Abstract
Objective
To examine the association between gestational weight gain and maternal body mass index (BMI) among Vietnamese women and the risk of delivering an infant too small or too large for gestational age.
Methods
A prospective health-facility-based study of 2989 pregnant Vietnamese women was conducted in the city of Nha Trang in 2007–2008. Cubic logistic regression was used to investigate the association of interest. Infants were classified into weight-for-gestational-age categories according to weight centiles for the Asian population. Gestational age was based on the date of last menstrual period and adjusted by the results of first-trimester ultrasound.
Findings
BMI was low (< 18.5), normal (18.5–22.9) and high (≥ 23.0) in 26.1%, 65.4% and 8.5% of the women, respectively. In each of these BMI categories, the percentage of women who delivered infants too small for gestational age was 18.1, 10.0 and 9.4, respectively, and the mean gestational weight gain was 12.5 kg (standard deviation, SD: ± 3.6), 12.2 kg (SD: ± 3.8) and 11.5 kg (SD: ± 4.7), respectively. Among women with low BMI, the risk of delivering an infant too small for gestational age ranged from approximately 40% if the gestational weight gain was < 5 kg to 20% if it was 5–10 kg.
Conclusion
Having a low BMI, commonly found in Viet Nam, puts women at risk of delivering an infant too small for gestational age, especially when total maternal gestational weight gain is < 10 kg.
ملخص
الغرض
فحص العلاقة بين زيادة وزن الأمهات الفيتناميات أثناء الحمل ومنسب كتلة الجسم لديهن وخطر ولادتهن لطفل صغير جداً أو كبير جداً بالنسبة لعمره الحملي.
الطريقة
أُجريت دراسةٌ استباقية مستندة إلى المرفق الصحي لـ 2989 امرأة من الحوامل الفيتناميات في مدينة "نها-ترانغ" في عامي 2007-2008. واستُخدِم التحوف اللوجستي المُكعّب لتقصي الارتباطات موضع الاهتمام. وجرى تقسيم الرضّع إلى فئات حسب وزنهم لقاء عمرهم الحملي وفقاً للشريحة المئوية للأسيويين. واستند تقدير العمر الحملي إلى تاريخ آخر دورة شهرية مع تصحيحه وفقاً لنتائج أول فحص بالموجات فوق الصوتية في الشهور الثلاثة الأولى من الحمل.
النتائج
كان منسب كتلة الجسم بين النساء منخفضاً (أقل من 18.5)، وطبيعياً (18.5-22.9)، ومرتفعاً (أكبر من أو يساوي 23.0)، بمقدار 26.1%، 65.4%، 8.5% بالترتيب. وفي كل فئة من فئات منسب كتلة الجسم، بلغت النسبة المئوية للنساء اللاتي ولدن أطفالاً صغاراً للغاية لقاء عمرهم الحملي 18.1%، 10.0%، 9.4% بالترتيب، وكان متوسط زيادة وزنهن أثناء الحمل 12.5 كيلوغرام (الانحراف المعياري: ± 3.6)، و 12.2 كيلوغرام (الانحراف المعياري: ± 3.8)، و 11.5 كيلوغرام (الانحراف المعياري: ± 4.7) بالترتيب. وتراوح خطر ولادة طفل صغير للغاية لقاء عمره الحملي، بين النساء في فئة منسب كتلة الجسم المنخفض، من حوالي 40% عندما تكون زيادة الوزن الحملي دون 5 كيلوغرامات، إلى 20% عندما تبلغ زيادة الوزن الحملي 5-10 كيلوغرام.
الاستنتاج
إن انخفاض منسب كتلة الجسم، الشائع في فيتنام، يعرّض النساء لخطر ولادة أطفال صغار الوزن لقاء عمرهم الحملي، ولاسيما عندما يكون إجمالي زيادة وزن الأم أثناء الحمل أقل من 10 كيلوغرام.
Résumé
Objectif
Examiner le rapport entre l'augmentation de poids en gestation et l'indice maternel de masse corporelle (IMC) chez les femmes vietnamiennes avec le risque d'accoucher d'un enfant trop petit ou trop grand pour son âge gestationnel.
Méthodes
Une étude prospective, en établissements de soins, a été menée auprès de 2 989 femmes enceintes vietnamiennes dans la ville de Nha Trang en 2007–2008. Une régression logistique cubique a été utilisée pour rechercher l'association pertinente. Les nouveau-nés ont été classés en plusieurs catégories selon le rapport/âge gestationnel, par référence aux centiles de poids de la population asiatique. L'âge gestationnel a été basé sur la date de la dernière période menstruelle et ajusté au vu des résultats de l'échographie du premier trimestre.
Résultats
L'IMC était faible (<18,5), normal (18,5 – 22,9) et élevé (≥23,0) chez 26,1%; 65% et 8,5% des femmes, respectivement. Dans chacune de ces catégories d'IMC, le pourcentage de femmes qui ont accouché d'enfants trop petits pour leur âge gestationnel était respectivement de 18,1, 10,0 et 9,4, et l'augmentation moyenne de poids a été respectivement de 12,5 kg (écart des normes, EN: ±3,6), 12,2 kg (EN: ±3,8), et 11,5 kg (EN: ±4.7). Pour les femmes avec un faible IMC, le risque d'accoucher d'un enfant trop petit pour son âge gestationnel s'étendait de 40% environ si l'augmentation gestationnelle de poids était <5 kg, à 20% s'il était entre 5 et 10 kg.
Conclusion
Un faible IMC, courant au Viet Nam, crée un risque pour les femmes d'accoucher d'un enfant trop petit pour son âge gestationnel, particulièrement quand l'augmentation gestationnelle du poids de la mère est <10 kg.
Resumen
Objetivo
Examinar la relación existente entre el aumento de peso durante el embarazo y el índice de masa corporal (IMC) de las madres en Vietnam y el riesgo de dar a luz a niños demasiado pequeños o demasiado grandes para su edad gestacional.
Métodos
Se desarrolló un estudio prospectivo en 2989 embarazadas vietnamitas en centros sanitarios de la ciudad de Nha Trang entre 2007 y 2008. Se empleó una regresión logística y cúbica para investigar la asociación de interés. Los niños se clasificaron en diversas categorías según el peso para la edad gestacional, de acuerdo con los centiles de peso para la población asiática. La edad gestacional se basó en la fecha de la última menstruación y se ajustó con los resultados de la ecografía del primer trimestre.
Resultados
El IMC fue bajo (< 18,5), normal (18,5–22,9) y alto (≥ 23,0) en un 26,1%, 65,4% y 8,5% de las mujeres, respectivamente. En cada una de estas categorías de IMC, el porcentaje de madres que dio a luz a niños demasiado pequeños para su edad gestacional fue del 18,1, 10,0 y 9,4 por ciento, respectivamente, y la media del aumento de peso durante el embarazo fue de 12,5 kg (desviación estándar, DE: ± 3,6), 12,2 kg (DE: ± 3,8) y 11,5 kg (DE: ± 4,7), respectivamente. Entre las mujeres con un IMC bajo, el riesgo de dar a luz a un niño demasiado pequeño para su edad gestacional osciló entre aproximadamente un 40% si el aumento de peso durante el embarazo fue inferior a 5 kg hasta un 20% si dicho aumento fue de entre 5 y 10 kg.
