SUMMARY
Worldwide, acute and chronic pain affects 20% of the adult population and represents an enormous financial and emotional burden. Using genome-wide neuronal-specific RNAi knock-down in Drosophila, we report a global screen for an innate behavior and identify hundreds of novel genes implicated in heat nociception, including the α2δ-family calcium channel subunit straightjacket (stj). Mice mutant for the stj ortholog CACNA2D3 (α2δ3) also exhibit impaired behavioral heat pain sensitivity. In addition, in humans, α2δ3 SNP variants associate with reduced sensitivity to acute noxious heat and chronic back pain. Functional imaging in α2δ3 mutant mice revealed impaired transmission of thermal pain evoked signals from the thalamus to higher order pain centers. Intriguingly, in α2δ3 mutant mice thermal pain and tactile stimulation triggered strong cross-activation or synesthesia of brain regions involved in vision, olfaction, and hearing.
INTRODUCTION
Acute and chronic pain affects millions of people after injury or surgery and those suffering from diseases like arthritis, cancer, and diabetes. Nociception (the detection of noxious or damaging stimuli) serves a crucial biological purpose: it alerts living organisms to environmental dangers, inducing the sensation of pain, reflex withdrawal and complex behavioural and emotional responses, which protect the organism from further damage (Cox et al., 2006). Noxious stimuli are detected by specialized high threshold primary sensory neurons (nociceptors) (Lumpkin and Caterina, 2007), which transfer signals to the spinal cord and then transmit them to the brain for higher level processing that results in the conscious awareness of the sensation called pain (Tracey and Mantyh, 2007). The functional importance of pain perception is exemplified by individuals with defects in nociception; patients with congenital insensitivity to pain do not survive past their twenties (Basbaum et al., 2009).
Drosophila (fruit flies) respond to noxious stimuli, and have become a powerful model organism for studying the genetics of nociception (Manev and Dimitrijevic, 2004; Tracey et al., 2003; Xu et al., 2006). For instance, the TRP channel PAINLESS was identified as a heat-responsive channel mediating thermal-based nociception in fly larvae (Sokabe et al., 2008; Tracey et al., 2003). Using genome-wide neuronal-specific RNAi knock-down, we report a global screen for an innate behavior and identify hundreds of novel genes implicated in heat nociception in the fly, including the α2δ-family calcium channel subunit straightjacket. Conservation of the mammalian straightjacket ortholog, α2δ3, in thermal nociception was confirmed in knock-out mice that exhibit significantly impaired basal heat pain sensitivity and delayed thermal hyperalgesia after inflammation. In humans, we found single nucleotide polymorphisms (SNPs) in α2δ3 that are associated with reduced acute heat pain sensitivity in healthy volunteers and chronic postsurgical back pain.
RESULTS
A genome-wide screen for thermal nociception
To identify genes required for nociception, we developed a high throughput behavioral assay to determine the response of adult Drosophila to noxious heat as a stimulus. When exposed to a surface at a constant temperature of 25°C, flies distribute evenly in the experimental chamber, but when given a choice between a noxious (46°C) and non-noxious (31°C) surface, flies rapidly avoid the harmful temperature (Fig. 1A,B). painless mutant flies respond normally to sub-noxious temperatures (≤39°C), but fail to avoid noxious heat (46°C) (Fig. 1B). Thus, adult flies can rapidly avoid noxious heat, and this complex innate behavior is dependent on painless.
Figure 1. Thermal nociception in adult Drosophila.
(A) Schematic representation of the thermal nociception assay in adult Drosophila. (B) Avoidance of noxious temperature of 46°C, but not avoidance of “sub-noxious” temperatures (25–35°C), is impaired in painless mutant (Painless(EP(2)2451) flies compared to the control strain Canton S (control). Data are presented as mean values +/− SEM. ~20 flies were tested per group, in replicates of at least four cohorts. Significant differences (P<0.001) were observed for temperature and strain responses. Further post hoc (Tukey’s) analysis showed a significant temperature avoidance response at 46 C for control (*, P<0.05) but not painless flies when compaired to responses at 25°C. (C) To set up the experimental screening system, w1118 (isogenic to the UAS-IR library) × elav-Gal4 flies (Control, grey; n = 1706) and painless mutant flies (painless, blue; n= 1816) were tested for avoidance to noxious heat (46°C). Based on these data a Z-score >= 1.65 was calculated as a specific cutoff to identify lines for further screening. Elav-Gal4 (also containing UAS-Dicer 2 (UAS-DRC2) for more efficient gene silencing) females were crossed to UAS-IR lines to knock-down the target genes in all neurons. All lines that exhibited a thermal avoidance defect (Z-score >= 1.65) were re-rested multiple times. (D) Results of the genome-wide screen. ~ 3% (622 transformants) of total lines tested (16051) exhibited a defect in thermal nociception, resulting in 580 candidate pain genes (622 transgenic lines). (E) Distribution of adult thermal nociception and developmental lethal hits for 16051 Drosophila UAS-IR lines. 1427 elav-Gal4 × UAS-IR lines were developmentally lethal (lethal). Among the 14624 viable lines, 562 lines exhibited defective thermal nociception (pain). Additional 60 lines that exhibited defective nociception as well as a semi-lethal phenotype were labeled as pain & lethal.
