Abstract
Stromal-vascular cells obtained from adult human subcutaneous adipose tissue were cultured in a chemically defined serum-free medium. In the presence of 0.2 nM triiodothyronine and 0.5 microM insulin, up to 25% of the cells were able to undergo terminal adipose differentiation within 18 d, as assessed by lipid accumulation and the expression of lipoprotein lipase (LPL) and glycerol-3-phosphate dehydrogenase (GPDH) activities. Addition of cortisol resulted in a potent dose-dependent stimulation of the adipose differentiation process. Cortisol could be replaced by dexamethasone and partly by aldosterone, but not by sex steroids. The proportion of differentiated cells was dependent upon the age of the donor; when isolated from young adults, up to 70% of the stromal-vascular cells expressed the adipocyte phenotype as compared with 5-10% when the cells were isolated from the oldest subjects. An inverse relationship was observed between the age of the 27 normal-weight donors and the extent of GPDH expression after maintenance of the cells for 18 d in chemically defined medium supplemented with insulin, triiodothyronine, and cortisol (r = -0.787, P less than 0.001). It is concluded that adult human adipose tissue still contains precursor cells that are able to undergo adipose differentiation in vitro. This improved culture system may offer the opportunity to characterize other adipogenic factors as well as antiadipogenic factors involved in the control of adipose tissue growth.
Full text
PDF

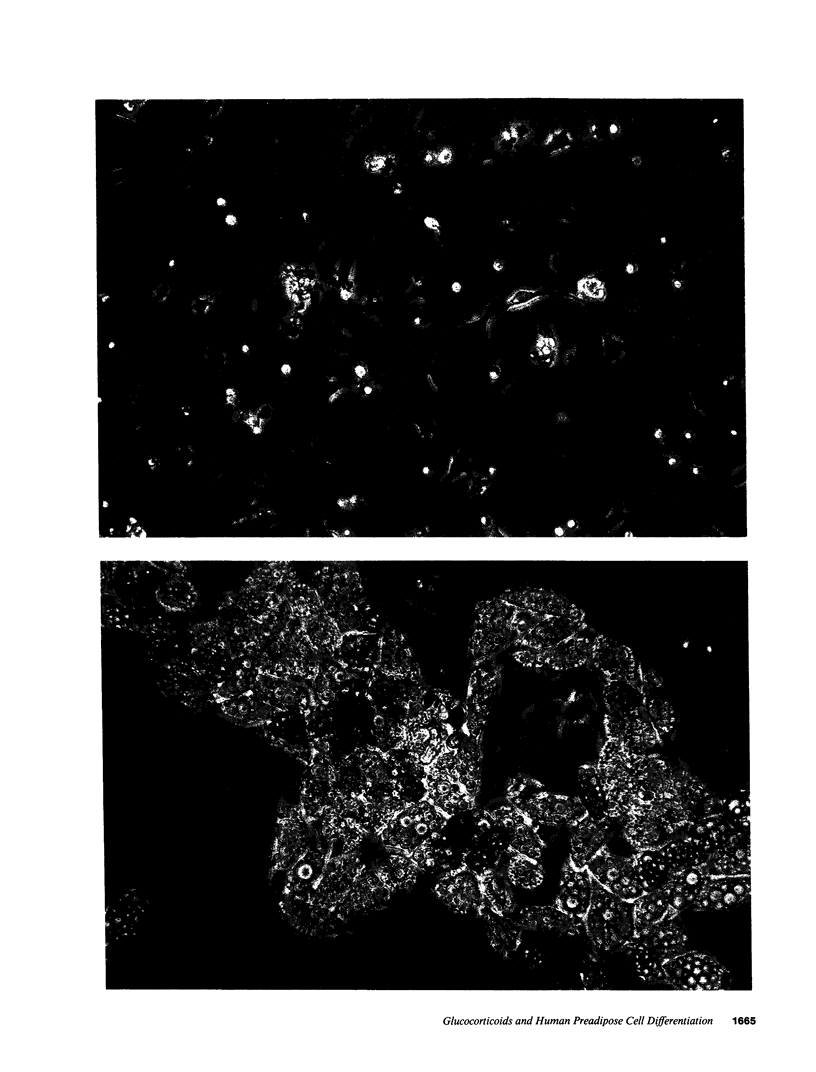
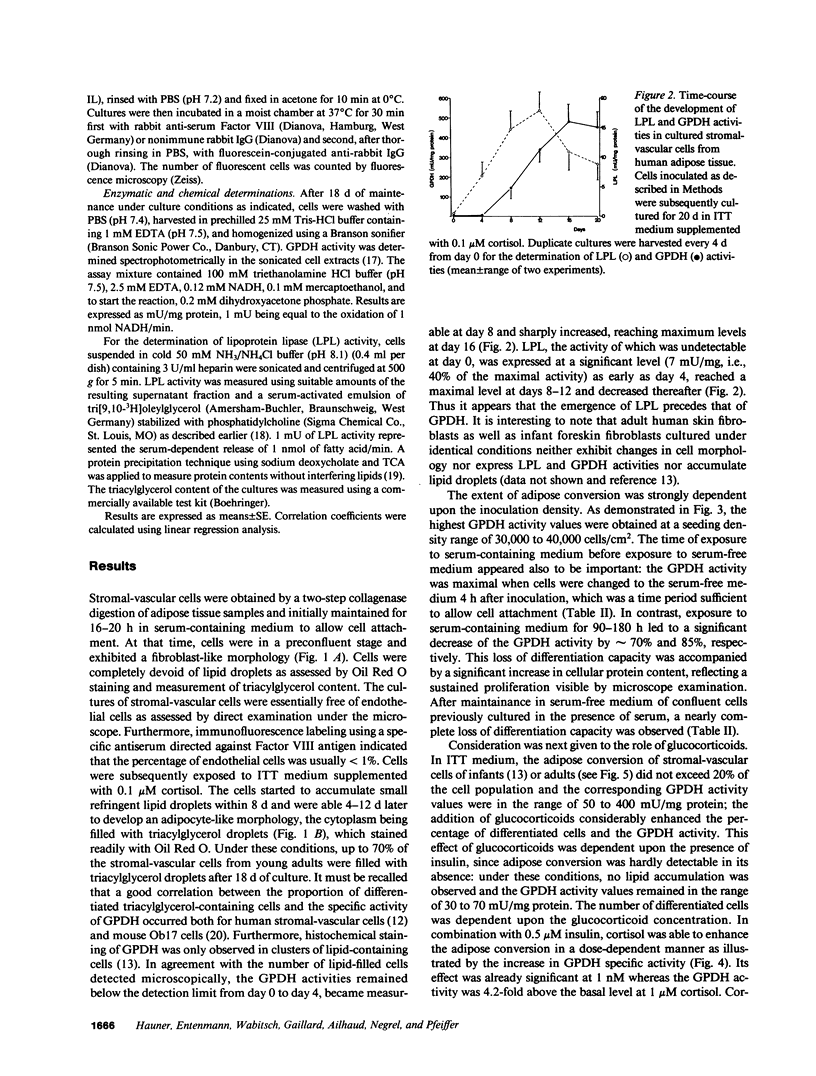
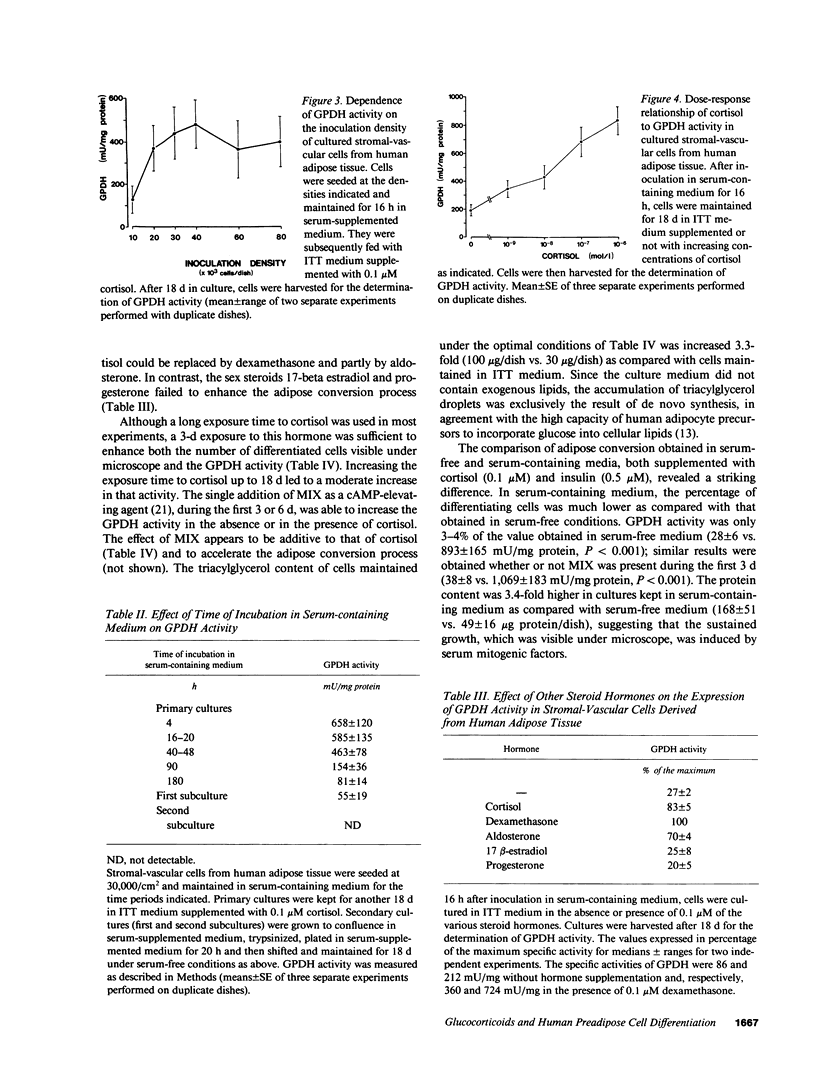
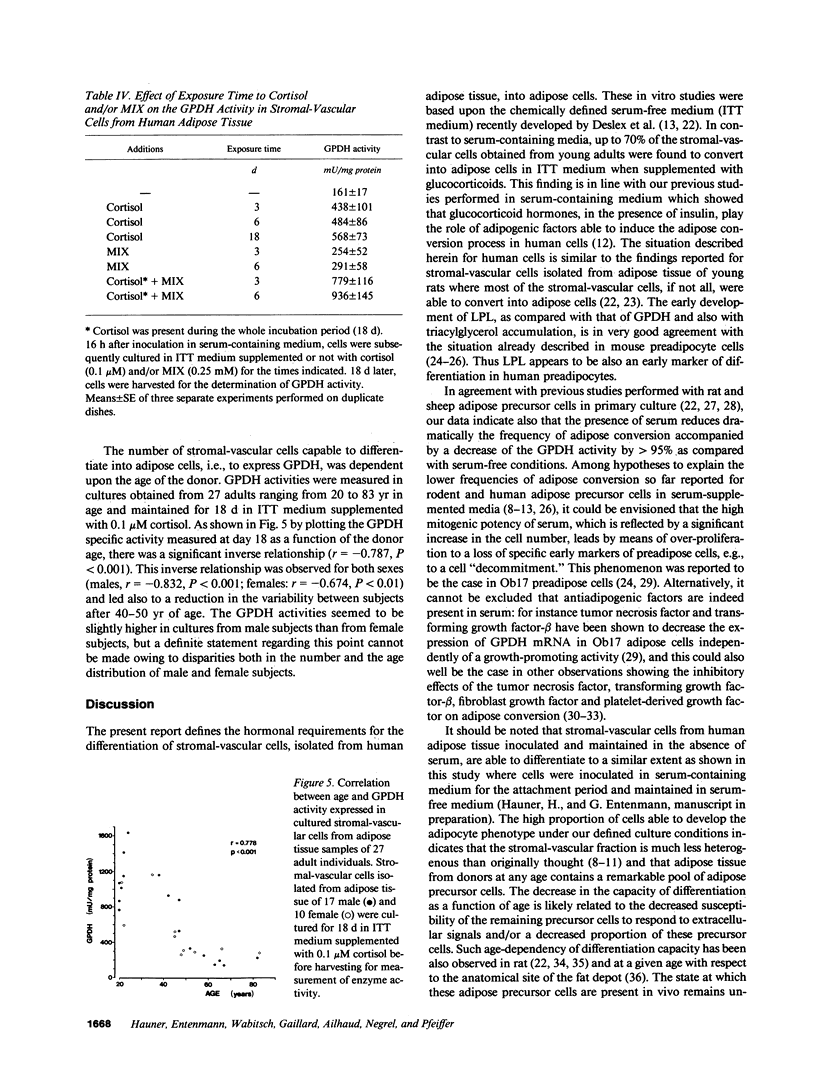


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amri E. Z., Dani C., Doglio A., Grimaldi P., Ailhaud G. Coupling of growth arrest and expression of early markers during adipose conversion of preadipocyte cell lines. Biochem Biophys Res Commun. 1986 Jun 13;137(2):903–910. doi: 10.1016/0006-291x(86)91165-4. [DOI] [PubMed] [Google Scholar]
- Bernlohr D. A., Bolanowski M. A., Kelly T. J., Jr, Lane M. D. Evidence for an increase in transcription of specific mRNAs during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1985 May 10;260(9):5563–5567. [PubMed] [Google Scholar]
