Abstract
Rationale: Acute asthma exacerbations, precipitated by viral infections, are a significant cause of morbidity, but not all patients with asthma are equally susceptible.
Objectives: To explore susceptibility factors for asthma exacerbations, we considered a role for histoblood group antigens because they are implicated in mechanisms of gastrointestinal viral infection, specifically the O-secretor mucin glycan phenotype. We investigated if this phenotype is associated with susceptibility to asthma exacerbation.
Methods: We performed two consecutive case-control studies in subjects with asthma who were either prone or resistant to asthma exacerbations. Exacerbation-prone cases had frequent use of prednisone for an asthma exacerbation and frequent asthma-related healthcare utilization, whereas exacerbation-resistant control subjects had rarely reported asthma exacerbations. The frequency of different mucin glycan phenotypes, defined by the presence or absence of H (O), A, B, or AB antigens, was compared in cases and control subjects.
Measurements and Main Results: In an initial study consisting of 49 subjects with asthma (23 cases and 26 control subjects), we found that having the O-secretor phenotype was associated with a 5.8-fold increase in the odds of being a case (95% confidence interval, 1.7–21.0; P = 0.006). In a replication study consisting of 204 subjects with asthma (101 cases and 103 control subjects), we found that having the O-secretor phenotype was associated with a 2.3-fold increased odds of being a case (95% confidence interval, 1.2–4.4; P = 0.02).
Conclusions: The O-secretor mucin glycan phenotype is associated with susceptibility to asthma exacerbation.
Clinical trial registered at www.clinicaltrials.gov (NCT00201266).
Keywords: asthma, mucins, fucosylation, H antigen, blood groups
AT A GLANCE COMMENTARY.
Current Knowledge on the Subject
The reasons why some patients with asthma are prone to exacerbations are not well understood, but factors underlying susceptibility to virus-induced asthma exacerbations are likely to be important. The expression of the O antigen (also called the H antigen) at mucosal epithelial surfaces confers risk for viral gastroenteritis, and we examined here if the H antigen is a risk for asthma exacerbations.
What This Study Adds to the Field
In two case control studies, we provide evidence that a specific mucin glycan phenotype (O-secretor) is associated with susceptibility to recurrent asthma exacerbations. These data show how blood group antigen structures on mucin glycans influence susceptibility to asthma exacerbation and suggest new approaches on how to prevent exacerbations.
There is mounting evidence that a limited subset of patients with exacerbation-prone asthma accounts for the majority of emergency department visits and hospitalizations for asthma (1). The reasons why some patients with asthma are prone to exacerbations and others are resistant are not well understood. Viral upper respiratory tract infections are frequent precipitants of asthma exacerbations (2–5), so factors underlying susceptibility to virus-induced asthma exacerbations are likely to be important. Susceptibility factors for viral infection at mucosal surfaces have been extensively studied in the gastrointestinal tract, and histoblood group antigens have emerged as important risk factors. For example, in the case of diarrhea caused by Norwalk virus, the initial observation that blood type O is a risk factor (6) was followed by studies showing conclusively that expression of the O antigen (also called the H antigen) at mucosal epithelial surfaces confers the risk and that absence of the H antigen is protective (7).
Histoblood group antigens, such as the O (“H”), A, and B antigens, form capping structures at the terminal ends of the carbohydrate side chains (glycans) on epithelial mucins. They are formed by stepwise addition of monosaccharide units through the action of a set of glycosyltransferases, including fucosyltransferases (FucTs) (8). In individuals of blood type O, the addition of fucose to terminal galactose in an α1,2 linkage forms the H antigen in a step catalyzed by α1,2 FucTs encoded by FUT1 (active in erythrocytes) and FUT2 (active in epithelial tissues) genes. Additional modifications to the H antigen depend on an individual's blood type and are controlled by genes at the FUT and ABO loci, which are highly polymorphic and give rise to considerable diversity in the profile of glycans on airway mucins. Approximately 25% of individuals are homozygous for nonsense mutations in FUT2 (9, 10), resulting in the inability to synthesize H antigens at epithelial surfaces and the absence of ABO structures on secreted mucins. These “nonsecretors” represent one of multiple possible mucin glycan phenotypes, including several secretor phenotypes characterized by the display and secretion of H (O), A, or B antigens on epithelial mucins.
