Abstract
AIM: To study the influence of CXCR4/stromal cell-derived factor-1 (SDF-1) axis on E-cadherin/β-catenin complex expression in HT29 colon cancer cells and its underlying mechanisms.
METHODS: Effect of SDF-1 on E-cadherin/β-catenin expression was detected by immunocytochemistry. E-cadherin and β-catenin mRNA expression levels were measured by reverse transcriptase-polymerase chain reaction. SDF-1-induced phosphorylation of phosphatidylinositol 3-kinase (PI3K)/AKT and β-catenin was detected by Western blotting.
RESULTS: The E-cadherin and β-catenin mRNA expression levels in HT29 cells were lower 48 h after incubated with SDF-1 at the concentrations of 20 and 40 ng/mL (P < 0.05). SDF-1-induced significant phosphorylation of PI3K/AKT and β-catenin. AMD3100 and LY294002 inhibited the phosphorylation of PI3K/AKT and β-catenin.
CONCLUSION: SDF-1 down-regulates the E-cadherin/β-catenin complex expression in HT29 cells by decreasing mRNA synthesis and increasing β-catenin phosphorylation.
Keywords: CXCR4, Stromal cell-derived factor-1, E-cadherin, β-catenin, Phosphatidylinositol 3-kinase/AKT, Colon cancer
INTRODUCTION
Colorectal cancer (CRC) is one of the most common cancers and the second leading cause of cancer-related death in the Western world. Death usually results from its uncontrolled metastasis. Although the 5-year survival rate approaches 90% for patients with local CRC, it has decreased to 19% for patients with distant metastasis[1]. The metastatic process of CRC consists of a series of individual steps, which are required to establish the diagnosis of metastatic lesions[2,3]. A number of molecules have been implicated in the metastatic process of CRC.
Chemokines are a group of chemoattractant cytokines that mediate several cellular functions. Stromal cell-derived factor-1 (SDF-1) is expressed in stromal cells, including fibroblasts and endothelial cells[4,5], and interacts specifically with the seven-transmembrane, G protein-coupled receptor CXCR4[6]. Recent studies showed that chemotaxis effect of CXCR4/SDF-1 axis is related with lymph node and liver metastasis of CRC[7-10]. Although there is evidence that the CXCR4/SDF-1 signaling pathway is involved in the metastatic process of CRC, the precise molecular mechanism underlying SDF-1-induced chemotaxis effect has not been completely elucidated.
E-cadherin, a transmembrane glycoprotein located at the adheren junction, mediates calcium-dependent cell-cell adhesion[11,12]. C terminus of E-cadherin is linked to α-catenin and actin cytoskeleton through the association with β-catenin. Strong cell-cell interactions result in a tight cell cluster as a community, and constrain cells from moving away. It has been shown that dysregulation of E-cadherin/β-catenin complex expression is responsible for the invasion and metastasis of CRC[13,14], indicating that the CXCR4/SDF-1 axis is correlated with E-cadherin/β-catenin complex expression in invasion and metastasis of CRC.
This study was to observe whether SDF-1 can alter E-cadherin/β-catenin expression in HT29 colon cancer cell line. In addition, the E-cadherin/β-catenin mRNA expression level was measured and the phosphorylation of phosphatidylinositol 3-kinase (PI3K)/AKT and β-catenin was examined to provide insights into the mechanism underlying the change in E-cadherin/β-catenin expression.
MATERIALS AND METHODS
Reagents
Antibodies against E-cadherin and β-catenin, and p-β-catenin antibody (Ser33/37) and p-AKT antibody (Ser473) were purchased from Cell Signaling Technology (Beverly, MA, USA). Peroxidase-conjugated goat anti-rabbit IgG and peroxidase-conjugated goat anti-mouse IgG were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). SDF-1 was bought from Pepro Tech Inc. (Rocky Hill, NJ, USA). AMD3100 was purchased from Sigma Aldrich, USA and LY29400 was bought from Beyotime, China.
Cell culture
Human colon cancer HT29 cell line was obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). Tumor cells were cultured in RPMI-1640 (Invitrogen product), supplemented with 10% newborn calf serum (Shanghai Mafa Corporation), 100 U/mL penicillin, and 100 mg/mL streptomycin in a humidified incubator containing 5% CO2 and 95% air at 37°C.