Conclusión
Tener un IMC bajo, algo muy común en Vietnam, aumenta el riesgo de dar a luz a un niño demasiado pequeño para su edad gestacional, especialmente cuando el aumento total de peso durante el embarazo es inferior a los 10 kilos.
Резюме
Цель
Проанализировать связь между прибавкой в весе во время беременности и индексом массы тела (ИМТ) матери у вьетнамских женщин, а также исследовать риск рождения слишком маленького или слишком большого ребенка для гестационного срока.
Методы
В 2007–2008 годах в городе Нячанг на базе поликлиники было проведено проспективное исследование 2989 беременных вьетнамских женщин. Для изучения интересующей нас корреляции использовалась кубическая логистическая регрессия. Новорожденные классифицировались по категориям веса для гестационного срока согласно центилям веса для азиатского населения. Гестационный срок определялся исходя из даты последней менструации и корректировался по результатам ультразвукового исследования в первом триместре.
Результаты
Низкий (<18.5), нормальный (18.5–22.9) и высокий (≥23.0) ИМТ был отмечен, соответственно, у 26,1 , 65,4 и 8,5% женщин. В каждой из этих категорий ИМТ процентная доля женщин, родивших слишком маленького ребенка для гестационного срока составила, соответственно, 18,1 , 10,0 и 9,4 , а средняя прибавка в весе во время беременности составила, соответственно, 12,5 кг (стандартное отклонение, СО: ±3,6), 12,2 кг (СО: ±3,8) и 11,5 кг (СО: ±4,7). Среди женщин с низким ИМТ риск родить слишком маленького ребенка для гестационного срока ранжировал примерно от 40%, если прибавка в весе во время беременности составляла менее 5 кг, до 20%, если она составляла 5–10 кг.
Вывод
Низкий ИМТ (явление, широко распространенное во Вьетнаме) подвергает женщин риску родить слишком маленького ребенка для гестационного срока, особенно когда общая прибавка веса матери в период беременности составляет менее 10 кг.
摘要
目的
旨在探讨越南妇女妊娠期体重增加与母亲体质指数的联系,及娩出巨大儿或低出生体重儿的风险。
方法
2007-2008年,我们对2989名越南芽庄孕妇进行了一次前瞻性的以医疗机构为基础的研究。应用三次逻辑回归研究所关注信息的关联。根据亚洲人群的体重百分位数,婴儿分为若干胎龄体重类型。胎龄是基于上一次的月经周期并结合早期妊娠的超声检测结果进行计算。
结果
体质指数低(<18.5)、正常(18.5-22.9)和高(23.0)的妇女分别为26.1%、65.4%和8.5%。每一体质指数类别中,娩出低出生体重儿的妇女的比例分别为18.1%、10.0%和9.4%,并且妊娠期平均体重增加分别为12.5 kg(标准差:±3.6)、12.2 kg(标准差:±3.8)和11.5 kg(标准差:±4.7)。体质指数低的妇女中,娩出低出生体重儿的风险从约40%(如果妊娠期体重增加<5 kg)变化到20%(如果妊娠期体重增加为5-10kg)。
结论
越南普遍存在的体质指数偏低的情况使得妇女娩出低出生体重儿的危险增加,特别是当妊娠期总体重增加<10kg的时候。
Introduction
The Global Safe Motherhood Initiative, launched in 1987, is designed to improve antenatal care and counselling throughout the world. Nutrient intake and weight gain during pregnancy are the two main modifiable factors influencing maternal and infant outcomes.1 Indeed, a low body mass index (BMI) and suboptimal weight gain during pregnancy are long-recognized risk factors for the delivery of infants too small for gestational age.2
Being born small for gestational age is a major predictor of neonatal mortality and morbidity,2 failure to grow, slow cognitive development and chronic diseases in adulthood.3 Infants too large for gestational age also experience higher perinatal and long-term health risks.4–7 In addition, both groups of infants are more likely to be delivered by Caesarean section. Thus, reducing the delivery of excessively small or large infants translates into fewer surgical risks for women.8 Appropriate antenatal management of maternal nutrition, as dictated by scientific evidence, is critical in reducing the delivery of these babies for whom both the intrauterine environment and the birth process can be life-threatening.8,9
Maternal anthropometry differs across populations.10 Women belonging to ethnic groups characterized by a small body size have been reported to gain less weight on average during pregnancy than larger women. In less-developed Asian countries, including Viet Nam, women generally have a lower BMI and/or a smaller gestational weight gain than in developed countries.11,12 In the United States of America, for example, 2% of pregnant women have a BMI < 18.5 and more than 50% have a BMI > 25.13 There is a need to assess whether the current anthropometric recommendations for pregnant women of the United States National Academy of Sciences Institute of Medicine (IOM), which are based on data from western countries, are appropriate for preventing adverse pregnancy outcomes across populations everywhere, including south-east Asia.1
The objectives of this study were: (i) to determine the prevalence of small and large size for gestational age among infants of Vietnamese women, and (ii) to estimate the risk of giving birth to an infant too small or too large for gestational age as a function of maternal gestational weight gain and BMI in Viet Nam.
Methods
Study setting and population
Viet Nam is a low-income country with an estimated total population of 87 million. According to data from the United Nations Children’s Fund, from 2003 to 2008 the average annual number of births in the country was 1 494 000 and the average annual prevalence of low birth weight was 7%. In 2008, the maternal mortality ratio was 150 per 100 000 live births, and the infant mortality rate was 12 per 1000 live births.14,15 We conducted a prospective health-facility-based study in Nha Trang city, in the province of Khanh Hoa. The study included women from eight community health centres and one provincial hospital in the catchment area of Nha Trang, whose total population is approximately 400 000.16
Trained midwives conducted semi-structured interviews with women residents of the study catchment area with singleton pregnancies who were admitted to the aforementioned health institutions for delivery from 3 July 2007 to 15 June 2008. Women who delivered a stillborn or who gave birth before the 22nd week of gestation were excluded from the study. A stillborn was defined as a fetus with a gestational age of at least 22 full weeks that did not breathe, cry or show minimal movement of the chest or limbs at birth.
Ethical approval
The institutional review boards of the University of Tokyo (Tokyo, Japan) and the National Institute of Hygiene and Epidemiology (Hanoi, Viet Nam) approved the study. All participants received detailed information about the study and were asked to give written informed consent.