The importance of mucins in innate defense in the lung and the fact that blood group antigens on mucins play roles in viral infection in the gastrointestinal tract led us to consider that ABH variation in mucin glycan phenotypes may influence susceptibility to asthma exacerbation. Because of the association between Norwalk virus infection and the secretor phenotype, we hypothesized that O-secretors may be more susceptible to asthma exacerbation.
METHODS
Subjects
Adults (18–75 yr of age) with a history of asthma were recruited using internet-based community advertising. Cases were defined as individuals having experienced an acute exacerbation of asthma requiring prednisone within the previous 2 years; we reasoned that 2 years was a period in which recall for a prednisone course would be accurate and that a relatively recent exacerbation would identify subjects with asthma who had experienced multiple prior exacerbations in their lifetime. Control subjects were defined as individuals having no history of prednisone use for an asthma exacerbation since the age of 12 years. The rationale for this definition was that a lack of severe exacerbations since age 12 would definitively identify adults with exacerbation-resistant asthma. The 12-year-old cut off has been used in other studies of asthma (11, 12). Exclusion criteria included history of prednisone use for asthma after the age of 12 years but not within the past 2 years and history of current cigarette smoking. Former smokers were included if they had not smoked tobacco in the previous year and if their total smoking history was less than 10 pack-years. The study was approved by the Committee on Human Research at the University of California, San Francisco, and all participants gave informed consent.
Study Overview
Our hypothesis, based on data from viral infection in the gastrointestinal tract (13), was that patients with asthma who are O-secretors would be more prone to asthma exacerbation than nonsecretors. A previous case control study had found that secretors are significantly overrepresented among patients with virus infections of the respiratory tract (14), but it did not specifically investigate the O-secretor phenotype. Therefore, we examined our hypothesis first in an initial study consisting of 49 consecutively enrolled subjects with asthma (23 cases and 26 control subjects). When we analyzed the frequency of all five major mucin glycan phenotypes (nonsecretors, O secretors, A secretors, B secretors, and AB secretors) among cases and control subjects, we found the O-secretor phenotype to be more common among cases than control subjects (see Results). We calculated that confirming this initial O-secretor finding required an additional 100 cases and 100 control subjects (assuming an odds ratio of 2.5 and 80% power). We then conducted a second (replication) study in which we recruited 101 exacerbation-prone cases and 103 exacerbation-resistant control subjects, so that the initial and replication studies had 49 and 204 subjects, respectively.
Study Protocol
Asthma diagnosis was confirmed by a positive methacholine challenge test or by documentation of airflow obstruction reversible with albuterol. Methacholine challenge was done if baseline FEV1 was greater than or equal to 50% predicted. If FEV1 was less than 50% predicted, the subject was given inhaled albuterol (360 μg) to document reversible airflow obstruction according to ATS standards (15). Characterization procedures also included medical history and allergen skin tests as previously described (16, 17). Most subjects underwent a single characterization visit, but those taking combination inhalers, such as fluticasone/salmeterol, had a screening visit to assess whether their asthma was stable enough to tolerate a 48-hour hold of the combination medication so as to allow characterization studies with a limited influence from these medications. If a subject could not hold their combination medication, characterization was conducted without the hold. The majority (75%) of subjects were able to hold their combination medication for 48 hours. All subjects completed a self-administered asthma characterization questionnaire, which included four questions relating to the frequency and severity of upper respiratory tract infections (colds) and how these infections typically impacted their asthma. Subjects were asked how many colds they typically got on an annual basis, and they completed a series of Likert scale questions about their colds, including how frequently colds led to worsening asthma, and the severity of their asthma during colds.
Subjects were asked to spit into a 50-ml tube for 5 minutes to collect a saliva sample. Saliva was processed and stored in an −80°C freezer. Subjects were also asked to provide a blood sample.
Determination of Mucin Glycan Phenotype
We characterized five mucin glycan phenotypes in our study populations (nonsecretors, O (H)-secretors, A-secretors, B-secretors, and AB-secretors) using data from ABO and Lewis typing in blood complemented by ABO typing in saliva and analysis of FUT2 polymorphisms in peripheral blood DNA.
