Abstract
Inflammatory bowel disease (IBD) arises from disruption of immune tolerance to the gut commensal microbiota, leading to chronic intestinal inflammation and mucosal damage in genetically predisposed hosts. In healthy individuals the intestinal microbiota have a symbiotic relationship with the host organism and possess important and unique functions, including a metabolic function (i.e. digestion of dietary compounds and xenobiotics, fermentation of undigestible carbohydrates with production of short chain fatty acids), a mucosal barrier function (i.e. by inhibiting pathogen invasion and strengthening epithelial barrier integrity), and an immune modulatory function (i.e. mucosal immune system priming and maintenance of intestinal epithelium homeostasis). A fine balance regulates the mechanism that allows coexistence of mammals with their commensal bacteria. In IBD this mechanism of immune tolerance is impaired because of several potential causative factors. The gut microbiota composition and activity of IBD patients are abnormal, with a decreased prevalence of dominant members of the human commensal microbiota (i.e. Clostridium IXa and IV groups, Bacteroides, bifidobacteria) and a concomitant increase in detrimental bacteria (i.e. sulphate-reducing bacteria, Escherichia coli). The observed dysbiosis is concomitant with defective innate immunity and bacterial killing (i.e. reduced mucosal defensins and IgA, malfunctioning phagocytosis) and overaggressive adaptive immune response (due to ineffective regulatory T cells and antigen presenting cells), which are considered the basis of IBD pathogenesis. However, we still do not know how the interplay between these parameters causes the disease. Studies looking at gut microbial composition, epithelial integrity and mucosal immune markers in genotyped IBD populations are therefore warranted to shed light on this obscure pathogenesis.
Keywords: Microbiota, Inflammatory bowel disease, Microbial dysbiosis, Immune tolerance, Innate immunity, Mucosal barrier
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic, relapsing inflammatory disorder affecting the gastrointestinal tract which involves an imbalanced host-commensal microbiota interaction. Crohn’s disease (CD) and ulcerative colitis (UC) are commonly included in the collective term IBD, although the two diseases present with distinct pathogenesis, symptomatology, inflammatory profiles and gut microbiota composition. Inflammation associated with CD is discontinuous, may extend deeply into the submucosal regions and occurs anywhere along the alimentary canal. In UC, inflammation involves only the superficial layers of the intestinal mucosa and is localised to regions of the gut most highly colonized by bacteria, starting at the distal colon and moving proximally along the large bowel[1]. CD is predominantly associated with a type 1 helper-T-cell (Th1) and type 17 helper-T-cell (Th17) immune responses, characterized by increased production of interleukin (IL)-12, IL-23, IL-27, interferon-γ (IFN-γ) and tumor necrosis factor (TNF)-α. Diversely, UC seems to be associated with a type 2 helper-T cell (Th2) immune response, mainly leading to raised levels of IL-5 and transforming growth factor-β (TGF-β)[2]. The etiology of IBD is complex and multifactorial, where environmental, genetic and immunological components appear to play a role[3].
A consistent body of evidence implicates the gut microbiota in the pathogenesis of IBD, including the consideration that inflammation mainly occurs in the intestinal sites with the highest bacterial concentration (in UC), that antibiotic treatment often results in amelioration of disease symptoms[4], and that germ-free mice do not spontaneously initiate colitis[5]. The most extensively investigated hypothesis is that IBD development might be due to an altered immune response and a disrupted mechanism of host tolerance to the non-pathogenic resident microbiota, leading to an elevated inflammatory response.
THE HUMAN INTESTINAL MICROBIOTA
The adult human gut contains around 1014 bacterial cells and up to an estimated 1000 different bacterial species, thus constituting the largest microbial community associated with the human body[6]. Recent studies using culture-independent molecular microbiological techniques have shown that the most abundant bacterial phyla found in the healthy human large intestine are the Gram-negative Bacteroidetes and the Gram-positive, low GC% Firmicutes[6,7]. Proteobacteria, Actinobacteria, Fusobacteria and Verrucomicrobia phyla are relatively less abundant, but nonetheless are known to play important roles in human health[6]. The same studies have described the vast diversity of bacterial species and identified the dominant bacterial groups to be Clostridium coccoides (C. coccoides)-Eubacterium rectale, Clostridium leptum (C. leptum), Bacteroides-Prevotella, Bifidobacterium species and Atopobium species[8]. The gut microbial species composition varies greatly between individuals, with each individual harboring a unique collection of bacterial species, which is highly stable over time[9]. Zoetendal et al[10] also showed that the gut microbiota composition of spouses, who were living in the same environment and had similar eating habits, showed the least degree of species similarity, while siblings showed increased similarity in species make-up. Interestingly, the gut microbiota profiles of identical twins showed a high degree of similarity, but were yet distinct. These findings highlight that genetic factors play an important role in gut microbiota development, although environment also drives species acquisition. Studies have shown that the vast majority of intestinal bacteria are novel, new to science and so far resist cultivation using traditional culture techniques, necessitating the use of culture-independent molecular microbiology techniques, such as 16S rRNA gene probing and polymerase chain reaction (PCR)-based strategies.
Recently, the human body together with its gut microbiota has been referred to as a “superorganism” comprised of human and bacterial genes[11]. It has been estimated that the human gut microbiome consists of 100 times more genes than the human genome. Therefore, the presence of the intestinal microbiota enriches the human organism with important functions, especially functions involved in deriving energy from nutrients which escape digestion in the upper gut and the metabolism of xenobiotics. The gut microbiota acts as a “metabolic organ”, through breakdown of complex indigestible dietary carbohydrates and proteins, with consequent generation of fermentation end-products (short chain fatty acids, ethanol and gas) and also through production of vitamins, ion absorption and conversion of dietary polyphenolic compounds into their active form[12,13]. The commensal microbiota contribute to the “barrier effect”, which constitutes a real obstacle to pathogen invasion of the intestinal mucosa. Recent studies have shown that a modulation of the gut microbiota through dietary supplementation with a prebiotic (i.e. oligofructose) increases epithelial barrier integrity by increasing the expression of tight junctions proteins (i.e. ZO-1 and occludin), with a mechanism that is dependent on the augmented secretion of the GLP-2 gut hormone[14]. The immune regulatory function of the intestinal microbiota consists of priming the mucosal immune system and maintenance of intestinal epithelium homeostasis. Studies in germ-free animals have demonstrated that the normal functioning of intestinal epithelial cells (IEC) and of the underlying immune cells are impaired in the absence of the gut microbiota. IEC expression of microbial recognition receptors, defensins and antimicrobial peptides are reduced in germ-free animals[15,16]. Defective development of gut-associated lymphoid tissues, antibody production (i.e. sIgA) and maturation of isolated lymphoid follicles have also been shown in germ-free animals, together with reduced Peyer’s patches and mesenteric lymph node number and dimension[17,18].