Enrolment and participation
Overall, 3022 pregnant women were recruited for the study: 2867 pregnant women were attended in the hospital and 393 in the eight community health centres, but 238 of them were excluded from the study because their weight and height had not been obtained. This left a total of 3022 recruits, of whom an additional 33 were excluded because other data were found to be missing. Hence, 2989 participants were included in the analysis. Prior to the study (in June and July 2006), we had conducted census surveys in 16 communes of the Khan Hoa Provincial Health Service area to assess the potential bias generated by using facility-based records.17,18 From the number of children less than 5 years of age identified through the census (13 935) we calculated the average number of children delivered annually (2750) over the past five years, and from this and the number of pregnant women in our study who were from the census area we concluded that our study covered approximately 92% of all children delivered in the study area during the study period (2524/2750).
Anthropometric measurements
The weight and height of all mothers were measured before delivery by midwives trained before the study. Measurements in the hospital were taken using a DC320 weight scale (Tanita Corp., Tokyo, Japan) and a SECA206 height scale (Seca Corp., Hamburg, Germany), which measures height to within 1 millimetres (mm). Measurements in the community health centres were taken using a TZ120 scale for weight and height (Yuyao Balance Instrument Factory, Yuyao, China) with minimum resolutions of 50 grams (g) and 0.5 centimetre (cm), respectively.
Midwives weighed unclothed newborn infants immediately after delivery using a D-8310 electronic baby scale (Soehnle-Waagen GmbH & Co. KG, Murrhardt, Germany), precise to within 10 g. Infants were measured supine with a SECA210 length-measuring strip mat (Seca Corp.), precise to within 1 mm.
Maternal BMI and perinatal outcomes
Neonates were classified as small, normal or large for gestational age in accordance with the following criteria, based on gender-specific centiles for the Asian population,19 as recommended by Clausson et al.20: small if the birth weight was below the 10th percentile; normal if it was between the 10th and 90th percentiles and large if it was above the 90th percentile.19
Low birth weight and macrosomia were defined as a weight at birth of < 2500 g and > 4000 g, respectively. These western definitions were used because no Asian standards exist. Pre-eclampsia was defined as antenatal systolic blood pressure ≥ 140 mmHg, or antenatal diastolic blood pressure ≥ 90 mmHg with proteinuria.21
We used the self-reported pre-gestational weight in kilograms (kg) and the height measured during the interview in metres squared (m2) to calculate maternal BMI (kg/m2). In western societies BMI is classified as low if < 18.5; normal if 18.5–24.9 and high if ≥ 25.0. Landmann et al. have suggested a BMI cut-off point of 23.0 for obesity in Viet Nam.22 The World Health Organization also recommends using this last criterion for Asian populations23 and this is the cut-off we used. Thus, we classified women into low-, normal- and high-BMI groups as follows: low, BMI < 18.5; normal, BMI 18.5–22.9 and high, BMI ≥ 23.0. Gestational age was estimated on the basis of the last menstrual period and/or a first-trimester ultrasound (performed on 86% of the women, since only the hospital performed ultrasound). If the age obtained by the two methods differed by more than 5 days, we used the age by ultrasound for the analysis.
Data management and analysis
Interview forms were completed, collected and inspected for completeness and consistency daily by two midwives specifically trained for this study. Data were double-entered and validated by trained data management staff using FoxPro 9.0 (Microsoft, Redmond, USA).
Crude and adjusted odds ratios (ORs) and their 95% confidence intervals (CIs) were generated by univariate and multivariate logistic regression analyses, with maternal gestational weight gain and BMI category as the main independent variables and small or large size for gestational age as dependent variables. Other covariables included in the model were maternal age, parity, household income, years of schooling, total physical activity,24,25 exposure to indoor smoking by a family member, infant gender and gestational age at delivery. To quantify physical activity we applied to all pregnant women at delivery the Vietnamese version of the Pregnancy Physical Activity Questionnaire (PPAQ),25 an instrument designed to assess the duration, frequency and intensity of total physical activity in pregnant women. In this way we quantified their physical activity over the three most recent months (i.e. during the third trimester of pregnancy). Because the PPAQ is short, self-administered and easily understood by respondents in a variety of settings, it lends itself to epidemiologic research. Details of the calculations and the validation study are in the original references.24 ORs were adjusted for these covariables by logistic regression using SPSS for Windows version 16 for Japan (SPSS, Chicago, USA). The criteria for categorical variables were defined a priori.
Cubic spline logistic regression analysis26 was performed to fit nonlinear curves smoothly using R version 2.7.1 for Windows (R Foundation for Statistical Computing, Vienna, Austria). This was done to examine potential nonlinear associations between small or large size for gestational age and gestational weight gain in each maternal BMI category. Principal component analysis was used to reduce the number of covariables in the multivariate model.27 Optimal weight gains were determined from the intersection on the regression graph of maternal weight gain and the probability of delivering an infant too small or too large for gestational age. We used bootstrapping to estimate the corresponding 95% CIs by employing bias-corrected and accelerated methods28 based on R resampling nonparametric methods.
Results
Table 1 shows the characteristics of the 2989 women who met the eligibility criteria. Those with low, normal and high BMI accounted for 26.1%, 65.4% and 8.5% of all participating women, respectively. The overall mean gestational weight gain was 12.2 kg (standard deviation, SD: ± 3.9). In the low BMI group, 78% of the pregnant women gained > 10 kg (Table 1).
Table 1. Maternal and infant characteristics for different categories of maternal body mass index (BMI), Nha Trang, Viet Nam, 2007–2008.