Using this assay, we performed a genome-wide behavioral screen using the Vienna global Drosophila RNAi library (Dietzl et al., 2007). The pan-neuronal specific elav-Gal4 driver line was crossed to flies containing UAS-IR (IR, inverted repeat) transgenes covering the expressed genome (Fig. 1C). Testing control flies (n = 1706) over many different days revealed that the vast majority avoided the noxious surface, with a mean avoidance response of 92 ± 6.4 % SD, whereas painless mutants (n= 1816) exhibited a markedly reduced avoidance response (51 ± 9.97 % SD). Based on these data, we set our primary cut-off at the 95 percentile of probability, corresponding to a Z-score of > 1.65 (Fig. 1C). At this threshold, we consistently observed impaired thermal nociception in painless mutant flies.
To identify novel genes regulating pain, we tested 16051 elav-Gal4>UAS-IR combinations targeting 11664 different Drosophila genes (82% of the Drosophila genome version 5.7) for effects on noxious temperature avoidance (Fig. 1D; Fig. S1B). Positive hits were retested, and 622 specific transgenic UAS-IR lines corresponding to 580 genes were identified as candidate thermal nociception genes (Fig. 1E; Tables S1A–C). Approximately 9% of the neuronal elav-Gal4 driven UAS-IR lines were lethal, yielding no or few progeny (Fig. 1E, Table S1D). Gene Ontology (GO) and GO gene set enrichment analysis of the total screen data (Fig. S1C–D; Table S1E,F) showed a significant enrichment of genes involved in ATP synthesis, neurotransmission and secretion. We further annotated 80 nociception hits with previously unknown functions (Table S1G). KEGG pathway analyses of the primary thermal nociception hits and their respective binding partners (Table S1H) revealed significant enrichment for oxidative phosphorylation, amino acid and fatty acid metabolism, ubiquitin-mediated proteolysis, and various signaling pathways such as Wnt, ErbB-, hedgehog-, JAK-Stat-, Notch-, mTOR, or TGFβ signaling (Table S1I). Thus, our thermal nociception screen and the subsequent bioinformatic analyses have revealed multiple genes and pathways that relate to the expression of an innate nociceptive behavior, many of which had no previous functional annotations.
straightjacket is a novel thermal nociception gene in Drosophila
One of the genes that we picked up in this screen was straightjacket (CG12295, stj), a member of the α2δ family of genes that function as subunits of voltage-gated Ca2+ channels (Fig. 2A) and control the function and development of synapses (Catterall, 2000; Dickman et al., 2008; Eroglu et al., 2009; Kurshan et al., 2009; Ly et al., 2008). The fly stj ortholog in mammals is α2δ3, a close homolog of α2δ1 which is the molecular target of gabapentin and pregabalin (Field et al., 2006), widely used analgesics for neuropathic pain in humans (Dworkin et al., 2007).
Figure 2. Straightjacket controls thermal nociception in adult Drosophila.
(A) Diagram of the α2-δ family encoding peripheral subunits of Ca2+ channels. (B) RNAi knock-down of stj impairs noxious thermal avoidance in adult Drosophila (% avoidance of noxious temperature). stj-IR1 = Inverted repeat 1, stj-IR2 = Inverted repeat 2, both crossed to elav-Gal4;UAS-DCR2. (C) Q-PCR for stj-Knock-down efficiency in elav-Gal4>UAS-stj-IR1/2 adult fly brains. (D) Kinetics of temperature-induced paralysis for control and elav-Gal4>UAS-stj flies. (E) stj-Gal4 driving expression of lamin:GFP to label nuclei and cell surface CD8:GFP to visualize axonal projections in the brain of adult flies. The pars intercerebralis is marked with an arrow. (F) Co-localization of anti-STJ immunostaining and stj-Gal4>UAS-lamin:GFP. The pars intercerebralis is marked with an arrow. (G) stj in situ hybridization in the leg of wild type (w1118) flies. Of note, the sense control did not show any signal. DAPI counterstaining is shown as mark nuclei. All data are presented as mean ± sem. * P < 0.05, ** P < 0.01 (Student’s t-test).
We confirmed that stj is required for noxious heat avoidance in adult Drosophila using a second independent hairpin (Fig. 2B). The two stj hairpins resulted in about 90% and 60% reduction of stj mRNA expression, respectively (Fig. 2C). Importantly, when flies were exposed to 46°C without given a choice to escape, stj knock-down did not alter the kinetics of temperature-induced paralysis (Fig. 2D), indicating a specific deficit in the nociception response. Of note, stj knock-down had no overt effect on brain morphology (Fig. S2A). Using a stj-Gal4 line (Ly et al., 2008) driving UAS-Lamin:GFP to mark nuclei (Stuurman et al., 1999), we found GFP expression primarily in neurons in the pars intercerebralis and surrounding the subesophageal ganglion (Fig. 2E; Fig. S2B). stjGAL4>UAS-CD8:GFP labeling of axons (Lee and Luo, 1999) revealed broad projections throughout the central brain (Fig. 2E; Fig. S2B).
Using antibodies raised against the Drosophila STJ protein, we confirmed STJ expression in stjGAL4>UAS-Lamin:GFP positive cells of the pars intercerebralis (Fig. 2F and data not shown). We also found GFP+ nuclei and projections in the ventral nerve cord (VNC) and ascending/descending axons from the VNC that innervate the central brain (Fig. S2C). stj-specific in situ hybridization revealed stj transcripts in the sensory organ (sensilla) of the leg (Fig. 2G) indicating expression in the peripheral and central nervous system of the fly. Further studies are required to fine map the site of stj action in the Drosophila pain circuit.
