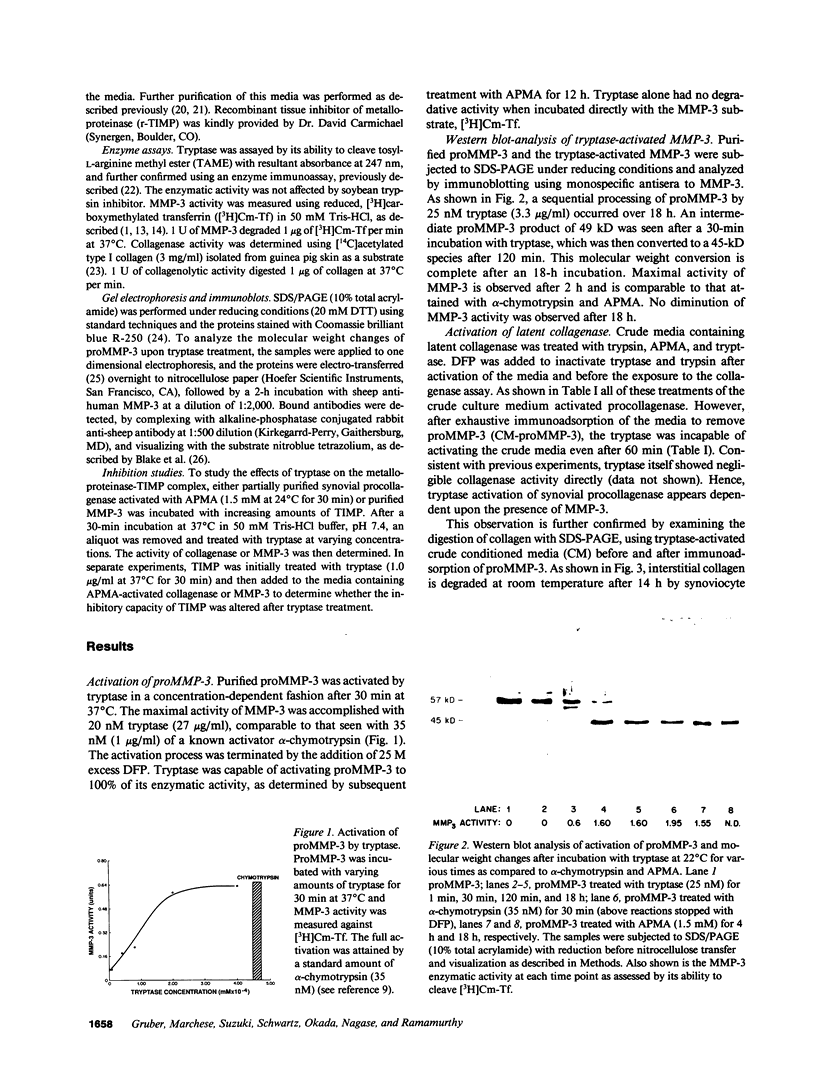
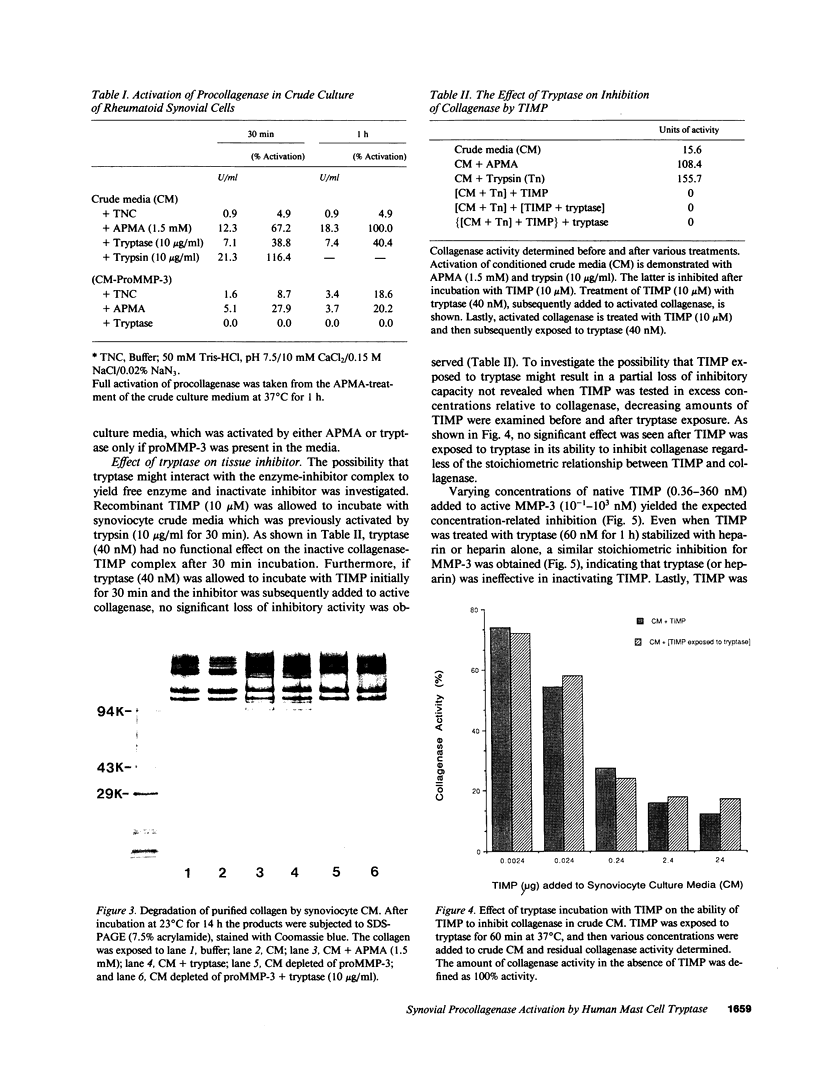
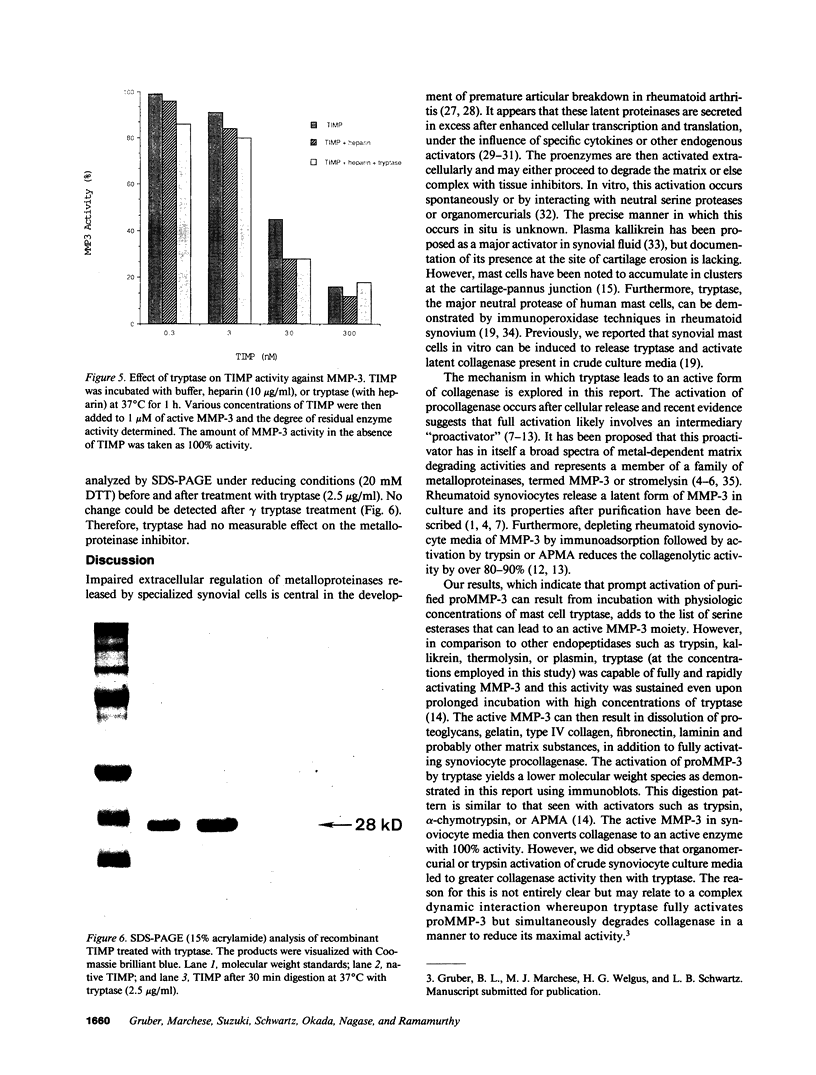
Abstract
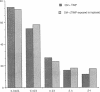
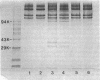
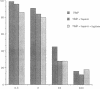
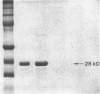
Mast cells have been implicated in the pathogenesis of the matrix degradation observed in the cartilaginous and osseous structures of the rheumatoid joint. We previously reported that human mast cell tryptase, a 134-kD granule-associated neutral protease, is present in rheumatoid synovium and can activate collagenase in crude culture medium in vitro. the present study attempts to depict the precise mechanism of this activation. To express full activation of latent collagenase, matrix metalloproteinase 3 (MMP-3) or stromelysin, can be activated by tryptase in a time and dose-dependent manner. Tryptase was not capable of generating active collagenase in the crude media from cultured rheumatoid synoviocytes depleted of proMMP-3 by immunoadsorption. In addition, the function of the tissue inhibitor of metalloproteinases (TIMP) was not altered by tryptase, and SDS-PAGE analysis revealed no degradation of TIMP by tryptase. The tryptase dependent activation of synoviocyte procollagenase thereby appears to be entirely dependent upon its ability to activate proMMP-3.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bejarano P. A., Noelken M. E., Suzuki K., Hudson B. G., Nagase H. Degradation of basement membranes by human matrix metalloproteinase 3 (stromelysin). Biochem J. 1988 Dec 1;256(2):413–419. doi: 10.1042/bj2560413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., Mitchell T. I., Karmilowicz M. J., Kluve-Beckerman B., Benson M. D. Autocrine induction of collagenase by serum amyloid A-like and beta 2-microglobulin-like proteins. Science. 1989 Feb 3;243(4891):655–657. doi: 10.1126/science.2536953. [DOI] [PubMed] [Google Scholar]