ABO and Lewis antigen typing in peripheral blood was done by the UCSF Blood Bank and Donor Center using The American Association of Blood Banks technical standards (18). An individual's ABO mucin glycan phenotype can be determined by phenotyping Lewis and ABO antigens on erythrocytes because erythrocytes acquire preformed Le antigens that are synthesized at epithelial surfaces, rather than synthesize them. α1,3 fucosyltransferase (α1,3 FucT) catalyzes the addition of fucose to the H antigen in the α1,3 position to form Leb antigen. Lea and Leb formed in epithelial tissues are reabsorbed and become available in blood for attachment to erythrocyte glycans. Subjects whose erythrocytes are Leb have active α1,2 FucT and α1,3 FucT in epithelial cells and can assemble the H antigen, which can be further modified with A and/or B antigens. These subjects are therefore “secretors” of mucins decorated with blood group antigens. In contrast, subjects whose erythrocytes are Lea have α1,3 FucT activity in epithelial cells but lack α1,2 FucT activity, cannot assemble the H antigen, and are nonsecretors. Nonsecretors can have O, A, or B antigens on their peripheral blood erythrocytes because inactive FUT2 has no influence on FUT1 activity. Lea and Leb antigens in peripheral blood serve as biomarkers of α1,2 FucT activity at epithelial mucosal surfaces, and subjects with the Leb erythrocyte phenotype can be further characterized as being O-, A-, B-, or AB- secretors using peripheral blood ABO typing.
ABO antigens are detectable in saliva from secretors and absent in saliva from nonsecretors. We assayed for the presence of ABO antigens in saliva using a commercially available kit that relies on red blood cell agglutination (Immucor, Norcross, GA). Peripheral blood DNA was collected from subjects to enable assay for the G428A mutation in FUT2. G428A is a nonsense mutation that results in an early stop codon, yielding a truncated α1,2 FucT protein that is nonfunctional. The G428A mutation in FUT2 was genotyped using iPLEX reagents and protocols from Sequenom (San Diego, CA). Additional details on FUT2 genotyping are provided in the online supplement.
We found excellent concordance between the mucin glycan phenotypes determined by Lewis and ABO typing in blood and those determined by ABO typing in saliva.
Statistical Analysis
Data are expressed in means and standard deviation for continuous variables and as counts and percentages for categorical variables. Differences between cases and control subjects were assessed by Fisher's exact test for categorical variables and Mann-Whitney test for continuous variables. Our analysis plans proposed adjusted analyses for the primary analyses. Logistic regression was used to assess the odds ratio of exacerbation-prone asthma associated with the O-secretor phenotype. To adjust for imbalances in baseline characteristics of the cases and control subjects, we applied propensity score methods (19). Propensity score analysis simulates the effects of a randomized comparison by creating subgroups of subjects with similar baseline characteristics. We decided a priori to include age, body mass index (BMI), race, use of inhaled corticosteroid (ICS), and FEV1 percent predicted category as predictors in our propensity score model. To be more flexible, we included age and BMI with linear and quadratic terms. Predicted values from a logistic regression model were used to calculate the propensity scores. The scores were divided into quintiles and were used in a final model with O-secretor status. Analyses were conducted using SAS version v9.1.3 (SAS Institute, Cary, NC) and Stata version 11 (Statacorp, College Station, TX). Two-sided tests were used to determine significance; P values less than 0.05 were considered statistically significant.
RESULTS
Characteristics of the Asthma Cases and Control Subjects
Characterization data for the 49 subjects in the initial study are presented in Tables 1 and 2. Our definitions of case and control succeeded in recruiting two distinct groups of subjects with asthma, one exacerbation-prone and one exacerbation-resistant. The cases had histories of multiple severe exacerbations, whereas control subjects had very few severe exacerbations, all occurring before the age of 12 years. Characterization in the replication study again revealed significant differences between these subgroups (Tables 1 and 2). In addition to the history of prednisone use in the past 2 years, cases were characterized by a lifetime history of nearly 20 prednisone treatments for asthma. Cases also had a history of more frequent emergency room visits, hospitalizations, and airway intubations for asthma, further confirming an exacerbation-prone phenotype among cases. There were no significant differences between cases and control subjects with respect to race, but cases were older, had higher body mass index, and more frequently used ICS (Table 1).
TABLE 1.