We next assessed whether stj also controls thermal nociception in the larval heat pain paradigm (Tracey et al., 2003). In larvae, we found expression of stj-Gal4>UAS-CD8:GFP in multidendritic sensory neurons (Fig. 3A). Pan-neuronal knock-down of stj (UAS-stj-IR x elav-Gal4) abrogated the larval response to noxious heat to an extent even greater than painless (Fig. 3B). Larvae carrying a stj point mutation and the stj point mutation over a corresponding deficiency (stj/def) also exhibited impaired thermal nociception. The extent of the defective thermal pain response was greater at higher temperatures (Fig. S3A). Restoring a functional copy of stj using the P[acman] system (Venken et al., 2009) rescued the thermal nociception defects in stj2 mutant larvae, confirming the requirement for stj in this behavior (Fig. 3B; Fig. S3A). stj2 mutant larvae showed wild-type responses to non-noxious touch (Kernan et al., 1994), indicating that these larvae are capable of responding to innocuous stimuli (Fig. S3B). Collectively, these data show that stj is required for avoidance of noxious heat in Drosophila.
Figure 3. Straightjacket controls thermal nociception in Drosophila larvae.
(A) stj-GAL4 driven expression of UAS-CD8:GFP in larval body wall sensory neurons co-stained with anti-Futsch as a marker for sensory neurons. CD8:GFP expression co-localizes with sensory neurons (Futsch) in larval abdominal hemi-segments (A3) (top panels), and multidendritic sensory neurons (bottom panels). (B) Pan-neuronal knock-down of stj (UAS-stj-IR x elav), a mutant of stj (stj2), and stj mutant larvae over a corresponding deficiency Df(2R)Exel7128 (stj2/def) show severely impaired thermal nociception responses compared to w1118 x elav controls. painless larvae are shown as a control. The impaired larval thermal responses of a stj/def was rescued by reintroducing a wild type stj allele using the P[acman] system (stj2/def, stj+) (Venken et al., 2009). Percent responses ± sem to a 46°C heated probe are shown for the indicated time points. Mean response latency ± sem. P value was generated using a Kruskal-Wallis non-parametric test for median comparison with the Dunn’s post-hoc test. All P values depicted highlight significance relative to control responses. stj rescue was also significantly difference from stj2 and stj2/def, (P < 0.001). At least 20 animals were tested three times per genotype.
Thermal analgesia in α2δ3 mutant mice
We next tested whether the fly stj data is predictive of altered nociceptive behavior in mammals. The closest stj ortholog in mammals is α2δ3 (mouse α2δ3 is 33% identical and 60% similar to the STJ protein and the domain structures are conserved throughout evolution (Ly et al., 2008). To examine the role of α2δ3 in vivo we studied α2δ3 mutant mice generated by homologous recombination. Correct recombination and loss of protein expression were confirmed by Southern (Fig. 4A) and Western blotting (Fig. 4B). α2δ3 mutant mice are born at the expected Mendelian frequency and are fertile. Extensive characterization of these mice showed no obvious anatomical or histological abnormalities, including apparently normal brain morphology (Tables S2A,B). There were also no genotype-related or biologically significant differences noted between age and gender matched mutant and wild-type control mice for any of the parameters evaluated at necropsy or by serum chemistry and haematology (Tables S2C–E). Moreover, normal growth and body weights were recorded for mice at 49, 90, 180, and 300 days of age (Table S2F). Hence, by all anatomical and physiological parameters assessed, α2δ3 mutant mice appear normal.
Figure 4. α2δ3 is required for thermal pain responses in mice.
(A) Southern blotting of genomic DNA in α2δ3 wild type (+/+) and α2δ3 heterozygous (+/−) ES cells to confirm successful gene targeting. The endogenous wild type and targeted alleles are indicated. A 5′ probe was used on Nhe I digested genomic DNA. (B) α2δ3 and α2δ1 protein expression in brain and isolated DRG lysated from α2δ3+/+ (+/+), α2δ3+/− (+/−), and α2δ3−/− (−/−) mice. Actin is shown as a loading control. (C) Using the hot plate assay, α2δ3 mutant mice (n=16) show a delayed acute thermal nociception response as compared to control α2δ3+/+ mice (n=12). Littermate mice were used as a control. Values represent the latency (seconds) to respond to the indicated temperatures. (D) CFA-induced inflammatory pain behavior. CFA (20 μl) was injected into the hindpaw of α2δ3+/− (+/+, n=10) and α2δ3−/− (−/−, n=21) littermates and mice were tested for thermal pain (54°C) using the hot plate assay on the indicated days. Days 1, 3, 5, and 7 indicate days after CFA injection. All data are presented as mean values ± sem. *p < 0.05; **p < 0.01; *** P < 0.001 comparing mutant versus control mice. # P< 0.05 comparing sensitization to baseline (day -2) of the same genotype (Student’s t-test).
Importantly, similar to Drosophila stj mutants, α2δ3 mutant mice showed a defect in acute thermal nociception in the hot plate assay, with diminished responsiveness at 50, 52, 54 and 56°C (Fig. 4C). In addition, α2δ3 mutant mice exhibited delayed thermal sensitization in the Complete Freund’s Adjuvant (CFA) model of peripheral inflammatory pain (Fig. 4D), indicating that α2δ3 contributes to the acute phase of heat hyperalgesia. CFA induced inflammation, as determined by paw swelling, was comparable between α2δ3 mutant and control mice (Fig. S4A). By contrast, mechanical sensitivities (von Frey test) were unaffected in α2δ3 mutant mice (Fig. S4B). α2δ3 mutant mice were also evaluated for other behavioral tasks (Crawley, 2008): open field test to assess locomotor activity, general exploratory behavior, intra-session habituation, and general anxiety; tail suspension to assess behavioral despair; and a rotarod test to assess basic motor skills and coordination. In these assays no significant differences were observed between α2δ3 mutant and control mice (Fig. S4C–F; Table S2G). Thus, genetic deletion of α2δ3 in mice results in substantially impaired acute heat pain responses and a delay in inflammatory heat hyperalgesia.