- Björntorp P., Hansson G. K., Jonasson L., Pettersson P., Sypniewska G. Isolation and characterization of endothelial cells from the epididymal fat pad of the rat. J Lipid Res. 1983 Feb;24(2):105–112. [PubMed] [Google Scholar]
- Björntorp P., Karlsson M., Pertoft H., Pettersson P., Sjöström L., Smith U. Isolation and characterization of cells from rat adipose tissue developing into adipocytes. J Lipid Res. 1978 Mar;19(3):316–324. [PubMed] [Google Scholar]
- Björntorp P., Karlsson M., Pettersson P., Sypniewska G. Differentiation and function of rat adipocyte precursor cells in primary culture. J Lipid Res. 1980 Aug;21(6):714–723. [PubMed] [Google Scholar]
- Broad T. E., Ham R. G. Growth and adipose differentiation of sheep preadipocyte fibroblasts in serum-free medium. Eur J Biochem. 1983 Sep 1;135(1):33–39. doi: 10.1111/j.1432-1033.1983.tb07614.x. [DOI] [PubMed] [Google Scholar]
- Chapman A. B., Knight D. M., Ringold G. M. Glucocorticoid regulation of adipocyte differentiation: hormonal triggering of the developmental program and induction of a differentiation-dependent gene. J Cell Biol. 1985 Oct;101(4):1227–1235. doi: 10.1083/jcb.101.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. S., Lucas J. J., Sibley E., Bolanowski M. A., Christy R. J., Kelly T. J., Lane M. D. Expression of the differentiation-induced gene for fatty acid-binding protein is activated by glucocorticoid and cAMP. Proc Natl Acad Sci U S A. 1988 May;85(9):2949–2953. doi: 10.1073/pnas.85.9.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardick I., Poznanski W. J., Waheed I., Setterfield G. Ultrastructural observations on differentiating human preadipocytes cultured in vitro. Tissue Cell. 1976;8(3):561–571. doi: 10.1016/0040-8166(76)90013-6. [DOI] [PubMed] [Google Scholar]
- Deslex S., Negrel R., Ailhaud G. Development of a chemically defined serum-free medium for differentiation of rat adipose precursor cells. Exp Cell Res. 1987 Jan;168(1):15–30. doi: 10.1016/0014-4827(87)90412-5. [DOI] [PubMed] [Google Scholar]