IMMUNE TOLERANCE TO THE COMMENSAL MICROBIOTA
In health, finely balanced mechanisms regulate the host’s immunological tolerance to the continuous stimulus of the resident gut microbiota and their metabolic end-products. Microbial recognition by antigen presenting cells (i.e. dendritic cells, DC) and epithelial cells is mainly carried out through sensing of conserved microbial-associated molecular patterns (MAMPs) by toll-like receptors (TLR), capable of detecting a variety of bacterial components, such as lipopolysaccharide (LPS), lipoproteins, CpG DNA[19], and by nucleotide-binding oligomerisation domain (NOD)-like receptors (NLR), which recognise peptidoglycan molecules on the bacterial cell wall[20]. In healthy hosts the pro-inflammatory pathways associated with TLR and NLR are suppressed by inhibitory molecules of both human and bacterial origin [i.e. cyclooxygenase-2 (COX-2) inhibitors; LPS; A20; peroxisome proliferator-activated receptor-γ (PPAR-γ); nuclear factor-κB (NF-κB) inhibitor IκB-α; interferon-α/β (IFN-α/β); interleukin-10 (IL-10); TGF-β; eicosanoids][21,22]. Activated innate immune cells, such as mucosal DC, constantly sample luminal microbial antigens and present them to adaptive immune cells. Recent studies have shown that the intestine is home to specialised DC, whose function it is to induce a highly tolerogenic response from T and B cells, through induction of regulatory T cells (Treg) and secretion of IgA, respectively[23,24]. Commensal bacteria actively coordinate the host tolerogenic response, either through DC-mediated conversion of naïve T cells into Treg, or through direct ligation of TLRs on the surface of Treg. Certain resident bacterial populations, often referred to as “beneficial bacteria” (i.e. lactobacilli and bifidobacteria) can influence DC differentiation towards a more undifferentiated and monocyte-like phenotype, which may account for DC immune tolerance[25]. Moreover, incubation of monocyte-derived DC with probiotic bacteria was shown to induce DC maturation and cytokine secretion, with strain-specific cytokine secretive profiles[26]. Repetitive TLR stimulation due to commensal bacterial exposure induces down-regulation of the NF-κB pathway and stimulates production of antimicrobial peptides (i.e. defensins)[27]. Also, chronic NOD-2 stimulation has been demonstrated to lead to down-regulation of pro-inflammatory cytokines (TNF-α, IL-8, IL-1β) in primary human monocyte-derived macrophages after pre-treatment with muramyl dipeptide (MDP) and re-stimulation with NOD-2, TLR-2 and TLR-4 ligands[28]. Therefore, the host’s mechanism of tolerance to the resident microbiota offers, at the same time, protection from unwanted inflammatory responses and from pathogen invasion. Microbial ligands have also been shown to modulate the expression levels of miR-155, a miRNA that is involved in immune homeostasis and whose absence causes a reduction in Treg numbers in miR-155-deficient mice[29]. However, since commensal and pathogenic bacteria possess many common motifs that are immunologically recognised by the host, how the host can tolerate resident bacteria whilst being able to mount an effective inflammatory response to invading pathogens is still not fully understood. Nonetheless, pathogenic bacteria do differentiate themselves from commensals by their behaviour; breaching the intestinal epithelial barrier and, in healthy individuals, eliciting strong inflammatory reactions when they trigger MAMPs basolaterally on epithelial cells[30].
In IBD, the homeostatic mechanisms that allow coexistence of the host organism and the commensal microbiota are disrupted. Polymorphisms in TLR (TLR4 D299G associated with CD and UC; TLR1 L80P and TLR2 R753G, associated with pancolitis) and NLR (i.e. three mutations in NOD 2/CARD15 gene, Arg702Trp, Gly908Arg, and a frameshift deletion mutation at Leu1007, accounting for about 80% of all CD-associated mutations) have been implicated in increased susceptibility to IBD[19,31-33]. However, not everyone who carries these mutations develops IBD, indicating that other etiologic mechanisms might underlie IBD pathogenesis.
INTESTINAL MICROBIOTA IN IBD
Evidence from several recent studies has highlighted that gut microbiota composition and activity in IBD patients are abnormal. In particular, several studies have demonstrated that IBD patients are characterized by a reduced abundance of dominant members of the gut microbiota. Through a combination of PCR of total bacterial genomic DNA with universal bacterial primers and clone sequencing of 16S rRNA genes, Frank et al[34] showed that in mucosal biopsies taken from CD and UC patients there was reduced abundance of rRNA sequences associated with Firmicutes and Bacteroidetes, and a concomitant increase in 16S rRNA sequences of Proteobacteria and Actinobacteria, compared to non-IBD controls. In particular, the decreased relative abundance of the Firmicutes phylum was due to decreases in populations of Clostridium IXa and IV groups. As a consequence of this dysbiosis, the relative abundance of Enterobacteriaceae was increased in IBD patients compared to healthy controls, although their absolute numbers remained unaltered. No differences were observed in fecal and mucosal bacterial population numbers between CD and UC patients. These findings are common to several other studies, which also observed decreased clostridia concentrations in IBD[35,36], although not always accompanied by a decrease in Bacteroides[34,37].
