes |
| Adalimumab (Humira®) | 36/36 | No RCT | No RCT | Yes/Yes | No/No | No/No |
| Certolizumab (Cimzia®) | 35/48 | No RCT | No RCT | Yes/No | No/No | No/No |
CD: Crohn’s disease; UC: Ulcerative colitis; FDA: US food and drug administration; RCT: Randomized controlled trial.
Regarding the safety of anti-TNF agents, the Crohn’s Therapy, Resource, Evaluation, and Assessment Tool registry, including 3179 CD patients who received infliximab, demonstrated that this agent was not an independent predictive factor of serious infections[45]. In a meta-analysis of 21 placebo-controlled trials enrolling 5356 individuals, anti-TNF therapy did not increase the risk of death, malignancy or serious infection when compared to control arms[9]. However, a longer duration of follow-up and a larger number of patients are required to better assess the safety profile of anti-TNF agents in CD.
Mechanisms of action of anti-TNF-α agents remain poorly known. Neutralization of TNF-α in the inflamed mucosa is unlikely to be a sufficient explanation. Antibody-dependent cytotoxicity also induces apoptosis or lysis of TNF-α-producing cells. This mechanism involves the Fc portion of antibodies that increases the pro-apoptotic factor caspase-3[46].
IL-12, p40/IL-23, p40
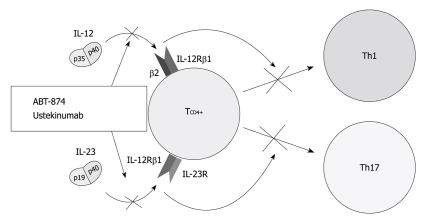
Mechanisms of action (Figure 2): IL-12 is a key cytokine that drives the inflammatory response mediated by Th1 cells[47,48]. As such, it underlies both normal host responses to a variety of intracellular bacterial, fungal and protozoan pathogens, and abnormal inflammatory responses linked to many autoimmune diseases, such as CD[49]. Indeed CD is characterized by increased production of IL-12 by antigen-presenting cells in intestinal tissue[50,51]. IL-23, secreted by antigen-presenting cells, is also a central cytokine involved in the differentiation and function of Th17 cells[2]. The IL-23-Th17 interaction mediates microbial defenses and intestinal inflammation[52,53]. Individual properties of IL-23 are also underscored by identification of the gene encoding the receptor for this cytokine as modifying host susceptibility[8,54,55]. These 2 most potent Th1- and Th17-activating cytokines, IL-12 and IL-23 are both composed of a p40 subunit and therefore, a p40 antibody may have therapeutic potential in inhibiting both Th1-activating IL-12 and Th17-activating IL-23[21].
Figure 2.
Therapeutic blockade of the interleukin-12/interleukin-23 pathway at the common p40 subunit of both cytokines. IL: Interleukin.
Results of clinical trials (Figure 2 and Table 2): IL-12 and IL-23 are targeted by one humanized IL-12/23 antibody, ABT-874. It has shown promising results in a phase II dose-ranging study comprising 79 patients with CD[49]. Seven weeks of uninterrupted treatment with 3 mg/kg ABT-874 resulted in higher response rates than placebo (75% vs 25%, P = 0.03). Another dose-ranging study comparing efficacy, safety and pharmacokinetic of intravenous infusions of ABT-874 vs placebo in subjects with active CD is ongoing. A double-blind, placebo-controlled, parallel-group, crossover study, assessing ustekinumab in 104 patients with CD has been completed[56]. The clinical response to ustekinumab was significantly greater than the group given placebo at weeks 4 and 6 (52%-54% vs 22%-39%, P < 0.05) but not at week 8 (49% vs 40%, P = 0.34). Interestingly, the effect was most prominent in patients treated previously with infliximab at weeks 4, 6 and 8 (59% in the ustekinumab group vs 25%-26% in the placebo group, P < 0.05). A phase 2, randomized, double-blinded, placebo-controlled study has evaluated the efficacy of apilimod mesylate, an oral IL-12 and IL-23 inhibitor in treating 220 patients with moderate-to-severe CD. The enrollment was closed early because it did not demonstrate efficacy over placebo[57].
Table 2.
Summary of safety and efficacy of anti-cytokine therapies in randomized, controlled trials
| Study | Drug name | Targeted cytokine | Indication | No. of patients | Follow-up | Clinical response (%) | Clinical remission (%) | SAE (%) |
| Mannon et al[49] | ABT-874 | IL-12/23 | CD | 79 | 8 wk | 69 | 38 | 10 |
| Sandborn et al[56] | Ustekinumab | IL-12/23 | CD | 131 | 8 wk | 49 | 19 | 4 |
| Sands et al[57] | Apilimod mesylate | IL-12/23 | CD | 220 | 168 d | 25.7 | NA | NSb |
| Ito et al[64] | Tocilizumab | IL-6 | CD | 36 | 12 wk | 80 | 20 | 13 |
| Hommes et al[68] | Fontolizumab | IFN-γ | CD | 133 | 28 d | 38-44 | 19-31 | 4.5 |
| Reinisch et al[69] | Fontolizumab | IFN-γ | CD | 201 | 29 d | 31-38 | 21 | 13.6 |
| Van assche et al[79] | Daclizumab | IL-2 | UC | 159 | 8 wk | 25-33 | 2-7 | 4.3-12.5 |
| Schreiber et al[85] | rhuIL-10 | IL-10 | CD | 320 | 29 d | NA | 23.5 | 7 |
| Schreiber et al[86] | rhuIL-10 | IL-10 | UC | 94 | 28 d | NA1 | NA1 | 7.5 |
| Colombel et al[87] | Tenovil | IL-10 | Postoperative CD | 65 | 12 wk | 46% of patients with endoscopic recurrence | 9 | |
| Sands et al[95] | rhIL-11 | IL11 | CD | 148 | 8 wk | 31.54 | 36.74 | NS2 |
| Herrlinger et al[90] | rhIL-11 | IL-11 | CD | 51 | 12 wk | 193 | 43 | 8 |
| Pena-Rossi et al[96] | IFN-β1a | IFN-β1a | UC | 194 | 12 wk | NA | 20-29.2 | 12.3 |
| Tilg et al[97] | PEG-IFN-α | IFN-α | UC | 60 | 12 wk | NA | 40 | 15 |
No difference between placebo and rhuIL-10 treatment;
More headache, edema and increased platelet count;
Significantly inferior than prednisolone;
Significantly superior than placebo at a dose of 15 microg/kg weekly. SAE: Severe adverse event; IL: Interleukin; IFN: Interferon; CD: Crohn’s disease; UC: Ulcerative colitis; NA: Not available; NS: Not significant.