Cell proliferation assay
Exponentially growing HT29 cells were seeded in 96-well plates in RPMI-1640 containing 3% newborn calf serum at a density of 2 × 104 cells/well. After 24 h, either PBS or AMD3100 (100 ng/mL) was added and incubated for 2 h. SDF-1 was added into three wells daily at different concentrations (10, 20 and 40 ng/mL). MTT assay (Amersham Biosciences, USA) was performed after 24, 48 and 72 h. Absorbance was measured at 570 and 630 nm (630 nm as the reference wave length). The results were expressed as a mean of three wells in each group. Proliferation rate of HT29 cells was calculated by the absorbance of experimental groups divided by that of the control group. The results were expressed as a mean of three individual experiments.
Cell chemotaxis and migration assay
Migration of HT29 cells was assessed in a HTS transwell-24 system (Corning, Acton, MA, USA) with 8-μm membrane pores. After rehydration for 2 h, RPMI-1640 and different SDF-1 concentrations (10, 20 and 40 ng/mL) were added into the lower chamber (0.5 mL per well) and 2 × 104 cells treated with PBS or AMD3100 (100 ng/mL) were added into the upper chamber 30 min before assay. After incubated at 37°C for 24 h, Matrigel and cells on the upper side of the membrane were wiped off with PBS-rinsed cotton swabs and invading cells migrated to the lower surface of the membrane were photographed and counted under an inverted light microscope at 100 × magnification. Six random fields were counted for each well. Migration of HT29 cells was assayed in triplicate.
Immunocytochemistry
To observe the effect of SDF-1 on E-cadherin/β-catenin complex expression, HT29 cells were seeded in 24-well plates with glass slides at a density of 2 × 105 cells/well in RPMI-1640 medium in the absence of serum. After incubated overnight, either PBS or CXCR4 antagonist AMD3100 (100 ng/mL) was added and incubated for 2 h. Then, SDF-1 was added at different concentrations (10, 20 and 40 ng/mL). Glass slides were collected after incubated for 24 and 48 h with SDF-1. Cells were washed thrice with PBS prior to fixation with 4% formaldehyde in PBS. Then, the cells were covered with 3% H2O2-methanol for 30 min at room temperature to inactivate the endogenous peroxidase. A methanol permeabilisation step was needed for β-catenin, during which cells were covered with ice-cold 100% methanol for 10 min in a freezer. After rinsed thrice with PBS, the cells were covered with 5% normal goat serum for 30 min at room temperature to block the non-specific binding. Primary rabbit anti-E-cadherin antibodies and mouse anti-β-catenin antibodies were applied to the slides and incubated overnight at 4°C. Slides were washed three times with PBS. Secondary antibodies were applied for 1 h at room temperature. Finally, the slides were stained with 0.025% diaminobenzidine tetrahydrochloride containing 4% H2O2 for 1-20 min, counterstained with hematoxylin for an appropriate period of time, and analyzed under a light microscope. The level of nonspecific background staining was established for each measurement using control cells processed in the same way without exposure to primary antibodies.
Reverse transcriptase-polymerase chain reaction analysis
HT29 cells (1 × 106) treated as in immunocytochemistry were collected and washed three times with 1 × PBS. Total RNA was extracted using trizol reagents and quantified by ND-1000 UV-visible spectrophotometry. cDNA was synthesized from 1 μg of total RNA using Revert Aid™ M-MuLV reverse transcriptase. Reaction mixture contained 4 μL of 5 × reaction buffer, 2 μL of 10 mmol/L dNTP mix, 1 μg of Oligo(dT)18, 1 μg of total RNA, 0.5 μL of ribonuclease inhibitor, and 200 U Aid™ M-MuLV reverse transcriptase in a total volume of 20 μL. PCR contained 2.5 μL of 10 × PCR buffer, 1.5 μL of 25 mmol/L MgCl2, 0.5 μL of 10 mmol/L dNTP mix, 0.8 umol/L β-catenin primer, 0.04 μmol/L GAPDH primer (for β-catenin) and 0.8 μmol/L E-cadherin primer, 0.1 μmol/L β-actin primer (for E-cadherin), 3 μL of cDNA and 25 U Taq polymerase in a total volume of 25 μL. The sequences of gene-specific primers are 5′-TTTGCGTGAGCAGGGTGC-3′ (forward) and 5′-GCTGCATATGTCGCCACACC-3′ (reverse) for β-catenin, 5′-CCACCCATGGCAAATTCCATGG-CA-3′ (forward) and 5′-TCTAGACGGCAGGTCAGGTCCACC-3′ (reverse) for GAPDH, 5′-GATTCTGCTGCTCTTGCTGT-3′ (forward) and 5′-CCTGGT-CTTTGTCTGACTCTG-3′ (reverse) for E-cadherin, 5′-CCTTCCTGGGCATGGAGTCCT-3′ (forward) and 5′-GGAGCAATGATCTTGATCTT-3′ (reverse) for β-actin, respectively. The PCR conditions for β-catenin were as follows: denaturing at 94°C for 5 min, followed by 35 cycles at 94°C for 30 s, at 63°C for 30 s, at 72°C for 1 min, and a final extension at 72°C for 7 min. The PCR conditions for E-cadherin were as follows: annealing at 58°C for 5 min, followed by 35 cycles at 94°C for 30 s, at 63°C for 30 s, at 72°C for 1 min, and a final extension at 72°C for 7 min. The PCR products were separated on a 2% agarose gel in 1 × TAE, visualized with ethidium bromide staining by BIO-RAD Gel Dos1000, and quantified using the Molecular Analyst software version 1.5 using GAPDH as an internal control for β-catenin, and β-actin as an internal control for E-cadherin. The hue value was calculated as the ratio of each group and the internal control group. Results were expressed as the mean value of three individual experiments.