Aberrancies in Bifidobacterium populations in IBD have also been previously observed in another study, where significantly lower counts of bifidobacteria were found in rectal biopsies of patients with UC compared to patients without UC[38]. By employing fluorescent in situ hybridization (FISH), Macfarlane et al[38] showed that bacteria belonging to the C. leptum phylogenetic group were significantly less abundant in fecal samples of CD patients compared to healthy individuals. Moreover, through a metagenomic approach, the same authors reported a conspicuous loss of microbial diversity in CD, mainly due to a reduction of operational taxonomic units (OTU) within the C. coccoides group and the C. leptum group. A reduction in bacterial diversity was also previously observed by Ott et al[39] after analysis of mucosa-associated microbiota of CD and UC patients through a combination of single strand conformation polymorphism (SSCP) fingerprint, cloning and real time PCR. Additionally, Zhang et al[40] more recently showed that bacterial diversity of lactobacilli and C. leptum group as determined by denaturing gradient gel electrophoresis (DGGE) analysis was also lower in ulcerated tissues compared to the non-ulcerated tissues within the same UC individual. These results suggest that microbial alteration in IBD patients might be caused by the physiological state of the intestinal mucosa. However, little is known about how inflammatory mediators (e.g. pro-inflammatory cytokines and chemokines) on the gut wall affect bacterial populations in vivo. We do know, however, that altered microbial composition may impact on important physiological processes in the intestinal environment. Clostridium and Bacteroides species are the main producers of short chain fatty acids (SCFA) in the human colon. Decreased clostridia of groups IV and XIVa, the main butyrate-producing bacteria in the gut, could therefore explain the decreased SCFA concentrations found in fecal samples of IBD patients. Among the SCFA produced upon carbohydrate fermentation, butyrate serves as a major source of energy for colonic epithelial cells[41] and as an inhibitor of pro-inflammatory cytokine expression in the intestinal mucosa, through a mechanism that involves hyperacetylation of histones and suppression of NF-κB signaling[42]. Moreover, butyrate reinforces the mucosal barrier by inducing production of mucin and antimicrobial peptides, and by strengthening epithelial barrier integrity through directly increasing the expression of tight junction proteins[43]. A decrease of butyrate levels could therefore be involved in the increased inflammatory state characteristic of IBD, and butyrate is already considered to be of possible therapeutic value in treating IBD[44-46]. Stimulation of butyric acid production could be achieved through repopulation of clostridial clusters IV and XIVa, or even through probiotic therapy with lactic acid bacteria, by increasing butyrate production through enhancement of carbohydrate fermentation (i.e. by supplementation with butyrogenic prebiotics such as inulin or oligofructose). Lactic acid can be employed as substrate for the production of high concentrations of butyrate by clostridial cluster XIVa, in a process also known as cross-feeding[47]. Faecalibacterium prausnitzii (F. prausnitzii), a prevalent member of the human gut microbiota belonging to clostridial cluster IV and an important butyrate producer, has been recently shown to be less abundant in the intestinal microbiota of IBD patients[48,49]. In vitro and in vivo animal studies have also demonstrated the anti-inflammatory and anti-colitic properties of supernatants from F. prausnitzii cultures in peripheral blood mononuclear cells or in mouse models of colitis, respectively[48]. This effect appeared to be due to an as yet unidentified metabolite produced by the microorganism, but was shown to be independent of butyrate production.
Overgrowth of a class of microorganisms referred to as sulphate-reducing bacteria (SRB) was also previously observed in IBD gut microbiota in concomitance with a decrease in clostridia of groups IV and XIVa, especially in UC and pouchitis patients[50]. SRB metabolize sulphate into hydrogen sulphide, which is toxic to colonocytes, blocks butyrate utilization, induces cell hyperproliferation, and inhibits phagocytosis and bacterial killing[51]. It was previously demonstrated that the presence of intestinal microorganisms is necessary for induction of dextran sodium sulphate (DSS) colitis in animal models, thus emphasizing the possible role of SRB in IBD, through their reduction of sulphate in DSS into the cytotoxic and inflammatory trigger molecule H2S[52]. SRB numbers or their metabolic activity were found to be significantly higher in studies comparing UC patients to controls or to UC patients in remission[53-55].
In the search for a putative microbial cause of IBD, the theory of bacterial pathogen-induced intestinal inflammation has also been put forward. A wide range of microorganisms have been suggested as etiologic agents of IBD, including mycobacteria, Listeria monocytogenes (L. monocytogenes), Chlamydia, Enterobacteriaceae [including strains of Escherichia coli (E. coli) and Helicobacter] and also reoviruses and paramyxovirus[56-58]. However, when considering the diversity of IBD lesions and disease course, and the fact that no single pathogenic agent can routinely be isolated from diseased tissue, there is no conclusive evidence that a single pathogen is the cause of the disease. Among the Enterobacteriaceae genus, E. coli is the bacterium most commonly related to IBD. It was observed that IBD patients harbor increased Enterobacteriaceae, in particular E. coli belonging to the B2+D group (i.e. with increased virulent potential), compared to controls[59]. Adherent invasive E. coli was commonly found in ileal CD patients, particularly associated with ileal mucosal lesions[60,61]. On the other hand, E. coli isolated from UC patients was less invasive compared to CD[62]. Mycobacterium avium subspecies paratuberculosis (MAP) is an obligate intracellular pathogen that causes spontaneous granulomatous enterocolitis in cattle by evading phagocytosis. Therefore, MAP infection would be favored in those individuals with defective innate immunological defenses, such as CD patients. MAP presence was found with significantly higher frequency in CD patients compared to non-IBD controls, but not in all individuals[63]. No significant correspondence was found between CD-associated NOD-2 polymorphisms, especially in ileal CD, and MAP infection[64,65]. Moreover, clinical studies failed to demonstrate the efficacy of antimycobacterium triple antibiotic therapy in inducing persistent response in CD patients[66]. Detection of MAP by molecular techniques (i.e. detection of insertion element-900 (IS-900) by PCR) has the limitation of picking up environmental mycobacteria and presents high variability among laboratories[67-69]. Hence, the etiologic role of MAP in IBD pathogenesis remains to be demonstrated.
Therefore, microbial dysbiosis consisting of a decrease in beneficial bacteria and their metabolic end-products, together with an increase of detrimental bacterial populations and their toxic metabolites, might alter gut luminal environment; thus contributing to the pathogenesis of IBD.