IL-6
Mechanisms of action: IL-6 is produced by various cells such as T cells, B cells, monocytes, fibroblasts, osteoblasts, keratinocytes, endothelial cells, mesangial cells and some tumor cells[58]. This cytokine specifically binds to the IL-6 receptor (IL-6R) or a soluble IL-6R, forming the IL-6/IL-6R complex that binds to gp130 and activates intracellular pathways including JAK/STAT signaling, tyrosine phosphatase SHP2 and NF-κB[59]. Many cells express gp130, hence IL-6 is a pleiotropic multi-functional cytokine acting as both a proinflammatory and an antiinflammatory cytokine[12,59]. It is involved in terminal differentiation of B cells, differentiation and activation of T cells, induction of a hepatic acute-phase response, hematopoiesis and fever[60,61]. Thus activated IL-6 plays a major role in its own amplification and then in the chronic phase of inflammation helped by mononuclear cell accumulation at the site of injury, through continuous monocyte chemoattractant protein-1 secretion, angioproliferation and antiapoptotic functions of T cells[59,62]. Plasma soluble IL-6R is increased in patient with CD and IL-6 plasma concentrations increase in active CD[63].
Results of clinical trials (Table 2): Tocilizumab binds to both the membrane-bound and the soluble forms of human IL-6R with high affinity and specificity[3,64]. Tocilizumab has shown promising results in a small phase I/II study (n = 36) that met its primary endpoint. At 12 wk, the response rate was higher in patients given an 8 mg/kg infusion of tocilizumab every 2 wk than in those given placebo (80% vs 31%, P = 0.019) and is accompanied by a decrease in C-reactive protein concentration[3,64]. However, only 2 of 10 patients went into remission, compared with none of 13 in the placebo group (P = 0.092), without significant improvement in mucosal healing[3,64]. Improvement in disease activity in a patient with UC associated with Takayasu arteritis has been reported after treatment with tocilizumab[65]. A placebo-controlled phase I study on the safety and biological effects of c326, an inhibitor of IL-6, in CD is ongoing.
IFN-γ
Mechanisms of action: Type II INF, also called IFN-γ, is a proinflammatory cytokine secreted by Th1-cells[66]. IFN-γ drives expression of major histocompatibility complex class II on antigen-presenting cells, modulates lipopolysaccharide responsiveness in intestinal epithelial cells, and increases chemokine secretion. It also activates macrophages, Th1 lymphocytes in a positive feedback loop, NK cells and endothelial cells[12,66,67]. Concentrations of IFN-γ are increased both in UC and CD.
Results of clinical trials (Table 2): Fontolizumab has been assessed in 3 phase I/II dose-ranging studies enrolling a total of 374 patients with moderate to severe CD[68-70]. Fontolizumab at doses of up to 4 mg/kg improved endoscopic lesions and decreased concentrations of C-reactive protein[68-70], but no study met its primary endpoint, which was defined as induction of clinical response at 1 mo[68-70]; thus the development of fontolizumab for CD has been stopped[3].
IL-2 family
Mechanisms of action: IL-2 is produced mainly by activated T cells[71]. In addition to promoting T cell proliferation and activation, IL-2 increases cytokine production and modifies the functional properties of B cells, NK cells, and macrophages. Thus, it improves the activated macrophage microbicidal and cytotoxic activities and promotes secretion of hydrogen peroxide, TNF-α and IL-6[72]. IL-2 signals through a heterodimeric (αγ) or trimeric αβγ high-affinity receptor complex[72]. Studies have proved a role for IL-2 in IBD pathogenesis, for example the calcineurin inhibitor cyclosporin, which inhibits IL-2 production, is effective in the treatment of severely active UC[73]. IL-21, an IL-2 cytokine family member expressed by activated CD4+ T cells and NK T cells, is a key regulator in production of Th17 cells. It also increases the proliferation of Th1 cells, CD4+ and CD8+ lymphocytes and regulates the profile of cytokines secreted by these cells[19,74]. Indeed, IL-21-deficient mice are protected from experimental colitis, possibly through the failure to generate the Th17 response[75]. Furthermore, blockade of endogenous IL-21, with an antagonisitic IL-21R/Fc, ameliorated dextran sulphate sodium colitis in mice[75]. No studies have been performed in humans as yet.
