Abstract
Waterpipe tobacco smoking is increasing in popularity worldwide and available evidence point to its addictive and harmful potential. This study is conducted to assess nicotine exposure in daily waterpipe users smoking the waterpipe according to their usual routine. The correlation between nicotine exposure and puff topography parameters has also not been explored systematically. Sixty-one waterpipe tobacco smokers (56 males; mean age ± SD, 30.9 ± 9.5 years; mean number of weekly waterpipe smoking episodes 7.8 ± 5.7) abstained from smoking for at least 24 hrs, and then smoked tobacco from a waterpipe ad libitum in a laboratory setting. During the session puff topography parameters were monitored continuously, and pre- and post-smoking expired-air CO was measured. Before and after smoking, venous blood was sampled for the assessment of plasma nicotine using Gas Chromatography-Mass Spectrometry. Average pre- and post-smoking expired-air CO was 4 ± 1.7 and 35.5 ± 32.7 ppm, respectively (i.e., a CO boost of 31.5 ppm, p < .01). Mean plasma nicotine concentration increased from 3.07±3.05 ng/ml pre-smoking to 15.7 ± 8.7 ng/ml post-smoking (p < .01). Plasma nicotine boost was correlated with total session time (Pearson correlation coefficient r = .31, p = .04), cumulative puff duration (r = .37, p = .01), mean puff duration (r = .34, p = .02), and total smoke inhaled in the session (r = .34, p = .02. These data show considerable nicotine exposure in daily waterpipe smokers, and that nicotine exposure is a function of waterpipe smoking patterns.
1. Introduction
The waterpipe (WP; a.k.a. hookah, shisha, narghile, arghile) is a centuries old tobacco use device that recently has surged in popularity among youth worldwide (Cobb et al., 2010). Data from over a half million 13-15 year olds who participated in the Global Tobacco Youth Survey (Warren et al., 2009) show that while cigarette smoking is stable or declining, other forms of tobacco use are rising in prevalence, mainly as a result of WP tobacco smoking (Warren et al., 2009).
In a common WP configuration, perforated aluminum foil separates burning charcoal from flavored tobacco (a.k.a. maassel) that is attached to a water bowl. Drawing air through a mouthpiece attached to the bowl causes tobacco and charcoal smoke to bubble through the water, whereupon they are inhaled by the smoker. Passing the smoke through water is believed to produce a “filtering” effect that contributes to the misperception of reduced harm/addiction of this tobacco use method compared to other tobacco use methods such as cigarette smoking (Maziak, 2008). In fact, WP tobacco smoking exposes smokers to tobacco toxicants (e.g. carbon monoxide, nicotine; Eissenberg and Shihadeh, 2009) and is associated with substantial harm (Akl et al., 2010). In addition, WP tobacco smoking is associated with features of tobacco/nicotine dependence, such as drug-seeking behavior, inability to quit despite repeated attempts, and abstinence-induced withdrawal/craving that is suppressed by subsequent WP use (Maziak et al., 2009; Ward et al., 2006). Toxicant exposure data correlate strongly with self-reported indices of dependence among WP users, and more frequent use leads to greater dependence (Salameh et al., 2008). Thus, there is a growing awareness that WP tobacco smoking involves a level of nicotine exposure that supports dependence at least in some users. More information about nicotine exposure in frequent (daily) WP smokers would add to the evidence about the addictive properties of this emerging tobacco use method. This study is conducted to assess nicotine exposure in daily WP users as they smoke the WP according to their usual routine.
2. Methods
2.1. Subjects and design
A detailed description of study method and participants has been published elsewhere (Maziak et al., 2009). Briefly, 61 adults who reported daily WP smoking on average (56 males; mean age 30.9 years; mean number of WP smoked/week = 7.8; mean duration of waterpipe smoking 8.5 ± 6.1 years) abstained from smoking for 24 hours (verified by exhaled air CO =7 ppm) and then smoked tobacco from a WP ad libitum in a laboratory setting. Participants were provided with a WP and necessary materials including their preferred tobacco and loaded the WP themselves (mean loaded tobacco ± SD 8.4 ± 2.6 g; mean consumed tobacco during session 4.1 ± 5.6 g). All participants used traditional lump charcoal (i.e. not manufactured easy-light briquettes), and they manipulated the charcoal during smoking according to their preference. Puff topography parameters were measured continuously during the smoking session using a portable topography unit (Shihadeh et al., 2005; Shihadeh et al., 2004), and pre- and post-smoking (5 minutes after last puff) expired-air CO was measured. Blood was sampled (though a venous catheter) just before and after the WP smoking session for later analysis of plasma nicotine concentration.