COMPROMISED EPITHELIAL BARRIER FUNCTION, DEFECTIVE INNATE IMMUNE RESPONSE TO BACTERIA AND LOSS OF IMMUNOTOLERANCE
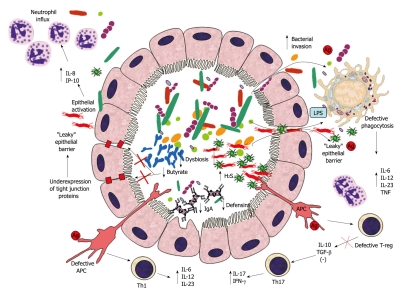
Efficient functioning of the gut mucosa is achieved by means of a combination of intact epithelial barrier and effective bacterial killing through secretion of antimicrobial peptides (e.g. defensins), secretory IgA and phagocytosis. In IBD these mechanisms of mucosal defence are compromised at all levels and they all contribute to disease progression. A potential mechanism of pathogenesis of IBD is summarized in Figure 1. Disease arises from the initial epithelial barrier dysfunction that brings about increased bacterial translocation through the lamina propria, where microbial antigens elicit a strong inflammatory response, due to ectopic (i.e. basolateral) TLR stimulation, activation of the NF-κB pathway and consequent induction of pro-inflammatory chemokine and cytokine secretion. This inflammatory process is aggravated by the decreased innate immune defense (i.e. reduced luminal defensin and IgA, defective phagocytosis in IBD), which amplifies the magnitude of bacterial translocation through the “leaky” epithelial layer. Disease progression mainly results from a more global defective immunoregulation and immunotolerance in response to the initial inflammatory insult, due to overaggressive T cell reaction, dysfunctional regulatory T cells and antigen presenting cells (APC) (Figure 1).
Figure 1.
Suggested mechanism of inflammatory bowel disease pathogenesis. Intestinal dysbiosis in inflammatory bowel disease (IBD) consists of decreased prevalence of putative beneficial bacteria (e.g. bifidobacteria) and concomitant increase in detrimental bacterial (e.g. sulphate-reducing bacteria). This microbial imbalance causes reduced intraluminal levels of butyrate (because of decreased production through fermentation and decreased utilization due to increased H2S levels), thus contributing to down-regulation of epithelial tight junction protein expression and increased epithelial permeability. Epithelial barrier dysfunction brings about increased bacterial translocation through the lamina propria, which is worsened by decreased luminal IgA and defensin concentrations. Killing of bacteria reaching the lamina propria through the “leaky” epithelium is also impaired by a genetically predisposed defective phagocytosis by macrophages. Ineffective bacterial clearance leads to excessive toll-like receptor (TLR) stimulation, secretion of pro-inflammatory cytokines and activation of innate and T-cell mediated immune responses. The disrupted mechanism of tolerance in epithelial cells and antigen presenting cells (APC) amplifies innate immune cell recruitment (i.e. neutrophils). Additionally, defective T-reg and APC cause excessive T-cell response (Th1 and Th17), with consequential intensification of the inflammatory response and granulomatous reaction. IL: Interleukin; IFN-γ: Interferon-γ; TNF: Tumor necrosis factor; TGF-β: Transforming growth factor-β; LPS: Lipopolysaccharide.
IBD, and especially CD, presents with a characteristic increased epithelial permeability, due to underexpression of certain tight junction proteins [e.g. claudins, junction adhesion molecule-A (JAM-A)] concomitant with up-regulation of other pore-forming proteins (i.e. claudin-2)[70,71]. Defective bacterial clearance due to impaired defensin and IgA production contributes to increased bacterial translocation from the gut lumen across the lamina propria. α-Defensins (i.e. human defensin 5 and 6 (HD5 and HD6)) are antibactericidal compounds produced by Paneth cells efficacious against Enterobacteriaceae (e.g. E. coli, Salmonella typhimurium, L. monocytogenes) and Bacteroides vulgatus, and were found significantly reduced in association with ileal CD, in particular in patients with NOD-2 mutations[72,73]. On the other hand, colonic CD, but not UC, was observed to be associated with lower copy number of β-defensins 2 and 3, which are the main antimicrobial peptides found in the colon. This reduction in β-defensins was shown to be due to a chromosomic polymorphism, since chromosome 8 presented with a lower copy number of β-defensin 2 in colonic CD[74,75].
Microbial clearance can also be impaired because of reduced levels of protective secretory IgA (SIgA) in IBD. IgA constitutes the most abundant immunoglobulin phenotype present in the human body[76]. In the gut, IgA is produced by lamina propria B cells, then translocates to the lumen by attaching to a basolateral receptor on epithelial cells, and finally is transported to the luminal surface of epithelial cells, where it forms SIgA clusters that elicit multiple roles in the intestinal lumen. Firstly, IgA in the mucus layer entraps bacteria and dietary antigens, down-regulates epitope expression on the bacterial cell surface and, therefore, regulates microbial intestinal colonization[77-79]. Moreover, SIgA prevents pathogen attachment and invasion of epithelial cells and removes bacteria breaching the epithelial barrier by translocating them back to the lumen and by promoting their clearance by dendritic cells, neutrophils and phagocytes[80-82]. In IBD, intestinal IgA is usually reduced and this is compensated for by increased secretion of IgG, which induces pro-inflammatory cytokine production and mounting of adaptive immune responses to the resident microbiota[83]. Mucosal secretory IgG was found to be significantly higher in UC and CD patients compared to control patients with irritable bowel syndrome[84]. In addition, the same study showed that both CD and UC patients presented with increased mucosal IgG bound to fecal bacterial cytoplasmic antigens compared to control patients with irritable bowel syndrome and to non-IBD controls with intestinal inflammation[84].