Results of clinical trials (Table 2): Two antibodies against the α-chain of the IL-2 receptor (CD25), namely daclizumab and basiliximab, have been studied to mimic the activity of cyclosporine[76-78]. Despite promising response rates observed in an uncontrolled trial, a randomized, double-blind, placebo-controlled, dose-ranging trial failed to demonstrate an increased remission or clinical response both at high (2 mg/kg intravenously at weeks 0, 2, 4, and 6) and low doses (1 mg/kg intravenously at weeks 0 and 4) in 159 treated patients with daclizumab for active UC[79].
ANTIINFLAMMATORY CYTOKINES
IL-10
Mechanisms of action: IL-10 is secreted by a wide variety of cells and has pleiotropic effects on T cells, B cells, myeloid cells, and other cell types[80]. IL-10 has suppressive antiinflammatory activity on T cells, macrophages, and dendritic cells (among other cells) in humans, as well as in animal models of inflammatory diseases[80]. In particular, mice deficient in IL-10 or the IL-10 receptor undergo spontaneous development of intestinal inflammation, similar to human disease[81,82]. Even though IL-10 effectively treats colitis in mouse models and suppresses inflammatory cytokine production in vitro in intestinal cells from IBD patients[83], unfortunately clinical trials using recombinant IL-10 to treat IBD in humans have been largely disappointing[84].
Results of clinical trials: A placebo-controlled study was conducted in 329 patients with moderate-to-severe CD and in 94 patients with UC and did not demonstrate any significant improvement in response and remission rates compared to placebo[85,86]. Also, no evidence of prevention of endoscopic recurrence in CD by subcutaneous IL-10 injections was observed in a placebo-controlled trial of 65 CD patients[87]. Animal studies showed that local administration of IL-10 to the colon via genetically engineered Lactococcus lactis bacteria administered orally allowed for the achievement of high colonic mucosal concentrations of IL-10, potentially resulting in increased efficacy[12,88].
IL-11
Mechanisms of action: IL-11 is a pleiotropic cytokine from mesenchymal cell origin[89]. It exhibits potent antiinflammatory activity on macrophages and T cells by inhibiting the secretion of pro-inflammatory cytokines[90-92] and has shown beneficial effects on intestinal mucosa in several animal IBD models[89,90]. However one study suggested that the expression of the IL-11 receptor α-chain in the mucosa was restricted to epithelial cells, and although reducing apoptosis, it had no antiinflammatory effects on these cells[93].
Results of clinical trials: In a placebo-controlled study in 76 active CD patients, subcutaneously administered recombinant human IL-11 was shown to be safe and well tolerated[94]. In a second placebo-controlled study in 148 patients comparing 2 doses of subcutaneously administered recombinant human IL-11, it was significantly superior in inducing remission after 6 wk when compared to placebo[95]. In contrast, a recent trial showed significant inferiority of recombinant human IL-11 when compared to prednisolone in inducing remission in active CD and in obtaining a clinical response[90].
Type I IFNs
Mechanisms of action: Type I IFNs consist of 14 α isoforms and β, ε, ω, and κ isoforms[66]. Immunoregulatory therapy with type I IFNs such as IFN-α or IFN-β can inhibit production of TNF-α and IFN-γ, antagonize the IFN-γ signaling pathway and increase production of the antiinflammatory cytokine IL-10. It has also been shown to be immunoregulated by enhanced regulatory T lymphocyte and NK cell activity[66].
Results of clinical trials: Several type I IFNs have been studied in UC. A phase 2 placebo-controlled, dose-ranging trial, studied IFN-β1a in 194 patients with moderately active UC. Clinical outcomes, including the proportion of patients achieving endoscopically confirmed remission, were not statistically significantly superior in the IFN-β1a treatment groups over placebo[96]. A randomized, placebo-controlled trial of pegylated IFN-α in 60 patients with active UC did not show any efficacy in clinical response and response rate despite a significant decrease in levels of C reactive protein[97].
WHERE DO WE GO FROM HERE?
In 2010, infliximab represents the pinnacle of the therapeutic pyramid of IBD treatment. However, this anti-TNF agent has several limitations. First, despite its widespread use in IBD, 20% of patients still require surgery[98]. Second, about 10% of patients are primary non-responders to infliximab and only one-third of IBD patients are in clinical remission at 1 year[9,98]. Third, the annual risk of loss of response is 13% per patient-year[99]. Finally, infliximab treatment optimization with combination therapy can be considered, but this must be weighed against the increased risk of serious infections and perhaps lymphoma. These data underscore the urgent need to develop new drug classes.
Humanized IL-12/23 antibodies seem the most promising therapy for the future: (1) IL-23 is an essential mediator for the differentiation and amplification of the proinflammatory Th17 pathway; (2) its role is underscored by the increased host susceptibility for IBD in cases of polymorphism of the gene encoding the receptor for this cytokine; and (3) the effective results observed in a recent randomized, controlled trial, particularly in cases of infliximab withdrawal. Phase III trials are ongoing in IBD patients.
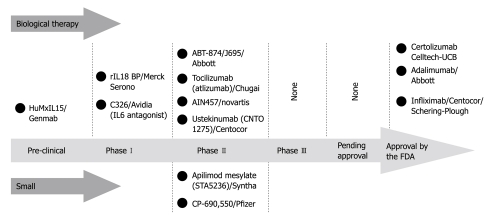
Recent advances in the pathophysiology of IBD have led to the identification of additional cytokine pathways representing potential therapeutic targets. Numerous other cytokines are currently under investigation: IL-27, produced mainly by dendritic cells, acting in the differentiation of both Th1 and Th2 cells; IL-32, produced by NK cell-activated lymphocytes and epithelial cells, providing a proinflammatory amplification pathway in the innate immune responses to bacteria[7]; IL-31, preferentially produced by T cells skewed towards a Th2 phenotype, playing a role in the acute phase of inflammation by maintaining proliferation of B and T cells[6]. Further studies are needed to fully explore their different roles in human IBD, and their biological significance, to eventually determine the therapeutic implications (Figure 3).