- Bromley M., Fisher W. D., Woolley D. E. Mast cells at sites of cartilage erosion in the rheumatoid joint. Ann Rheum Dis. 1984 Feb;43(1):76–79. doi: 10.1136/ard.43.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston T. E., Barrett A. J. A rapid and reproducible assay for collagenase using [1-14C]acetylated collagen. Anal Biochem. 1979 Nov 1;99(2):340–345. doi: 10.1016/s0003-2697(79)80017-2. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Mercer E., Tyler J. A. The activation of latent pig synovial collagenase. Biochim Biophys Acta. 1981 Jan 15;657(1):73–83. doi: 10.1016/0005-2744(81)90131-5. [DOI] [PubMed] [Google Scholar]
- Chin J. R., Murphy G., Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985 Oct 5;260(22):12367–12376. [PubMed] [Google Scholar]
- Crisp A. J., Chapman C. M., Kirkham S. E., Schiller A. L., Krane S. M. Articular mastocytosis in rheumatoid arthritis. Arthritis Rheum. 1984 Aug;27(8):845–851. doi: 10.1002/art.1780270802. [DOI] [PubMed] [Google Scholar]
- Dabbous M. K., Walker R., Haney L., Carter L. M., Nicolson G. L., Woolley D. E. Mast cells and matrix degradation at sites of tumour invasion in rat mammary adenocarcinoma. Br J Cancer. 1986 Sep;54(3):459–465. doi: 10.1038/bjc.1986.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., de Rochemonteix B., Burrus B., Demczuk S., Dinarello C. A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhout Y., Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977 Jul 15;166(1):21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemont A. J., Denton J. Disease distribution of synovial fluid mast cells and cytophagocytic mononuclear cells in inflammatory arthritis. Ann Rheum Dis. 1985 May;44(5):312–315. doi: 10.1136/ard.44.5.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey H. P., Ilardi C., Engber W., Graziano F. M. Quantitation of human synovial mast cells in rheumatoid arthritis and other rheumatic diseases. Arthritis Rheum. 1984 Aug;27(8):852–856. doi: 10.1002/art.1780270803. [DOI] [PubMed] [Google Scholar]
- Goto T., Befus D., Low R., Bienenstock J. Mast cell heterogeneity and hyperplasia in bleomycin-induced pulmonary fibrosis of rats. Am Rev Respir Dis. 1984 Nov;130(5):797–802. doi: 10.1164/arrd.1984.130.5.797. [DOI] [PubMed] [Google Scholar]
- Gruber B. L., Schwartz L. B., Ramamurthy N. S., Irani A. M., Marchese M. J. Activation of latent rheumatoid synovial collagenase by human mast cell tryptase. J Immunol. 1988 Jun 1;140(11):3936–3942. [PubMed] [Google Scholar]