2.2. Analysis
Plasma nicotine was analyzed using solid phase extraction and liquid chromatography-atmospheric pressure chemical ionization- mass spectrometry (Kim and Huestis, 2006). The nicotine analysis was done at the American University of Beirut's Environmental Core Lab. When nicotine was not detected in the plasma of subjects, the limit of quantitation (LOQ) for our adopted method of analysis was used (LOQ = 2.5 ng/ml). Topography data were averaged within each session to obtain summary parameters for all subjects (e.g. puff number, volume, duration).
Pre- and post WP smoking plasma nicotine concentration was analyzed with a paired t test. In addition, Pearson's correlation coefficients (r) were calculated to assess the strength of the relationship between nicotine boost (change in plasma nicotine pre-post) and puff topography parameters, as well as CO boost (change in exhaled air CO pre-post).
3. Results
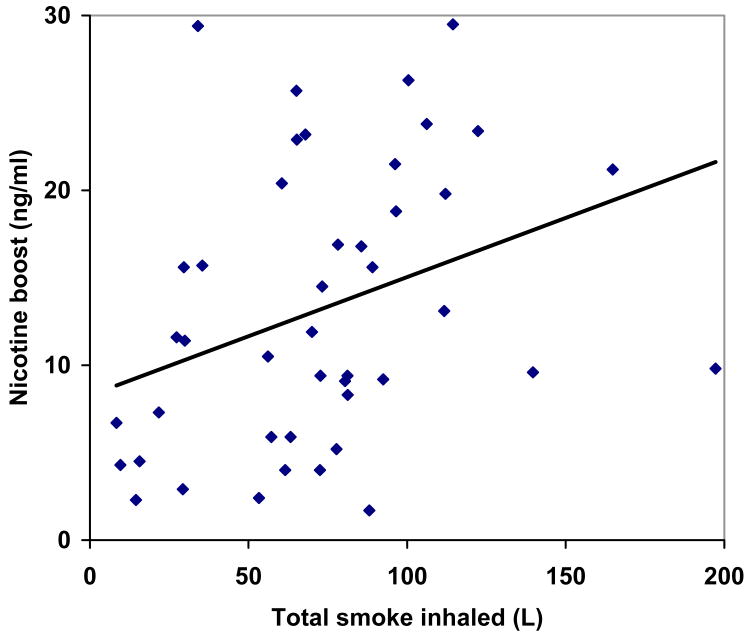
Mean (±SD) time spent smoking was 33± 13.1 minutes. Mean plasma nicotine concentration increased from 3.07±3.05 ng/ml pre-smoking to 15.7 ± 8.7 ng/ml post-smoking (p < .01). Plasma nicotine boost (12.6 ng/ml) was correlated with total session time (Pearson correlation coefficient r = .31, p = .04), total puffing time (r = .37, p = .01), mean puff duration (r = .34, p = .02), and total smoke inhaled in the session (r = .34, p = .02) (insert Table 1 and Figure 1 here). Average pre- and post-smoking expired-air CO was 4 ± 1.7 and 35.5 ± 32.7 ppm, respectively (i.e., a CO boost of 31.5 ppm, p < .01). Plasma nicotine boost was not correlated with expired air CO boost (r = .19, P = .19).
Table 1.
Correlation of plasma nicotine boost with puff parameters and CO boost.
| Nicotine boost | ||
|---|---|---|
| Pearson correlation coefficient | P | |
| Total session time (second) | .31 | .04 |
| Total puff time (second) | .37 | .01 |
| Number of puffs | .13 | .40 |
| Mean puff duration (second) | .34 | .02 |
| Mean puff volume (liter) | .29 | .06 |
| Mean inter-puff interval (second) | .26 | .08 |
| Total smoke inhaled per session (liter) | .34 | .02 |
| CO boost (31.5 ppm) | .19 | .19 |
Figure 1.
Correlation between plasma nicotine boost and total smoke inhaled (log transformed, r = .34).