Figure 3.
Cytokine therapies and inflammatory bowel disease: pipeline compounds.
To overcome anti-TNF therapy failure in IBD, one way would be to develop more targeted therapy[100]. A humanized TNF receptor-1 specific antagonistic antibody for selective inhibition of TNF action has shown interesting results in animal experiments[100]. Avimer proteins or nanobodies look promising, offering multiple advantages with a low immunogenicity, a high ligand affinity, a high specificity, oral bioavailability and a low cost[101]. Another way would be to use cytokine therapy in association with other anti-cytokine agents. The efficacy of TNF-α antagonist agents alone reflects probably the pleiotropic effects of TNF-α[2]. An effective treatment strategy for patients might therefore involve the blockade of multiple cytokines in order to intervene in several pathways[102]. Animal studies in rheumatoid arthritis showed that anti-CD4 therapy acts synergistically with anti-TNF-α in improving established collagen-induced arthritis[103]. In IBD, a safety study suggested several positive trends in improving efficacy when natalizumab was added to infliximab treatment[104]. Further investigations are necessary to better evaluate the cost-effectiveness and long-term safety profile of these associations.
CONCLUSION
Despite recent advances in the pathophysiology of IBD, leading to the identification and understanding of several cytokine pathways, anti-TNF-α agents still represent the pinnacle of the therapeutic pyramid of IBD treatment. The humanized IL-12/23 antibodies appear to be the most promising therapy. Future directions could include the development of more targeted therapy or therapeutic blockade of multiple cytokines in order to intervene in several pathways.
Footnotes
Peer reviewer: Takayuki Yamamoto, MD, Inflammatory Bowel Disease Center, Yokkaichi Social Insurance Hospital, 10-8 Hazuyamacho, Yokkaichi 510-0016, Japan
S- Editor Tian L L- Editor Cant MR E- Editor Lin YP
References
- 1.Monteleone G, Caprioli F. Why are molecular mechanisms of immune activation important in IBD? Inflamm Bowel Dis. 2008;14 Suppl 2:S106–S107. doi: 10.1002/ibd.20678. [DOI] [PubMed] [Google Scholar]
- 2.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peyrin-Biroulet L, Desreumaux P, Sandborn WJ, Colombel JF. Crohn's disease: beyond antagonists of tumour necrosis factor. Lancet. 2008;372:67–81. doi: 10.1016/S0140-6736(08)60995-2. [DOI] [PubMed] [Google Scholar]
- 4.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 5.Bamias G, Nyce MR, De La Rue SA, Cominelli F. New concepts in the pathophysiology of inflammatory bowel disease. Ann Intern Med. 2005;143:895–904. doi: 10.7326/0003-4819-143-12-200512200-00007. [DOI] [PubMed] [Google Scholar]
- 6.Andoh A, Yagi Y, Shioya M, Nishida A, Tsujikawa T, Fujiyama Y. Mucosal cytokine network in inflammatory bowel disease. World J Gastroenterol. 2008;14:5154–5161. doi: 10.3748/wjg.14.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fantini MC, Monteleone G, Macdonald TT. New players in the cytokine orchestra of inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:1419–1423. doi: 10.1002/ibd.20212. [DOI] [PubMed] [Google Scholar]
- 8.Podolsky DK. Beyond tumor necrosis factor: next-generation biologic therapy for inflammatory bowel disease. Dig Dis. 2009;27:366–369. doi: 10.1159/000228575. [DOI] [PubMed] [Google Scholar]
- 9.Peyrin-Biroulet L, Deltenre P, de Suray N, Branche J, Sandborn WJ, Colombel JF. Efficacy and safety of tumor necrosis factor antagonists in Crohn's disease: meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol. 2008;6:644–653. doi: 10.1016/j.cgh.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 11.Korzenik JR, Podolsky DK. Evolving knowledge and therapy of inflammatory bowel disease. Nat Rev Drug Discov. 2006;5:197–209. doi: 10.1038/nrd1986. [DOI] [PubMed] [Google Scholar]
- 12.Rutgeerts P, Vermeire S, Van Assche G. Biological therapies for inflammatory bowel diseases. Gastroenterology. 2009;136:1182–1197. doi: 10.1053/j.gastro.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–267. [PubMed] [Google Scholar]
- 14.Armstrong AM, Gardiner KR, Kirk SJ, Halliday MI, Rowlands BJ. Tumour necrosis factor and inflammatory bowel disease. Br J Surg. 1997;84:1051–1058. [PubMed] [Google Scholar]
- 15.Scaldaferri F, Fiocchi C. Inflammatory bowel disease: progress and current concepts of etiopathogenesis. J Dig Dis. 2007;8:171–178. doi: 10.1111/j.1751-2980.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 16.Targan SR, Karp LC. Defects in mucosal immunity leading to ulcerative colitis. Immunol Rev. 2005;206:296–305. doi: 10.1111/j.0105-2896.2005.00286.x. [DOI] [PubMed] [Google Scholar]
- 17.Hvas CL, Kelsen J, Agnholt J, Höllsberg P, Tvede M, Møller JK, Dahlerup JF. Crohn's disease intestinal CD4+ T cells have impaired interleukin-10 production which is not restored by probiotic bacteria. Scand J Gastroenterol. 2007;42:592–601. doi: 10.1080/00365520601013754. [DOI] [PubMed] [Google Scholar]