- Deslex S., Negrel R., Vannier C., Etienne J., Ailhaud G. Differentiation of human adipocyte precursors in a chemically defined serum-free medium. Int J Obes. 1987;11(1):19–27. [PubMed] [Google Scholar]
- Dixon-Shanies D., Rudick J., Knittle J. L. Observatons on the growth and metabolic functions of cultured cells derived from human adipose tissue. Proc Soc Exp Biol Med. 1975 Jun;149(2):541–545. doi: 10.3181/00379727-149-38846. [DOI] [PubMed] [Google Scholar]
- Djian P., Roncari A. K., Hollenberg C. H. Influence of anatomic site and age on the replication and differentiation of rat adipocyte precursors in culture. J Clin Invest. 1983 Oct;72(4):1200–1208. doi: 10.1172/JCI111075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djian P., Roncari D. A., Hollenberg C. H. Adipocyte precursor clones vary in capacity for differentiation. Metabolism. 1985 Sep;34(9):880–883. doi: 10.1016/0026-0495(85)90114-3. [DOI] [PubMed] [Google Scholar]
- Elks M. L., Manganiello V. C. Selective effects of phosphodiesterase inhibitors on different phosphodiesterases, adenosine 3',5'-monophosphate metabolism, and lipolysis in 3T3-L1 adipocytes. Endocrinology. 1984 Oct;115(4):1262–1268. doi: 10.1210/endo-115-4-1262. [DOI] [PubMed] [Google Scholar]
- Faust I. M., Johnson P. R., Stern J. S., Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am J Physiol. 1978 Sep;235(3):E279–E286. doi: 10.1152/ajpendo.1978.235.3.E279. [DOI] [PubMed] [Google Scholar]
- Gaillard D., Ailhaud G., Négrel R. Fetuin modulates growth and differentiation of Ob17 preadipose cells in serum-free hormone-supplemented medium. Biochim Biophys Acta. 1985 Jul 30;846(1):185–191. doi: 10.1016/0167-4889(85)90125-9. [DOI] [PubMed] [Google Scholar]
- Gaillard D., Négrel R., Lagarde M., Ailhaud G. Requirement and role of arachidonic acid in the differentiation of pre-adipose cells. Biochem J. 1989 Jan 15;257(2):389–397. doi: 10.1042/bj2570389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauner H., Schmid P., Pfeiffer E. F. Glucocorticoids and insulin promote the differentiation of human adipocyte precursor cells into fat cells. J Clin Endocrinol Metab. 1987 Apr;64(4):832–835. doi: 10.1210/jcem-64-4-832. [DOI] [PubMed] [Google Scholar]