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS
| Initial Study |
Replication Study |
|||
|---|---|---|---|---|
| Characteristic | Asthma Control Subjects (n = 26) | Asthma Cases (n = 23) | Asthma Control Subjects (n = 103) | Asthma Cases (n = 101) |
| Age, yr | 36.3 ± 12.7* | 38.1 ± 13.4 | 32.5 ± 11.3 | 39.4 ± 14.3† |
| Age of diagnosis, yr (median ± IQR) | 15.0 ± 12.6 | 17 ± 14.9 | 9 (4–13) | 8 (3–19) |
| Race/ethnicity | ||||
| Asian | 3 (12) | 2 (9) | 22 (21) | 12 (12) |
| Black/African decent | 2 (8) | 5 (22) | 15 (15) | 16 (16) |
| Caucasian, non-Hispanic | 18 (69) | 10 (43) | 54 (52) | 56 (55) |
| Caucasian, Hispanic | 3 (12) | 6 (26) | 12 (12) | 17 (17) |
| Body mass index | 31.3 ± 10.4 | 27.9 ± 6.2 | 27.5 ± 7.7 | 30.8 ± 8.4† |
| Gender, female | 16 (62) | 14 (64) | 71 (69) | 67 (66) |
| Smoking history, former | 8 (31) | 4 (18) | 23 (22) | 26 (26) |
| ICS therapy | ||||
| Combination (ICS/long-acting beta-agonist) | 3 (12) | 9 (39)† | 17 (17) | 59 (58)† |
| Monotherapy (ICS only) | 5 (19) | 7 (30)† | 20 (19) | 20 (20)† |
| FEV1 % predicted (median/range) | 86 (57–111) | 78 (28–100)† | 87 (41–127) | 78 (31–114)† |
| Severity by FEV1 % predicted | ||||
| > 80% | 17 (65) | 11 (48)† | 70 (68) | 44 (44)† |
| 60–80% | 8 (31) | 6 (26)† | 29 (28) | 33 (33)† |
| < 60% | 1 (4) | 6 (26)† | 4 (4) | 24 (24)† |
| PC20 methacholine, mg/ml‡ | 1.0 ± 1.3 | 1.5 ± 1.8 | 1.2 ± 1.5 | 1.5 ± 2.5 |
| Serum IgE, IU/ml | 350.4 ± 503.4 | 381.0 ± 374.9 | 439.1 ± 578.2 | 398.0 ± 480.9 |
| Blood eosinophils, 109/l | 0.25 ± 0.15 | 0.40 ± 0.31 | 0.29 ± 0.24 | 0.35 ± 0.28 |
| Positive allergen skin test‡ mite | 17 (65) | 14 (61) | 86 (89) | 57 (66)† |
| Cockroach | 8 (31) | 7 (30) | 37 (38) | 22 (26) |
| Furred animals (cat, dog, mouse, rat) | 16 (62) | 13 (57) | 71 (72) | 60 (70) |
| Fungi (alternaria, aspergillus, cladosporium, penicillium) | 14 (54) | 10 (43) | 41 (42) | 41 (48) |
Definition of abbreviation: ICS = inhaled corticosteroid; IQR = interquartile ratio.
Values are n (%) or mean ± SD.
P < 0.05 for comparison with control subjects.
Ninety-eight control subjects and 77 cases had methacholine challenge testing; 98 control subjects and 86 cases had complete, valid allergen skin test results; 26 control subjects and 20 cases had methacholine challenge testing; and 25 control subjects and 22 cases had complete, valid allergen skin test results.
TABLE 2.
ASTHMA HEALTHCARE UTILIZATION AND TRENDS IN VIRAL INFECTION
| Initial Study |
Replication Study |
|||
|---|---|---|---|---|
| Characteristic | Asthma Control Subjects (n = 26) | Asthma Cases (n = 23) | Asthma Control Subjects (n = 103) | Asthma Cases (n = 101) |
| Prednisone treatments for asthma in previous 2 yr | 0* | 1.5 ± 1.1† | 0 | 2.0 ± 1.9† |
| Prednisone treatments for asthma in lifetime | 0.04 ± 0.2 | 9.9 ± 19.1† | 0.5 ± 0.26 | 18.2 ± 31.3† |
| Emergency department visits for asthma, lifetime | 1.54 ± 3.20 | 10.54 ± 22.77† | 2.3 ± 6.3 | 17.9 ± 32.8† |
| Hospitalized for asthma in lifetime | 3 (12) | 10 (45)† | 14 (14) | 45 (45)† |
| Intubated for asthma in lifetime | 0 | 4 (18)† | 0 | 13 (13) † |
| Number of upper respiratory tract infections (“colds”) per year | 2.0 ± 1.1 | 2.0 ± 1.1 | 2.5 ± 1.4 | 2.5 ± 1.5 |
| Colds characterized by subjects as being severe, very severe, or extremely severe | 8 (31) | 5 (24) | 35 (34) | 46 (46) |
| Colds reported to usually or always make asthma worse | 15 (58) | 17 (81)† | 61 (60) | 79 (80)† |
| Asthma reported to be severe, very severe, or extremely severe with colds | 11 (44) | 13 (62)† | 40 (40) | 77 (79)† |
Values are mean ± SEM or n (%).
