SUMMARY
Metabolic disorders including obesity, type 2 diabetes and atherosclerosis have been viewed historically as lipid storage disorders brought about by overnutrition. It is now widely appreciated that chronic low-grade inflammation plays a key role in the initiation, propagation and development of metabolic diseases. Consistent with its central role in coordinating inflammatory responses, numerous recent studies have implicated the transcription factor NF-κB in the development of such diseases, thereby further establishing inflammation as a critical factor in their etiology and offering hope for the development of new therapeutic approaches for their treatment.
INTRODUCTION
The competing need to protect the body from infection while maintaining proper energy metabolism represents a fundamental physiological challenge. Mounting an immune response to infection is energy intensive, but essential for life (Demas et al., 1997; Romanyukha et al., 2006). Both immunity to disease and economical use of energy reserves have been heavily favored throughout human evolution. Yet in the modern era, when overnutrition is more common than starvation, metabolic diseases have become the leading cause of deaths in the United States, with an incidence skyrocketing worldwide. Furthermore, as the risk of infectious diseases recedes, an immune system poised to respond vigorously to all inflammatory challenges has itself become a threat. Indeed, inappropriate triggering of such responses may account for the prevalence of inflammatory diseases such as allergy, asthma, diabetes and cancer.
Metabolic syndrome encompasses a cluster of conditions that result from nutrient excess, hyperglycemia, hyperlipidemia, insulin resistance, obesity, and hepatic steatosis, which together affect a quarter of Americans adults and over a million children (Iyer et al., 2010). Metabolic diseases track together, and obese patients are at increased risk for type-2 diabetes, while insulin-resistant patients frequently suffer from cardiovascular diseases such as atherosclerosis. This review will examine the role of inflammatory signaling pathways in metabolic diseases, focusing on the central regulator of inflammation, nuclear factor κB (NF-κB). The NF-κB pathway unites the inflammatory and metabolic responses, and as a well-studied mediator of inflammation and immunity, represents an entry point for better understanding metabolic diseases with an eye towards developing novel treatment strategies.
NF-κB directs the inflammatory response
The transcription factor NF-κB promotes immunity by controlling the expression of genes involved in inflammation (http://www.nf-kb.org/target/index.html). Cytokines and pathogen-associated molecular patterns (PAMPs) stimulate cell surface receptors including toll-like receptors (TLRs) to initiate a signaling cascade resulting in the activation of NF-κB. NF-κB drives expression of target genes that mediate cell proliferation and release of anti-microbial molecules and cytokines to activate the immune response (Hayden and Ghosh, 2008). Although NF-κB was first characterized in cells of the hematopoietic system, subsequent research has revealed NF-κB activation can occur in most cell types. Indeed, a number of recent high profile reports have demonstrated a key role for the NF-κB signaling pathway in the liver, adipose tissue, and central nervous system in the development of inflammation-associated metabolic diseases.
NF-κB activation in macrophages initiates the inflammatory cascade
Microbial infection or damage to the tissue triggers an inflammatory response characterized by an influx of white blood cells, redness, pain, swelling and, ultimately, organ dysfunction. Macrophages, mononuclear phagocytic leukocytes, provide a first line of defense against invading microorganisms by organizing the inflammatory response. The inflammatory cascade begins when recognition of PAMPs by local cells results in the release of cytokines that cause blood vessels to display adhesion molecules that promote entry of leukocytes into the tissue. Local cytokines activate NF-κB to promote macrophage relocalization and activation at the site of infection. Activated macrophages produce antimicrobial molecules and release chemokines and cytokines to augment macrophage activation and recruitment to the tissue. Together, antimicrobial molecules and recruited leukocytes cooperate to kill pathogens, clear infection, and remove dead cells. Termination of the inflammatory response is vital to maintain overall health, as chronic macrophage activation is associated with rheumatoid arthritis, type 2 diabetes and atherosclerosis (Baumgartl et al., 2006; Jimi et al., 2004; Rocha and Libby, 2009).
The IKK complex regulates NF-κB Activation
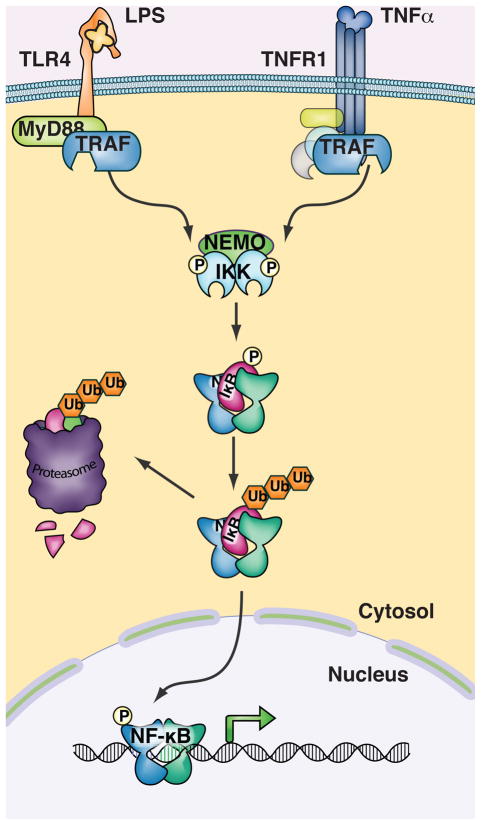
NF-κB activity is under the control of signaling from extracellular stimuli (Figure 1). Inhibitor of κB (IκB) molecules sequester NF-κB in the cytosol of resting cells and prevent its nuclear localization and transcriptional function. Cytokine or PAMP ligation of cell surface receptors initiates signaling cascades that converge on the activation of the Inhibitor of κB kinase (IKK) complex (Hayden and Ghosh, 2008). IKK phosphorylation of IκB molecules promotes their degradation and releases NF-κB, which translocates to the nucleus to promote transcription of target genes. The IKK complex consists of three subunits, the catalytic subunits IKKα(IKK1) and IKKβ(IKK2) and the regulatory subunit NEMO(IKKγ). Extensive research has defined IKKβ and NEMO as essential mediators of the inflammatory and immune response. Loss of IKKβ or NEMO function is not compatible with life, as IKK mediates multiple essential pathways including cell survival and metabolic functions described later in this review.
Figure 1. Inflammatory Triggers activate expression of pro-inflammatory genes through IKK and NF-κB.
Following engagement of Toll-like receptors and cytokine receptors, signaling through MyD88 and death domain adapter molecules (respectively) and then via TRAFs results in phosphorylation of IKK and activation of the IKK complex kinase activity. NF-κB is constitutively bound to IκB molecules which confine its localization to the cytosol. IKK complex phosphorylation of serine residues on IκB promotes its polyubiquitination and degradation, thereby freeing NF-κB to enter the nucleus and activate transcription of target genes.
NF-κB regulates inflammation through cytokine-directed cell differentiation
NF-κB regulated genes direct the differentiation of distinct immune cell types. Macrophage cells differentiate from monocytes following recruitment to sites of infection and exposure to the local cytokine milieu (Mantovani et al., 2005). Differentiated macrophages can be categorized into two broad types based both on function and cytokine expression. M1 macrophages produce IL-1, IL-6, TNF-α and other proinflammatory cytokines and, as a result mobilize neutrophils and help initiate the innate immune response to pathogens. These and other NF-κB-driven cytokines produced by M1 macrophages also activate lymphocytes and communicate infection to the adaptive immune system. T-lymphoctyes are stimulated by pro-inflammatory cytokines and ligands specific for their individual T-cell antigen receptors (TCRs). Activated T-cells proliferate and release more cytokines, including IFN-γ, IL-6 and TNF-α, further driving differentiation of M1 macrophages, recruitment of more lymphocytes, and initiation of the adaptive immune response. Both T-cells and M1 macrophages are associated with chronic inflammation in metabolic diseases, and have been found to accumulate in the adipose tissue of obese mice and in the liver and pancreas of mice with insulin resistance (Weisberg et al., 2003).