- Hauner H., Wabitsch M., Pfeiffer E. F. Differentiation of adipocyte precursor cells from obese and nonobese adult women and from different adipose tissue sites. Horm Metab Res Suppl. 1988;19:35–39. [PubMed] [Google Scholar]
- Hayashi I., Nixon T., Morikawa M., Green H. Adipogenic and anti-adipogenic factors in the pituitary and other organs. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3969–3972. doi: 10.1073/pnas.78.6.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J., Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab. 1976 Jul;5(2):299–311. doi: 10.1016/s0300-595x(76)80023-0. [DOI] [PubMed] [Google Scholar]
- Hirsch J., Knittle J. L. Cellularity of obese and nonobese human adipose tissue. Fed Proc. 1970 Jul-Aug;29(4):1516–1521. [PubMed] [Google Scholar]
- Hollenberg C. H., Vost A. Regulation of DNA synthesis in fat cells and stromal elements from rat adipose tissue. J Clin Invest. 1969 Nov;47(11):2485–2498. doi: 10.1172/JCI105930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Type beta transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8530–8534. doi: 10.1073/pnas.82.24.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navre M., Ringold G. M. A growth factor-repressible gene associated with protein kinase C-mediated inhibition of adipocyte differentiation. J Cell Biol. 1988 Jul;107(1):279–286. doi: 10.1083/jcb.107.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Négrel R., Gaillard D., Ailhaud G. Prostacyclin as a potent effector of adipose-cell differentiation. Biochem J. 1989 Jan 15;257(2):399–405. doi: 10.1042/bj2570399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pairault J., Green H. A study of the adipose conversion of suspended 3T3 cells by using glycerophosphate dehydrogenase as differentiation marker. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5138–5142. doi: 10.1073/pnas.76.10.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pettersson P., Van R., Karlsson M., Björntorp P. Adipocyte precursor cells in obese and nonobese humans. Metabolism. 1985 Sep;34(9):808–812. doi: 10.1016/0026-0495(85)90103-9. [DOI] [PubMed] [Google Scholar]
- Pilgrim C. DNA synthesis and differentiation in developing white adipose tissue. Dev Biol. 1971 Sep;26(1):69–76. doi: 10.1016/0012-1606(71)90108-4. [DOI] [PubMed] [Google Scholar]