- 18.Saruta M, Yu QT, Fleshner PR, Mantel PY, Schmidt-Weber CB, Banham AH, Papadakis KA. Characterization of FOXP3+CD4+ regulatory T cells in Crohn's disease. Clin Immunol. 2007;125:281–290. doi: 10.1016/j.clim.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Shih DQ, Targan SR. Insights into IBD Pathogenesis. Curr Gastroenterol Rep. 2009;11:473–480. doi: 10.1007/s11894-009-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 21.Brand S. Crohn's disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut. 2009;58:1152–1167. doi: 10.1136/gut.2008.163667. [DOI] [PubMed] [Google Scholar]
- 22.Braegger CP, Nicholls S, Murch SH, Stephens S, MacDonald TT. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89–91. doi: 10.1016/0140-6736(92)90999-j. [DOI] [PubMed] [Google Scholar]
- 23.Breese EJ, Michie CA, Nicholls SW, Murch SH, Williams CB, Domizio P, Walker-Smith JA, MacDonald TT. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994;106:1455–1466. doi: 10.1016/0016-5085(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 24.Esposito E, Cuzzocrea S. TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr Med Chem. 2009;16:3152–3167. doi: 10.2174/092986709788803024. [DOI] [PubMed] [Google Scholar]
- 25.Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705–1709. doi: 10.1136/gut.34.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul AT, Gohil VM, Bhutani KK. Modulating TNF-alpha signaling with natural products. Drug Discov Today. 2006;11:725–732. doi: 10.1016/j.drudis.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Pober JS, Lapierre LA, Stolpen AH, Brock TA, Springer TA, Fiers W, Bevilacqua MP, Mendrick DL, Gimbrone MA Jr. Activation of cultured human endothelial cells by recombinant lymphotoxin: comparison with tumor necrosis factor and interleukin 1 species. J Immunol. 1987;138:3319–3324. [PubMed] [Google Scholar]
- 28.Slowik MR, De Luca LG, Fiers W, Pober JS. Tumor necrosis factor activates human endothelial cells through the p55 tumor necrosis factor receptor but the p75 receptor contributes to activation at low tumor necrosis factor concentration. Am J Pathol. 1993;143:1724–1730. [PMC free article] [PubMed] [Google Scholar]
- 29.Estrada C, Gómez C, Martín C, Moncada S, González C. Nitric oxide mediates tumor necrosis factor-alpha cytotoxicity in endothelial cells. Biochem Biophys Res Commun. 1992;186:475–482. doi: 10.1016/s0006-291x(05)80832-0. [DOI] [PubMed] [Google Scholar]
- 30.Yoshizumi M, Perrella MA, Burnett JC Jr, Lee ME. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ Res. 1993;73:205–209. doi: 10.1161/01.res.73.1.205. [DOI] [PubMed] [Google Scholar]
- 31.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 32.Pappu BP, Borodovsky A, Zheng TS, Yang X, Wu P, Dong X, Weng S, Browning B, Scott ML, Ma L, et al. TL1A-DR3 interaction regulates Th17 cell function and Th17-mediated autoimmune disease. J Exp Med. 2008;205:1049–1062. doi: 10.1084/jem.20071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tremelling M, Berzuini C, Massey D, Bredin F, Price C, Dawson C, Bingham SA, Parkes M. Contribution of TNFSF15 gene variants to Crohn's disease susceptibility confirmed in UK population. Inflamm Bowel Dis. 2008;14:733–737. doi: 10.1002/ibd.20399. [DOI] [PubMed] [Google Scholar]
- 34.Yamazaki K, McGovern D, Ragoussis J, Paolucci M, Butler H, Jewell D, Cardon L, Takazoe M, Tanaka T, Ichimori T, et al. Single nucleotide polymorphisms in TNFSF15 confer susceptibility to Crohn's disease. Hum Mol Genet. 2005;14:3499–3506. doi: 10.1093/hmg/ddi379. [DOI] [PubMed] [Google Scholar]
- 35.Bamias G, Martin C 3rd, Marini M, Hoang S, Mishina M, Ross WG, Sachedina MA, Friel CM, Mize J, Bickston SJ, et al. Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J Immunol. 2003;171:4868–4874. doi: 10.4049/jimmunol.171.9.4868. [DOI] [PubMed] [Google Scholar]
- 36.Takedatsu H, Michelsen KS, Wei B, Landers CJ, Thomas LS, Dhall D, Braun J, Targan SR. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology. 2008;135:552–567. doi: 10.1053/j.gastro.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 38.Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333; quiz 591. doi: 10.1053/j.gastro.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 39.Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 40.Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 41.Sandborn WJ, Feagan BG, Stoinov S, Honiball PJ, Rutgeerts P, Mason D, Bloomfield R, Schreiber S. Certolizumab pegol for the treatment of Crohn's disease. N Engl J Med. 2007;357:228–238. doi: 10.1056/NEJMoa067594. [DOI] [PubMed] [Google Scholar]
- 42.Schreiber S, Khaliq-Kareemi M, Lawrance IC, Thomsen OØ, Hanauer SB, McColm J, Bloomfield R, Sandborn WJ. Maintenance therapy with certolizumab pegol for Crohn's disease. N Engl J Med. 2007;357:239–250. doi: 10.1056/NEJMoa062897. [DOI] [PubMed] [Google Scholar]