Western blotting
To study the effect of SDF-1 on phosphorylation of various signaling proteins, HT29 cells were treated with 20 ng/mL SDF-1 for different periods of time (from 1 min to 2 d) or with different concentrations of SDF-1 (5-100 ng/mL) for 30 min after overnight starvation of growth factors. Cell lysates were analyzed for the presence of phosphorylated AKT and β-catenin by phospho-specific antibodies to the specific phosphorylation sites of AKT (Ser473) and β-catenin (Ser33/37). For experiments using inhibitors, HT29 cells were pretreated with AMD3100 (100 ng/mL) or PI3K/AKT inhibitor LY294002 (20 μmol/L) for 2 h, followed by stimulation with 20 ng/mL SDF-1. After each treatment, 4 × 106 HT29 cells were collected and washed three times with 1 × PBS. Cytoplasm and membrane extracts were acquired according to the instruction datasheet of Pierce Biotechnology Corporation and quantified by Bradford protein assay. Different extraction proteins were separated by SDS-PAGE and transferred onto the PVDF membrane. Membranes were blocked with 5% BSA for 2 h at room temperature, incubated overnight at 4°C with primary antibodies (p-β-catenin 1:1000, p-AKT 1:1000 and β-actin 1:400) and washed three times prior to incubation with HRP-conjugated secondary antibodies (peroxidase conjugated goat anti rabbit IgG 1:10 000) for 1 h at room temperature. Protein expression was visualized by chemiluminescence and quantified using the multigauge software. β-actin was used as a loading control. Data were shown as the ratio of phosphorylation and loading control. Western blotting assay was performed in triplicate.
Statistical analysis
All data were analyzed using the SPSS 16.0 and expressed as mean ± SD. Statistical significance of differences was determined by Student’s t-test in two groups and one-way ANOVA among multiple groups. P < 0.05 was considered statistically significant.
RESULTS
SDF-1 enhanced viability of HT29 colon cancer cells
The viability of HT29 cells in any experiment groups was not different from that in control group 48 h after incubated with SDF-1. The cells grew much faster with a proliferation rate of 129% and 135%, respectively (P < 0.05) 72 h after incubated with SDF-1 at the concentrations of 20 and 40 ng/mL. AMD3100 plus SDF-1 inhibited the cell growth. AMD3100 alone had no effect on cell proliferation.
SDF-1 promoted migration of HT29 colon cancer cells
MTT assay revealed no significant difference in viability of HT29 colon cancer cells in any experiment groups within 24 h after incubated with SDF-1. Therefore, the migration of HT29 colon cancer cells was assayed 24 h after incubated with SDF-1 to exclude the influence of cell viability. The migration ability of HT29 cells was significantly greater in experiment groups than in control group 24 h after incubated with SDF-1 at the concentration of 10 ng/mL (149 ± 13.3 vs 92.3 ± 12.4, P = 0.041), 20 ng/mL (161 ± 13.5 vs 92.3 ± 12.4, P = 0.023), and 40 g/mL (187.5 ± 14 vs 92.3 ± 12.4, P < 0.001). AMD3100 plus SDF-1 inhibited the migration of HT29 cells. AMD3100 alone had no effect on cell migration.