A second class of macrophages, M2, participates in wound healing responses. M2 macrophages secrete the anti-inflammatory cytokine IL-10. IL-10 is also released by CD4+FoxP3+ regulatory T-cells (Tregs). Tregs and M2 macrophages reside in the adipose tissue of lean mice, and in healthy peripheral tissues of insulin-responsive animals. NF-κB-dependent differentiation of monocytes into either M1 or M2 macrophages, in response to cytokines produced by lymphocytes and other immune cells, is a critical factor in the development of inflammatory metabolic diseases. Therefore, NF-κB regulates expression of the inflammatory mediators that recruit monocytes, drive differentiation to macrophages and direct macrophage cell fate determination, further involving NF-κB in inflammation-associated metabolic disease.
Downregulation of the Immune response and chronic Inflammation
Both cell-intrinsic and cell-extrinsic mechanisms work in a coordinated manner to regulate the inflammatory response. Among cell extrinsic mechanisms, the short half-life of pro-inflammatory cytokines limits the duration of the inflammatory response. The anti-inflammatory cytokine IL-10 counters immune cell activation, and attenuates the inflammatory response. Following release of anti-microbial molecules, macrophages clear dead cells by phagocytosis to prevent tissue necrosis. By removing dead cells, macrophages eliminate the source of pro- inflammatory mediators at the cellular level.
Cell intrinsic regulatory mechanisms are many and include those that attenuate the NF-κB inflammatory gene program. Numerous negative regulators of NF-κB have been identified (Hayden & Ghosh 2008). For example, de novo synthesis of IκB molecules directly reinstates control over NF-κB in the cytosol and the deubiqutinase A20 targets upstream components of the NF-κB signaling pathway. Interestingly, both A20 and IκBs are targets of NF-κB transcriptional activity, indicating that the NF-κB transcriptional program induces its own signal decay and termination. Specificity within this process helps to fine tune responses and limit deleterious effects. Thus, rather than representing a global shutdown of NF-κB, selective inactivation of the transcription allows continued expression of anti-microbial effectors while limiting tissue destruction resulting from unchecked inflammation (Foster et al., 2007).
Both cell extrinsic and intrinsic mechanisms are dysregulated under conditions of chronic inflammatory disease. Macrophages recruited to clear away dead cells can themselves be activated by local cells to produce pro-inflammatory mediators. Indeed, successive cycles of cell death, macrophage recruitment, activation, and release of IL-6 and TNF-α can lead to a state of chronic unresolved inflammation. Uncoupling NF-κB from its intracellular negative regulatory networks by knocking-out A20, or by enforced expression of a constitutively active IKK complex, has been associated with arthritis, type-2 diabetes, atherosclerosis and cancer (Idel et al., 2003; Lee et al., 2000; Wolfrum et al., 2007).
The NF-κB inflammatory program has been shown to participate in the development of multiple metabolic diseases. A number of recent high-profile reports have demonstrated a requirement for components of the NF-κB signaling apparatus in the development of metabolic disease in genetic mouse models. This review will summarize the recent literature and highlight unanswered questions regarding the role of NF-κB in three common metabolic disorders: obesity, insulin resistance, and atherosclerosis.
Inflammation and Obesity
65% of American adults are overweight or obese and face decreased life expectancy and numerous additional health risks as a result, including dementia, degenerative neurological disease, airway disease, insulin resistance, type 2 diabetes, cardiovascular disease, atherosclerosis and cancer. As an epidemic of obesity spreads across the globe, a better understanding of how obesity develops and contributes to associated diseases is an imperative public health objective.
A major advance in understanding the molecular mechanisms of obesity was the discovery of leptin. An adipokine, leptin is secreted by adipocytes and communicates energy needs to the central nervous system. Following feeding leptin release signals satiety to the hypothalmus triggering loss of appetite and appropriate energy storage in the liver and muscle. Thus mice deficient in leptin exhibit voracious appetite and unregulated glucose production despite feeding. These mice rapidly gain weight leading to morbid obesity at only four weeks of age (Zhang et al., 1994). In cases of diet-induced obesity, excess metabolites and lipid consumed in diet result in high levels of circulating leptin, but dysregulated leptin signaling maintains adipocyte hypertrophy and obesity. The mechanism of leptin dysregulation in obesity remains unclear.
For years, genome-wide association studies have sought to identify obesity susceptibility genes. Recent studies of gene expression networks of adipose tissue of obese mice and human subjects have revealed extensive inflammatory gene networks associated with obesity. Two recent reports took unbiased approaches to investigate how complex gene expression networks vary in obese and lean subjects. Obesity-associated traits including body mass index (BMI) and body fat percentage were correlated with gene transcripts from hundreds of human adipose tissue samples (Emilsson et al., 2008). The transcriptional program in obese adipose tissue contained a single module of co-expressed genes that was found to overlap with a murine genetic network defined in a companion report. In this study, gene networks in liver and adipose tissue perturbed by loci conferring susceptibility to metabolic syndrome were used to define an expression module highly enriched in genes controlling inflammation, immunity and macrophage activation (Chen et al., 2008).
The mechanistic link between these macrophage gene networks and obesity is not clear. However, a few possibilities that have emerged in recent literature may provide clues to how the immune system can influence the development of obesity. First, central nervous system inflammation may directly facilitate diet-induced obesity. Second, although controversial, TLR4 may directly contribute to the development of obesity, particularly in the context of diets high in saturated fats (Nguyen et al., 2004; Tsukumono et al., 2007). Third, the established association of gut microbiota with obesity (Ley et al., 2006) is supported by recent findings causally linking innate immunity to obesity through the regulation of gut microbiota (Vijay-Kumar et al., 2010). The growing body of work reviewed below suggests that not only does overnutrition promote inflammation, but that the immune system may at least be complicit, if not directly involved, in the development of obesity as well.
Inflammation in obese adipose tissue
Obesity is associated with chronic activation of inflammatory pathways in both adipocytes and in macrophages residing in or infiltrating the adipose tissue. Adipocytes are not only lipid storage depots, but also secretory cells that produce proinflammatory cytokines and adipokines. Adipocytes monitor energy storage levels and release pro-inflammatory cytokines to report overnutrition to the rest of the body. Adipocytes from obese mice produce the chemokines and cytokines such as CCL-2, MIPs, IL-6, IL-1β and TNF-α. Together, these proinflammatory mediators recruit monocytes into adipose tissue and activate their differentiation to M1 macrophages (Xu et al., 2003). Adipocytes also release adipokines such as Sfrp1 that counter proinflammatory signaling (Ouchi et al., 2010). Compared to lean mice, the adipose tissue of obese mice is greatly enriched in proinflammatory M1 macrophages. The fraction of adipose tissue that is adipose tissue macrophage (ATM) of the M1 type increases commensurately with the severity of obesity, and can be up to 40% in obese mice (Weisberg et al., 2003; Lumeng 2007a and b). It should be noted that similarities between adipocytes and macrophage cells might obscure the source of inflammatory mediators in the adipose tissue. Both cells can consume lipid, and lipid engorged macrophage cells may be difficult to distinguish from adipocytes in some cases (Weisberg et al., 2003). Intermuscular adipose tissue also contains significant numbers of macrophages, which increase in frequency in obese mice (Weisberg et al., 2003) and are of the pro-inflammatory M1 type (Nguyen et al., 2007; Patsouris et al., 2008).
A number of recent reports have suggested that T-lymphocytes play an important role in the production of pro-inflammatory cytokines and recruitment of macrophage to obese adipose tissue. Like monocytes, T-cells circulate through the body and infiltrate peripheral tissues in response to chemokine and cytokine signals. Infiltrating lymphocytes precede macrophage populations in response to high-fat diet, and may therefore provide an important source of pro-inflammatory mediators to promote macrophage recruitment and activation. Cytotoxic T lymphocytes of the CD8 lineage are greatly enriched in the adipose tissue of mice fed a diet high in fat, a finding consistent with increased CD8 cells in obese human patients (Nishimura et al., 2009). Mice deficient in CD8 cells were partially protected from diet-induced obesity, whereas adoptive transfer of CD8 cells aggravated adipose inflammation (Nishimura et al., 2009). In another recent study, a unique pattern of T-cell antigen receptors (TCRs) was identified among adipose tissue T cells of obese mice, a subset of the full TCR repertoire in this tissue(Yang et al., 2010). Skewing of T-cell populations and TCR suggests T-cells contribute to inflammation in obesity.