Malfunctioning bacterial killing in IBD has also recently been linked to dysfunctional autophagy. Autophagy is a constitutive pathway of cellular homeostasis and organelle turnover. However, it has recently been demonstrated that autophagy plays a key role in innate and adaptive immunity. Macrophages use autophagy to capture and effectively kill intracellular and extracellular invading bacterial pathogens, including Legionella, E. coli, Streptococcus and Mycobacterium species, by fusion of the phagocytic compartment with the lysosome[85,86]. Epithelial cells also employ autophagy to kill invading bacteria and the gene ATG 16L1 has been shown to be necessary for starting the autophagic process against the cytoplasmic invasion of Salmonella typhimurium[87]. Mutations in ATG 16L1 have recently been associated with CD, thus implicating defective bacterial killing by autophagy in IBD[87]. Autophagy impairment might also influence the adaptive immune response to bacteria, since autophagy is involved in major histocompatibility complex (MHC) class II loading in the lysosome, where the autophagic cytoplasmic content is also delivered[88]. Therefore, a defect in the autophagy pathway could influence antigen presentation by APC, epithelial cells and immune surveillance. Finally, autophagy has been implicated in the regulation of T cell death and proliferation, and ATG 16L1 is central to these autophagy-regulated processes[89]. Alteration of ATG 16L1 in CD might therefore, at least in part, explain the pathologic behaviour of T cells in IBD. In IBD the coexistence of compromised epithelial barrier and defective innate immunity aggravates the impaired mechanism of tolerance to the resident microbiota and causes inflammatory granulomatous reaction (Figure 1). Defective interaction between regulatory T lymphocytes in the lamina propria and epithelial cells is central to the process of loss of tolerance, through a mechanism that involves NF-κB signaling. Epithelial NF-κB activation in healthy hosts is normally suppressed by anti-inflammatory cytokines produced by the underlying T lymphocytes, such as TGF-β and IL-10, while in IBD Th1- and Th17-type immune responses are predominant and lead to chronic inflammation and worsening of the epithelial layer damage[90]. Perpetuation of the epithelial damage causes increased basolateral as opposed to physiological apical stimulation of TLR-9 receptors, thus causing activation, rather than blockade, of NF-κB signaling[30]. This leads to a vicious cycle of aberrant immune response, mucosal inflammation, altered microbiota composition and/or activity and increased mucosal permeability, which would explain the persistent and recurrent nature of IBD.
THERAPEUTIC IMPLICATIONS OF GUT MICROBIOTA-HUMAN HOST INTERACTION
The increasing understanding of the gut microbiota-host immune system interaction has recently drawn interest towards a modulation of intestinal bacterial communities as a novel potential adjuvant in IBD therapy. Although antibiotic therapy constitutes an established therapeutic tool for the treatment of specific IBD-associated symptoms (e.g. abscesses and fistulae), as well as a possible preventive measure, research studies that demonstrate antibiotic efficacy in IBD are still limited[91]. Promising outcomes have been observed after gut microbiota modulation through probiotic, prebiotic and synbiotic supplementation in CD and UC to change IBD-associated dysbiosis. Treating CD patients with the probiotic strain E. coli Nissle 1917 has been shown to induce remission more rapidly than untreated control patients, although it did not influence the number of patients achieving remission[92]. In UC, E. coli Nissle 1917 was proven as effective as mesalazine in maintaining remission[93,94]. Maintenance of remission after probiotic supplementation was observed in a study with the yeast probiotic Saccharomyces boulardii (reduced percentage of relapses in probiotic + mesalamine-treated CD patients, compared to control mesalamine-treated CD patients), although the significance of the study is somewhat restricted because of the low number of subjects involved (n = 32)[95,96]. Positive results were also observed in a double-blind, randomised controlled trial with Bifidobacterium breve and Bifidobacterium bifidum fermented milk supplementation in 20 UC subjects for 12 wk, where a significant decrease of clinical indices was observed compared to unsupplemented controls[97]. The probiotic mixture VSL#3 showed convincing effects in the maintenance of remission in UC patients[98-100], and it was later shown to prevent the onset of pouchitis[101]. On the other hand, the data with regard to VSL#3 supplementation in CD are still preliminary. VSL#3 supplementation did not result in a reduction in post-surgical relapse when administered to pediatric CD patients, compared to control mesalamine-treated patients[102]. In general, it appears that this probiotic supplementation is more effective in reducing disease onset or recurrence, rather than diminishing active inflammatory symptoms.
Prebiotic supplementation with inulin was shown to improve clinical condition in pouchitis patients, and to increase tolerance (i.e. through decreased TLR-2 and TLR-4 expression on DC) and fecal bifidobacteria levels in CD patients[103-105]. Synbiotics (i.e. a synergy of pro- and pre-biotics in a single preparation) also showed potential therapeutic effect, although the number of studies in IBD is still limited. Supplementation of the inulin-derived prebiotic, Synergy-1, together with Bifidobacterium longum, in 18 UC patients for 4 wk significantly decreased rectal pro-inflammatory cytokine levels and down-regulated the expression of inflammation-associated β-defensins[106].
In summary, some evidence has already indicated a promising therapeutic effect of pro-, pre- and synbiotics in IBD. However, the studies are still very few, underpowered and their design and selection of active agent are sometimes less than optimal. Indeed, this topic deserves further investigation in studies using an adequate number of subjects and employing functional food products targeting the gut microbiota, that have been specifically selected for their anti-inflammatory properties from preliminary in vitro and animal studies.
CONCLUSION
Despite the observation that IBD is associated with an abnormal gut microbiota composition, the question as to whether the altered gut microbial dysbiosis is a cause of disease or a consequence of the inflammatory state of the intestinal environment still remains unanswered. Although several studies implicate the gut microbiota in IBD pathogenesis, so far no pathogenic/infectious microorganism has been identified as sole disease causing agent. It is more likely that microbial dysbiosis and lack of beneficial bacteria, together with genetically predisposed increased epithelial permeability, bacterial translocation into the lamina propria, defective innate immunity and loss of tolerance to the resident microbiota, may lead to the abnormal inflammatory response and granulomatous reaction characteristic of IBD. A modulation of the gut microbiota through pro-, pre- and synbiotics, specifically designed to reduce IBD-associated dysbiosis and inflammation, constitutes an interesting approach in the field of novel therapeutic approaches for IBD.