P < 0.05 for comparison with control subjects.
Cases and control subjects reported similar frequencies and severity of upper respiratory tract infections, but a significantly larger proportion of cases reported more frequent and more severe asthma symptoms associated with viral infections (Table 2). Specifically, 80% of cases reported that upper respiratory tract infections “usually” or “always” led to worsening asthma symptoms, compared with 60% of control subjects (P < 0.001) (Table 2). Similarly, 79% of cases reported “severe,” “very severe,” or “extremely severe” asthma symptoms associated with upper respiratory tract infections, compared with 40% of control subjects (P < 0.001).
Blood Types and Mucin Glycan Phenotypes among Study Subjects
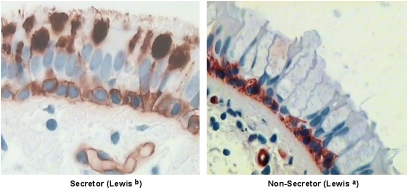
ABO and Lewis blood typing results are shown in Table 3. It is well established that FUT2 controls the expression of ABO antigens on epithelial mucin glycans, and we confirmed abundant H antigen staining in stored mucins in goblet cells from a subject with the O-secretor mucin glycan phenotype, whereas lectin staining was absent in stored mucins in goblet cells from a subject who was a nonsecretor (Figure 1). Both subjects showed staining for the H antigen on the surface of basal cells, indicating that FUT1 controls the expression of H antigens in basal cells in the airway epithelium.
TABLE 3.
ABO, LEWIS, AND MUCIN GLYCAN PHENOTYPES IN CASES AND CONTROL SUBJECTS
| Initial Study |
Replication Study |
|||
|---|---|---|---|---|
| Characteristic | Asthma Control Subject (n = 26) | Asthma Cases (n = 23) | Asthma Control Subjects (n = 103) | Asthma Cases (n = 101) |
| Blood group phenotype, n (%) | ||||
| O | 7 (27) | 18 (78)* | 38 (37) | 38 (38) |
| A | 14 (54) | 4 (17) | 41 (40) | 39 (39) |
| B | 3 (11) | 1 (4) | 19 (18) | 14 (14) |
| AB | 2 (8) | 0 | 5 (5) | 10 (10) |
| Lewis antigen phenotype,† n (%) | ||||
| Lewisa | 3 (11) | 3 (13) | 28 (27) | 18 (18) |
| Lewisb | 21 (81) | 15 (68) | 59 (57) | 66 (65) |
| Mucin glycan phenotype, n (%) | ||||
| O secretor | 6 (23) | 15 (65)* | 22 (21) | 34 (34)* |
| A secretor | 12 (46) | 3 (13)* | 30 (29) | 31 (31) |
| B secretor | 2 (8) | 1 (4) | 16 (16) | 8 (8) |
| AB secretor | 3 (11) | 0 | 4 (4) | 7 (7) |
| Nonsecretor | 3 (11) | 4 (17) | 31 (30) | 21 (21) |
< 0.05 for comparison with control subjects.
Lewis antigen typing results were not available for one case subject and one control subject. Eighteen cases and 16 control subjects were negative for Lewisa and Lewisb antigens.
Figure 1.
Influence of secretor status on expression of the H antigen in airway epithelial cells. (A) Lectin staining for the H antigen in a subject with asthma with the O-secretor mucin phenotype shows strong expression in goblet cell mucins. (B) Lectin staining for the H antigen in an O nonsecretor phenotype shows absent expression in goblet cell mucins. In contrast, the H antigen is detectable in basal cells in secretors and nonsecretors, demonstrating that basal cells can synthesize the H antigen (presumably using an α1,2 fucosyltransferases encoded by FUT1 rather than FUT2).
In the initial study, we analyzed the frequency of all five major mucin glycan phenotypes among cases and control subjects and found that the O-secretor phenotype was much more common in cases than in control subjects (Table 3) and that being an O-secretor, compared with all other mucin glycan phenotypes combined, was associated with a 5.83-fold increased risk of being a case (Table 4). In the replication study, we also found that the O-secretor phenotype was more common in cases than in control subjects (Table 3) and that the O-secretor was associated with a 1.87-fold increased risk of being exacerbation prone (Table 4). This odds ratio was lower than in the initial cohort, and the lower endpoint of the confidence interval was 1.0 (P = 0.05). However, this analysis was unadjusted for known confounders such as airflow obstruction, use of ICS, obesity, and race. When we adjusted for FEV1 in a logistic regression analysis, the O-secretor phenotype was associated with a 2-fold increased risk of being exacerbation prone (P = 0.04). Furthermore, when we controlled for the effects of ICS use, race, BMI, and FEV1 together in a propensity score analysis, the O-secretor phenotype was associated with a 2.25-fold increased risk of being exacerbation prone (P = 0.02) (Table 4). This propensity score analysis was a predefined analysis.