SDF-1 down-regulated activation of CXCR4 and E-cadherin expression at protein and mRNA levels
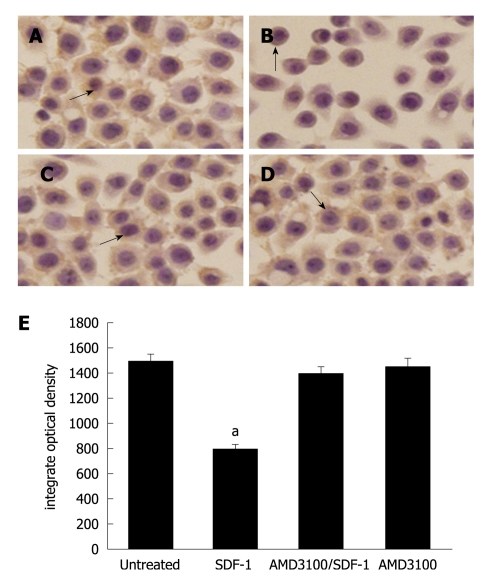
Immunocytochemistry assay showed that E-cadherin was significantly expressed in HT29 cells (Figure 1A). No change was found in E-cadherin expression 24 h after incubated with SDF-1. The E-cadherin expression level was significantly lower 48 h after incubated with SDF-1 at the concentrations of 20 and 40 ng/mL (P < 0.05, Figure 1B). HT29 cells treated with AMD3100 prior to administration of SDF-1 did not decrease the E-cadherin expression level. However, HT29 cells treated SDF-1 decreased the E-cadherin expression level (Figure 1C). AMD3100 alone had no effect on the E-cadherin expression (Figure 1D). The changes of E-cadherin expression in HT29 cells are demonstrated in Figure 1E.
Figure 1.
Effect of stromal cell-derived factor-1 (20 ng/mL) on E-cadherin expression (× 400). A: E-cadherin expression in HT29 cells; B: Significantly lower E-cadherin expression level 48 h after incubated with stromal cell-derived factor-1 (SDF-1) (P < 0.05); C: AMD3100- inhibited E-cadherin expression; D: No effect of AMD3100 alone on E-cadherin expression. Arrows mean the expression of E-cadherin; Bars indicate mean ± SD of six random fields. aP < 0.05.
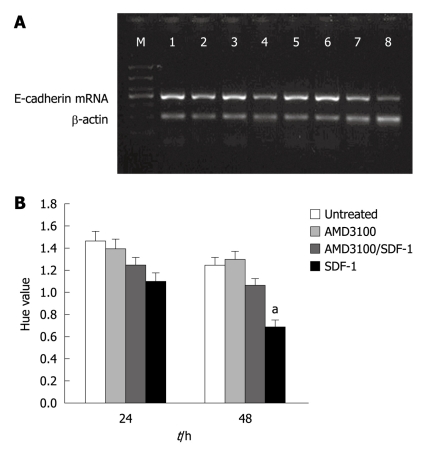
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis demonstrated that the E-cadherin mRNA expression level was lower 48 h after incubated with SDF-1 at the concentrations of 20 and 40 ng/mL (P < 0.05). AMD3100 plus SDF-1 inhibited the E-cadherin mRNA expression. AMD3100 alone had no influence on E-cadherin mRNA expression (Figure 2A and B).
Figure 2.
Effect of stromal cell-derived factor-1 (20 ng/mL) on E-cadherin mRNA expression after 24 h (A) and 48 h (B) in different groups. Bars represent mean ± SD of three individual experiments. aP < 0.05. SDF-1: Stromal cell-derived factor-1.
SDF-1 down-regulated β-catenin expression at protein and mRNA levels
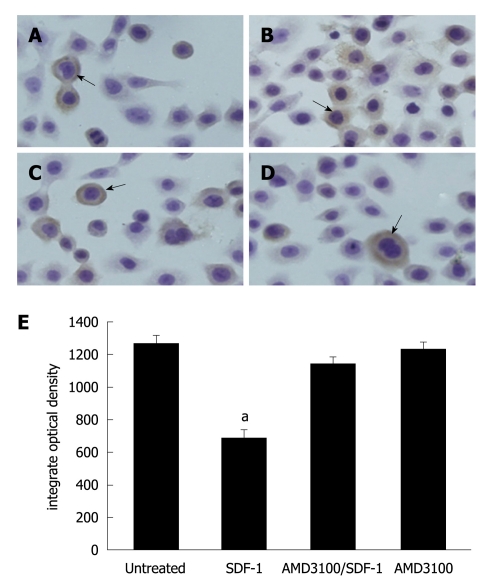
β-catenin was strongly stained in HT29 cells not incubated with SDF-1 (Figure 3A). The β-catenin expression level was lower 24 and 48 h after incubated with SDF-1 at the concentrations of 20 and 40 ng/mL (P < 0.05, Figure 3B). The β-catenin expression level was slightly lower in AMD3100- treated HT29 cells (Figure 3C). AMD3100 alone had no influence on β-catenin expression (Figure 3D). The changes of β-catenin expression in HT29 cells are demonstrated in Figure 3E.