- 43.Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, Podolsky DK, Sands BE, Braakman T, DeWoody KL, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398–1405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 44.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 45.Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Chen DM, Pritchard ML, Sandborn WJ. Serious infections and mortality in association with therapies for Crohn's disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621–630. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Stokkers PC, Hommes DW. New cytokine therapeutics for inflammatory bowel disease. Cytokine. 2004;28:167–173. doi: 10.1016/j.cyto.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 47.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 48.Trinchieri G. Proinflammatory and immunoregulatory functions of interleukin-12. Int Rev Immunol. 1998;16:365–396. doi: 10.3109/08830189809043002. [DOI] [PubMed] [Google Scholar]
- 49.Mannon PJ, Fuss IJ, Mayer L, Elson CO, Sandborn WJ, Present D, Dolin B, Goodman N, Groden C, Hornung RL, et al. Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med. 2004;351:2069–2079. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- 50.Monteleone G, Biancone L, Marasco R, Morrone G, Marasco O, Luzza F, Pallone F. Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997;112:1169–1178. doi: 10.1016/s0016-5085(97)70128-8. [DOI] [PubMed] [Google Scholar]
- 51.Parronchi P, Romagnani P, Annunziato F, Sampognaro S, Becchio A, Giannarini L, Maggi E, Pupilli C, Tonelli F, Romagnani S. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn's disease. Am J Pathol. 1997;150:823–832. [PMC free article] [PubMed] [Google Scholar]
- 52.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 53.McGeachy MJ, Cua DJ. The link between IL-23 and Th17 cell-mediated immune pathologies. Semin Immunol. 2007;19:372–376. doi: 10.1016/j.smim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 54.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet. 2008;40:955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sandborn WJ, Feagan BG, Fedorak RN, Scherl E, Fleisher MR, Katz S, Johanns J, Blank M, Rutgeerts P. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn's disease. Gastroenterology. 2008;135:1130–1141. doi: 10.1053/j.gastro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 57.Sands BE, Jacobson EW, Sylwestrowicz T, Younes Z, Dryden G, Fedorak R, Greenbloom S. Randomized, double-blind, placebo-controlled trial of the oral interleukin-12/23 inhibitor apilimod mesylate for treatment of active Crohn's disease. Inflamm Bowel Dis. 2010;16:1209–1218. doi: 10.1002/ibd.21159. [DOI] [PubMed] [Google Scholar]
- 58.Kishimoto T. The biology of interleukin-6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 59.Fonseca JE, Santos MJ, Canhão H, Choy E. Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmun Rev. 2009;8:538–542. doi: 10.1016/j.autrev.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 61.Veldhuis GJ, Willemse PH, Sleijfer DT, van der Graaf WT, Groen HJ, Limburg PC, Mulder NH, de Vries EG. Toxicity and efficacy of escalating dosages of recombinant human interleukin-6 after chemotherapy in patients with breast cancer or non-small-cell lung cancer. J Clin Oncol. 1995;13:2585–2593. doi: 10.1200/JCO.1995.13.10.2585. [DOI] [PubMed] [Google Scholar]
- 62.Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 63.Nancey S, Hamzaoui N, Moussata D, Graber I, Bienvenu J, Flourie B. Serum interleukin-6, soluble interleukin-6 receptor and Crohn's disease activity. Dig Dis Sci. 2008;53:242–247. doi: 10.1007/s10620-007-9849-6. [DOI] [PubMed] [Google Scholar]
- 64.Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, Matsumoto T, Yamamura T, Azuma J, Nishimoto N, et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn's disease. Gastroenterology. 2004;126:989–996; discussion 947. doi: 10.1053/j.gastro.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Nishimoto N, Nakahara H, Yoshio-Hoshino N, Mima T. Successful treatment of a patient with Takayasu arteritis using a humanized anti-interleukin-6 receptor antibody. Arthritis Rheum. 2008;58:1197–1200. doi: 10.1002/art.23373. [DOI] [PubMed] [Google Scholar]
- 66.Ghosh S, Chaudhary R, Carpani M, Playford R. Interfering with interferons in inflammatory bowel disease. Gut. 2006;55:1071–1073. doi: 10.1136/gut.2005.090134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suzuki M, Hisamatsu T, Podolsky DK. Gamma interferon augments the intracellular pathway for lipopolysaccharide (LPS) recognition in human intestinal epithelial cells through coordinated up-regulation of LPS uptake and expression of the intracellular Toll-like receptor 4-MD-2 complex. Infect Immun. 2003;71:3503–3511. doi: 10.1128/IAI.71.6.3503-3511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hommes DW, Mikhajlova TL, Stoinov S, Stimac D, Vucelic B, Lonovics J, Zákuciová M, D'Haens G, Van Assche G, Ba S, et al. Fontolizumab, a humanised anti-interferon gamma antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn's disease. Gut. 2006;55:1131–1137. doi: 10.1136/gut.2005.079392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reinisch W, de Villiers W, Bene L, Simon L, Rácz I, Katz S, Altorjay I, Feagan B, Riff D, Bernstein CN, et al. Fontolizumab in moderate to severe Crohn's disease: a phase 2, randomized, double-blind, placebo-controlled, multiple-dose study. Inflamm Bowel Dis. 2010;16:233–242. doi: 10.1002/ibd.21038. [DOI] [PubMed] [Google Scholar]