- Gruber B., Poznansky M., Boss E., Partin J., Gorevic P., Kaplan A. P. Characterization and functional studies of rheumatoid synovial mast cells. Activation by secretagogues, anti-IgE, and a histamine-releasing lymphokine. Arthritis Rheum. 1986 Aug;29(8):944–955. doi: 10.1002/art.1780290802. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr Recent insights into the pathogenesis of the proliferative lesion in rheumatoid arthritis. Arthritis Rheum. 1976 Jan-Feb;19(1):68–72. doi: 10.1002/art.1780190111. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Welgus H. G., Krane S. M. Regulation of the mammalian collagenases. Coll Relat Res. 1984 Dec;4(6):493–512. doi: 10.1016/s0174-173x(84)80015-1. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Claman H. N., Clark R. A., Steigerwald J. C. Increased dermal mast cell populations in progressive systemic sclerosis: a link in chronic fibrosis? Ann Intern Med. 1985 Feb;102(2):182–186. doi: 10.7326/0003-4819-102-2-182. [DOI] [PubMed] [Google Scholar]
- Ishibashi M., Ito A., Sakyo K., Mori Y. Procollagenase activator produced by rabbit uterine cervical fibroblasts. Biochem J. 1987 Jan 15;241(2):527–534. doi: 10.1042/bj2410527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A., Nagase H. Evidence that human rheumatoid synovial matrix metalloproteinase 3 is an endogenous activator of procollagenase. Arch Biochem Biophys. 1988 Nov 15;267(1):211–216. doi: 10.1016/0003-9861(88)90025-2. [DOI] [PubMed] [Google Scholar]
- Jeffcoat M. K., Williams R. C., Johnson H. G., Wechter W. J., Goldhaber P. Treatment of periodontal disease in beagles with lodoxamide ethyl, an inhibitor of mast cell release. J Periodontal Res. 1985 Sep;20(5):532–541. doi: 10.1111/j.1600-0765.1985.tb00837.x. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Cawston T. E. Human lung mast cell tryptase fails to activate procollagenase or degrade proteoglycan. Biochem Biophys Res Commun. 