- Poznanski W. J., Waheed I., Van R. Human fat cell precursors. Morphologic and metabolic differentiation in culture. Lab Invest. 1973 Nov;29(5):570–576. [PubMed] [Google Scholar]
- Rubin C. S., Hirsch A., Fung C., Rosen O. M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem. 1978 Oct 25;253(20):7570–7578. [PubMed] [Google Scholar]
- Salans L. B., Cushman S. W., Weismann R. E. Studies of human adipose tissue. Adipose cell size and number in nonobese and obese patients. J Clin Invest. 1973 Apr;52(4):929–941. doi: 10.1172/JCI107258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiwek D. R., Löffler G. Glucocorticoid hormones contribute to the adipogenic activity of human serum. Endocrinology. 1987 Feb;120(2):469–474. doi: 10.1210/endo-120-2-469. [DOI] [PubMed] [Google Scholar]
- Serrero G., Mills D. Differentiation of newborn rat adipocyte precursors in defined serum-free medium. In Vitro Cell Dev Biol. 1987 Jan;23(1):63–66. doi: 10.1007/BF02623495. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Frank M., Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983 Aug 25;258(16):10083–10089. [PubMed] [Google Scholar]
- Spooner P. M., Chernick S. S., Garrison M. M., Scow R. O. Development of lipoprotein lipase activity and accumulation of triacylglycerol in differentiating 3T3-L1 adipocytes. Effects of prostaglandin F2alpha, 1-methyl-3-isobutylxanthine, prolactin, and insulin. J Biol Chem. 1979 Feb 25;254(4):1305–1311. [PubMed] [Google Scholar]
- Torti F. M., Dieckmann B., Beutler B., Cerami A., Ringold G. M. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985 Aug 30;229(4716):867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]
- Van R. L., Bayliss C. E., Roncari D. A. Cytological and enzymological characterization of adult human adipocyte precursors in culture. J Clin Invest. 1976 Sep;58(3):699–704. doi: 10.1172/JCI108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederer O., Löffler G. Hormonal regulation of the differentiation of rat adipocyte precursor cells in primary culture. J Lipid Res. 1987 Jun;28(6):649–658. [PubMed] [Google Scholar]