Populations of CD4+FoxP3+ Tregs are also altered as CD8 effectors increase in obese adipose tissue. Tregs are highly enriched in adipose tissue of normal mice (Feuerer et al., 2009). Adipose resident Tregs increase expression of numerous chemokines and chemokine receptors, integrin adhesion molecules and components of the IL-10 anti-inflammatory pathway. Intriguingly, restricted TCR diversity was found in adipose tissue Tregs as well (Feuerer et al., 2009). The selection of Treg TCR, through the recognition of cognate antigen, suggests that both Treg and effector T-cells of the adaptive immune system participate in the development of metabolic inflammation. Indeed, unlike normal mice, Tregs were lost from the abdominal adipose tissue in obese mice (Feuerer et al., 2009). These data suggest that Treg cells may repress adipose tissue inflammation directly in non-obese animals, yet the mechanism of Treg dysregulation in obesity is unclear.
What triggers the inflammatory response in adipose tissue remains unclear. The prevailing hypothesis is that adipocyte-generated cytokines recruit monocytes and T-cells to the obese adipose tissue and promote the differentiation of monocytes to M1 macrophage to initiate systemic inflammation. But what induces inflammation in adipose tissue? It is agreed that metabolic stress, both the extracellular and intracellular build-up of metabolites from overnutrition, can activate inflammatory pathways. For instance, an excess of metabolic building blocks in the endoplasmic reticulum (ER) or increased levels of unmetabolized free fatty acids can initiate inflammatory pathways in a variety of cell types. Alternatively, increased adipocyte death in obese tissue could recruit macrophages to clear away dead cells. Indeed, adipose tissue macrophages organize crown-like structures around necrotic cells, thereby forming a locus of chronic inflammation (Medina Gomez et al., 2007). In yet another potential mechanism, the possible ligation of Toll-like receptors on adipocytes or macrophages by dietary lipids could exploit the canonical inflammatory signaling pathway to activate NF-κB and produce inflammatory mediators. The reports discussed below employ mouse genetic models in which signaling pathways responsible for coupling nutrient excess to inflammation during obesity were interrogated.
The non-canonical IKK kinase IKKε was recently shown to be required for high fat diet induced obesity (Chiang et al., 2009). IKKε is not expressed in most resting cells, but is transcriptionally induced by NF-κB downstream of inflammatory stimuli. IKKε contributes to the late phase of the NF-κB transcriptional activity but primarily plays an important role in interferon signaling, and is therefore required to combat certain viral infections (Adil et al., 2006; Tenoever et al., 2007). IKKε expression is dramatically upregulated in response to nutrient excess, up to 40-fold in adipocytes and fat-infiltrating macrophages (Chiang et al., 2009). IKKε deficiency uncoupled obesity from high fat diet by increasing energy usage, oxygen respiration, and thermogenesis. In part as a likely consequence of reduced weight gain and perhaps also due to direct defects in cytokine production and signaling, IKKε KO mice were protected against diet-induced insulin resistance, chronic inflammation in liver and fat, and activation of additional proinflammatory pathways. Therefore, IKK family signaling has a crucial role in both overnutrition-driven obesity and metabolic disease.
Extensive research has shown that the IKKα/IKKβ complex activates NF-κB-mediated gene expression downstream of TLRs and cytokine receptors (Hayden and Ghosh, 2008). To investigate whether canonical NF-κB signaling pathways are responsible for inflammation associated with obesity, mice deficient in IKKβ in individual tissue compartments and cell types were evaluated. Using this approach, expression of IKKβ in both the liver and in myeloid cells was shown to be dispensable for induction of obesity by a high fat diet or leptin deficiency (Arkan et al., 2005). These findings suggested that although inflammation is required for perturbation of central metabolism by nutrient excess, inflammatory signaling pathways in individual tissues of the liver and macrophages can be bypassed during systemic overnutrition and development of obesity in mice.
Central nervous system inflammation and obesity
Overnutrition-associated chronic inflammation also affects metabolic regulation in the central nervous system (CNS). The mediobasal hypothalamus regulates energy balance and prevents obesity by adjusting appetite and food intake in response to signals of metabolic status including insulin and leptin. To investigate how inflammatory gene expression contributes to central control of nutrient metabolism, the Cai group sought to define IKKβ action in the hypothalamus. IKKβ is constitutively expressed in the hypothalamus, and directs NF-κB activation in the CNS of mice exposed to a high-fat diet (Zhang et al., 2008). Forced expression of IKKβ in the CNS interrupted leptin and insulin signaling, resulting in increased intake of high-fat food and weight gain compared to WT mice. By contrast, targeted disruption of IKKβ in the hypothalamus lowered food intake, and protected mice from obesity as well as diet-induced insulin resistance and glucose intolerance (Zhang et al., 2008).
IKKβ activity in the CNS was associated with augmented IL-6 production, and JNK and cytokine signaling, suggesting that NF-κB target genes mediate the metabolic changes observed in the CNS of IKKβ KO animals. Several reports have linked overnutrition with stressed protein assembly pathways in the ER leading to inflammation and even the development of hepatic insulin resistance (Hotamisligil 2010; Ozcan et al., 2009; Sabio et al., 2008; Ozcan et al., 2004). Pharmacological inhibition of ER stress pathways diminished NF-κB activation by high-fat diet (Ozcan et al., 2004; Ozcan et al., 2006). IKKβ interfered with leptin signaling in the brain, food intake and body weight, indicating that NF-κB coordinates metabolic stress responses to overnutrition in non-canonical cell types.
NF-κB activation by overnutrition also results from detection of extracellular inflammatory triggers by classical inflammatory pathways. TLR4 recognition of LPS molecules on the outer membrane of gram-negative bacteria initiates a robust innate immune response through NF-κB. In a highly interesting observation, nutritional free fatty acids were found to activate signaling downstream of TLR4 (Shi et al., 2006). TLR4 deficiency uncouples lipid excess and high fat diet from inflammatory signaling in adipocytes, insulin resistance in the muscle and glucose intolerance (Shi et al., 2006). A loss of function mutation in TLR4 protects against insulin resistance in adipose tissue as well as diet-induced obesity (Poggi et al., 2007; Tsukumo et al., 2007; Davis et al., 2008). A similar role to that of TLR4 has also been reported for TLR2. Activation of ATM through either TLR4 or TLR2 in response to free fatty acids induces inflammatory cytokine production and promotes local insulin resistance (Nguyen et al., 2007). Mice lacking TLR2 are protected from increased abdominal adiposity following high fat diet, and display reduced inflammatory cytokine expression (Davis et al., 2010). How dietary lipids, often oxidized and rendered more immunogenic by overnutrition, activate NF-κB remains incompletely understood. Recent studies have demonstrated that excess nutritional fatty acids can be sensed by TLR2/4, triggering activation of IKKβ and NF-κB, as well as JNK and other TLR-activated pathways, in a MyD88 dependent manner both in hematopoietic cells and in the CNS.
The adapter molecule MyD88 associates with the cytoplasmic domains of TLRs to organize the initiation of a signaling cascade (Figure 1). MyD88 deficient mice uncouple TLR4 ligation from activation of NF-κB, and the innate immune response to bacterial pathogens. A recent study found mice containing a targeted deletion of MyD88 exclusively in the CNS were protected from obesity and leptin resistance induced by dietary lipids or a high-fat diet (Kleinridders et al., 2009). CNS-specific MyD88 KO mice respond to high-fat diet by reducing food intake to maintain body weight, insulin responsiveness and glucose tolerance. Interestingly, activation of ER stress mediators including JNK was equivalent in MyD88 KO and WT mice. Likewise, inflammatory mediators TNF-α and IL-6 were not downregulated in the CNS-specific MyD88 KO mice. The authors therefore postulate that, rather than ER or inflammatory mechanisms, direct TLR4 ligation by oxidized lipids stimulates MyD88-dependent activation of IKK without activating a secondary cytokine signaling cascade. In a similar finding, TLR4 mutant mice uncouple high-fat diet from obesity and metabolic syndrome, as discussed later in this review (Tsukumo et al., 2007). How LPS or other TLR triggers might access the CNS is not known. Yet free fatty acids may induce NF-κB to disrupt leptin signaling in the CNS during nutrient excess.