4. Discussion
This study provides a further demonstration that WP tobacco smoking is associated with toxicant exposure (i.e., nicotine and carbon monoxide) and also suggests that nicotine intake in WP smokers is directly related to smoking time and volume of smoke inhaled. It is also the first study to investigate nicotine exposure in daily WP smokers, who smoked tobacco in the WP in a laboratory according to their own preferences (i.e. time spent smoking, tobacco amount, charcoal manipulation etc., were all under control of the smoker). To date, quantification of plasma nicotine in WP smokers has been limited to two published studies. In one report involving 14 Jordanian men who smoked tobacco in a WP an average of 3 times/week (Shafagoj et al., 2002), mean plasma nicotine increased from 1.1 ng/ml before smoking to 60.3 ng/ml after 45 minute of WP tobacco smoking. This increase in plasma nicotine concentration is much greater than that reported here (i.e., from 3.07 to 15.7 ng/ml), and may reflect the greater amount of WP tobacco loaded in the head (20 g; determined by the investigators), differences in nicotine content of the tobacco, and individual differences in smoking behavior. In the other report involving 31 U.S. university students (21 men) who smoked tobacco in a WP an average of 5 times/month (Eissenberg and Shihadeh, 2009) mean plasma nicotine increased from 2 ng/ml to 8.5 ng/ml after pre-defined 45 minutes of WP tobacco smoking (15 g tobacco; determined by the investigators), and was associated with a corresponding rise in heart rate. Our observed increase in plasma nicotine is about twice that reported by Eissenberg and Shihadeh, perhaps due to our smokers being mostly daily WP smokers compared to the occasional smokers studied by Eissenberg and Shihadeh. In fact, topography data show that total smoke inhaled in the session is higher in this study (79.1 liter; Maziak et al., 2009) compared to the Eissenberg and Shihadeh (2009) study (48.6 liter). Thus, the current report is consistent with existing literature demonstrating that WP tobacco smoking exposes users to a physiologically active nicotine dose.
We observed in this study that nicotine boost was correlated significantly with session time, total puff time, mean puff duration, and total smoke inhaled, but not expired air carbon monoxide boost. In our previous report, expired air carbon monoxide boost was correlated significantly with virtually every puff topography outcome, including total session time, total puff time, number of puffs, puff volume, and total smoke inhaled (Maziak et al., 2009). The failure to observe a significant correlation between plasma nicotine and expired air carbon monoxide boost may be due to several factors, including the various sources of the two toxicants in WP smoke. It has been previously found that while tobacco is the source of nicotine in the smoke, charcoal is the main source of carbon monoxide (Monzer et al., 2008) in the smoke.
The laboratory setting of this and other studies (i.e., Shafagoj et al., 2002; Eissenberg and Shihadeh, 2009; Maziak et al., 2009) may have influenced smoking behavior in WP tobacco smokers who often engage in this behavior as part of a group (Asfar et al., 2005; Smith-Simone et al., 2008). However, even when WP tobacco smokers are in their natural environment, WP tobacco smoking produces dramatic increases in expired air carbon monoxide (e.g., Bacha et al., 2007) and apart from total volume inhaled and session duration, puff topography data collected in the laboratory (Maziak et al., 2009) are consistent with those collected in the natural environment (e.g., Shihadeh et al., 2004). Future laboratory research comparing the effects of individual and group WP use on toxicant exposure, topography, and other outcomes may be able to address this issue empirically. Overall, this study adds to the accumulating evidence regarding nicotine exposure associated with WP tobacco smoking and provides evidence in support of policy initiatives designed to address the emerging global WP epidemic.
Acknowledgments
This study was supported by grants from the U.S. National Institutes of Health (DA024876 and CA120142) and Research for International Tobacco Control, a secretariat of the International Development Research Centre (IDRC Canada). Revelations regarding IDRC Chairperson Barbara McDougall's unfortunate ties to the tobacco industry were not known to the authors of this study at the time it was funded and executed.
Footnotes
Maziak, Shihadeh, and Eissenberg designed the study. Rastam, Bazzi, Ibrahim, and Zaatari conducted the study and performed the analysis. Maziak wrote the first draft of the manuscript and all authors contributed to and have approved the final manuscript.
All other authors declare that they have no conflicts of interest*.