Figure 3.
Effect of stromal cell-derived factor-1 (20 ng/mL) on β-catenin expression after 48 h (× 400). A: β-catenin expression in HT29 cells; B: Significantly lower β-catenin expression level 48 h after incubated with stromal cell-derived factor-1 (SDF-1) (P = 0.031); C: AMD310-inhibited β-catenin expression; D: No effect of AMD3100 alone on β-catenin expression. Arrows mean the expression of β-catenin; Bars indicate mean ± SD of six random fields. aP < 0.05.
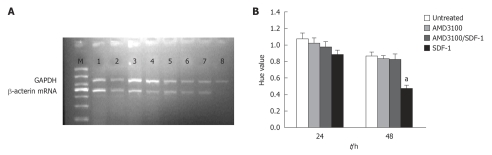
RT-PCR analysis demonstrated that the β-catenin mRNA expression level was lower 24 and 48 h after incubated with SDF-1 at the concentrations of 20 and 40 ng/mL (P < 0.05, Figure 4A and B). AMD3100 plus SDF-1 inhibited the β-catenin mRNA expression. AMD3100 alone did not influence the β-catenin mRNA expression.
Figure 4.
Effect of stromal cell-derived factor-1 (20 ng/mL) on β-catenin mRNA expression after 24 h (A) and 48 h (B) in different groups. Bars represent mean ± SD of three individual experiments. aP < 0.05. SDF-1: Stromal cell-derived factor-1.
Involement of phosphorylation of PI3K/AKT and β-catenin in SDF-1-induced down-regulation of E-cadherin/β-catenin expression
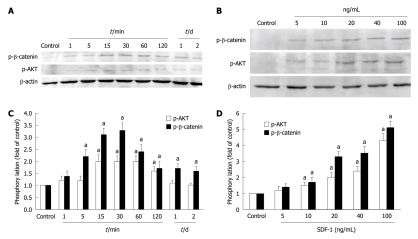
The phosphorylation of PI3K/AKT and β-catenin was detected to observe whether phosphorylation is involved in down-regulation of E-cadherin/β-catenin complex expression, which demonstrated that SDF-1 increased the phosphorylation of AKT and β-catenin. The β-catenin was evidently activated at 5 min and the AKT was significantly phosphorylated at 15 min after incubated with SDF-1. The phosphorylation of β-catenin reached its peak 30 min after incubated with SDF-1. Then, β-catenin was evidently phosphorylated for 2 d (Figure 5A and C). The phosphorylation of AKT reached its peak at 15-60 min after incubated with SDF-1, remained there for 2 h, and then slowly declined. To determine the dose-dependent effect of SDF-1 on the phosphorylation of PI3K/AKT and β-catenin, HT29 cells were treated with SDF-1 at different concentrations (0, 5, 10, 20, 40 and 100 ng/mL) for 30 min. Then, cell lysates were analyzed for the phosphorylation of PI3K/AKT and β-catenin. Administration of SDF-1 for 30 min increased the phosphorylation of PI3K/AKT and β-catenin in a dose-dependent manner. PI3K/AKT and β-catenin were phosphorylated after incubated with SDF-1 at the concentration of 5 ng/mL and reached its peak after incubated with SDF-1 at the concentration of 100 ng/mL (Figure 5B and D).
Figure 5.
Effect of stromal cell-derived factor-1 on the phosphorylation of PI3K/AKT and β-catenin. A: Phosphorylation of PI3K/AKT and β-catenin after incubated with stromal cell-derived factor (SDF) at different periods of time (1, 5, 10, 15, 30, 60, 120 min and on days 1 and 2); B: Phosphorylation of PI3K/AKT and β-catenin after incubated with SDF-1 at different concentrations (5, 10, 20, 40 and 100 ng/mL) for 30 min; C and D: SDF-1 increases phosphorylation of PI3K/AKT and β-catenin in HT29 cells in a time- and dose-dependent manner. Bars represent mean ± SD of triplicate experiments. aP < 0.05.
Inhibition of PI3K/AKT prevented phosphorylation of SDF-1-induced β-catenin
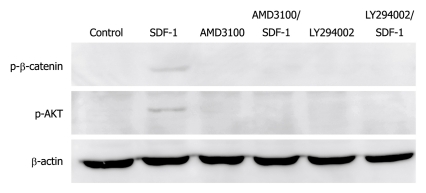
To investigate the relation between PI3K/AKT and β-catenin, AMD3100 and LY294002 were used to inhibit the effect of SDF-1 and the signal transmission through PI3K/AKT, which showed that AMD3100 inhibited the phosphorylation of PI3K/AKT and β-catenin (Figure 6). Further study demonstrated that administration of LY294002 prior to SDF-1 also prevented the phosphorylation of PI3K/AKT and β-catenin (Figure 6), suggesting that β-catenin is phosphorylated via the PI3K/AKT, and may be the downstream signaling molecule of PI3K/AKT.