TLRs, gut microbiota and obesity
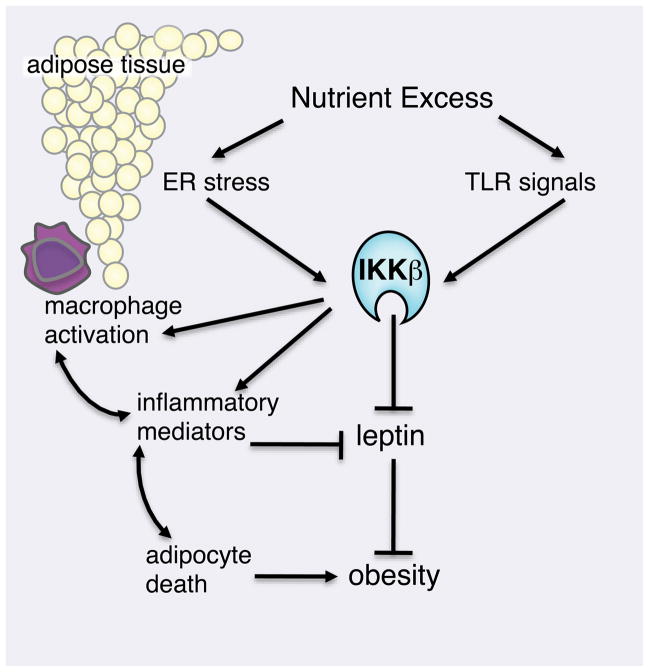
TLRs themselves play important roles in the development of obesity. A significant factor in the relationship between inflammation and obesity is the influence of TLRs on the gut microbiota. It has long been known that the gut flora of obese patients differ dramatically from healthy ones: circulating LPS is dramatically elevated in obese patients compared to lean patients. A recent study demonstrates that mice deficient in TLR5 possess unique gut microflora that sensitize them to obesity and metabolic syndrome (Vijay-Kumar et al., 2010). Remarkably, transfer of the gut flora from TLR5 KO animals to lean recipients recapitulates an obese phenotype. Yet whereas TLR5 deficiency in the gut permits outgrowth of pro-obesity flora, TLR4 deficiency in hematopoietic cells reduces inflammatory signaling required for diet-induced insulin resistance. Using a bone- marrow transfer system, TLR4-deficient macrophages were shown to protect against diet-induced insulin resistance (Saberi et al., 2009). These studies suggest that innate immune receptors influence immunity in numerous ways in different cell types, underscoring a potential role for NF-κB and the immune system in the development of obesity (Figure 2).
Figure 2. Overweight and obesity induces activation of inflammatory mediators in adipocytes and infiltrating macrophage cells.
Metabolism of excess nutrients imposes stress on adipose tissue. Production of pro- inflammatory cytokines by adipocytes and adipocyte hypertrophy and expiration recruits M1 pro-inflammatory macrophages into the adipose tissue. Pro-inflammatory signaling in central nervous system and macrophage-derived cytokines promote systemic dysregulation of leptin signaling and perturbed energy balance.
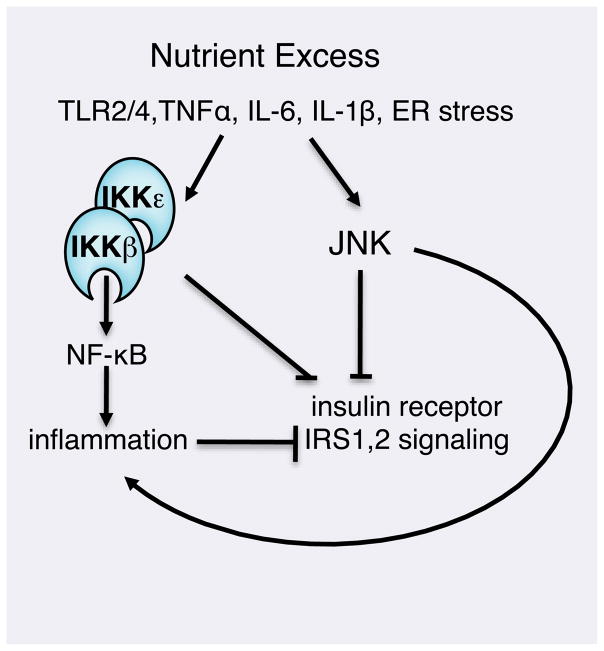
While growing evidence suggests that inflammatory signaling pathways can contribute to obesity, it remains unclear how the two processes are linked. A global mechanism whereby excess metabolites induce CNS inflammation via IKK and ER stress is supported by studies of another positive regulator of NF-κB, 3-phoshoinositide dependent kinase 1 (PDK-1). Mice in which PDK-1 was specifically inactivated in pro-opiomelanocortin CNS cells demonstrate altered energy balance and increased adiposity compared to WT mice (Belgardt et al., 2008). Moreover, selective ablation of JNK in the CNS normalizes adiposity in mice subjected to a high-fat diet (Belgardt et al., 2010). The role of NF-κB was not examined in these reports, and signaling cross-talk to IKK may underlie the effects of ER stress on pro-inflammatory signaling. Alternatively, PAMP-Rs and oxidized lipids could activate IKK via MyD88. Or both mechanisms may operate in the CNS, collaborating to activate IKK and NF-κB during nutrient excess and obesity (Figure 3).
Figure 3. TLR and ER stress responses in insulin resistance.
Signaling from cytokine receptors and Toll-like receptors on the cell surface, as well as ER stress can activate the IKK complex and NF-κB to activate expression of pro-inflammatory cytokines and disable insulin signaling. Defective insulin signaling contributes to insulin resistance and the development of Type 2 diabetes.
NF-κB, Insulin Resistance and Type 2 Diabetes
Insulin resistance and type 2 diabetes now affect over sixty million Americans and are predicted to be the leading killer worldwide by 2050. Type 2 diabetes begins with insulin resistance, the impaired responsiveness of target organs to circulating insulin. An anabolic hormone released by beta (β) cells in the pancreas, insulin regulates energy metabolism in liver, skeletal muscle and adipose tissues. Ligation of the insulin receptor in target cells induces tyrosine phosphorylation of insulin receptor substrates and a signaling cascade to direct changes in energy metabolism. Excess glucose and lipid metabolites impair insulin sensitivity, causing desensitized signaling from the insulin receptor, abnormal translocation of glucose transporters, and altered activities of intracellular glucose metabolism enzymes. Because insulin promotes glucose uptake in the muscle and reduces gluconeogenesis in the liver and lipolysis in adipose tissue, insulin resistance results in defective nutrient metabolism and glucose intolerance. With increasing insulin resistance in muscle, liver and adipose tissues, there comes a point at which pancreatic β-cells can no longer compensate by secreting more insulin and type 2 diabetes with elevated circulating glucose develops.
Insulin resistance and type 2 diabetes are now recognized as chronic inflammatory diseases. As β-cells in the pancreas work to produce more and more insulin, metabolic stress signals recruit monocytes to clear dying β-cells. These recruited monocytes differentiate into TNF-α, IL-6 and IL-1 producing macrophages that promote further β-cell dysfunction and death. TNF-α disrupts insulin signaling and sensitivity (Hotamisligil et al., 1993). Elevated levels of TNF-α are detected in the bloodstream and in the peripheral tissues of insulin-resistant mice (Hotamisligil et al., 1993). Insulin-responsive cells including adipocytes and hepatocytes produce TNF-α, IL-6, and IL-1, and both tissue and circulating cytokine levels are increased by a high-fat diet. In mice, both TNF-α neutralization and deficiency each prevent high-fat diet-induced insulin resistance (Hotamisligil et al., 1993; Uysal et al., 1997).
The role of NF-κB in the pathology of type 2 diabetes was first suggested when aspirin and other salicylates, used extensively in the early 20th century to treat rheumatic disease, proved beneficial in patients with glucose intolerance (Williamson R, et al., 1901; Hotamisligil and Erbay, 2008). The discovery that salicylates target NF-κB (Kopp & Ghosh, 1994) was followed by the demonstration that insulin resistance is effectively reversed by high dose salicylates in murine models of obesity (Yuan et al., 2001). In a landmark study, mice heterozygous for IKKβ (IKKβ +/−) were protected against insulin resistance from both diet-induced and genetic obesity (Yuan et al., 2001). Finally, salicylates, which may directly target IKKβ within the NF-κB pathway (Yin et al., 1998), were also shown to inhibit fat-induced insulin resistance in skeletal muscle (Kim et al., 2001; Yuan et al., 2001). Together, these findings suggest IKKβ is not only an essential mediator of obesity-induced diabetes, but also a promising therapeutic target for insulin resistance and glucose intolerance. They also raise the question of whether NF-κB activity is directly responsible for diet-induced insulin resistance. Because NF-κB pathways are activated in a variety of cell types at different stages of insulin resistance, follow-up studies sought to address the signaling pathways and cell types responsible for IKKβ-mediated metabolic disease.