α2δ3 SNPs associate with human pain sensitivity
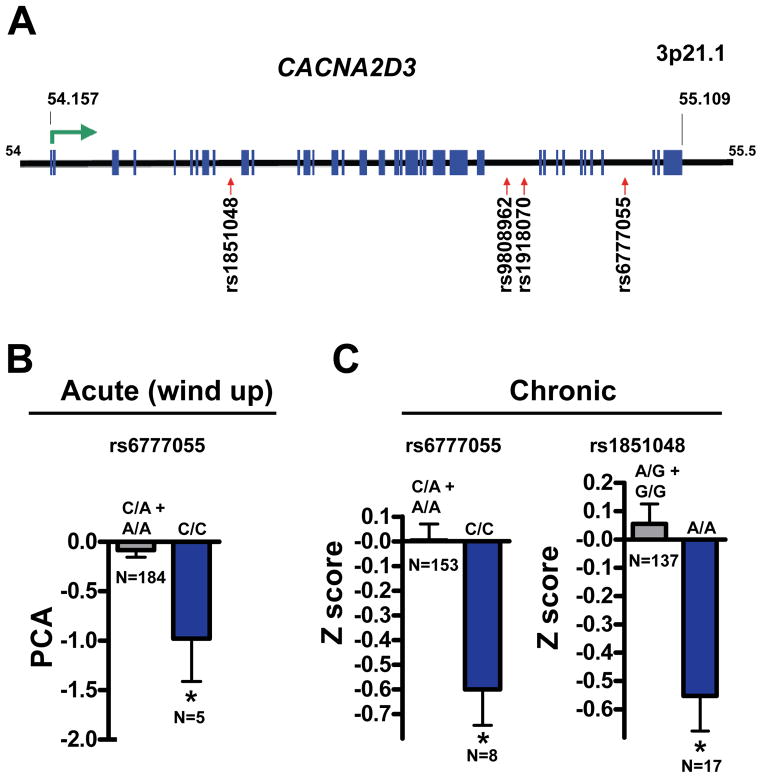
Since knock-down of straightjacket in Drosophila and knock-out of α2δ3 in mice results in impaired sensitivity to thermal pain, we speculated that polymorphisms at the α2δ3 (CACNA2D3) locus might be associated with heat pain variance in humans. To assay for potential association of α2δ3 haplotypes relative to pain sensitivity we screened 4 single nucleotide polymorphisms (SNPs) within or close to the human CACNA2D3 gene (Fig. 5A) in a cohort of 189 healthy volunteers subjected to a battery of experimental pain sensitivity tests (Diatchenko et al., 2005). Of these, the minor allele of the SNP rs6777055 was significantly associated with reduced thermal pain sensitivity, i.e, heat wind-up pain (Fig. 5B, recessive model). Wind-up measures successive increases in perceived pain intensity to a repeated noxious heat stimulus (ten heat pulses of 1.5 seconds each at 50°C each separated by 3 seconds).
Figure 5. Polymorphisms in CACNA2D3 (α2δ3) associate with decreased acute and chronic pain in humans.
(A) Schematic representation of the human CACNA2D3 gene locus on chromosome 3p21.1. The positions of the SNPs assayed are indicated. Blue boxes represent exons. The relative gene position is given in megabases (Mb). (B) Homozygous carriers of the rs6777055 minor allele (C/C) were significantly less sensitive to heat wind-up induced sensitivity relative to the other genotypes (C/A or A/A). (C) Of 169 lumbar chronic root pain patients 1 year post discectomy those homozygous for the minor allele C/C at SNP rs6777055 and A/A at SNP rs1851048 were less sensitive than the other allele combinations. In each case, the homozygous minor allele is associated with significantly less pain. Note that genotyping was not always successful for every individual, hence, the slightly different total numbers in the chronic pain group. All data are presented as mean values ± sem. *p < 0.05 (Student’s t-test).
Thermosensitive neurons have been also implicated in chronic pain in humans (Premkumar, 2010). To explore this we compared pain levels in 169 Caucasian adults who participated in a prospective observational study of surgical discectomy for persistent lumbar root pain, caused by an intervertebral disc herniation (Atlas et al., 2001) for an association with CACNA2D3 SNPs. The minor alleles of two CACNA2D3 SNPs (rs1851048 and rs6777055) were associated with less pain within the first year following surgery (Fig. 5C, recessive model). Importantly, the rs6777055 SNP C/C was significantly associated with less pain in both healthy volunteer and chronic pain cohorts showing a recessive mode of inheritance. In both the experimental and lumbar pain groups the minor allele frequency for rs6777055 was 0.2, that is ~ 4% of the human population is homozygous for this genetic variant. These data show that minor variants within the human CACNA2D3 gene are associated with less heat induced pain in healthy volunteers and reduced chronic pain in lumbar back pain patients.