Figure 6.
MD3100 and LY294002 inhibit stromal cell-derived factor-1-induced phosphorylation of PI3K/AKT and β-catenin while AMD3100 or LY294002 alone has no effect on phosphorylation of PI3K/AKT and β-catenin. SDF-1: Stromal cell-derived factor-1.
DISCUSSION
Although the CXCR4/SDF-1 biological axis contributes to organ-selective metastasis of tumors[15-17], the mechanism underlying the effect of chemotaxis remains unclear. In this study, the relation between CXCR4/SDF-1 axis and E-cadherin/β-catenin complex expression was observed. Experiment on HT29 colon cancer cell line was performed because it shows a high expression level of CXCR4[18]. SDF-1 promoted the proliferation of HT29 cells, thereby contributing to primary tumor formation and down-regulated the E-cadherin/β-catenin expression by reducing the mRNA expression levels and decomposing their complex formation by phosphorylating the PI3K/AKT and β-catenin. AMD3100 and LY294002 blocked the two processes mediated by SDF-1, suggesting that they may be effective anti-metastatic agents, at least against CRC.
It was reported that CXCR4/SDF-1 axis-induced chemotaxis effect plays a role in the invasion of CRC cells[16,17]. A number of molecules, such as vascular endothelial growth factor, matrix metalloproteinase (MMP)9, and MMP2, have been implicated in SDF-1-induced CRC invasion[19]. E-cadherin is not only an adhesion molecule but also a tumor suppressor as well as the most important epithelial marker[20]. In most cancers of epithelial origin, E-cadherin mediates cell-cell adhesion. Loss of E-cadherin-mediated cell-cell adhesion is implicated in tumor invasion and metastasis[21,22]. Recent studies showed that Krüppel-like factor 4 inhibits epithelial to mesenchymal transition by regulating E-cadherin gene expression[23]. In our study, the E-cadherin expression level at protein and mRNA levels was lower 48 h after incubated with SDF-1 at the concentrations of 20 and 40 ng/mL, suggesting that E-cadherin is involved in SDF-1-induced chemotaxis effect. In this study, the molecular mechanism underlying SDF-1-induced chemotaxis effect was studied. The E-cadherin mRNA expression level was lower after incubated with SDF-1, which may account for the down-regulation of E-cadherin expression.
Tumor invasion is a complex process and loss of E-cadherin-mediated cell adhesion is not sufficient to confer an invasive phenotype to tumor cells. Cell migration requires precise control, which is altered or lost when tumors become invasive and metastatic. It has been shown that decreased β-catenin expression is often related with the absent or reduced E-cadherin, which contributes to the development of several cervical carcinoma cell lines[24,25]. A recent study revealed that β-catenin membrane/cytosolic expression level is significantly lower in primary tumors than in corresponding matched metastases, suggesting that the low β-catenin expression level may be a prognostic factor for the occurrence of metastasis and a worse outcome[26]. In this study, the β-catenin protein and mRNA expression levels were significantly lower 48 h after incubated with SDF-1 at the concentrations of 20 and 40 ng/mL, suggesting that β-catenin is involved in SDF-1-induced chemotaxis effect. Changes in β-catenin may also lead to the degradation of E-cadherin/β-catenin complex and alter cytoskeleton, thus promoting cell migration.
Growth factors, such as epidermal growth factor, induce β-catenin and plakoglobin phosphorylation, resulting in breakage of E-cadherin binding to actin cytoskeleton and contact disassembly[26]. In this study, the phosphorylation of β-catenin was increased after incubated with SDF-1, indicating that SDF-1 induces phosphorylation of β-catenin. Phosphorylation of β-catenin reduces β-catenin and may lead to decomposition of E-cadherin/β-catenin complex. G protein-coupled receptor activation results in PI3K and downstream AKT activation[27]. In this study, SDF-1- induced phosphorylation of PI3K/AKT and β-catenin in a time- and dose-dependent manner. Finally, whether SDF-1 induces β-catenin phosphorylation via PI3K/AKT was also studied. Given that both AMD3100 and LY294002 inhibited the phosphorylation of PI3K/AKT and β-catenin, β-catenin may be the downstream signaling molecule of PI3K/AKT, indicating that the phosphorylation of β-catenin may account for the down-regulation of β-catenin, and the breakage of E-cadherin/β-catenin complex. The phosphorylation of β-catenin may exert its effect via the PI3K/AKT pathway.