TABLE 4.
ODDS RATIOS OF EXACERBATION-PRONE ASTHMA ASSOCIATED WITH THE O-SECRETOR PHENOTYPE
| Predictor of Interest | Adjustments | OR | 95% CI | P Value |
|---|---|---|---|---|
| O secretor status, (initial study, n = 49) | ||||
| Model 1 | Unadjusted | 5.83 | 1.66–20.56 | 0.006 |
| O secretor status (replication study, n = 204) | ||||
| Model 1 | Unadjusted | 1.87 | 1.00–3.50 | 0.05 |
| Model 2 | Adjusted for FEV1 % predicted | 2.00 | 1.03–3.87 | 0.04 |
| Model 3 | Adjusted for propensity score* | 2.25 | 1.16–4.36 | 0.02 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
Developed using race, body mass index, inhaled corticosteroid use, and FEV1 % predicted.
Although FEV1 was significantly lower in cases than in control subjects (Table 1), we noted that 44% of the cases had FEV1 values in the normal range. Conversely, 32% of control subjects had FEV1 values in the abnormal range. These findings emphasize that some subjects with asthma with normal or near normal airflow can suffer severe exacerbations and can do so repeatedly, whereas some patients with asthma who have significant airflow obstruction are not prone to these events.
The H antigen, which represents α1,2 fucose occurring in glycolipids and glycoproteins in multiple cellular locations, is present in erythrocytes and epithelial cells. We can conclude that the α1,2 fucose structure at epithelial surfaces confers the asthma exacerbation risk because the blood group O-phenotype, as distinct from the O-secretor phenotype, was not overrepresented in the cases (Table 3) and was not associated with an increased risk of being a case in the replication study (odds ratio, 1.03; 95% confidence interval, 0.58–1.82; P = 0.91). Although there was an association between the blood group O-phenotype and risk of being a case in the initial study, this was clearly a function of the high incidence of the O-secretor phenotype among the cases because 15 of the 18 (83%) subjects who were blood group O had the O-secretor phenotype.
The addition of fucose to terminal galactose in α1,2 linkage forms the H antigen in a step catalyzed by α1,2 FucT, whereas the addition of fucose in α1,3 linkage forms the Lea antigen in a step catalyzed by α1,3 FucT. Our data show that it is the α1,2 fucose structure, not α1,3 fucose, that is associated with increased risk for asthma exacerbation because the Lea phenotype was not overrepresented among the cases (Table 3).
DISCUSSION
We investigated whether the O-secretor mucin glycan phenotype is associated with susceptibility to asthma exacerbations. Because the ABO glycan phenotype of mucins can be determined from measures of Lewis and ABO blood type in peripheral blood and of ABO antigens in saliva, we were able to noninvasively determine the airway mucin glycan phenotype of a large number of subjects with asthma who were also carefully characterized by exacerbation history. Using this approach, we found that having the O-secretor mucin glycan phenotype significantly increases the odds of the risk for severe asthma exacerbations.
In the larger replication study, we found that the O-secretor phenotype is associated with a 2.3-fold increased risk of having exacerbation-prone asthma. This is a sizeable odds ratio given the many precipitants of severe exacerbation. Chief among these precipitants are viral upper respiratory tract infections, and a plausible explanation for our findings is that the O-secretor phenotype confers a risk for virus-induced asthma exacerbations. Although we did not identify specific susceptibility of O-secretors to virally induced exacerbations, it is notable that the exacerbation-prone cases differed from control subjects in consistently reporting that upper respiratory tract infections usually or always led to worsening asthma symptoms and that these symptoms were severe. The frequency of upper respiratory tract infections was not different in cases than in controls subjects, however. Thus, patients with asthma who are prone to severe exacerbation experience upper respiratory tract infections about as frequently as patients with asthma who are resistant to exacerbations, but the consequences of these infections for their asthma is much more severe. Our data suggest that expression of the H antigen in the lower airway confers risk for more severe lower airway consequences from upper airway infections.