α2δ3 controls central transmission of pain signals to the sensory cortex and other higher order pain centers
Nociceptive processing involves the relay of sensory information from primary nociceptor neurons to second order neurons in the dorsal horn of the spinal cord which then transfer nociceptive information to the brain stem, thalamus, and higher order brain centres (Costigan et al., 2009; Lumpkin and Caterina, 2007; Tracey and Mantyh, 2007). Since our α2δ3 knock-out mice carry a LacZ reporter, we used β-Gal staining as a marker to assess α2δ3 expression. In the brain, β-Gal labeled the thalamus, pyramidal cells of the ventro-posterior paraflocculus of the cerebellum, caudate, putamen, the dentate gyrus of the hippocampus, the olfactory bulb and olfactory tubercle, as well as diffusely throughout the cortex (Fig. 6A and not shown). The LacZ expression profiles were confirmed by Western blotting and quantitative PCR (not shown) and matched in situ data from the Allen brain atlas (not shown and ref. (Koester and Insel, 2007)). We failed to detect LacZ expression in the spinal cord and DRG (not shown). Absence of α2δ3 expression in primary sensory DRG neurons was confirmed by Western blotting (Fig. 4B). In line with these expression data, our behavioral experiments showed that loss of α2δ3 does not affect the noxious heat-induced tail flick response (Fig. S4G), a pain behavior mediated by a spinal reflex circuit (Pitcher et al., 1995). Finally, patch clamping showed that calcium current and kinetics were comparable among DRG neurons from control and α2δ3 mutant mice (Fig. S4H–K) indicating no requirement for α2δ3 in calcium channel function in these cells. These data suggest that α2δ3 is not required for thermal pain processing in nociceptors and the spinal cord, but α2δ3 may regulate thermal pain processing in the brain.
Figure 6. α2δ3 is expressed in the brain and relays the pain signal to higher order brain centers.
(A) β-Gal staining of whole brain slices from α2δ3+/− mice that carry the LacZ cassette. Different brain regions that are positive for LacZ expression are indicated. White lines indicate the brain slices displayed in Fig. 7A. (B–C) Quantification of % BOLD change and mean activation volume (in voxels) for (B) the thalamus and (C) the S1 somato-sensory cortex of α2δ3+/+ and α2δ3−/− mice. Of note, it has been proposed that the S1 cortical region is involved in the localisation of nociception (Treede et al., 1999). The different stimulation temperatures are indicated. Data are presented as mean +/− sem. *p < 0.05, **p < 0.01 (Student’s t-test comparing the respective control and α2δ3−/− groups). (D) Cross-correlation matrix of time profiles. Whereas the pain signal spreads from the thalamus to other higher order pain centers in α2δ3+/+ mice (red areas), in α2δ3−/− mice correlated activation can be only observed up to the level of the thalamus. Very weak activity is found in somato-sensory cortex (SC) for α2δ3−/− mice (green stripes). Data from the structures of left side of the brain are shown following challenge with noxious heat (55°C) at the right hindpaw. SI: sensory input; Th: thalamus; SC: somato-sensory cortex; AC: association cortex; LL: link to limbic system; LS: limbic system; HT: hypothalamus; BG: basal ganglia; C. cerebellum; M: motor cortex, P: periaquaeductal gray. Correlation-coefficients (cc) are given in the range from 0 (green), to +1 (red). n=20 for α2δ3+/+; n = 18 for α2δ3−/−.
To address this, we employed non-invasive functional magnetic resonance imaging (fMRI) using the blood oxygenation level dependent (BOLD) signal to generate a activation maps of brain regions affected by noxious topical heat stimuli (Knabl et al., 2008; Ogawa et al., 1990; Thulborn et al., 1982). In wild type mice (n=20), noxious thermal stimuli activate brain structures known as the “pain matrix” (Melzack, 1999) such as the thalamus (Fig. 6B), the S1 and S2 somatosensory cortex (Fig. 6C), the cingulum, amygdala, hypothalamus, or the motor cortex (Fig. S5). These areas are also involved in pain perception in human subjects (Tracey and Mantyh, 2007). In both wild type (n=20) and α2δ3 mutant mice (n=18), thermal pain-induced activation of the thalamus (Fig. 6B), the key sub-cortical pain relay centre (Price, 2000). Intriguingly, loss of α2δ3 expression interrupted the normal engagement of pain circuitry in the brain resulting in markedly reduced BOLD peak amplitudes and activation volumes in higher order pain centers such as the somato-sensory S1 and S2 cortices, cingulate, or motor cortex after exposure to noxious temperatures (Fig. 6C; Fig. S5). Impaired activation of higher order pain centers, i.e. sensory and motor cortices, were confirmed by calculation of Euclidian distances (not shown).
To additionally assess temporal information flow of the pain signal within different cerebral structures, we calculated a cross-correlation matrix of the response time profiles for each predefined region of the somato-sensory pain matrix (Fig. 6D). In control mice, the sensory input relays thermal-evoked neural signals to the thalamus, where it effectively spreads to other central brain centers such as the sensory and association cortex, limbic system, cerebellum, basal ganglia, and motor cortex. α2δ3 mutant mice again exhibited normal activation of the thalamus but a reduced flow to nearly all the higher order pain centers, in particular the somato-sensory cortex (SC) (Fig. 6D). Moreover, whereas the pain signal spreads from the left (i.e. contra-lateral to the side of stimulation) in control mice, we found a considerable reduction in correlation coefficients of the pain signal from the left to the right brain in α2δ3 mutant mice (Fig. S6A). In addition, we observed increased negative BOLD signals in the S1 somato-sensory, motor and the cingulate cortex on both hemispheres of α2δ3 mutant mice (Fig. S6B), suggesting that genetic inactivation of α2δ3 not only results in impaired transmission of the signal to higher pain structures, but also in intra-cortical inhibition (Arthurs and Boniface, 2002; Shmuel et al., 2002). Thus, loss of α2δ3 leads to impaired transmission of noxious heat evoked signals from the thalamus to higher pain centers.
Loss of α2δ3 results in sensory cross-activation
Although we found a marked impairment in the activation of known higher order pain centers, one puzzling finding from our fMRI data was that we did not find statistically significant differences between control and α2δ3 mutant mice in total activation volume and peak height when neuronal activity was surveyed in the entire brain (Fig. S7A). Since we had initially only focused on the pain matrix, we speculated that therefore loss of α2δ3 may result in hyperactivation of additional brain regions. Remarkably, noxious heat stimulation of all α2δ3 mutant mice triggered significantly enhanced activation of the visual cortex, the auditory cortex, as well as olfactory brain regions (Fig. 7A,B). Thus, in α2δ3 mutant mice, noxious heat stimulation results in a significant sensory cross-activation of brain regions involved in vision, hearing, and olfaction.