In conclusion, down-regulation of E-cadherin/β-catenin complex expression is involved in SDF-1-induced chemotaxis. The decreased E-cadherin mRNA expression and the down-regulated β-catenin expression may account for the down-regulation of E-cadherin. CXCR4/SDF-1 axis stimulates the phosphorylation of PI3K/AKT and β-catenin. The down-regulation of β-catenin may be induced by the decreased β-catenin mRNA expression and the increased phosphorylation of PI3K/AKT. PI3K inhibitor LY294002 inhibits the SDF-1-induced phosphorylation of PI3K/AKT and β-catenin. β-catenin may be the downstream signaling molecule of PI3K/AKT.
COMMENTS
Background
CXCR4/stromal cell-derived factor-1 (SDF-1) axis-mediated chemotaxis effect is crucial in organ-selective metastasis. E-cadherin/β-catenin complex plays an important role in colorectal tumorigenesis. Relatively little is known about the role of E-cadherin/β-catenin in CXCR4/SDF-1 axis-mediated chemotaxis effect.
Research frontiers
Recent studies demonstrated that the CXCR4/SDF-1 signaling pathway is involved in the metastatic process of colorectal cancer (CRC). In addition, inhibiting the interaction of SDF-1 and CXCR4 with CXCR4 antagonist AMD3100 prevents the chemotactic migration of CRC cells. However, the underlying molecular mechanism has not been elucidated.
Innovations and breakthroughs
Down-regulation of E-cadherin/β-catenin complex is involved in SDF-1-induced chemotaxis effect in HT29 colon cancer cells. Moreover, decreased mRNA synthesis and increased β-catenin phosphorylation may down-regulate E-cadherin/β-catenin. To the best of our knowledge, this is the first study that analyzes the relation between CXCR4/SDF-1 axis and E-cadherin/β-catenin complex.
Applications
This study demonstrated the relation between E-cadherin/β-catenin complex and SDF-1-induced chemotaxis effect in HT29 colon cancer cells, thus providing a possible molecular mechanism underlying the SDF-1-induced chemotaxis effect.
Terminology
SDF-1, also know as CXCL12, belongs to the CXC chemokine family and interacts specifically with the seven-transmembrane, G protein-coupled receptor CXCR4. E-cadherin is not only an adhesion molecule but also a tumor suppressor as well as the most important epithelial marker. β-catenin is a key component of adheren junctions, necessary for homophilic cell-cell adhesion. In addition to the membrane-associated pool, β-catenin plays a role in cell-signaling and gene transcription.
Peer review
This is a well designed study, which may explain the development of colorectal metastasis in vitro.
Acknowledgments
The authors thank Cui-Ling Li and Hai-Peng Yin for their excellent technical support in this study.
Footnotes
Supported by National Natural Science Foundation of China, No. 30571712 and 30810403081
Peer reviewer: Julio Mayol, MD, PhD, Department of Digestive surgery, Hospital Clinico San Carlos, MARTIN-LAGOS S/n, Madrid, 28040, Spain
S- Editor Sun H L- Editor Wang XL E- Editor Zheng XM
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3:65–71. [PubMed] [Google Scholar]
- 3.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 4.Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002;99:2703–2711. doi: 10.1182/blood.v99.8.2703. [DOI] [PubMed] [Google Scholar]
- 5.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 6.Federsppiel B, Melhado IG, Duncan AM, Delaney A, Schappert K, Clark-Lewis I, Jirik FR. Molecular cloning of the cDNA and chromosomal localization of the gene for a putative seven-transmembrane segment (7-TMS) receptor isolated from human spleen. Genomics. 1993;16:707–712. doi: 10.1006/geno.1993.1251. [DOI] [PubMed] [Google Scholar]
- 7.Matsusue R, Kubo H, Hisamori S, Okoshi K, Takagi H, Hida K, Nakano K, Itami A, Kawada K, Nagayama S, et al. Hepatic stellate cells promote liver metastasis of colon cancer cells by the action of SDF-1/CXCR4 axis. Ann Surg Oncol. 2009;16:2645–2653. doi: 10.1245/s10434-009-0599-x. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Takeuchi H, Lam ST, Turner RR, Wang HJ, Kuo C, Foshag L, Bilchik AJ, Hoon DS. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–2753. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 9.Schimanski CC, Schwald S, Simiantonaki N, Jayasinghe C, Gönner U, Wilsberg V, Junginger T, Berger MR, Galle PR, Moehler M. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11:1743–1750. doi: 10.1158/1078-0432.CCR-04-1195. [DOI] [PubMed] [Google Scholar]