Revelations regarding IDRC Chairperson Barbara McDougall's unfortunate ties to the tobacco industry were not known to the authors of this study at the time it was funded and executed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akl EA, Gaddam S, Gunukula SK, Honeine R, Jaoude PA, Irani J. The effects of waterpipe tobacco smoking on health outcomes: a systematic review. Int J Epidemiol. 2010;39(3):834–857. doi: 10.1093/ije/dyq002. [DOI] [PubMed] [Google Scholar]
- Asfar T, Ward K, Eissenberg T, Maziak W. Comparison of patterns of use, beliefs, and attitudes related to waterpipe between beginning and established smokers. BMC Public Health. 2005;5:19. doi: 10.1186/1471-2458-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacha ZA, Salameh P, Waked M. Saliva Cotinine and Exhaled Carbon Monoxide Levels in Natural Environment Waterpipe Smokers. Inhalation Toxicology. 2007;19:771–777. doi: 10.1080/08958370701401699. [DOI] [PubMed] [Google Scholar]
- Cobb C, Ward K, Maziak W, Shihadeh A, Eissenberg T. Waterpipe tobacco smoking: An emerging health crisis in the united states. American Journal of Health Behavior. 2010;34:275–285. doi: 10.5993/ajhb.34.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg T, Shihadeh A. Waterpipe tobacco and cigarette smoking: direct comparison of toxicant exposure. Am J Prev Med. 2009;37:518–23. doi: 10.1016/j.amepre.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Huestis M. A validated method for the determination of nicotine, cotinine, trans-3′-hydroxycotinine, and norcotinine in human plasma using solid-phase extraction and liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry. Journal of Mass Spectrometry. 2006;41:815–821. doi: 10.1002/jms.1039. [DOI] [PubMed] [Google Scholar]
- Maziak W. The waterpipe: time for Action. Addiction. 2008;103(11):1763–1767. doi: 10.1111/j.1360-0443.2008.02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziak W, Rastam S, Ward K, Shihadeh A, Eissenberg T. CO exposure, Puff Topography, and Subjective Effects in Waterpipe Tobacco Smokers. Nicotine & Tobacco Research. 2009;11:806–811. doi: 10.1093/ntr/ntp066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzer B, Sepetdjian E, Saliba N, Shihadeh A. Charcoal emissions as a source of CO and carcinogenic PAH in mainstream narghile waterpipe smoke. Food Chem Toxicol. 2008;46:2991–2995. doi: 10.1016/j.fct.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Salameh P, W M, Aoun Z. Waterpipe smoking: Construction and validation of the Lebanon Waterpipe Dependence Scale (LWDS-11) Nicotine Tob Res. 2008;10:149–158. doi: 10.1080/14622200701767753. [DOI] [PubMed] [Google Scholar]
- Shafagoj Y, Mohammed F, Hadidi K. Hubble-bubble (water pipe) smoking: levels of nicotine and cotinine in plasma, saliva and urine. Int J Clin Pharmacol Ther. 2002;40:249–255. doi: 10.5414/cpp40249. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Antonios C, Azar S. A portable, low-resistance puff topography instrument for pulsating, high-flow smoking devices. Behavior Research Methods. 2005;37:186–191. doi: 10.3758/bf03206414. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Azar S, Antonios C, Haddad A. Towards a topographical model of narghile water-pipe café smoking: a pilot study in a high socioeconomic status neighborhood of Beirut, Lebanon. Pharmacol Biochem Behav. 2004;79:75–82. doi: 10.1016/j.pbb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Smith-Simone S, Maziak W, Ward K, Eissenberg T. Waterpipe tobacco smoking: knowledge, attitudes, beliefs and behavior in two U.S. samples. Nicotine & Tobacco Research. 2008;10:393–398. doi: 10.1080/14622200701825023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward K, Vander Weg M, Relyea G, Debon M, Klesges R. Waterpipe smoking among American military recruits. Prev Med. 2006;43:92–97. doi: 10.1016/j.ypmed.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Warren CW, Lea V, Lee J, Jones NR, Asma S, McKenna M. Change in tobacco use among 13--15 year olds between 1999 and 2008: findings from the Global Youth Tobacco Survey. Global Health Promotion. 2009;16:38–90. doi: 10.1177/1757975909342192. [DOI] [PubMed] [Google Scholar]