1985 Oct 30;132(2):453–459. doi: 10.1016/0006-291x(85)91155-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malone D. G., Wilder R. L., Saavedra-Delgado A. M., Metcalfe D. D. Mast cell numbers in rheumatoid synovial tissues. Correlations with quantitative measures of lymphocytic infiltration and modulation by antiinflammatory therapy. Arthritis Rheum. 1987 Feb;30(2):130–137. doi: 10.1002/art.1780300202. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Stephens P. E., Smith B. J., Docherty A. J. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987 Nov 15;248(1):265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., McAlpine C. G., Poll C. T., Reynolds J. J. Purification and characterization of a bone metalloproteinase that degrades gelatin and types IV and V collagen. Biochim Biophys Acta. 1985 Sep 20;831(1):49–58. doi: 10.1016/0167-4838(85)90148-7. [DOI] [PubMed] [Google Scholar]
- Murphy G., Nagase H., Brinckerhoff C. E. Relationship of procollagenase activator, stromelysin and matrix metalloproteinase 3. Coll Relat Res. 1988 Jul;8(4):389–391. doi: 10.1016/s0174-173x(88)80009-8. [DOI] [PubMed] [Google Scholar]
- Nagase H., Cawston T. E., De Silva M., Barrett A. J. Identification of plasma kallikrein as an activator of latent collagenase in rheumatoid synovial fluid. Biochim Biophys Acta. 1982 Mar 18;702(1):133–142. doi: 10.1016/0167-4838(82)90036-x. [DOI] [PubMed] [Google Scholar]
- Okada Y., Harris E. D., Jr, Nagase H. The precursor of a metalloendopeptidase from human rheumatoid synovial fibroblasts. Purification and mechanisms of activation by endopeptidases and 4-aminophenylmercuric acetate. Biochem J. 1988 Sep 15;254(3):731–741. doi: 10.1042/bj2540731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Konomi H., Yada T., Kimata K., Nagase H. Degradation of type IX collagen by matrix metalloproteinase 3 (stromelysin) from human rheumatoid synovial cells. FEBS Lett. 1989 Feb 27;244(2):473–476. doi: 10.1016/0014-5793(89)80586-1. [DOI] [PubMed] [Google Scholar]
- Okada Y., Nagase H., Harris E. D., Jr A metalloproteinase from human rheumatoid synovial fibroblasts that digests connective tissue matrix components. Purification and characterization. J Biol Chem. 1986 Oct 25;261(30):14245–14255. [PubMed] [Google Scholar]