The liver is a vital tissue in energy metabolism, the site of amino acid, glucose and lipid biosynthesis. In hepatocytes, insulin signaling causes the arrest of gluconeogenesis and increased storage of glucose as glycogen. Expression of constitutively-active (CA) IKKβ in the liver resulted in defective insulin signaling in hepatocytes and muscle cells, elevated resting levels of insulin and free fatty acids, and systemic insulin resistance and glucose intolerance (Cai et al., 2005). Interestingly, although nutrient excess can be sensed in various tissues, IKKβ activation in liver was able to communicate overnutrition pathways to peripheral tissues. IKKβ activation was associated with increased expression of NF-κB target genes including IL-6. IL-6 blockade reversed CA-IKKβ-induced inflammation in both liver and in muscle, supporting a role for pro-inflammatory cytokines in the transmission of insulin resistance among tissues (Cai et al., 2005). Although muscle is clearly an essential insulin responsive tissue (Petersen & Shulman, 2006), constitutively active IKKβ in muscle produced severe muscle wasting but no change in insulin signaling (Cai et al., 2004). However, deletion of IKKβ in macrophages protected mice from the development of muscle insulin resistance in the context of obesity (Arkan et al., 2005). These results suggest that NF-κB activation in ATM of both muscle and liver adipose tissue can contribute to development of insulin resistance in these tissues in the context of obesity.
Unregulated expression of IKKβ in liver promotes diet-induced insulin resistance. To determine whether IKK is required for diet-induced insulin resistance, IKKβ was specifically deleted in the liver. Liver-specific ablation of IKKβ protected mice against diet-induced insulin resistance and glucose intolerance among hepatocytes, and reduced lipid deposition in liver (Arkan et al., 2005). Yet despite local glucose tolerance, central insulin signaling in IKKβ liver KO mice remained susceptible to overnutrition: muscle and adipose tissue developed insulin resistance in response to high fat diet, obesity and aging. Modest decreases in circulating IL-1β and IL-6 levels suggested that levels of cytokines in the liver are likely significantly diminished by the absence of IKKβ. Further studies demonstrating that ablation of IL-6 in the liver exacerbates obesity-induced insulin resistance suggest that a fine balance of inflammatory mediators are required for optimal metabolic control in the liver (Wunderlich et al., 2010). Together, these findings indicate that although the pro-inflammatory signaling pathways initiated by overnutrition in hepatocytes can be sufficient to induce systemic insulin resistance, IKKβ signaling in the liver is not required for insulin resistance, as it can be bypassed by release of pro-inflammatory mediators by other tissues and cell types.
NEMO (IKKγ) expression in the liver is also required for development of obesity-induced insulin resistance. Mice deficient in NEMO in the liver were somewhat protected from diet-induced obesity, glucose intolerance and insulin resistance (Wunderlich et al., 2008). Interestingly, liver NEMO KO caused deposition of fatty acids in the liver, causing increased hepatocyte death compared to WT. Therefore, both pro-inflammatory and pro-survival NF-κB functions (Luo et al., 2005) may be activated alongside each other during overnutrition. This synergy may account for the increased incidence of cancer in obese patients (Calle et al., 2003), although different nutritional states and TLR signaling mechanisms are also likely at play.
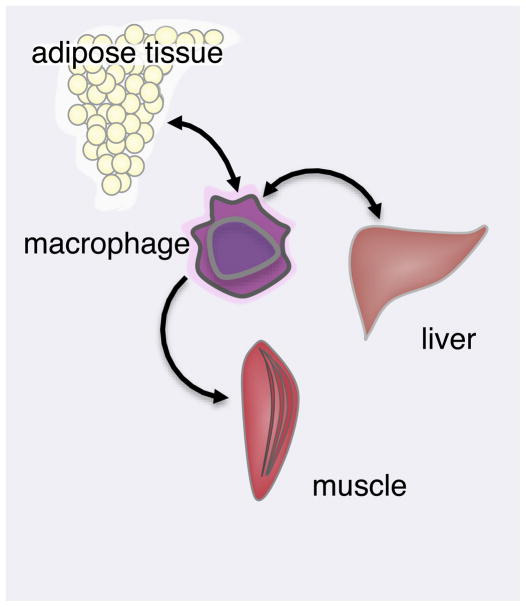
Analyses of IKK action in the liver suggested that pro-inflammatory signaling initiated by overnutrition in insulin-sensitive tissues requires propagation by additional cell types to produce a systemic inflammatory response. Monocytes circulate through the bloodstream and tissue where they sense cytokines through surface receptors, and secrete large amounts of cytokines upon activation. Macrophage infiltration of the pancreas and adipose tissue is associated with the development of insulin resistance. To determine the requirement for myeloid inflammation in insulin resistance, IKKβ was deleted in monocytes. Myeloid IKKβ KO mice uncoupled overnutrition by high fat diet or genetic obesity from pro-inflammatory pathways resulting in insulin resistance, glucose intolerance in all insulin-sensitive tissues (Arkan et al., 2005). Thus, while liver may provide an initial source of inflammatory mediators directly affecting liver insulin resistance, NF-κB signaling in specialized innate immune cells, the ATMs, is required to propagate these signals to the rest of the organism and promote systemic insulin resistance in muscle and other normally insulin sensitive tissues (Figure 4).
Figure 4. Macrophage cells are central mediators of insulin resistance.
Nutrient excess is sensed in peripheral metabolic tissues to result in production of pro- inflammatory cytokines IL-6, IL-1 and TNF-α. These cytokines drive macrophage activation and propagation of pro-inflammatory signals throughout the organism.
T-lymphocytes may collaborate with monocytic leukocytes to regulate insulin responsiveness during diet-induced obesity. Lymphocytes regulate macrophage differentiation and activation by producing IL-6 and TNF-α. Obese adipose tissue is enriched in CD8 cytotoxic T lymphocytes and TH1-type helper CD4 T-cells, and depleted in CD4+CD25+ regulatory T-cells (Feuerer et al., 2009; Nishimura et al., 2009; Yang et al., 2010). Additionally, IFNγ-producing TH1 CD4 helper T-cells infiltrate adipose tissue of mice on a high-fat diet (Winer et al., 2009). T-cell deficient Rag−/− animals appear hyper-susceptible to diet-induced obesity and insulin resistance, suggesting that T cells mediate inflammation resulting in obesity and metabolic disease.
Restoration of energy balance in Rag−/− mice by adoptive transfer of CD4+FoxP3+ Tregs suggested that Tregs promote glucose tolerance, insulin sensitivity and diminished weight gain despite a diet high in fat. Induction of Tregs using TGF-β alleviated insulin resistance and glucose intolerance through IL-10, providing further support for immunosupression by Tregs and IL-10 in insulin-sensitive tissue (Ilan et al. 2010). The finding that both effector and regulatory CD4 T cells attenuate inflammatory signaling in adipose tissue suggests that the adaptive immune system is intimately intertwined with the progression of metabolic disease. Although overnutrition may generate molecules capable of driving TCR-specific T cell activation and expansion, the identities of such specific metabolic antigens remain obscure.
Studies of genetic mutant mice demonstrate an essential role for NF-κB in the inflammatory response required for the development of insulin resistance. Yet, NF-κB controls the expression of wide array of target genes, including many that regulate cell growth and survival. Pro-survival functions of NF-κB also contribute to insulin sensitivity in pancreas. During the onset of insulin resistance, β cells grow and expand in an NF-κB dependent manner to compensate for insulin insensitivity in individual cells. Furthermore, as insulin insensitivity and β-cell stress increases production of inflammatory mediators, NF-κB protects pancreatic β cells from TNF-α mediated apoptosis (Chang et al., 2003). In spite of these functions, NF- κB blockade can protect pancreatic islets from diabetogenic agents (Eldor et al., 2006). In a similar finding, an NF-κB super-repressor protected pancreatic islet cells from IL-1β induced cell death (Giannoukakis et al., 2000). NF-κB may also regulate normal glucose uptake and insulin secretion. Specific attenuation of NF-κB activation in β-cells diminished glucose-stimulated insulin secretion (Norlin et al., 2005). Together, these reports suggest that multiple functions of NF-κB operate in β-cells, and their contribution to insulin resistance depends on both inflammatory and cell survival signaling pathways.