- 10.Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63:3833–3839. [PubMed] [Google Scholar]
- 11.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 12.Brembeck FH, Rosário M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16:51–59. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Li JN, Li X, Qian JM, Lu XQ, Yang H. [Effects of K-ras gene mutation on colon cancer cell line Caco-2 metastasis by regulating E-cadherin/beta-catenin/p120 protein complex formation and RhoA protein activity] Zhongguo Yixue Kexueyuan Xuebao. 2010;32:46–50. doi: 10.3881/j.issn.1000-503X.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Choi HN, Kim KR, Lee JH, Park HS, Jang KY, Chung MJ, Hwang SE, Yu HC, Moon WS. Serum response factor enhances liver metastasis of colorectal carcinoma via alteration of the E-cadherin/beta-catenin complex. Oncol Rep. 2009;21:57–63. [PubMed] [Google Scholar]
- 15.Tan CT, Chu CY, Lu YC, Chang CC, Lin BR, Wu HH, Liu HL, Cha ST, Prakash E, Ko JY, et al. CXCL12/CXCR4 promotes laryngeal and hypopharyngeal squamous cell carcinoma metastasis through MMP-13-dependent invasion via the ERK1/2/AP-1 pathway. Carcinogenesis. 2008;29:1519–1527. doi: 10.1093/carcin/bgn108. [DOI] [PubMed] [Google Scholar]
- 16.Kajiyama H, Shibata K, Terauchi M, Ino K, Nawa A, Kikkawa F. Involvement of SDF-1alpha/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int J Cancer. 2008;122:91–99. doi: 10.1002/ijc.23083. [DOI] [PubMed] [Google Scholar]
- 17.Leelawat K, Leelawat S, Narong S, Hongeng S. Roles of the MEK1/2 and AKT pathways in CXCL12/CXCR4 induced cholangiocarcinoma cell invasion. World J Gastroenterol. 2007;13:1561–1568. doi: 10.3748/wjg.v13.i10.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richard CL, Tan EY, Blay J. Adenosine upregulates CXCR4 and enhances the proliferative and migratory responses of human carcinoma cells to CXCL12/SDF-1alpha. Int J Cancer. 2006;119:2044–2053. doi: 10.1002/ijc.22084. [DOI] [PubMed] [Google Scholar]
- 19.Brand S, Dambacher J, Beigel F, Olszak T, Diebold J, Otte JM, Göke B, Eichhorst ST. CXCR4 and CXCL12 are inversely expressed in colorectal cancer cells and modulate cancer cell migration, invasion and MMP-9 activation. Exp Cell Res. 2005;310:117–130. doi: 10.1016/j.yexcr.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 21.Sánchez-Tilló E, Lázaro A, Torrent R, Cuatrecasas M, Vaquero EC, Castells A, Engel P, Postigo A. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene. 2010;29:3490–3500. doi: 10.1038/onc.2010.102. [DOI] [PubMed] [Google Scholar]
- 22.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yori JL, Johnson E, Zhou G, Jain MK, Keri RA. Kruppel-like factor 4 inhibits epithelial-to-mesenchymal transition through regulation of E-cadherin gene expression. J Biol Chem. 2010;285:16854–16863. doi: 10.1074/jbc.M110.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denk C, Hülsken J, Schwarz E. Reduced gene expression of E-cadherin and associated catenins in human cervical carcinoma cell lines. Cancer Lett. 1997;120:185–193. doi: 10.1016/s0304-3835(97)00308-x. [DOI] [PubMed] [Google Scholar]
- 25.Gloushankova NA. Changes in regulation of cell-cell adhesion during tumor transformation. Biochemistry (Mosc) 2008;73:742–750. doi: 10.1134/s000629790807002x. [DOI] [PubMed] [Google Scholar]
- 26.Pancione M, Forte N, Fucci A, Sabatino L, Febbraro A, Di Blasi A, Daniele B, Parente D, Colantuoni V. Prognostic role of beta-catenin and p53 expression in the metastatic progression of sporadic colorectal cancer. Hum Pathol. 2010;41:867–876. doi: 10.1016/j.humpath.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Jiang B, Guo Z, Sardet C, Zou B, Lam CS, Li J, He M, Lan HY, Pang R, et al. Four-and-a-half LIM protein 2 promotes invasive potential and epithelial-mesenchymal transition in colon cancer. Carcinogenesis. 2010;31:1220–1229. doi: 10.1093/carcin/bgq094. [DOI] [PubMed] [Google Scholar]