The reasons why the O-secretor phenotype confers risk for asthma exacerbation is not revealed by our study. Fucoslyation is known to confer unique properties to oligosaccharides, even if these properties remain incompletely understood (20); additionally, work that describes the role of specific sialic acids in viral binding in the airway epithelia provides precedence for a plausible mechanism (21). One possibility is that the mucin fucosylation promotes lower airway infection with upper respiratory tract viruses such as rhinoviruses. These viruses use cell membrane receptors for cell entry; major group rhinoviruses bind to intercellular adhesion molecule 1 (ICAM-1), and minor group serotypes bind the low-density lipoprotein receptor (22). Two recent studies have found an association between blood group antigens and serum levels of ICAM-1 (23, 24). If expression of the H antigen at epithelial surfaces regulates the expression of membrane-bound or soluble ICAM-1 in the airway, then this mechanism could explain the association we find for the O-secretor mucin glycan phenotype and susceptibility to asthma exacerbation.
Despite the many behavioral and socioeconomic factors that contribute to asthma-related urgent healthcare utilization and prednisone use (e.g., low self-efficacy, low literacy), our definitions of “case” and “control” enabled us to recruit two distinct groups of subjects with asthma: an exacerbation-prone group and an exacerbation-resistant group. The exacerbation-prone group is well recognized, but less well recognized is the patient with asthma who consistently remains completely free of severe asthma exacerbations during adulthood. Medications such as corticosteroids contribute to this resistance, but only a minority of our subjects with exacerbation-resistant asthma was taking corticosteroids, indicating that other resistance factors must be operating in this important asthma subgroup. A better understanding of the mechanisms of resistance and susceptibility to exacerbation in asthma is needed, including the mechanism of the O-secretor association described here, so that novel treatment approaches can be developed to convert exacerbation-prone asthma into the exacerbation-resistant phenotype.
Supplementary Material
Acknowledgments
The authors thank Homer Boushey, M.D., Stephen Lazarus, M.D., and Peggy Cadbury, RN, for assistance in clinical characterization of the study subjects; Pearl Toy, M.D., Chief of the UCSF Blood Bank and Donor Center, for the Lewis and ABO blood typing studies; and Esteban Burchard, M.D., M.P.H., for assistance with FUT2 genotyping.
Supported by the National Institutes of Health grant HL080414 (J.V.F.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201003-0488OC on August 23, 2010
Author Disclosure: A.L.I. received grant support from the National Institutes of Health (NIH) (more than $100,001). K.W.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.H.D. received grant support from the NIH ($50,001–$100,000). C.E.M. received grant support from Amgen ($10,001–$50,000) and the NIH (more than $100,001). P.G.W. received grant support from Genentech and the NIH/NHLBI (more than $100,001). He owns a patent through the University of California, San Francisco (blood-based diagnostic test for sarcoidosis). M.A.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.S.O. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.J.I. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.C.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.D.C. received grant support from the NIH (more than $100,001). J.V.F. was on the Board or Advisory Board of Amira, Oxagen ($1,001–$5,000), Gilead (up to $1,000), GlaxoSmithKline, and Amgen ($1,001–$5,000). He received grant support from Genentech (more than $100,001), Boehringer Ingelheim ($50,001–$100,000), and Aerovance ($10,001–$50,000) and has a pending patent through Genentech (Provisional patent application submitted for a gene signature for Th2 high asthma (with Genentech). No money has been received from this patent). He received grant support from the NHLBI (more than $100,001).