NF-κB Function in the Development of Atherosclerosis
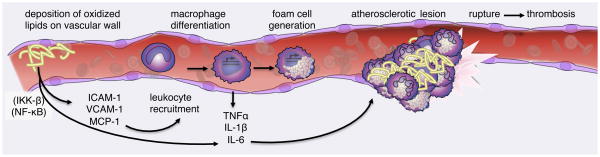
In addition to their role in insulin resistance in both liver and adipose tissue, pro-inflammatory pathways regulated by NF-κB also contribute to vascular disease associated with metabolic excess. Atherosclerosis is a disease of the arterial wall, now recognized to be driven by inflammatory processes (Rocha and Libby, 2009). Atherosclerosis begins when excess unmetabolized lipoproteins collect in the bloodstream. Oxidized lipoproteins trigger secretion of chemokines MIP-1α and MCP-1 by vascular endothelia, recruiting leukocytes to the site of inflammation (Weisberg et al., 2006). There, adhesion molecules ICAM-1 and VCAM-1 on the vascular surface promote slow rolling and arrest of circulating monocytes on the vascular wall. Once in contact with vascular endothelia, monocytes differentiate into macrophages, ingest deposited lipoproteins to become foam cells and create a site of local inflammation. Macrophage foam cells secrete IL-6, TNF-α and other cytokines to recruit more immune cells, resulting in the formation of atherosclerotic plaques (Figure 5). These lesions, containing lipid molecules and immune cells, narrow the arterial wall and promote cardiovascular disease and thrombosis.
Figure 5. NF-κB mediates pro-inflammatory signaling in endothelia and leukocytes that drives atherogenesis.
Pro-inflammatory signaling networks through NF-κB in vascular endothelia respond to deposition of oxidized lipoproteins by expression of adhesion molecules on the cell surface and leukocyte cytokines. Circulating monocytes are arrested at the site of inflammation and differentiate into cytokine-producing macrophage cells. Macrophage cells also consume lipids on the vascular wall swelling into foam cells that nucleate the atherosclerotic plaque. Continued recruitment, activation of leukocytes, lipid consumption and cell death results in the formation of a large atherosclerotic lesion impairing arterial blood flow and promoting thrombosis.
NF-κB integrates multiple processes in the formation of atherosclerotic plaques. NF-κB controls expression of genes directing the initiation and progression of atherosclerosis, including cytokines TNF-α, IL-1β, IL-6, chemokine MCP-1, and the adhesion molecule ICAM-1. The detection of NF-κB in nuclei of macrophages in atherosclerotic lesions (Brand et al., 1996) suggests that NF-κB activation is associated with atherogenesis. Together, numerous genetic studies reveal a sophisticated role for NF-κB in the pathology of atherosclerosis, in which different mechanisms and cell-specific actions coordinate its initiation, progression and resolution.
Because inflammatory programs in both endothelia and recruited myeloid cells contribute to atherotic plaques, numerous studies have set out to define the individual contribution of each cell type in atherogenesis. Mice deficient in apolipoprotein E (ApoE), a ligand important in lipoprotein clearance, exhibit greatly elevated levels of circulating very low-density lipoprotein (VLDL) cholesterol. Because hyperlipidemia accelerates the formation of atherosclerotic plaques, these mice are commonly employed to model atherosclerosis (Plump et al., 1992; Zhang et al., 1992). Mice deficient in the receptor for low-density lipoprotein (LDLR) are predisposed to atherosclerosis for similar reasons (Ishibashi et al., 1993).
Hyperlipidemic mice were used to investigate the requirement for IKK mediated NF-κB activation in endothelial cells in atherogenesis (Gareus et al., 2008). NEMO deletion in vascular endothelial cells by Tie2-CRE protects against atherosclerosis induced by high-fat diet in ApoE−/− mice. Endothelial NEMO deletion abrogated expression of adhesion molecules ICAM-1 and VCAM-1. Diminished expression of cytokines TNF-α, IL-1β, and IL-6 and chemokines MIP-1α and MCP-1 by NEMO-deficient endothelial cells also reduced infiltration of both macrophage and T-lymphocytes into atherosclerotic plaques. Together, decreased expression of adhesion molecules and pro-inflammatory mediators reduced both the size and severity of lesions in endothelial NEMO-deficient animals. These findings support an essential role for NF-κB signaling in vascular endothelial cells in atherosclerosis initiation, as both macrophage recruitment and plaque formation were greatly reduced when the NF-κB inflammatory program is disabled in these cells.
As described above, macrophages play an essential role in communicating inflammation among metabolic tissues in response to overnutrition and insulin resistance. Type-I interferon signaling promotes macrophage recruitment and accumulation within the atherosclerotic plaque and is required for atherosclerosis in LDLR−/− mice (Goossens et al., 2010). Yet the role of NF-κB mediated inflammatory functions by macrophage cells in atherosclerosis remains unclear. Bone marrow transplant studies found that hematopoietic cells deficient in the NF-κB subunit p50 diminished number and decreased size of atherosclerotic lesions in LDLR−/− mice (Kanters et al., 2004). Surprisingly, lesions in hematopoietic p50 knockout mice exhibited a highly inflammatory phenotype indicating intact production of proinflammatory cytokines. Lesions in these mice exhibited a near complete absence of foam cells, supporting previous findings that NF-κB targets mediate lipid ingestion by macrophage scavenger receptors and foam cell biogenesis (Ferreira et al., 2007). Yet how p50 and other NF-κB family members cooperate to support pro-inflammatory macrophage function in atherogenesis remains to be established. Metabolic stress in lipid-consuming macrophages has been proposed to drive cell death and plaque formation. Indeed, pharmacological inhibition of ER stress uncouples high-fat diet from atherosclerosis, implicating metabolic stress in the development of atherosclerosis (Erbay et al., 2009).
In addition to control of inflammatory cell activation, NF-κB plays an important role in cell survival. In macrophage foam cells, whose necrotic death creates the core of atherosclerotic plaques, the pro-inflammatory and pro-survival functions of NF-κB may act at cross-purposes. Using ApoE−/− or LDLR−/− models, several studies have shown that IKKβ deletion from the myeloid compartment fails to prevent atherogenesis in mice fed a high-fat diet and, instead, increased atherosclerosis in LDLR deficient mice (Kanters et al., 2003). However, incomplete deletion of IKKβ, and a 50% reduction of both NF-κB protein and activity may factor into these observations. Completely ablated expression of IL-10, in contrast to only modestly reduced IL-6 expression, suggests that different thresholds for NF-κB target genes may underlie the described knockout phenotypes.
Furthermore, NF-κB pro-survival functions may also contribute to development of atherosclerosis in IKKβ myeloid KO mice. Severe atherosclerosis results when foam cells unable to clear accumulated lipids perish within the necrotic core of an atherotic plaque. Absent pro-survival functions of IKKβ and NF-κB in lipid-engorged foam cells, increased and unregulated foam cell death likely facilitates necrosis within the plaque. The relative threshold of IKKβ function for foam cell survival is not known. Thus although NF-κB action in endothelial cells and macrophage foam cells drives the production of pro-atherogenic cytokines, its pro-survival functions and its role in the resolution of inflammation appear to limit plaque size and inflammatory pathology in mouse models of atherosclerosis. Future studies will define which NF-κB family members, target genes and functions, in which cell types coordinate the formation of the necrotic core and the destructive consequences of chronic inflammation.