Footnotes
Peer reviewers: Tauseef Ali, MD, Assistant Professor, Section of Digestive Diseases and Nutrition, University of Oklahoma Health Sciences Center, 920 SL Young Blvd, Oklahoma City, OK 73104, United States; Jürgen Büning, MD, Internal Medicine I, Department of Gastroenterology, University Hospital of Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany
S- Editor Shi ZF L- Editor Logan S E- Editor Ma WH
References
- 1.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 2.Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 3.Danese S, Fiocchi C. Etiopathogenesis of inflammatory bowel diseases. World J Gastroenterol. 2006;12:4807–4812. doi: 10.3748/wjg.v12.i30.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg GR. Antibiotics should be used as first-line therapy for Crohn's disease. Inflamm Bowel Dis. 2004;10:318–320. doi: 10.1097/00054725-200405000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis P, Scott KP, Duncan SH, Flint HJ. Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol. 2007;102:1197–1208. doi: 10.1111/j.1365-2672.2007.03322.x. [DOI] [PubMed] [Google Scholar]
- 8.Zoetendal EG, Vaughan EE, de Vos WM. A microbial world within us. Mol Microbiol. 2006;59:1639–1650. doi: 10.1111/j.1365-2958.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- 9.Zoetendal EG, Akkermans AD, De Vos WM. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl Environ Microbiol. 1998;64:3854–3859. doi: 10.1128/aem.64.10.3854-3859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoetendal EG, Akkermans ADL, Akkermans-van Vliet WM, de Visser JAGM, de Vos WM. The host genotype affects the bacterial community in the human gastronintestinal tract. Microb Ecol Health Dis. 2001;13:129–134. [Google Scholar]
- 11.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 13.Falony G, Vlachou A, Verbrugghe K, De Vuyst L. Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl Environ Microbiol. 2006;72:7835–7841. doi: 10.1128/AEM.01296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 15.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bry L, Falk PG, Midtvedt T, Gordon JI. A model of host-microbial interactions in an open mammalian ecosystem. Science. 1996;273:1380–1383. doi: 10.1126/science.273.5280.1380. [DOI] [PubMed] [Google Scholar]
- 17.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 18.Bouskra D, Brézillon C, Bérard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 19.Cario E. Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2. Gut. 2005;54:1182–1193. doi: 10.1136/gut.2004.062794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 21.Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V, Rao AS, Madara JL. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000;289:1560–1563. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 22.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ, et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tezuka H, Abe Y, Iwata M, Takeuchi H, Ishikawa H, Matsushita M, Shiohara T, Akira S, Ohteki T. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 25.Davies JM, Sheil B, Shanahan F. Bacterial signalling overrides cytokine signalling and modifies dendritic cell differentiation. Immunology. 2009;128:e805–e815. doi: 10.1111/j.1365-2567.2009.03086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Latvala S, Pietila TE, Veckman V, Kekkonen RA, Tynkkynen S, Korpela R, Julkunen I. Potentially probiotic bacteria induce efficient maturation but differential cytokine production in human monocyte-derived dendritic cells. World J Gastroenterol. 2008;14:5570–5583; discussion 5581-5582. doi: 10.3748/wjg.14.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng H, Wu H, Sloane V, Jones R, Yu Y, Lin P, Gewirtz AT, Neish AS. Flagellin/TLR5 responses in epithelia reveal intertwined activation of inflammatory and apoptotic pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G96–G108. doi: 10.1152/ajpgi.00273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedl M, Li J, Cho JH, Abraham C. Chronic stimulation of Nod2 mediates tolerance to bacterial products. Proc Natl Acad Sci USA. 2007;104:19440–19445. doi: 10.1073/pnas.0706097104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, Loeb GB, Lee H, Yoshimura A, Rajewsky K, et al. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30:80–91. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 31.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 32.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 33.Pierik M, Joossens S, Van Steen K, Van Schuerbeek N, Vlietinck R, Rutgeerts P, Vermeire S. Toll-like receptor-1, -2, and -6 polymorphisms influence disease extension in inflammatory bowel diseases. Inflamm Bowel Dis. 2006;12:1–8. doi: 10.1097/01.mib.0000195389.11645.ab. [DOI] [PubMed] [Google Scholar]
- 34.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gophna U, Sommerfeld K, Gophna S, Doolittle WF, Veldhuyzen van Zanten SJ. Differences between tissue-associated intestinal microfloras of patients with Crohn's disease and ulcerative colitis. J Clin Microbiol. 2006;44:4136–4141. doi: 10.1128/JCM.01004-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55:205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, Yantiss R, Orsi RH, Wiedmann M, McDonough P, Kim SG, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum. ISME J. 2007;1:403–418. doi: 10.1038/ismej.2007.52. [DOI] [PubMed] [Google Scholar]
- 38.Macfarlane S, Furrie E, Cummings JH, Macfarlane GT. Chemotaxonomic analysis of bacterial populations colonizing the rectal mucosa in patients with ulcerative colitis. Clin Infect Dis. 2004;38:1690–1699. doi: 10.1086/420823. [DOI] [PubMed] [Google Scholar]
- 39.Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Fölsch UR, Timmis KN, Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang M, Liu B, Zhang Y, Wei H, Lei Y, Zhao L. Structural shifts of mucosa-associated lactobacilli and Clostridium leptum subgroup in patients with ulcerative colitis. J Clin Microbiol. 2007;45:496–500. doi: 10.1128/JCM.01720-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fleming LL, Floch MH. Digestion and absorption of fiber carbohydrate in the colon. Am J Gastroenterol. 1986;81:507–511. [PubMed] [Google Scholar]
- 42.Segain JP, Raingeard de la Blétière D, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottière HM, Galmiche JP. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanhoutvin SA, Troost FJ, Hamer HM, Lindsey PJ, Koek GH, Jonkers DM, Kodde A, Venema K, Brummer RJ. Butyrate-induced transcriptional changes in human colonic mucosa. PLoS One. 2009;4:e6759. doi: 10.1371/journal.pone.0006759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breuer RI, Soergel KH, Lashner BA, Christ ML, Hanauer SB, Vanagunas A, Harig JM, Keshavarzian A, Robinson M, Sellin JH, et al. Short chain fatty acid rectal irrigation for left-sided ulcerative colitis: a randomised, placebo controlled trial. Gut. 1997;40:485–491. doi: 10.1136/gut.40.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanauchi O, Suga T, Tochihara M, Hibi T, Naganuma M, Homma T, Asakura H, Nakano H, Takahama K, Fujiyama Y, et al. Treatment of ulcerative colitis by feeding with germinated barley foodstuff: first report of a multicenter open control trial. J Gastroenterol. 2002;37 Suppl 14:67–72. doi: 10.1007/BF03326417. [DOI] [PubMed] [Google Scholar]