| Characteristica | BMI categoryb |
|||
|---|---|---|---|---|
| All | Low BMI | Normal BMI | High BMI | |
| n = 2 989 (100%) | n = 780 (26.1%) | n = 1955 (65.4%) | n = 254 (8.5%) | |
| Mean age (years) | 27.9 ± 5.3 | 26.2 ± 5.0 | 28.2 ± 5.3 | 30.7 ± 5.1 |
| Parity | ||||
| Primipara | 1496 (50.1) | 484 (62.1) | 935 (47.8) | 77 (30.3) |
| Multipara | 1493 (49.9) | 296 (37.9) | 1020 (52.2) | 177 (69.7) |
| Mean height (cm) | 154.2 ± 4.8 | 154.6 ± 4.7 | 154.0 ± 4.8 | 154.0 ± 5.2 |
| Mean BMI | 20.0 ± 2.2 | 17.4 ± 0.8 | 20.3 ± 1.2 | 24.7 ± 1.7 |
| Mean gestational weight gain (kg) | 12.2 ± 3.9 | 12.5 ± 3.6 | 12.2 ± 3.8 | 11.5 ± 4.7 |
| < 10.0 | 776 (25.9) | 177 (22.7) | 509 (26.0) | 90 (35.3) |
| ≥ 10.0 but < 15.0 | 1546 (51.8) | 414 (53.1) | 1028 (52.6) | 104 (41.2) |
| ≥ 15.0 but < 20.0 | 587 (19.6) | 174 (22.3) | 362 (18.5) | 51 (20.0) |
| ≥ 20 | 80 (2.7) | 15 (1.9) | 56 (2.9) | 9 (3.5) |
| Gestational weight gain (kg) recommended by medical staff | 11.6 ± 0.9 | 11.6 ± 0.9 | 11.6 ± 0.8 | 11.6 ± 0.9 |
| Smoking | ||||
| During pregnancy | 1 (0.03) | 0 | 1 (0.03) | 0 |
| Passive indoor smoking | 1053 (35.2) | 306 (39.2) | 653 (33.4) | 94 (37.0) |
| Drinking during pregnancy | 9 (0.3) | 3 (0.4) | 5 (0.3) | 1 (0.4) |
| Marital status | ||||
| Single | 21 (0.7) | 4 (0.5) | 15 (0.8) | 2 (0.8) |
| Married | 2934 (98.1) | 765 (98.1) | 1918 (98.0) | 251 (98.8) |
| Divorced/separated | 9 (0.3) | 3 (0.4) | 6 (0.3) | 0 |
| Widowed | 10 (0.4) | 3 (0.4) | 7 (0.4) | 0 |
| Others | 15 (0.5) | 5 (0.6) | 9 (0.5) | 1 (0.4) |
| Years of schooling | ||||
| < 12 | 1645 (55.0) | 397 (50.9) | 1093 (55.9) | 155 (61.0) |
| 12 | 791 (26.5) | 218 (27.9) | 512 (26.2) | 61 (24.0) |
| > 12 | 553 (18.5) | 165 (21.2) | 350 (17.9) | 38 (15.0) |
| Household income ( × 1 000 Vietnamese dong) | ||||
| < 1 500 | 273 (9.1) | 77 (9.9) | 177 (9.0) | 19 (7.5) |
| ≥ 1 500 but < 2 500 | 1082 (36.2) | 274 (35.1) | 707 (36.2) | 101 (39.8) |
| ≥ 2 500 but < 3 500 | 873 (29.2) | 235 (30.1) | 566 (28.9) | 72 (28.3) |
| ≥ 3 500 | 761 (25.5) | 194 (24.9) | 505 (25.8) | 62 (24.4) |
| No. of previous gestational care visits | 5.3 ± 2.6 | 5.4 ± 2.5 | 5.2 ± 2.5 | 5.1 ± 2.8 |
| Morning sickness | 1522 (50.9) | 410 (52.6) | 993 (50.8) | 119 (46.9) |
| Occupation | ||||
| Yes | 1842 (61.6) | 481 (61.7) | 1209 (61.8) | 151 (59.4) |
| No | 1147 (38.4) | 298 (38.3) | 745 (38.2) | 103 (40.6) |
| Physical activity during pregnancy (MET h/wk) | ||||
| Total in the 3rd trimester | 127.2 ± 76.1 | 124.1 ± 75.8 | 127.8 ± 75.2 | 132.1 ± 83.5 |
| Sedentary (< 1.5) | 42.6 ± 34.9 | 42.0 ± 35.0 | 43.1 ± 34.8 | 40.3 ± 34.6 |
| Light (≥ 1.5 but < 3.0) | 63.6 ± 40.3 | 62.9 ± 40.0 | 63.6 ± 40.2 | 66.1 ± 42.5 |
| Moderate (3.0–6.0) | 19.2 ± 32.2 | 17.3 ± 32.4 | 19.3 ± 31.1 | 24.2 ± 39.2 |
| Vigorous (> 6.0) | 1.7 ± 7.1 | 1.8 ± 6.7 | 1.8 ± 7.4 | 1.4 ± 6.5 |
| Duration of pregnancy (weeks) | ||||
| < 37 | 41 (1.3) | 14 (1.8) | 25 (1.3) | 2 (0.8) |
| 37–39 | 1374 (46.0) | 388 (49.7) | 877 (44.9) | 109 (42.9) |
| 40–42 | 1565 (52.4) | 376 (48.2) | 1046 (53.5) | 143 (56.3) |
| ≥ 43 | 9 (0.3) | 2 (0.3) | 7 (0.3) | 0 |
| Infant sex | ||||
| Male | 1592 (53.3) | 408 (52.3) | 1044 (53.4) | 140 (55.1) |
| Female | 1397 (46.7) | 372 (47.7) | 911 (46.6) | 114 (44.9) |
| Infant birth weight (g) | 3227 ± 423 | 3105 ± 396 | 3258 ± 415 | 3365 ± 490 |
| Infant birth height (cm) | 50.4 ± 1.8 | 50.2 ± 1.8 | 50.5 ± 1.8 | 50.7 ± 1.7 |
MET h/wk, metabolic equivalent hours per week.
a All values are either the mean ± the standard deviation or the number and percentage of women or infants with the characteristic.
b BMI represents body weight in kilograms divided by body surface area in square metres. Categories: low BMI: < 18.5; normal BMI, ≥ 18.5 but < 23.0; high BMI, ≥ 23.0.
The proportion of infants born small for gestational age varied in accordance with maternal BMI. Among women with low, normal and high BMI, the percentage whose infants were small for gestational age was 18.1, 10.0 and 9.4, respectively, and the percentage whose infants were large for gestational age was 4.7%, 10.5% and 21.2%. Overall, 12.1% and 9.9% of all infants born to participant mothers were too small or too large for gestational age, respectively.
The crude and adjusted ratio of the odds of having an infant too small for gestational age and of having one too large for gestational age (relative to the odds of having one of normal size) are shown in Table 2 and Table 3, respectively. On multiple logistic regression, a gestational weight gain of < 10 kg, a low BMI and pre-eclampsia were associated with a higher risk of having an infant too small for gestational age. A monthly income of ≥ 3 500 000 Vietnamese dong (approximately 180 US dollars) and a later week of delivery were associated with a lower risk of having an infant too small for gestational age. A gestational weight gain of more than 15 kg, a high BMI and multiparity were associated with a higher risk of having an infant too large for gestational age; being younger (< 24 years of age) and having a low BMI were associated with a lower risk of having such an infant.