References
- 1.Dougherty RH, Fahy JV. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy 2009;39:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atmar RL, Guy E, Guntupalli KK, Zimmerman JL, Bandi VD, Baxter BD, Greenberg SB. Respiratory tract viral infections in inner-city asthmatic adults. Arch Intern Med 1998;158:2453–2459. [DOI] [PubMed] [Google Scholar]
- 3.Heymann PW, Carper HT, Murphy DD, Platts-Mills TA, Patrie J, McLaughlin AP, Erwin EA, Shaker MS, Hellems M, Peerzada J, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol 2004;114:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O'Toole S, Myint SH, Tyrrell DA, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ 1995;310:1225–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ 1993;307:982–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutson AM, Atmar RL, Graham DY, Estes MK. Norwalk virus infection and disease is associated with abo histo-blood group type. J Infect Dis 2002;185:1335–1337. [DOI] [PubMed] [Google Scholar]
- 7.Lindesmith L, Moe C, Marionneau S, Ruvoen N, Jiang X, Lindblad L, Stewart P, LePendu J, Baric R. Human susceptibility and resistance to norwalk virus infection. Nat Med 2003;9:548–553. [DOI] [PubMed] [Google Scholar]
- 8.Hounsell EF, Lawson AM, Feizi T. Structural and antigenic diversity in mucin carbohydrate chains. Adv Exp Med Biol 1982;144:39–41. [DOI] [PubMed] [Google Scholar]
- 9.Carlsson B, Kindberg E, Buesa J, Rydell GE, Lidon MF, Montava R, Abu Mallouh R, Grahn A, Rodriguez-Diaz J, Bellido J, et al. The g428a nonsense mutation in fut2 provides strong but not absolute protection against symptomatic gii.4 norovirus infection. PLoS ONE 2009;4:e5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor-Cousar JL, Zariwala MA, Burch LH, Pace RG, Drumm ML, Calloway H, Fan H, Weston BW, Wright FA, Knowles MR. Histo-blood group gene polymorphisms as potential genetic modifiers of infection and cystic fibrosis lung disease severity. PLoS ONE 2009;4:e4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horner CC, Bacharier LB. Diagnosis and management of asthma in preschool and school-age children: focus on the 2007 naepp guidelines. Curr Opin Pulm Med 2009;15:52–56. [DOI] [PubMed] [Google Scholar]
- 12.Main C, Shepherd J, Anderson R, Rogers G, Thompson-Coon J, Liu Z, Hartwell D, Loveman E, Green C, Pitt M, et al. Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in children under the age of 12 years. Health Technol Assess 2008;12:1–174, iii–iv. [DOI] [PubMed] [Google Scholar]
- 13.Marionneau S, Airaud F, Bovin NV, Le Pendu J, Ruvoen-Clouet N. Influence of the combined abo, fut2, and fut3 polymorphism on susceptibility to norwalk virus attachment. J Infect Dis 2005;192:1071–1077. [DOI] [PubMed] [Google Scholar]
- 14.Raza MW, Blackwell CC, Molyneaux P, James VS, Ogilvie MM, Inglis JM, Weir DM. Association between secretor status and respiratory viral illness. BMJ 1991;303:815–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948–968. [DOI] [PubMed] [Google Scholar]
- 16.Innes AL, Woodruff PG, Ferrando RE, Donnelly S, Dolganov GM, Lazarus SC, Fahy JV. Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest 2006;130:1102–1108. [DOI] [PubMed] [Google Scholar]
- 17.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, Ellwanger A, Sidhu SS, Dao-Pick TP, Pantoja C, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA 2007;104:15858–15863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roback J, Combs MR, Grossman B, Hillyer C. Aabb technical manual. Bethesda, MD: American Association of Blood Banks (AABB); 2008.
- 19.Mansson R, Joffe MM, Sun W, Hennessy S. On the estimation and use of propensity scores in case-control and case-cohort studies. Am J Epidemiol 2007;166:332–339. [DOI] [PubMed] [Google Scholar]
- 20.Becker DJ, Lowe JB. Fucose: biosynthesis and biological function in mammals. Glycobiology 2003;13:41R–53R. [DOI] [PubMed] [Google Scholar]
- 21.Walters RW, Pilewski JM, Chiorini JA, Zabner J. Secreted and transmembrane mucins inhibit gene transfer with aav4 more efficiently than aav5. J Biol Chem 2002;277:23709–23713. [DOI] [PubMed] [Google Scholar]
- 22.Querol-Audi J, Konecsni T, Pous J, Carugo O, Fita I, Verdaguer N, Blaas D. Minor group human rhinovirus-receptor interactions: geometry of multimodular attachment and basis of recognition. FEBS Lett 2009;583:235–240. [DOI] [PubMed] [Google Scholar]
- 23.Barbalic M, Dupuis J, Dehghan A, Bis JC, Hoogeveen RC, Schnabel RB, Nambi V, Bretler M, Smith NL, Peters A, et al. Large-scale genomic studies reveal central role of abo in sp-selectin and sicam-1 levels. Hum Mol Genet 2010;19:1863–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pare G, Chasman DI, Kellogg M, Zee RY, Rifai N, Badola S, Miletich JP, Ridker PM. Novel association of abo histo-blood group antigen with soluble ICAM-1: results of a genome-wide association study of 6,578 women. PLoS Genet 2008;4:e1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.