- 70.Reinisch W, Hommes DW, Van Assche G, Colombel JF, Gendre JP, Oldenburg B, Teml A, Geboes K, Ding H, Zhang L, et al. A dose escalating, placebo controlled, double blind, single dose and multidose, safety and tolerability study of fontolizumab, a humanised anti-interferon gamma antibody, in patients with moderate to severe Crohn's disease. Gut. 2006;55:1138–1144. doi: 10.1136/gut.2005.079434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gordon J, MacLean LD. A lymphocyte-stimulating factor produced in vitro. Nature. 1965;208:795–796. doi: 10.1038/208795a0. [DOI] [PubMed] [Google Scholar]
- 72.Chavez AR, Buchser W, Basse PH, Liang X, Appleman LJ, Maranchie JK, Zeh H, de Vera ME, Lotze MT. Pharmacologic administration of interleukin-2. Ann N Y Acad Sci. 2009;1182:14–27. doi: 10.1111/j.1749-6632.2009.05160.x. [DOI] [PubMed] [Google Scholar]
- 73.Lichtiger S, Present DH, Kornbluth A, Gelernt I, Bauer J, Galler G, Michelassi F, Hanauer S. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841–1845. doi: 10.1056/NEJM199406303302601. [DOI] [PubMed] [Google Scholar]
- 74.Strengell M, Sareneva T, Foster D, Julkunen I, Matikainen S. IL-21 up-regulates the expression of genes associated with innate immunity and Th1 response. J Immunol. 2002;169:3600–3605. doi: 10.4049/jimmunol.169.7.3600. [DOI] [PubMed] [Google Scholar]
- 75.Fina D, Sarra M, Fantini MC, Rizzo A, Caruso R, Caprioli F, Stolfi C, Cardolini I, Dottori M, Boirivant M, et al. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology. 2008;134:1038–1048. doi: 10.1053/j.gastro.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 76.Creed TJ, Norman MR, Probert CS, Harvey RF, Shaw IS, Smithson J, Anderson J, Moorghen M, Gupta J, Shepherd NA, et al. Basiliximab (anti-CD25) in combination with steroids may be an effective new treatment for steroid-resistant ulcerative colitis. Aliment Pharmacol Ther. 2003;18:65–75. doi: 10.1046/j.1365-2036.2003.01639.x. [DOI] [PubMed] [Google Scholar]
- 77.Creed TJ, Probert CS, Norman MN, Moorghen M, Shepherd NA, Hearing SD, Dayan CM. Basiliximab for the treatment of steroid-resistant ulcerative colitis: further experience in moderate and severe disease. Aliment Pharmacol Ther. 2006;23:1435–1442. doi: 10.1111/j.1365-2036.2006.02904.x. [DOI] [PubMed] [Google Scholar]
- 78.Van Assche G, Dalle I, Noman M, Aerden I, Swijsen C, Asnong K, Maes B, Ceuppens J, Geboes K, Rutgeerts P. A pilot study on the use of the humanized anti-interleukin-2 receptor antibody daclizumab in active ulcerative colitis. Am J Gastroenterol. 2003;98:369–376. doi: 10.1111/j.1572-0241.2003.07239.x. [DOI] [PubMed] [Google Scholar]
- 79.Van Assche G, Sandborn WJ, Feagan BG, Salzberg BA, Silvers D, Monroe PS, Pandak WM, Anderson FH, Valentine JF, Wild GE, et al. Daclizumab, a humanised monoclonal antibody to the interleukin 2 receptor (CD25), for the treatment of moderately to severely active ulcerative colitis: a randomised, double blind, placebo controlled, dose ranging trial. Gut. 2006;55:1568–1574. doi: 10.1136/gut.2005.089854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 81.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 82.Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, Sordat B, Gibbs VC, Aguet M. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. J Exp Med. 1998;187:571–578. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schreiber S, Heinig T, Thiele HG, Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995;108:1434–1444. doi: 10.1016/0016-5085(95)90692-4. [DOI] [PubMed] [Google Scholar]
- 84.Kelsall B. Interleukin-10 in inflammatory bowel disease. N Engl J Med. 2009;361:2091–2093. doi: 10.1056/NEJMe0909225. [DOI] [PubMed] [Google Scholar]
- 85.Schreiber S, Fedorak RN, Wild G, Gangl A, Targan S, Jacyna M, Wright JP, Kilian A, Cohard M, Lebeaut A, Tremaine WJ, the Ulcerative Colitis IL-10 Cooperative Study Group. Safety and tolerance of rHuIL-10 treatment in patients with mild/moderate active ulcerative colitis. Gastroenterology. 1998;114:A1080–A1081. [Google Scholar]
- 86.Schreiber S, Fedorak RN, Nielsen OH, Wild G, Williams CN, Nikolaus S, Jacyna M, Lashner BA, Gangl A, Rutgeerts P, et al. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn's disease. Crohn's Disease IL-10 Cooperative Study Group. Gastroenterology. 2000;119:1461–1472. doi: 10.1053/gast.2000.20196. [DOI] [PubMed] [Google Scholar]