Table 2. Ratio of the odds of delivering an infant too small for gestational age relative to the odds of delivering an infant with appropriate size for gestational age as a function of maternal characteristics, infant sex and gestational age at delivery, Nha Trang, Viet Nam, 2007–2008.
| Characteristic | No.a/totalb (%) | Crude OR | 95% CI | P | Adjusted OR | 95% CI | P |
|---|---|---|---|---|---|---|---|
| Gestational weight gain (kg) | |||||||
| < 10 | 151/729 (20.7) | 2.08 | 1.63–2.64 | < 0.001 | 1.90 | 1.48–2.44 | < 0.001 |
| ≥ 10 but < 15 | 161/1405 (11.5) | 1.00 | – | 1.00 | – | ||
| ≥ 15 | 49/558 (8.8) | 0.68 | 0.49–0.95 | 0.024 | 0.74 | 0.52–1.05 | 0.086 |
| BMIc | |||||||
| Low | 141/742 (19.0) | 1.86 | 1.47–2.35 | < 0.001 | 1.95 | 1.52–2.50 | < 0.001 |
| Normal | 196/1749 (11.2) | 1.00 | – | 1.00 | – | ||
| High | 24/201 (11.9) | 1.07 | 0.68–1.69 | 0.755 | 0.95 | 0.60–1.52 | 0.844 |
| Maternal age (years) | |||||||
| < 24 | 136/812 (16.7) | 1.55 | 1.18–2.03 | 0.002 | 1.13 | 0.84–1.52 | 0.431 |
| 25–29 | 109/949 (11.5) | 1.00 | – | 1.00 | – | ||
| 30–34 | 67/587 (11.4) | 0.99 | 0.72–1.37 | 0.966 | 1.02 | 0.73–1.44 | 0.904 |
| ≥ 35 | 49/344 (14.2) | 1.28 | 0.89–1.84 | 0.182 | 1.24 | 0.84–1.82 | 0.281 |
| Parity | |||||||
| Primipara | 204/1400 (14.6) | 1.00 | – | 1.00 | – | ||
| Multipara | 157/1292 (12.2) | 0.81 | 0.65–1.01 | 0.066 | 0.78 | 0.60–1.02 | 0.073 |
| Income ( × 1 000 Vietnamese dong) | |||||||
| < 1 500 | 49/255 (19.2) | 1.26 | 0.88–1.80 | 0.201 | 1.08 | 0.75–1.56 | 0.688 |
| ≥ 1 500 but < 2 500 | 157/989 (15.9) | 1.00 | – | 1.00 | – | ||
| ≥ 2 500 but < 3 500 | 97/779 (12.5) | 0.75 | 0.57–0.99 | 0.042 | 0.85 | 0.64–1.13 | 0.271 |
| ≥ 3 500 | 58/669 (8.7) | 0.50 | 0.37–0.69 | < 0.001 | 0.67 | 0.48–0.95 | 0.025 |
| Education (years) | |||||||
| < 12 | 243/1506 (16.1) | 1.43 | 1.10–1.87 | 0.008 | 1.30 | 0.98–1.74 | 0.068 |
| 12 | 84/708 (11.9) | 1.00 | – | 1.00 | – | ||
| > 12 | 34/478 (7.1) | 0.57 | 0.38–0.86 | 0.008 | 0.67 | 0.43–1.04 | 0.071 |
| Total physical activitydin MET h/wk | |||||||
| < 25th percentile | 98/671 (14.6) | 0.96 | 0.71–1.30 | 0.797 | 0.89 | 0.65–1.22 | 0.460 |
| ≥ 25th but < 50th percentile | 103/681 (15.1) | 1.00 | – | 1.00 | – | ||
| ≥ 50th but < 75th percentile | 79/666 (11.9) | 0.76 | 0.55–1.04 | 0.082 | 0.88 | 0.64–1.23 | 0.460 |
| ≥ 75th percentile | 81/673 (12.0) | 0.77 | 0.56–1.05 | 0.100 | 0.98 | 0.70–1.35 | 0.878 |
| Smoking exposure | |||||||
| None | 215/1737 (12.4) | 1.00 | – | 1.00 | – | ||
| Passive indoor smoking | 146/955 (15.3) | 1.28 | 1.02–1.60 | 0.034 | 1.06 | 0.84–1.34 | 0.638 |
| Pre-eclampsia | 10/35 (28.6) | 2.63 | 1.25–5.52 | 0.011 | 3.14 | 1.43–6.90 | 0.004 |
| Infant sex (male = 1, female = 0) | 207/1419 (14.6) | 1.24 | 0.99–1.55 | 0.059 | 1.21 | 0.96–1.52 | 0.114 |
| Gestational age at delivery | – | 0.87e | 0.79–0.95 | 0.002 | 0.89 | 0.81–0.97 | 0.012 |
BMI, body mass index; CI, confidence interval; MET h/wk, metabolic equivalent hours per week; OR, odds ratio.
a This represents the number of infants too small for gestational age (n = 361) born to women with the characteristic.
b This figure represents the number of infants of appropriate size plus the number of infants too small for gestational age (n = 2692) born to women with the characteristic.
c BMI represents body weight in kilograms divided by body surface area in square metres. Categories: low BMI: < 18.5; normal BMI, ≥ 18.5 but < 23.0; high BMI, ≥ 23.0.
d There is one case with missing data for physical activity.
e The OR represents the change in the risk of delivering a small baby with each additional week of gestation.
Table 3. Ratio of the odds of delivering an infant too large for gestational age relative to the odds of delivering an infant with appropriate size for gestational age as a function of maternal characteristics, infant sex and gestational age at delivery, Nha Trang, Viet Nam, 2007–2008.
| Characteristic | No.a/totalb (%) | Crude OR | 95% CI | P | Adjusted OR | 95% CI | P |
|---|---|---|---|---|---|---|---|
| Gestational weight gain (kg) | |||||||
| < 10 | 47/625 (7.5) | 0.65 | 0.46–0.92 | 0.014 | 0.72 | 0.50–1.02 | 0.067 |
| ≥ 10 but < 15 | 139/1383 (10.1) | 1.00 | – | – | 1.00 | – | – |
| ≥ 15 | 109/618 (17.6) | 1.97 | 1.51–2.59 | < 0.001 | 1.93 | 1.46–2.57 | < 0.001 |
| BMIc | |||||||
| Low | 37/638 (5.8) | 0.43 | 0.30–0.61 | < 0.001 | 0.51 | 0.35–0.74 | < 0.001 |
| Normal | 205/1758 (11.7) | 1.00 | – | – | 1.00 | – | – |
| High | 53/230 (23.0) | 2.25 | 1.61–3.15 | < 0.001 | 2.04 | 1.43–2.91 | < 0.001 |
| Maternal age (years) | |||||||
| < 24 | 39/715 (5.5) | 0.47 | 0.32–0.69 | < 0.001 | 0.64 | 0.43–0.96 | 0.030 |
| 25–29 | 103/943 (10.9) | 1.00 | – | – | 1.00 | – | – |
| 30–34 | 105/625 (16.8) | 1.65 | 1.23–2.21 | 0.001 | 1.24 | 0.91–1.70 | 0.175 |
| ≥ 35 | 48/343 (14.0) | 1.33 | 0.92–1.92 | 0.131 | 0.98 | 0.66–1.44 | 0.916 |
| Parity | |||||||
| Primipara | 95/1291 (7.4) | 1.00 | – | – | 1.00 | – | – |
| Multipara | 200/1335 (15.0) | 2.22 | 1.72–2.87 | < 0.001 | 1.93 | 1.43–2.61 | < 0.001 |