In further evidence that NF-κB critically regulates atherogenesis, the negative regulator of NF-κB, A20 (tnfaip3) was mapped to a locus conferring sensitivity to atherosclerosis (Idel et al., 2003). Indeed, mice haploinsufficient for A20 (tnfaip3+/−) exhibited increased atherotic lesion size among mice deficient in ApoE. Enforced expression of A20 decreased lesion size compared to WT, indicating that NF-κB attenuation by A20 exerts an anti-atherogenic function in mice (Wolfrum et al., 2007). A20 haploinsufficiency was associated with increased expression of pro-atherogenic NF-κB target genes including VCAM1, ICAM1, MCF-1 and proinflammatory cytokines known to promote atherosclerosis (Wolfrum et al., 2007). It remains unclear whether A20-regulated signaling in endothelial cells, foam cells, undifferentiated monocytes, or in all of the above, is responsible for attenuating inflammatory atherogenesis. Nevertheless, these findings demonstrate that IKK signaling to NF-κB can be pro-atherogenic, and that modulation of the NF-κB expression programs offers potential in treating atherosclerosis and cardiovascular disease.
CONCLUDING REMARKS
NF-κB signaling in numerous cell types contributes to the pathology of metabolic disorders. Resident tissue cells activate NF-κB in response to stress associated with nutrient excess. Vascular endothelia induce NF-κB in response to binding oxidized lipids in the bloodstream. Specialized cells of the metabolic system including hepatocytes, adipocytes and neurons in the hypothalamus induce NF-κB in response to overnutrition, perhaps in response to metabolic or oxidative stress in the ER. Increased metabolic demands can induce necrotic cell death, itself a pro-inflammatory signal to NF-κB activation. And lastly, activation of innate immune receptors such as TLR4 through MyD88 could activate NF-κB in response to an excess of free fatty acids in a high fat diet.
It is clear that NF-κB inflammatory pathways promote metabolic disease, and therapeutic strategies might directly target macrophage IKK and NF-κB target genes. Although inhibitors of specific NF-κB pathways are not yet clinically available, there continues to be progress in the development of more selective anti-NF-κB pharmaceuticals and understanding the effects of existing therapies on the NF-κB pathway. NF-κB underlies the most prevalent type 2 diabetes therapeutic treatment thiazolidinedione (TZD) which targets the nuclear hormone receptor peroxisome proliferator-activated receptor gamma (PPARγ) and promotes insulin sensitivity in macrophages and adipocytes (Hevener et al., 2007). Blocking the action of inflammatory mediators is currently an attractive therapeutic approach. Although TNF-α blockade failed to alleviate insulin resistance in human patients (Martinez-Abundis et al., 2007; Dominguez et al., 2005), this failure may have resulted from insufficient penetration into the peripheral tissues. Treatment with IL-1βR antagonist reversed insulin resistance and glucose intolerance in diabetes patients (Sauter et al., 2008; Larsen et al., 2007). In a reprise of classical discoveries, recent clinical trials have found significant amelioration of insulin resistance and glucose homeostasis in human type 2 diabetes patients treated with salicylates which have been reported to inhibit NF-κB activation (Goldfine et al., 2010; Fleischman et al., 2008).
Together these studies demonstrate that interfering with NF-κB-driven inflammation can alleviate type 2 diabetes in patients and decrease hyperglycemia and insulin resistance. And yet a mechanistic understanding of how inflammatory signaling promotes metabolic disease remains tentative. In particular, the initiating events, those that link metabolic excess to inflammation are, as of yet, incompletely understood. Understanding these events is likely to yield more selectively druggable targets with the ultimate goal of breaking the link between obesity and the production of pro-inflammatory cytokines without broadly crippling the host’s ability to fight disease. The relatively specialized role of IKKε in NF-κB signaling may provide one potential site of intervention. Very recent and exciting work has uncovered a mechanistic link between the beneficial effects of omega-3 fatty acids and inhibition of the NF-κB pathway (Oh da et al., 2010). This work reveals new sites of potential intervention in the pathways between diet, NF-κB and insulin resistance. Continued investigations into lipid receptors, cell stress pathways and the networks of inflammatory propagation should provide new and better targets for therapeutic intervention in the future.
Acknowledgments
The work in the authors laboratory was supported by grants from NIH (R37-AI33443, RO1-AI068977 and RO1 AI066109).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adli M, Baldwin AS. IKK-i/IKKepsilon controls constitutive, cancer cell-associated NF-kappaB activity via regulation of Ser-536 p65/RelA phosphorylation. J Biol Chem. 2006;281:26976–26984. doi: 10.1074/jbc.M603133200. [DOI] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Baumgartl J, Baudler S, Scherner M, Babaev V, Makowski L, Suttles J, McDuffie M, Tobe K, Kadowaki T, Fazio S, Kahn CR, Hotamisligil GS, Krone W, Linton M, Bruning JC. Myeloid lineage cell-restricted insulin resistance protects apolipoproteinE-deficient mice against atherosclerosis. Cell Metab. 2006;3:247–256. doi: 10.1016/j.cmet.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgardt BF, Husch A, Rother E, Ernst MB, Wunderlich FT, Hampel B, Klockener T, Alessi D, Kloppenburg P, Bruning JC. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab. 2008;7:291–301. doi: 10.1016/j.cmet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Brand K, Page S, Rogler G, Bartsch A, Brandl R, Knuechel R, Page M, Kaltschmidt C, Baeuerle PA, Neumeier D. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97:1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Chang I, Kim S, Kim JY, Cho N, Kim YH, Kim HS, Lee MK, Kim KW, Lee MS. Nuclear factor kappaB protects pancreatic beta-cells from tumor necrosis factor-alpha-mediated apoptosis. Diabetes. 2003;52:1169–1175. doi: 10.2337/diabetes.52.5.1169. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhu J, Lum PY, Yang X, Pinto S, MacNeil DJ, Zhang C, Lamb J, Edwards S, Sieberts SK, Leonardson A, Castellini LW, Wang S, Champy MF, Zhang B, Emilsson V, Doss S, Ghazalpour A, Horvath S, Drake TA, Lusis AJ, Schadt EE. Variations in DNA elucidate molecular networks that cause disease. Nature. 2008;452:429–435. doi: 10.1038/nature06757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang SH, Bazuine M, Lumeng CN, Geletka LM, Mowers J, White NM, Ma JT, Zhou J, Qi N, Westcott D, Delproposto JB, Blackwell TS, Yull FE, Saltiel AR. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138:961–975. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity. 2008;16:1248–55. doi: 10.1038/oby.2008.210. [DOI] [PubMed] [Google Scholar]
- Davis JE, Braucher DR, Walker-Daniels J, Spurlock ME. Absence of Tlr2 protects against high-fat diet-induced inflammation and results in greater insulin-stimulated glucose transport in cultured adipocytes. J Nutr Biochem. 2010 doi: 10.1016/j.jnutbio.2009.12.008. online 29 April 2010. [DOI] [PubMed] [Google Scholar]
- Demas GE, Chefer V, Talan MI, Nelson RJ. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am J Physiol. 1997;273:R1631–1637. doi: 10.1152/ajpregu.1997.273.5.R1631. [DOI] [PubMed] [Google Scholar]
- Dominguez H, Storgaard H, Rask-Madsen C, Steffen Hermann T, Ihlemann N, Baunbjerg Nielsen D, Spohr C, Kober L, Vaag A, Torp-Pedersen C. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res. 2005;42(6):517–25. doi: 10.1159/000088261. [DOI] [PubMed] [Google Scholar]
- Eldor R, Yeffet A, Baum K, Doviner V, Amar D, Ben-Neriah Y, Christofori G, Peled A, Carel JC, Boitard C, Klein T, Serup P, Eizirik DL, Melloul D. Conditional and specific NF-kappaB blockade protects pancreatic beta cells from diabetogenic agents. Proc Natl Acad Sci U S A. 2006;103:5072–5077. doi: 10.1073/pnas.0508166103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emilsson V, Thorleifsson G, Zhang B, Leonardson AS, Zink F, Zhu J, Carlson S, Helgason A, Walters GB, Gunnarsdottir S, Mouy M, Steinthorsdottir V, Eiriksdottir GH, Bjornsdottir G, Reynisdottir I, Gudbjartsson D, Helgadottir A, Jonasdottir A, Styrkarsdottir U, Gretarsdottir S, Magnusson KP, Stefansson H, Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31:289–294. doi: 10.2337/dc07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossdal R, Kristjansson K, Gislason HG, Stefansson T, Leifsson BG, Thorsteinsdottir U, Lamb JR, Gulcher JR, Reitman ML, Kong A, Schadt EE, Stefansson K. Genetics of gene expression and its effect on disease. Nature. 2008;452:423–428. doi: 10.1038/nature06758. [DOI] [PubMed] [Google Scholar]
- Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, Hotamisligil GS. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira V, van Dijk KW, Groen AK, Vos RM, van der Kaa J, Gijbels MJ, Havekes LM, Pannekoek H. Macrophage-specific inhibition of NF-kappaB activation reduces foam-cell formation. Atherosclerosis. 2007;192:283–290. doi: 10.1016/j.atherosclerosis.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31:289–294. doi: 10.2337/dc07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- Gareus R, Kotsaki E, Xanthoulea S, van der Made I, Gijbels MJ, Kardakaris R, Polykratis A, Kollias G, de Winther MP, Pasparakis M. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell Metab. 2008;8:372–383. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Giannoukakis N, Rudert WA, Trucco M, Robbins PD. Protection of human islets from the effects of interleukin-1beta by adenoviral gene transfer of an Ikappa B repressor. J Biol Chem. 2000;275:36509–36513. doi: 10.1074/jbc.M005943200. [DOI] [PubMed] [Google Scholar]
- Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 152:346–357. doi: 10.1059/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens P, Gijbels MJ, Zernecke A, Eijgelaar W, Vergouwe MN, van der Made I, Vanderlocht J, Beckers L, Buurman WA, Daemen MJ, et al. Myeloid type I interferon signaling promotes atherosclerosis by stimulating macrophage recruitment to lesions. Cell Metab. 2010;12:142–153. doi: 10.1016/j.cmet.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, Leung HY, Watt MJ, Benner C, Febbraio MA, Nguyen AK, Folian B, Subramaniam S, Gonzalez FJ, Glass CK, Ricote M. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117:1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Idel S, Dansky HM, Breslow JL. A20, a regulator of NFkappaB, maps to an atherosclerosis locus and differs between parental sensitive C57BL/6J and resistant FVB/N strains. Proc Natl Acad Sci U S A. 2003;100:14235–14240. doi: 10.1073/pnas.1835672100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, Wu HY, Weiner HL. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer A, Fairlie DP, Prins JB, Hammock BD, Brown L. Inflammatory lipid mediators in adipocyte function and obesity. Nat Rev Endocrinol. 2010;6:71–82. doi: 10.1038/nrendo.2009.264. [DOI] [PubMed] [Google Scholar]
- Jimi E, Aoki K, Saito H, D’Acquisto F, May MJ, Nakamura I, Sudo T, Kojima T, Okamoto F, Fukushima H, Okabe K, Ohya K, Ghosh S. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004;10:617–624. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- Kanters E, Gijbels MJ, van der Made I, Vergouwe MN, Heeringa P, Kraal G, Hofker MH, de Winther MP. Hematopoietic NF-kappaB1 deficiency results in small atherosclerotic lesions with an inflammatory phenotype. Blood. 2004;103:934–940. doi: 10.1182/blood-2003-05-1450. [DOI] [PubMed] [Google Scholar]
- Kanters E, Pasparakis M, Gijbels MJ, Vergouwe MN, Partouns-Hendriks I, Fijneman RJ, Clausen BE, Forster I, Kockx MM, Rajewsky K, Kraal G, Hofker MH, de Winther MP. Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;112:1176–1185. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Kim YK, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437–446. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinridders A, Schenten D, Konner AC, Belgardt BF, Mauer J, Okamura T, Wunderlich FT, Medzhitov R, Bruning JC. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 2009;10:249–259. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JL, Kamata H, Karin M. The anti-death machinery in IKK/NF-kappaB signaling. J Clin Immunol. 2005;25:541–550. doi: 10.1007/s10875-005-8217-6. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Martínez-Abundis E, Reynoso-von Drateln C, Hernández-Salazar E, González-Ortiz M. Effect of etanercept on insulin secretion and insulin sensitivity in a randomized trial with psoriatic patients at risk for developing type 2 diabetes mellitus. Arch Dermatol Res. 2007;299(9):461–5. doi: 10.1007/s00403-007-0784-3. [DOI] [PubMed] [Google Scholar]
- Medina-Gomez G, Gray SL, Yetukuri L, Shimomura K, Virtue S, Campbell M, Curtis RK, Jimenez-Linan M, Blount M, Yeo GS, Lopez M, Seppänen-Laakso T, Ashcroft FM, Oresic M, Vidal-Puig A. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007;3(4):e64. doi: 10.1371/journal.pgen.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–92. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- Norlin S, Ahlgren U, Edlund H. Nuclear factor-B activity in cells is required for glucose-stimulated insulin secretion. Diabetes. 2005;54:125–132. doi: 10.2337/diabetes.54.1.125. [DOI] [PubMed] [Google Scholar]
- Oh da Y, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, Akasaki Y, Shimono A, Walsh K. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329:454–457. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Ozcan L, Yilmaz E, Duvel K, Sahin M, Manning BD, Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell. 2008;29:541–551. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119:S10–16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Poggi M, Bastelica D, Gual P, Iglesias MA, Gremeaux T, Knauf C, Peiretti F, Verdier M, Juhan-Vague I, Tanti JF, Burcelin R, Alessi MC. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia. 2007;50:1267–1276. doi: 10.1007/s00125-007-0654-8. [DOI] [PubMed] [Google Scholar]
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- Romanyukha AA, Rudnev SG, Sidorov IA. Energy cost of infection burden: an approach to understanding the dynamics of host-pathogen interactions. J Theor Biol. 2006;241:1–13. doi: 10.1016/j.jtbi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, Verma IM, Olefsky JM. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–429. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter NS, Schulthess FT, Galasso R, Castellani LW, Maedler K. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology. 2008;149:2208–2218. doi: 10.1210/en.2007-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC, Ganchi PA, Ballard DW, Greene WC. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- Tanti JF, Gremeaux T, van Obberghen E, Le Marchand-Brustel Y. Serine/threonine phosphorylation of insulin receptor substrate 1 modulates insulin receptor signaling. J Biol Chem. 1994;269:6051–6057. [PubMed] [Google Scholar]
- Tenoever BR, Ng SL, Chua MA, McWhirter SM, Garcia-Sastre A, Maniatis T. Multiple functions of the IKK-related kinase IKKepsilon in interferon-mediated antiviral immunity. Science. 2007;315:1274–1278. doi: 10.1126/science.1136567. [DOI] [PubMed] [Google Scholar]
- Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic Syndrome and Altered Gut Microbiota in Mice Lacking Toll-Like Receptor 5. Science. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfrum S, Teupser D, Tan M, Chen KY, Breslow JL. The protective effect of A20 on atherosclerosis in apolipoprotein E-deficient mice is associated with reduced expression of NF-kappaB target genes. Proc Natl Acad Sci U S A. 2007;104:18601–18606. doi: 10.1073/pnas.0709011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich FT, Luedde T, Singer S, Schmidt-Supprian M, Baumgartl J, Schirmacher P, Pasparakis M, Bruning JC. Hepatic NF-kappa B essential modulator deficiency prevents obesity-induced insulin resistance but synergizes with high-fat feeding in tumorigenesis. Proc Natl Acad Sci U S A. 2008;105:1297–1302. doi: 10.1073/pnas.0707849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich FT, Strohle P, Konner AC, Gruber S, Tovar S, Bronneke HS, Juntti-Berggren L, Li LS, van Rooijen N, Libert C, et al. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab. 2010;12:237–249. doi: 10.1016/j.cmet.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Deignan JL, Qi H, Zhu J, Qian S, Zhong J, Torosyan G, Majid S, Falkard B, Kleinhanz RR, Karlsson J, Castellani LW, Mumick S, Wang K, Xie T, Coon M, Zhang C, Estrada-Smith D, Farber CR, Wang SS, van Nas A, Ghazalpour A, Zhang B, Macneil DJ, Lamb JR, Dipple KM, Reitman ML, Mehrabian M, Lum PY, Schadt EE, Lusis AJ, Drake TA. Validation of candidate causal genes for obesity that affect shared metabolic pathways and networks. Nat Genet. 2009;41(4):415–23. doi: 10.1038/ng.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]