Figure 7. α2δ3 mutant mice exhibit sensory cross-activation in response to thermal and tactile stimuli.
(A) Second order statistical parameters maps showing only the significant differences of heat (55°C) and tactile (vibrissae) stimulation induced brain activation between α2δ3+/+ and α2δ3−/− mutant mice. Activation was assessed by BOLD-fMRI. The three planes correspond to the white lines shown in Fig. 6A. The green/blue scale indicates increased peak activation (55°C) in α2δ3+/+ control mice compared to α2δ3−/− mutant mice. The yellow/red scale indicates increased activation in α2δ3−/− mutant mice compared to α2δ3+/+ control mice. Images depict significant differences of second order group statistics corrected for multiple comparisons over all mice tested (n = 20 for α2δ3+/+ mice, n = 18 for α2δ3−/− mice). Arrows point to activated regions; note that for heat stimulation the S1/S2 somato-sensory cortex, the cingulate (Cg) cortex and the motor (M) cortex show significantly higher activity in α2δ3+/+ controls. In α2δ3−/− mice, heat stimulation leads to significantly higher activity auditory cortex (AC), the visual cortex (VC), and the olfactory tubercle (OT), as well as the amygdala (Amd) and the hypothalamus (HT). For tactile stimulation, only one small region in the S1 somatosensory cortex, ipsilateral to the side of stimulation (right), showed significantly higher activity in α2δ3+/+ controls, whereas α2δ3−/− mice again exhibited increased activation of the VC, AC, and OT, in addition to the caudate putamen (Cpu), S1 and S2 regions of the somato-sensory cortex, and the superior colliculus (SC). (B,C) % BOLD changes in the auditory cortex (AC), olfactory tubercle (OT), and visual cortex (VC) in control and α2 δ3 −/− mice following (B) heat (55°C) and (C) tactile vibrissal stimulation. Data is presented as mean values ± sem. *p < 0.05; **p < 0.01 (Student’s t-test).
To image basal neuronal activity integrated over a 24 hour time period, we employed manganese labeling (MEMRI) (Silva et al., 2004). We observed similar activity in all imaged brain regions among a group comparison of control and α2δ3 mutant mice indicating that the observed stimulus induced sensory cross-activations are not due to altered basal neuronal activity. Moreover, diffusion tensor imaging (DTI) showed no overt structural changes with respect to fractional anisotropy between the thalamus and higher order pain centers or the thalamus and the visual, auditory, and olfactory centers. Further, cross-correlation analysis of the time profiles of the structures of the the pain matrix from resting-state BOLD imaging showed no defects in spontaneous spreading from the thalamus to higher order pain centers in α2δ3 mutant mice (not shown). Finally, network analysis of this resting state brain activity showed no overt changes in total functional connectivity within the pain matrix (1087 connections in wild type versus 1040 connections in α2δ3 mutant mice) and also apparently normal multisensory-thalamo-cortical network connectivity (1922 connections in wild type versus 2099 connections in α2δ3 mutant mice). Although we cannot exclude subtle developmental changes in defined neuronal populations of α2δ3 mutant mice, these data suggest that the observed heat induced sensory cross-activations (and defective transmission of the thermal pain signal from the thalamus to higher order pain centers) are not due to altered basal neuronal connectivities.
To assess whether loss of α2δ3 also results in sensory cross-activation in other sensory modalities, we performed BOLD imaging in response to tactile vibrissal stimulation (Hess et al., 2000). Among control and α2δ3 mutant mice, we observed apparently normal activation of the brain region that encompasses the barrel field (not shown); the barrel field is the primary cortical somato-sensory brain centre for processing of vibrissal stimulation (Petersen, 2007). Remarkably, although we observed apparently normal activation of the barrel field, tactile stimulation again resulted in sensory cross-activation of visual, auditory, and olfactory brain centers in α2δ3 knock-out mice (Fig. 7A,C). Thus, in α2δ3 mutant mice, noxious heat stimulation as well as tactile stimulation trigger sensory cross-activation of brain regions involved in vision, hearing, and olfaction.
DISCUSSION
Our whole-genome, neuron-specific RNAi screen provides a global functional analysis of a complex, innate behavior. We have uncovered hundreds of novel candidate genes for thermal nociception, a large proportion of which had completely unknown functions until now. Since many of these novel genes are conserved across phyla, our data provide a starting point for large scale human genomics efforts to finding novel pain genes and defining the molecular mechanisms of nociception.
One of the screen hits was the calcium channel subunit straightjacket/α2δ3. In both larva and adult Drosophila, we show that straightjacket is indeed required for heat nociception. Further work is required to define the spatial and temporal requirements for stj in thermal nociception. Importantly, similar to the fly, genetic deletion of α2δ3 in mice also results in impaired acute heat pain responses. These results translate to humans, since we found α2δ3 polymorphisms that significantly associate with reduced heat pain sensitivity in healthy volunteers and lower levels of chronic pain in lumbar back pain patients. These data reinforce the extraordinary conservation of the neurobiological mechanisms of nociception, from its manifestation as avoidance of damage in primitive creatures like flies, to the complex sensation of pain in humans.