| Income ( × 1 000 Vietnamese dong) | |||||||
| < 1 500 | 17/223 (7.6) | 0.75 | 0.44–1.28 | 0.288 | 0.93 | 0.53–1.62 | 0.789 |
| ≥ 1 500 but < 2 500 | 92/924 (10.0) | 1.00 | – | – | 1.00 | – | – |
| ≥ 2 500 but < 3 500 | 94/776 (12.1) | 1.25 | 0.92–1.69 | 0.157 | 1.15 | 0.83–1.59 | 0.391 |
| ≥ 3 500 | 92/703 (13.1) | 1.36 | 1.00–1.85 | 0.049 | 1.13 | 0.79–1.61 | 0.507 |
| Education (years) | |||||||
| < 12 | 137/1400 (9.8) | 0.82 | 0.61–1.09 | 0.167 | 0.79 | 0.58–1.09 | 0.791 |
| 12 | 83/707 (11.7) | 1.00 | – | – | 1.00 | – | – |
| > 12 | 75/519 (14.5) | 1.27 | 0.91–1.78 | 0.162 | 1.20 | 0.83–1.72 | 1.197 |
| Total physical activitydin MET h/wk | |||||||
| < 25th percentile | 68/641 (10.6) | 1.01 | 0.71–1.44 | 0.954 | 1.17 | 0.81–1.71 | 0.400 |
| ≥ 25th but < 50th percentile | 68/646 (10.5) | 1.00 | – | – | 1.00 | – | – |
| ≥ 50th but < 75th percentile | 82/669 (12.3) | 1.19 | 0.85–1.67 | 0.319 | 1.09 | 0.76–1.56 | 0.658 |
| ≥ 75th percentile | 77/669 (11.5) | 1.11 | 0.78–1.57 | 0.563 | 0.90 | 0.63–1.30 | 0.587 |
| Smoking exposure | |||||||
| None | 198/1720 (11.5) | 1.00 | – | – | 1.00 | – | – |
| Passive indoor smoking | 97/906 (10.7) | 0.92 | 0.71–1.19 | 0.535 | 1.08 | 0.82–1.42 | 0.576 |
| Infant sex (male = 1, female = 0) | 171/1383 (12.4) | 1.27 | 1.00–1.63 | 0.053 | 1.22 | 0.95–1.58 | 0.120 |
| Gestational age at delivery | – | 1.01e | 0.91–1.13 | 0.797 | 1.00 | 0.89–1.12 | 0.964 |
BMI, body mass index; CI, confidence interval; MET h/wk, metabolic equivalent hours per week; OR, odds ratio.
a This represents the number of infants too large for gestational age (n = 295) born to women with the characteristic.
b This represents the number of infants of appropriate size plus the number of infants too large for gestational age (n = 2626) born to women with the characteristic.
c BMI represents body weight in kilograms divided by body surface area in square metres. Categories: low BMI: < 18.5; normal BMI, ≥ 18.5 but < 23.0; high BMI, ≥ 23.0.
d There is one case with missing data for physical activity.
e This OR represents the change in the risk of delivering a large baby with each additional week of gestation.
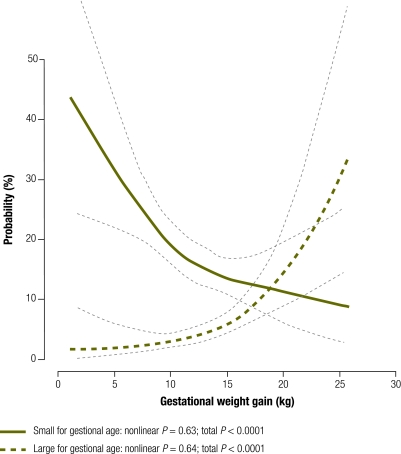
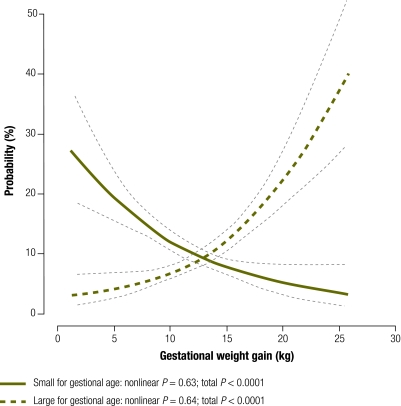
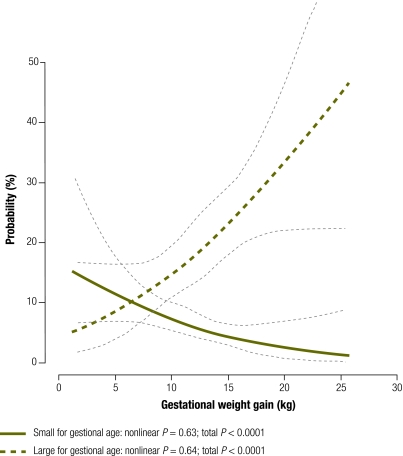
For each maternal BMI category we examined the association between gestational weight gain and the probability of delivering an infant too small or too large for gestational age (Fig. 1). The risk of having an infant too small for gestational age was much greater than the risk of having one too large for gestational age among women with low BMI (Fig. 1) or normal BMI (Fig. 2) up to pregnancy weight gains of 18.8 kg (95% CI: 16.5–29.5) and 12.8 kg (95% CI: 11.8–14.0), respectively. For women with high BMI, the risk of having an infant too small for gestational age exceeded the risk of having one too large for gestational age with pregnancy weight gains up to only 6.6 kg (95% CI: 0–9.0) (Fig. 3). A gestational weight gain of > 15 kg was associated with an increased risk of delivering an infant too large for gestational age in all three BMI groups, with the steepest increase noted among women with a high BMI. The greater the gestational weight gain, the lower the risk of delivering an infant too small for gestational age (P < 0.001) and the higher the risk of delivering an infant too large for gestational age (P < 0.001) (Fig. 1, Fig. 2 and Fig. 3). There was no statistical evidence of an interaction between BMI group and the probability of delivering an infant too small for gestational age (P = 0.8) or too large for gestational age (P = 0.6).
Fig. 1.
Probabilitiesa of delivering an infant too small or too large for gestational age by gestational weight gain in women with a lowb body mass index (BMI), Nha Trang, Viet Nam, 2007–2008
a Logistic regression analyses with cubic splines adjusted by maternal age, parity, household income, maternal education, exposure to environmental indoor smoking, total physical activity, infant sex and delivery week using the principal component method.
b BMI < 18.5 kg /m2.
Fig. 2.
Probabilitiesa of delivering an infant too small or too large for gestational age by gestational weight gain in women with a normalb body mass index (BMI), Nha Trang, Viet Nam, 2007–2008
a Logistic regression analyses with cubic splines adjusted by maternal age, parity, household income, maternal education, exposure to environmental indoor smoking, total physical activity, infant sex and delivery week using the principal component method.
b BMI ≥ 18.5 but < 23.0 kg/m2.
Fig. 3.
Probabilitiesa of delivering an infant too small or too large for gestational age by gestational weight gain in women with a highb body mass index (BMI), Nha Trang, Viet Nam, 2007–2008
a Logistic regression analyses with cubic splines adjusted by maternal age, parity, household income, maternal education, exposure to environmental indoor smoking, total physical activity, infant sex and delivery week using the principal component method.
b BMI ≥ 23.0 kg/m2.