- 46.Galvez J, Rodríguez-Cabezas ME, Zarzuelo A. Effects of dietary fiber on inflammatory bowel disease. Mol Nutr Food Res. 2005;49:601–608. doi: 10.1002/mnfr.200500013. [DOI] [PubMed] [Google Scholar]
- 47.Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004;70:5810–5817. doi: 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, Doré J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 50.Roediger WE, Duncan A, Kapaniris O, Millard S. Reducing sulfur compounds of the colon impair colonocyte nutrition: implications for ulcerative colitis. Gastroenterology. 1993;104:802–809. doi: 10.1016/0016-5085(93)91016-b. [DOI] [PubMed] [Google Scholar]
- 51.Gardiner KR, Halliday MI, Barclay GR, Milne L, Brown D, Stephens S, Maxwell RJ, Rowlands BJ. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut. 1995;36:897–901. doi: 10.1136/gut.36.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohkusa T, Yamada M, Takenaga T, Kitazume C, Yamamoto N, Sasabe M, Takashimizu I, Tamura Y, Sakamoto E, Kurosawa H. [Protective effect of metronidazole in experimental ulcerative colitis induced by dextran sulfate sodium] Nippon Shokakibyo Gakkai Zasshi. 1987;84:2337–2346. [PubMed] [Google Scholar]
- 53.Gibson GR, Macfarlane GT, Cummings JH. Sulphate reducing bacteria and hydrogen metabolism in the human large intestine. Gut. 1993;34:437–439. doi: 10.1136/gut.34.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loubinoux J, Bronowicki JP, Pereira IA, Mougenel JL, Faou AE. Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol Ecol. 2002;40:107–112. doi: 10.1111/j.1574-6941.2002.tb00942.x. [DOI] [PubMed] [Google Scholar]
- 55.Pitcher MC, Beatty ER, Cummings JH. The contribution of sulphate reducing bacteria and 5-aminosalicylic acid to faecal sulphide in patients with ulcerative colitis. Gut. 2000;46:64–72. doi: 10.1136/gut.46.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munro J, Mayberry JF, Matthews N, Rhodes J. Chlamydia and Crohn’s disease. Lancet. 1979;2:45–46. doi: 10.1016/s0140-6736(79)90213-7. [DOI] [PubMed] [Google Scholar]
- 57.Burnham WR, Lennard-Jones JE, Stanford JL, Bird RG. Mycobacteria as a possible cause of inflammatory bowel disease. Lancet. 1978;2:693–696. doi: 10.1016/s0140-6736(78)92699-5. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, van Kruiningen HJ, West AB, Cartun RW, Cortot A, Colombel JF. Immunocytochemical evidence of Listeria, Escherichia coli, and Streptococcus antigens in Crohn’s disease. Gastroenterology. 1995;108:1396–1404. doi: 10.1016/0016-5085(95)90687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kotlowski R, Bernstein CN, Sepehri S, Krause DO. High prevalence of Escherichia coli belonging to the B2+D phylogenetic group in inflammatory bowel disease. Gut. 2007;56:669–675. doi: 10.1136/gut.2006.099796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Darfeuille-Michaud A, Neut C, Barnich N, Lederman E, Di Martino P, Desreumaux P, Gambiez L, Joly B, Cortot A, Colombel JF. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology. 1998;115:1405–1413. doi: 10.1016/s0016-5085(98)70019-8. [DOI] [PubMed] [Google Scholar]
- 61.Rolhion N, Darfeuille-Michaud A. Adherent-invasive Escherichia coli in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:1277–1283. doi: 10.1002/ibd.20176. [DOI] [PubMed] [Google Scholar]
- 62.Sasaki M, Sitaraman SV, Babbin BA, Gerner-Smidt P, Ribot EM, Garrett N, Alpern JA, Akyildiz A, Theiss AL, Nusrat A, et al. Invasive Escherichia coli are a feature of Crohn's disease. Lab Invest. 2007;87:1042–1054. doi: 10.1038/labinvest.3700661. [DOI] [PubMed] [Google Scholar]
- 63.Abubakar I, Myhill D, Aliyu SH, Hunter PR. Detection of Mycobacterium avium subspecies paratuberculosis from patients with Crohn's disease using nucleic acid-based techniques: a systematic review and meta-analysis. Inflamm Bowel Dis. 2008;14:401–410. doi: 10.1002/ibd.20276. [DOI] [PubMed] [Google Scholar]
- 64.Bernstein CN, Wang MH, Sargent M, Brant SR, Collins MT. Testing the interaction between NOD-2 status and serological response to Mycobacterium paratuberculosis in cases of inflammatory bowel disease. J Clin Microbiol. 2007;45:968–971. doi: 10.1128/JCM.02062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sechi LA, Gazouli M, Ikonomopoulos J, Lukas JC, Scanu AM, Ahmed N, Fadda G, Zanetti S. Mycobacterium avium subsp. paratuberculosis, genetic susceptibility to Crohn's disease, and Sardinians: the way ahead. J Clin Microbiol. 2005;43:5275–5277. doi: 10.1128/JCM.43.10.5275-5277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Selby W, Pavli P, Crotty B, Florin T, Radford-Smith G, Gibson P, Mitchell B, Connell W, Read R, Merrett M, et al. Two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for Crohn's disease. Gastroenterology. 2007;132:2313–2319. doi: 10.1053/j.gastro.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 67.Englund S, Bölske G, Johansson KE. An IS900-like sequence found in a Mycobacterium sp. other than Mycobacterium avium subsp. paratuberculosis. FEMS Microbiol Lett. 2002;209:267–271. doi: 10.1111/j.1574-6968.2002.tb11142.x. [DOI] [PubMed] [Google Scholar]
- 68.Bull TJ, McMinn EJ, Sidi-Boumedine K, Skull A, Durkin D, Neild P, Rhodes G, Pickup R, Hermon-Taylor J. Detection and verification of Mycobacterium avium subsp. paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn's disease. J Clin Microbiol. 2003;41:2915–2923. doi: 10.1128/JCM.41.7.2915-2923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Collins MT, Lisby G, Moser C, Chicks D, Christensen S, Reichelderfer M, Høiby N, Harms BA, Thomsen OO, Skibsted U, et al. Results of multiple diagnostic tests for Mycobacterium avium subsp. paratuberculosis in patients with inflammatory bowel disease and in controls. J Clin Microbiol. 2000;38:4373–4381. doi: 10.1128/jcm.38.12.4373-4381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vetrano S, Rescigno M, Cera MR, Correale C, Rumio C, Doni A, Fantini M, Sturm A, Borroni E, Repici A, et al. Unique role of junctional adhesion molecule-a in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology. 2008;135:173–184. doi: 10.1053/j.gastro.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 71.Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nuding S, Fellermann K, Wehkamp J, Stange EF. Reduced mucosal antimicrobial activity in Crohn's disease of the colon. Gut. 2007;56:1240–1247. doi: 10.1136/gut.2006.118646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wehkamp J, Harder J, Weichenthal M, Schwab M, Schäffeler E, Schlee M, Herrlinger KR, Stallmach A, Noack F, Fritz P, et al. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658–1664. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wehkamp J, Harder J, Weichenthal M, Mueller O, Herrlinger KR, Fellermann K, Schroeder JM, Stange EF. Inducible and constitutive beta-defensins are differentially expressed in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2003;9:215–223. doi: 10.1097/00054725-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 75.Fellermann K, Stange DE, Schaeffeler E, Schmalzl H, Wehkamp J, Bevins CL, Reinisch W, Teml A, Schwab M, Lichter P, et al. A chromosome 8 gene-cluster polymorphism with low human beta-defensin 2 gene copy number predisposes to Crohn disease of the colon. Am J Hum Genet. 2006;79:439–448. doi: 10.1086/505915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 77.Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424–1427. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- 78.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 79.Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthésy B. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17:107–115. doi: 10.1016/s1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 80.Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pasquier B, Launay P, Kanamaru Y, Moura IC, Pfirsch S, Ruffié C, Hénin D, Benhamou M, Pretolani M, Blank U, et al. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: dual role of FcRgamma ITAM. Immunity. 2005;22:31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 82.Phalipon A, Corthésy B. Novel functions of the polymeric Ig receptor: well beyond transport of immunoglobulins. Trends Immunol. 2003;24:55–58. doi: 10.1016/s1471-4906(02)00031-5. [DOI] [PubMed] [Google Scholar]
- 83.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 84.Macpherson A, Khoo UY, Forgacs I, Philpott-Howard J, Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut. 1996;38:365–375. doi: 10.1136/gut.38.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T, Nara A, Funao J, Nakata M, Tsuda K, et al. Autophagy defends cells against invading group A Streptococcus. Science. 2004;306:1037–1040. doi: 10.1126/science.1103966. [DOI] [PubMed] [Google Scholar]
- 86.Amer AO, Byrne BG, Swanson MS. Macrophages rapidly transfer pathogens from lipid raft vacuoles to autophagosomes. Autophagy. 2005;1:53–58. doi: 10.4161/auto.1.1.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuballa P, Huett A, Rioux JD, Daly MJ, Xavier RJ. Impaired autophagy of an intracellular pathogen induced by a Crohn's disease associated ATG16L1 variant. PLoS One. 2008;3:e3391. doi: 10.1371/journal.pone.0003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schmid D, Pypaert M, Münz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Müller M, Kreymborg K, Altenberend F, Brandenburg J, Kalbacher H, et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci USA. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruiz PA, Shkoda A, Kim SC, Sartor RB, Haller D. IL-10 gene-deficient mice lack TGF-beta/Smad-mediated TLR2 degradation and fail to inhibit proinflammatory gene expression in intestinal epithelial cells under conditions of chronic inflammation. Ann N Y Acad Sci. 2006;1072:389–394. doi: 10.1196/annals.1326.023. [DOI] [PubMed] [Google Scholar]
- 91.Kruis W. Review article: antibiotics and probiotics in inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20 Suppl 4:75–78. doi: 10.1111/j.1365-2036.2004.02051.x. [DOI] [PubMed] [Google Scholar]
- 92.Malchow HA. Crohn's disease and Escherichia coli. A new approach in therapy to maintain remission of colonic Crohn's disease? J Clin Gastroenterol. 1997;25:653–658. doi: 10.1097/00004836-199712000-00021. [DOI] [PubMed] [Google Scholar]
- 93.Kruis W, Schütz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 1997;11:853–858. doi: 10.1046/j.1365-2036.1997.00225.x. [DOI] [PubMed] [Google Scholar]
- 94.Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, Kamm MA, Weismueller J, Beglinger C, Stolte M, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guslandi M, Mezzi G, Sorghi M, Testoni PA. Saccharomyces boulardii in maintenance treatment of Crohn's disease. Dig Dis Sci. 2000;45:1462–1464. doi: 10.1023/a:1005588911207. [DOI] [PubMed] [Google Scholar]
- 96.Rolfe VE, Fortun PJ, Hawkey CJ, Bath-Hextall F. Probiotics for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2006:CD004826. doi: 10.1002/14651858.CD004826.pub2. [DOI] [PubMed] [Google Scholar]
- 97.Kato K, Mizuno S, Umesaki Y, Ishii Y, Sugitani M, Imaoka A, Otsuka M, Hasunuma O, Kurihara R, Iwasaki A, et al. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment Pharmacol Ther. 2004;20:1133–1141. doi: 10.1111/j.1365-2036.2004.02268.x. [DOI] [PubMed] [Google Scholar]
- 98.Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–309. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 99.Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108–114. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Venturi A, Gionchetti P, Rizzello F, Johansson R, Zucconi E, Brigidi P, Matteuzzi D, Campieri M. Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther. 1999;13:1103–1108. doi: 10.1046/j.1365-2036.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 101.Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–1209. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 102.Campieri M, Rizzello F, Venturi A, Poggioli G, Ugolini F, Helwig U. Combination of antibiotic and probiotic treatment is efficacious in prophylaxis of post-operative recurrence of Crohn’s disease: a randomized controlled study versus mezalamine. Gastroenterology. 2000;118:A781. [Google Scholar]
- 103.Welters CF, Heineman E, Thunnissen FB, van den Bogaard AE, Soeters PB, Baeten CG. Effect of dietary inulin supplementation on inflammation of pouch mucosa in patients with an ileal pouch-anal anastomosis. Dis Colon Rectum. 2002;45:621–627. doi: 10.1007/s10350-004-6257-2. [DOI] [PubMed] [Google Scholar]
- 104.Hussey TA, Issenman RM, Persad R, Olley AR, Christensen BA. Nutrition therapy in pediatric Crohn’s disease patients improves nutrition status and decreases inflammation. J Pediatr Gastroenterol. 2003;37:A341. [Google Scholar]
- 105.Lindsay JO, Whelan K, Stagg AJ, Gobin P, Al-Hassi HO, Rayment N, Kamm MA, Knight SC, Forbes A. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut. 2006;55:348–355. doi: 10.1136/gut.2005.074971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O’neil DA, Macfarlane GT. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54:242–249. doi: 10.1136/gut.2004.044834. [DOI] [PMC free article] [PubMed] [Google Scholar]