- Okada Y., Watanabe S., Nakanishi I., Kishi J., Hayakawa T., Watorek W., Travis J., Nagase H. Inactivation of tissue inhibitor of metalloproteinases by neutrophil elastase and other serine proteinases. FEBS Lett. 1988 Feb 29;229(1):157–160. doi: 10.1016/0014-5793(88)80817-2. [DOI] [PubMed] [Google Scholar]
- Schwartz L. B., Bradford T. R. Regulation of tryptase from human lung mast cells by heparin. Stabilization of the active tetramer. J Biol Chem. 1986 Jun 5;261(16):7372–7379. [PubMed] [Google Scholar]
- Schwartz L. B., Lewis R. A., Austen K. F. Tryptase from human pulmonary mast cells. Purification and characterization. J Biol Chem. 1981 Nov 25;256(22):11939–11943. [PubMed] [Google Scholar]
- Schwartz L. B., Lewis R. A., Seldin D., Austen K. F. Acid hydrolases and tryptase from secretory granules of dispersed human lung mast cells. J Immunol. 1981 Apr;126(4):1290–1294. [PubMed] [Google Scholar]
- Sellers A., Reynolds J. J., Meikle M. C. Neutral metallo-proteinases of rabbit bone. Separation in latent forms of distinct enzymes that when activated degrade collagen, gelatin and proteoglycans. Biochem J. 1978 May 1;171(2):493–496. doi: 10.1042/bj1710493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadwell B. V., Neidel J., Pavia M., Towle C. A., Trice M. E., Mankin H. J. Purification and characterization of collagenase activator protein synthesized by articular cartilage. Arch Biochem Biophys. 1986 Dec;251(2):715–723. doi: 10.1016/0003-9861(86)90381-4. [DOI] [PubMed] [Google Scholar]
- Vaes G. Multiple steps in the activation of the inactive precursor of bone collagenase by trypsin. FEBS Lett. 1972 Dec 1;28(2):198–200. doi: 10.1016/0014-5793(72)80711-7. [DOI] [PubMed] [Google Scholar]
- Vater C. A., Nagase H., Harris E. D., Jr Purification of an endogenous activator of procollagenase from rabbit synovial fibroblast culture medium. J Biol Chem. 1983 Aug 10;258(15):9374–9382. [PubMed] [Google Scholar]
- Wenzel S., Irani A. M., Sanders J. M., Bradford T. R., Schwartz L. B. Immunoassay of tryptase from human mast cells. J Immunol Methods. 1986 Jan 22;86(1):139–142. doi: 10.1016/0022-1759(86)90277-2. [DOI] [PubMed] [Google Scholar]
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]