Discussion
This study is the first to quantify the prevalence of small and large size for gestational age and the risk factors involved in Viet Nam, and to estimate the optimal weight gain during pregnancy as a function of maternal BMI. Since our study covered most deliveries in the study facilities during the study period, our results can probably be generalized to pregnant women in the entire study area and perhaps similar areas in Viet Nam and south-eastern Asia.
Well designed population-based studies have been carried out, but primarily in developed countries with well nourished populations29,30 and with a focus on obesity instead of low BMI, given the high prevalence of obesity in their populations.13 Cnattingius et al. have warned against generalizing the results of studies conducted in developed countries to developing Asian countries where low BMI among women is common.31 In fact, women in south-eastern Asia, including Viet Nam, tend to be smaller and to gain less weight during pregnancy on average than Caucasian women in Europe or the United States.1,32 Thus, the relationship between maternal BMI and fetal growth may differ by race.
Whether the more decisive influence on perinatal risks comes from genes or the environment is still a matter of controversy. Perinatal outcomes in different Asian immigrant populations in the United States vary significantly. Vietnamese immigrant women, for example, have a lower incidence of gestational diabetes mellitus and a greater incidence of primary Caesarean delivery than other Asian women in the United States, and they give birth to fewer low-birth-weight infants.33,34 According to another study that compared immigrants born in the United States with foreign-born immigrants also suggests that among the latter the risk of infant death and of low birth weight is substantially lower.35 On the other hand, a population-based, within-family cohort study recently conducted in the United States has shown that maternal weight gain during pregnancy correlates with birth weight independent of genetic factors.36
Our finding that low maternal BMI and a weight gain of < 10 kg during pregnancy, especially in combination, put women at risk of having infants too small for gestational age is consistent with those from a series of studies conducted in western countries to the effect that pre-eclampsia, low maternal BMI (< 18.5) and a gestational weight gain lower than recommended by the IOM are associated with an increased risk of delivering an infant too small for gestational age.29
In study participants with a low BMI, the optimal weight gain during pregnancy was 18.8 kg. The IOM recommends a gain of 12.5 kg to 18.0 kg during pregnancy as optimal.37 In this study, the low prevalence of small or large size for gestational age among infants born to women whose gestational weight gain was within this range lends support to this recommendation. The fact that 78% of the study women with low BMI gained > 10 kg during pregnancy may explain why the prevalence of small size for gestational age was relatively low in infants born to women in this group. For those women who feel more comfortable having an infant too large for gestational age rather than one that is too small, a gain of a few kilograms above the recommended number may be optimal, although the risks of developing pre-eclampsia and of needing a Caesarean section must be considered, among others.
In the present study, a weight gain of 12.8 kg in women with a normal BMI (18.5–22.9) and of 6.6 kg in women with a high BMI (≥ 23.0) proved optimal. The IOM recommends a gain of 11.5–16.0 kg in the first group of women37 and of 7.0–11.5 kg in the second group,37 and the recommendation for women with normal BMI can be applied to the Vietnamese population. However, the upper-limit IOM recommendation for women with a high BMI may increase the risk of having an infant too large for gestational age.
In our study, gestational weight gain was inversely correlated with the risk of having an infant too small for gestational age and directly correlated with the risk of having one too large for gestational age. A previous study showing a similar nonlinear trend in African and Caucasian women belonging to various BMI categories suggested that the recommended weight gains represented acceptably low levels of risk for these outcomes rather than minimal risk.8 The study also revealed that compared with Caucasian women, African women were at greater risk of having infants too small and at lower risk of having infants too large for gestational age.8 The probability of delivering an infant too small for gestational age among women with low BMI in Nha Trang was twice as high as the risk among women in the same BMI group in the United States, although BMI categories and gestational weight gain ranges were defined differently in the two studies.8 Thus, racial differences may have mediated the effect of maternal BMI on fetal growth.
The present study has several limitations. First, pre-gestational weight was self-reported and was measured an average of 11.8 months before the interview. Thus, it may have been subject to recall bias. However, measured and self-reported pre-gestational weight have shown high agreement in developing country populations with a high school education.38 In our study, 2658 (88.9%) women had their weight measured and recorded at their initial perinatal visit during the first trimester of pregnancy, and the recorded weight correlated highly with the self-reported pre-gestational weight (r2 = 0.911; P < 0.001).
Second, the exclusion from the study of women with incomplete data who may have been at high risk could have resulted in an underestimation of premature births. We performed a sensitivity analysis using log book data on the birth weight and sex of missing neonates and found that the prevalence of small and large size for gestational age was 12.3% and 9.6%, respectively, versus 12.1% and 9.9% when the missing cases were excluded. Hence, the relationship between gestational weight gain and the probability of having an infant too small or too large for gestational age as a function of maternal BMI category remained the same irrespective of the inclusion of study participants with missing data.
Further research is required to determine what range of gestational weight gain minimized the risk of having infants too small or large for gestational age among women with a low BMI, especially in Asian countries where a low BMI is commonly found in the female population.
Acknowledgements
Authors thank the Khanh Hoa Health Service, especially Trunag Tan Minh, Phu Quoc Viet, Luu Trung Hieu, Trinh Thi Van Giang and participating commune health centres for their assistance during fieldwork. We also thank Lisa Kawazu and Wolf-Peter Schmidt.
Funding:
This study was part of a research project funded by grants from the Cooperative Research Grant of NEKKEN (2007-19-A-1), Nagasaki University; the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases, MEXT Japan; the Yamaji Fumiko Nursing Education and Research Foundation.
Competing interests:
None declared.