Our functional imaging data indicate that α2δ3 appears to be specifically required for central transmission of the thermal nociceptive signal from the thalamus to the sensory cortex and other higher order pain centers. Until now it has been assumed that the threshold for thermal pain sensitization is set exclusively in peripheral sensory neurons via thermosensitive TRP channels like TRPV1 and altered excitability of nociceptor terminals (Hucho and Levine, 2007). However, our results indicate that noxious heat evoked behavior is reduced even when nociception appears to be intact up to the thalamus. Whether the loss of α2δ3 results in defective signaling and/or subtle alterations in synaptic circuits that link the thalamus to higher order pain centers remains to be determined.
Intriguingly, in α2δ3 mutant mice thermal pain and tactile vibrissal stimulation triggered strong cross-activation of brain regions involved in vision, olfaction, and hearing. Sensory cross-activation or synesthesia is a neurological condition where a stimulus in one sensory modality triggers perception of another sense (Hubbard and Ramachandran, 2005). In humans, synesthesia can be only objectively verified using functional brain imaging (Aleman et al., 2001). Multiple forms of synesthesia exist including pain stimuli that trigger color (Dudycha, 1935). Synesthesia might affect up to 4% of the population, shows genetic linkage, and has been associated with intelligence and creativity (Hubbard and Ramachandran, 2005). In addition, thalamic lesions can also cause synesthesia (Beauchamp and Ro, 2008). Thus, α2δ3 mutant mice might provide an animal model to enable the phenomenon of sensory cross-activation to be experimentally dissected.
EXPERIMENTAL PROCEDURES
Detailed experimental procedures are provided in the Supplemental Data.
Fly stocks
All UAS-IR transgenic fly lines were obtained from the VDRC RNAi library (Dietzl et al., 2007) with the exception of the second stj hairpin, which was obtained from the Harvard trip stocks. elav and UAS-Dcr2 were gifts from B. Dickson (Dietzl et al., 2007). See supplemental materials for a complete list of stocks used.
Drosophila behavioral tests
For avoidance of noxious heat ~20 four day old flies were placed in a sealed experimental chamber. All tests were performed in the dark. The bottom of the chamber was heated to 46°C while the subnoxious zone was measured to be 31°C at the end of the 4 minute experiment. % Avoidance was calculated by counting the number of flies that failed to avoid the noxious temperature compared to the total number of flies in the chamber. Larval pain assays were performed as described (Tracey et al., 2003). Mechanosensation (Kernan score) was performed as described (Kernan et al., 1994).
Detection of stj expression
Brains and ventral nerve cords of adult flies and sensory nerves of larvae from stj-Gal4>UAS-Lamin:GFP (to detect nuclei) and stj-Gal4>UAS-CD8:GFP (to detect axonal projections) lines were imaged. Stj expressing neurons were further detected using antibody staining and in situ hybridization.
Generation of α2δ3 knock-out mice
For gene targeting of α2δ3 in mice, a targeting vector was inserted into exon 15 of the murine α2δ3 gene. Germline transmitted F1 mice were backcrossed onto a C57BL/6 background. All behavioral and fMRI mouse studies were conducted in accordance with guidelines of the European Union Council (86/609/EU) and following Austrian regulations for the use of laboratory animals.
Mouse behavioral experiments
For the hot plate assay, wild type and α2δ3 mutant littermate mice were tested for hot plate latency at 50–56°C. The mechanical pain test was performed by applying von Frey hairs to the dorsal surface of each hindpaw until a hindlimb withdrawal response was observed; the hair with the minimum bending force required to produce a response was recorded (Whishaw et al., 1999). Inflammatory thermal hyperalgesia was produced in the mouse right hind paw by intraplantar injection of Complete Freund’s Adjuvant (CFA). Before and after CFA injection, nociceptive responses to heat were measured using the hot-plate test (54°C). Paw swelling was measured to evaluate the inflammatory response elicited by CFA.
Western blotting
The following primary antibodies were used: mouse anti-Actin, dilution 1/1000; goat anti- α2δ1, dilution 1/500, and rabbit anti-α2δ3, dilution 1/500. To generate the anti-α2δ3 antibody, rabbits were immunized with the peptide VSERTIKETTGNIAC conjugated to KLH. Secondary antibodies were used at a dilution of 1 in 5000.
LacZ expression
Tissues from 7–12 week old heterozygote mice were analysed for LacZ expression using X-Gal staining followed by Nuclear Fast Red counterstaining. For whole mount brain staining, the brain was cut longitudinally, fixed, and stained using X-Gal. Tissues were fixed with buffered formaldehyde.
fMRI and BOLD imaging
Male mice were anesthetized with isoflurane and placed inside a MR machine (Bruker BioSpec 47/40, quadratur head coil) under extensive physiological monitoring. The contact heat stimuli (40°C, 45°C, 50°C, and 55°C, plateau for 5 sec after 15 sec of heat increase) were applied at the right hind paw (presented at 3 min 25 s intervals, 3 time each temperature) using a custom made computer controlled Peltier heating device. For tactile stimulation, the C1 vibrissa of the mice was moved with an air driven device integrated into a cradle shifting the vibrissa by an inverted comb with an amplitude of 5 mm at 7 Hz. A series of 750 sets of functional images (matrix 64 × 64, field of view 15 × 15 mm, slice thickness 0.5 mm, axial, 22 slices) were collected using the Echo Planar Technique (EPI, single shot: TR = 4000 ms, TEef = 24,38 ms).