- 87.Colombel JF, Rutgeerts P, Malchow H, Jacyna M, Nielsen OH, Rask-Madsen J, Van Deventer S, Ferguson A, Desreumaux P, Forbes A, et al. Interleukin 10 (Tenovil) in the prevention of postoperative recurrence of Crohn's disease. Gut. 2001;49:42–46. doi: 10.1136/gut.49.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–1355. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 89.Du X, Williams DA. Interleukin-11: review of molecular, cell biology, and clinical use. Blood. 1997;89:3897–3908. [PubMed] [Google Scholar]
- 90.Herrlinger KR, Witthoeft T, Raedler A, Bokemeyer B, Krummenerl T, Schulzke JD, Boerner N, Kueppers B, Emmrich J, Mescheder A, et al. Randomized, double blind controlled trial of subcutaneous recombinant human interleukin-11 vs prednisolone in active Crohn's disease. Am J Gastroenterol. 2006;101:793–797. doi: 10.1111/j.1572-0241.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- 91.Leng SX, Elias JA. Interleukin-11 inhibits macrophage interleukin-12 production. J Immunol. 1997;159:2161–2168. [PubMed] [Google Scholar]
- 92.Trepicchio WL, Ozawa M, Walters IB, Kikuchi T, Gilleaudeau P, Bliss JL, Schwertschlag U, Dorner AJ, Krueger JG. Interleukin-11 therapy selectively downregulates type I cytokine proinflammatory pathways in psoriasis lesions. J Clin Invest. 1999;104:1527–1537. doi: 10.1172/JCI6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kiessling S, Muller-Newen G, Leeb SN, Hausmann M, Rath HC, Strater J, Spottl T, Schlottmann K, Grossmann J, Montero-Julian FA, et al. Functional expression of the interleukin-11 receptor alpha-chain and evidence of antiapoptotic effects in human colonic epithelial cells. J Biol Chem. 2004;279:10304–10315. doi: 10.1074/jbc.M312757200. [DOI] [PubMed] [Google Scholar]
- 94.Sands BE, Bank S, Sninsky CA, Robinson M, Katz S, Singleton JW, Miner PB, Safdi MA, Galandiuk S, Hanauer SB, Varilek GW, Buchman AL, Rodgers VD, Salzberg B, Cai B, Loewy J, DeBruin MF, Rogge H, Shapiro M, Schwertschlag US. Preliminary evaluation of safety and activity of recombinant human interleukin 11 in patients with active Crohn's disease. Gastroenterology. 1999;117:58–64. doi: 10.1016/s0016-5085(99)70550-0. [DOI] [PubMed] [Google Scholar]
- 95.Sands BE, Winston BD, Salzberg B, Safdi M, Barish C, Wruble L, Wilkins R, Shapiro M, Schwertschlag US. Randomized, controlled trial of recombinant human interleukin-11 in patients with active Crohn's disease. Aliment Pharmacol Ther. 2002;16:399–406. doi: 10.1046/j.1365-2036.2002.01179.x. [DOI] [PubMed] [Google Scholar]
- 96.Pena-Rossi C, Schreiber S, Golubovic G, Mertz-Nielsen A, Panes J, Rachmilewitz D, Shieh MJ, Simanenkov VI, Stanton D, Graffner H. Clinical trial: a multicentre, randomized, double-blind, placebo-controlled, dose-finding, phase II study of subcutaneous interferon-beta-1a in moderately active ulcerative colitis. Aliment Pharmacol Ther. 2008;28:758–767. doi: 10.1111/j.1365-2036.2008.03778.x. [DOI] [PubMed] [Google Scholar]
- 97.Tilg H, Vogelsang H, Ludwiczek O, Lochs H, Kaser A, Colombel JF, Ulmer H, Rutgeerts P, Krüger S, Cortot A, et al. A randomised placebo controlled trial of pegylated interferon alpha in active ulcerative colitis. Gut. 2003;52:1728–1733. doi: 10.1136/gut.52.12.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, Hoffman I, Van Steen K, Vermeire S, Rutgeerts P. Long-term outcome of treatment with infliximab in 614 patients with Crohn's disease: results from a single-centre cohort. Gut. 2009;58:492–500. doi: 10.1136/gut.2008.155812. [DOI] [PubMed] [Google Scholar]
- 99.Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn's disease: a review. Am J Gastroenterol. 2009;104:760–767. doi: 10.1038/ajg.2008.88. [DOI] [PubMed] [Google Scholar]
- 100.Kontermann RE, Münkel S, Neumeyer J, Müller D, Branschädel M, Scheurich P, Pfizenmaier K. A humanized tumor necrosis factor receptor 1 (TNFR1)-specific antagonistic antibody for selective inhibition of tumor necrosis factor (TNF) action. J Immunother. 2008;31:225–234. doi: 10.1097/CJI.0b013e31816a88f9. [DOI] [PubMed] [Google Scholar]
- 101.He MM, Smith AS, Oslob JD, Flanagan WM, Braisted AC, Whitty A, Cancilla MT, Wang J, Lugovskoy AA, Yoburn JC, et al. Small-molecule inhibition of TNF-alpha. Science. 2005;310:1022–1025. doi: 10.1126/science.1116304. [DOI] [PubMed] [Google Scholar]
- 102.Cominelli F. Cytokine-based therapies for Crohn's disease--new paradigms. N Engl J Med. 2004;351:2045–2048. doi: 10.1056/NEJMp048253. [DOI] [PubMed] [Google Scholar]
- 103.Williams RO, Mason LJ, Feldmann M, Maini RN. Synergy between anti-CD4 and anti-tumor necrosis factor in the amelioration of established collagen-induced arthritis. Proc Natl Acad Sci USA. 1994;91:2762–2766. doi: 10.1073/pnas.91.7.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sands BE, Kozarek R, Spainhour J, Barish CF, Becker S, Goldberg L, Katz S, Goldblum R, Harrigan R, Hilton D, et al. Safety and tolerability of concurrent natalizumab treatment for patients with Crohn's disease not in remission while receiving infliximab. Inflamm Bowel Dis. 2007;13:2–11. doi: 10.1002/ibd.20014. [DOI] [PubMed] [Google Scholar]