References
- 1.Institute of Medicine. Nutrition during pregnancy. Washington: National Academy of Sciences; 1990. [Google Scholar]
- 2.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 3.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–41. doi: 10.1016/0140-6736(93)91224-A. [DOI] [PubMed] [Google Scholar]
- 4.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331:929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–6. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 6.Jarvis S, Glinianaia SV, Torrioli MG, Platt MJ, Miceli M, Jouk PS, et al. Cerebral palsy and intrauterine growth in single births: European collaborative study. Lancet. 2003;362:1106–11. doi: 10.1016/S0140-6736(03)14466-2. [DOI] [PubMed] [Google Scholar]
- 7.Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol. 2007;165:849–57. doi: 10.1093/aje/kwk071. [DOI] [PubMed] [Google Scholar]
- 8.Caulfield LE, Stoltzfus RJ, Witter FR. Implications of the Institute of Medicine weight gain recommendations for preventing adverse pregnancy outcomes in black and white women. Am J Public Health. 1998;88:1168–74. doi: 10.2105/AJPH.88.8.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maternal mortality in Viet Nam 2000-2001 Geneva: World Health Organization; 2005. [Google Scholar]
- 10.Kelly A, Kevany J, de Onis M, Shah PMAA. WHO collaborative study of maternal anthropometry and pregnancy outcomes. Int J Gynaecol Obstet. 1996;53:219–33. doi: 10.1016/0020-7292(96)02652-5. [DOI] [PubMed] [Google Scholar]
- 11.Siega-Riz AM, Adair LS. Biological determinants of pregnancy weight gain in a Filipino population. Am J Clin Nutr. 1993;57:365–72. doi: 10.1093/ajcn/57.3.365. [DOI] [PubMed] [Google Scholar]
- 12.Abrams B, Carmichael S, Selvin S. Factors associated with the pattern of maternal weight gain during pregnancy. Obstet Gynecol. 1995;86:170–6. doi: 10.1016/0029-7844(95)00119-C. [DOI] [PubMed] [Google Scholar]
- 13.Chu SY, Bachman DJ, Callaghan WM, Whitlock EP, Dietz PM, Berg CJ, et al. Association between obesity during pregnancy and increased use of health care. N Engl J Med. 2008;358:1444–53. doi: 10.1056/NEJMoa0706786. [DOI] [PubMed] [Google Scholar]
- 14.State of world population 2008. Reaching common ground: culture, gender and human rights New York: United Nations Population Fund; 2008. [Google Scholar]
- 15.At a glance: Viet Nam. Statistics [Internet]. New York: United Nations Children’s Fund; 2009. Available from: http://www.unicef.org/infobycountry/vietnam_statistics.html [accessed 25 October 2010].
- 16.Khanh Hoa Health Service. Provincial health statistics Nha Trang: KHHS; 2007.
- 17.Yanai H, Thiem VD, Matsubayashi T, Huong VTT, Suzuki M, Mai LP, et al. The Khahn Hoa Health Project: characterization of study population and field site development for clinical epidemiological research on emerging and re-emerging infectious diseases. Trop Med Health. 2007;35:61–3. doi: 10.2149/tmh.35.61. [DOI] [Google Scholar]
- 18.Suzuki M, Thiem VD, Yanai H, Matsubayashi T, Yoshida LM, Tho LH, et al. Association of environmental tobacco smoking exposure with an increased risk of hospital admissions for pneumonia in children under 5 years of age in Vietnam. Thorax. 2009;64:484–9. doi: 10.1136/thx.2008.106385. [DOI] [PubMed] [Google Scholar]
- 19.Hong JS, Yi SW, Han YJ, Park YW, Nam CM, Kang HC, et al. Fetal growth and neonatal mortality in Korea. Paediatr Perinat Epidemiol. 2007;21:397–410. doi: 10.1111/j.1365-3016.2007.00850.x. [DOI] [PubMed] [Google Scholar]
- 20.Clausson B, Gardosi J, Francis A, Cnattingius S. Perinatal outcome in SGA births defined by customised versus population-based birthweight standards. BJOG. 2001;108:830–4. doi: 10.1016/S0306-5456(00)00205-9. [DOI] [PubMed] [Google Scholar]
- 21.International statistical classification of diseases and related health problems, 10th revision: version for 2007 Geneva: World Health Organization; 2007. Available from: http://apps.who.int/classifications/apps/icd/icd10online/ [accessed 2 November 2010].
- 22.Landmann E, Reiss I, Misselwitz B, Gortner L. Ponderal index for discrimination between symmetric and asymmetric growth restriction: percentiles for neonates from 30 weeks to 43 weeks of gestation. J Matern Fetal Neonatal Med. 2006;19:157–60. doi: 10.1080/14767050600624786. [DOI] [PubMed] [Google Scholar]
- 23.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 24.Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sports Exerc. 2004;36:1750–60. doi: 10.1249/01.MSS.0000142303.49306.0D. [DOI] [PubMed] [Google Scholar]
- 25.Ota E, Haruna M, Yanai H, Suzuki M, Anh DD, Matsuzaki M, et al. Reliability and validity of the Vietnamese version of the Pregnancy Physical Activity Questionnaire (PPAQ). Southeast Asian J Trop Med Public Health. 2008;39:562–70. [PubMed] [Google Scholar]
- 26.Harrell FE. Regression modeling strategies with applications to linear models, logistic regression, and survival analysis 3rd printing ed. New York: Springer; 2001. [Google Scholar]
- 27.Radhakrishna Rao C. The use and interpretation of principal component analysis in applied research. Ind J Stat. 1964;26:329–58. [Google Scholar]
- 28.Efron B. Better bootstrap confidence intervals. J Am Stat Assoc. 1987;82:171–85. doi: 10.2307/2289144. [DOI] [Google Scholar]
- 29.Viswanathan M, Siega-Riz AM, Moos MK, Deierlein A, Mumford S, Knaack J, et al. Outcomes of maternal weight gain. Evid Rep Technol Assess (Full Rep) 2008;168:1–223. [PMC free article] [PubMed] [Google Scholar]
- 30.Langford A, Joshu C, Chang JJ, Myles T, Leet T. Does gestational weight gain affect the risk of adverse maternal and infant outcomes in overweight women? Matern Child Health J. 2008 doi: 10.1007/s10995-008-0318-4. [DOI] [PubMed] [Google Scholar]
- 31.Cnattingius S, Bergström R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338:147–52. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- 32.Urban nutrition. Assessment of nutritional status in urban areas. Analysis of body mass index for women [Internet]. Rome: Food and Agriculture Organization of the United Nations; 2010. Available from: http://www.fao.org/ag/agn/nutrition/urban_assessment_en.stm [accessed 2 November 2010].
- 33.Rao AK, Daniels K, El-Sayed YY, Moshesh MK, Caughey AB. Perinatal outcomes among Asian American and Pacific Islander women. Am J Obstet Gynecol. 2006;195:834–8. doi: 10.1016/j.ajog.2006.06.079. [DOI] [PubMed] [Google Scholar]
- 34.Wong LF, Caughey AB, Nakagawa S, Kaimal AJ, Tran SH, Cheng YW. Perinatal outcomes among different Asian-American subgroups. Am J Obstet Gynecol. 2008;199:382–, e1-6. doi: 10.1016/j.ajog.2008.06.073. [DOI] [PubMed] [Google Scholar]
- 35.Singh GK, Yu SM. Adverse pregnancy outcomes: differences between US- and foreign-born women in major US racial and ethnic groups. Am J Public Health. 1996;86:837–43. doi: 10.2105/AJPH.86.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ludwig DS, Currie J. The association between pregnancy weight gain and birthweight: a within-family comparison. Lancet. 2010;376:984–90. doi: 10.1016/S0140-6736(10)60751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasmussen K, Yaktine A, editors. Weight gain during pregnancy: reexamining the guidelines Washington: National Academies Press; 2009. [PubMed] [Google Scholar]
- 38.Lim LL, Seubsman SA, Sleigh A. Validity of self-reported weight, height, and body mass index among university students in Thailand: Implications for population studies of obesity in developing countries. Popul Health Metr. 2009;7:15. doi: 10.1186/1478-7954-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]