SNP mapping in humans
We genotyped 4 single nucleotide polymorphisms (SNPs) spaced evenly through α2δ3 using the 5′ exonuclease method (Tegeder et al., 2006). For acute pain studies, we genotyped 189 normal volunteers who had previously been phenotyped for ratings of experimental pain (Diatchenko et al., 2005). All subjects gave informed consent following protocols approved by the UNC Committee on Investigations Using Human Subjects. Volunteers were phenotyped with respect to temporal summation of heat pain (i.e., windup). For chronic pain studies, we collected DNA from 169 Caucasian adults who participated in a prospective observational study of surgical diskectomy for persistent lumbar root pain (Atlas et al., 2001). The primary endpoint was persistent leg pain over the first postoperative year. Genotype-phenotype associations for each SNP were sought by regression analysis. The collection of DNA and genetic analyses were carried out with the approval of the National Institute of Dental and Craniofacial Research institutional review board and informed consent was obtained from all subjects.
Statistical analyses
For analysis of adult heat-dose avoidance responses between and within control and painless flies, a two way ANOVA was performed, followed by Tukey’s post hoc test. For analysis of adult Drosophila avoidance response and RNAi knock down efficiency a Student’s t test (with correction for multiple comparison) was performed. For analysis of larval pain behavior, we have performed the Kruskal-wallis non-parametric test for median comparison followed by the Dunn’s post-hoc test. For mouse behavior, a Student’s t test was used. For fMRI, the mean activity of each activated brain structure was averaged across all significant activated voxels and subjected to t-tests comparing α2δ3 mutant and control mice. At the second level group analysis a standard analysis of variance (ANOVA) was performed for Z-score maps between the different mice genotypes. Areas of significant group activation differences (P < 0.05) were used as masks to only show the calculated peak activation maps in these regions. For human studies, genotype-phenotype associations for each SNP were sought by regression analysis. Unless otherwise indicated, data are represented as mean values ± sem.
Supplementary Material
Acknowledgments
We thank all members of our laboratories and the VDRC for helpful discussions and excellent technical support. We thank Ricardo de Matos Simoes for support with statistical analysis. We thank BJ Dickson for elav/UAS Dicer 2 stocks. α2δ3 mutant mice were generated by Deltagen (San Mateo, CA). JPM is supported by grants from IMBA, the Austrian Ministry of Sciences, the Austrian Academy of Sciences, GEN-AU (AustroMouse), ApoSys, and an EU ERC Advanced Grant. ACK is supported by National Institute of Health NRSA 1 F32GM086207-01. CJW is supported by NIH NS039518 and NS038253. GGN was supported by a Mary Curie IIF Fellowship and EuroThymaide. AH is supported by DFG 661/TP4, BMBF (01EM0514, 01GQ0731, 0314102) and KB by the Doerenkamp Foundation for Innovations in Animal and Consumer Protection. PAG is supported by NIH NS044232.
Footnotes
Supplemental data includes 7 supplemental figures, 2 supplemental tables, and extended experimental procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleman A, Rutten GJ, Sitskoorn MM, Dautzenberg G, Ramsey NF. Activation of striate cortex in the absence of visual stimulation: an fMRI study of synesthesia. Neuroreport. 2001;12:2827–2830. doi: 10.1097/00001756-200109170-00015. [DOI] [PubMed] [Google Scholar]
- Arthurs OJ, Boniface S. How well do we understand the neural origins of the fMRI BOLD signal? Trends in neurosciences. 2002;25:27–31. doi: 10.1016/s0166-2236(00)01995-0. [DOI] [PubMed] [Google Scholar]
- Atlas SJ, Keller RB, Chang Y, Deyo RA, Singer DE. Surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: five-year outcomes from the Maine Lumbar Spine Study. Spine. 2001;26:1179–1187. doi: 10.1097/00007632-200105150-00017. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Ro T. Neural substrates of sound-touch synesthesia after a thalamic lesion. J Neurosci. 2008;28:13696–13702. doi: 10.1523/JNEUROSCI.3872-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annual review of cell and developmental biology. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic Pain: A Maladaptive Response of the Nervous System to Damage. Annual review of neuroscience. 2009 doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444:894–898. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–818. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Human molecular genetics. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- Dickman DK, Kurshan PT, Schwarz TL. Mutations in a Drosophila alpha2delta voltage-gated calcium channel subunit reveal a crucial synaptic function. J Neurosci. 2008;28:31–38. doi: 10.1523/JNEUROSCI.4498-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Dudycha GJ, Dudycha Martha M. A Case of Synesthesia: Visual-Pain and Visual-Audition. Journal of Abnormal and Social Psychology. 1935;30:57–69. [Google Scholar]
- Dworkin RH, O’Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski C, Nurmikko TJ, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su TZ, Bramwell S, Corradini L, England S, Winks J, et al. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17537–17542. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A, Stiller D, Kaulisch T, Heil P, Scheich H. New insights into the hemodynamic blood oxygenation level-dependent response through combination of functional magnetic resonance imaging and optical recording in gerbil barrel cortex. J Neurosci. 2000;20:3328–3338. doi: 10.1523/JNEUROSCI.20-09-03328.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard EM, Ramachandran VS. Neurocognitive mechanisms of synesthesia. Neuron. 2005;48:509–520. doi: 10.1016/j.neuron.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 1994;12:1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- Knabl J, Witschi R, Hosl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, et al. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- Koester SE, Insel TR. Mouse maps of gene expression in the brain. Genome biology. 2007;8:212. doi: 10.1186/gb-2007-8-5-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurshan PT, Oztan A, Schwarz TL. Presynaptic alpha(2)delta-3 is required for synaptic morphogenesis independent of its Ca(2+)-channel functions. Nature neuroscience. 2009;12:1415–1423. doi: 10.1038/nn.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- Ly CV, Yao CK, Verstreken P, Ohyama T, Bellen HJ. straightjacket is required for the synaptic stabilization of cacophony, a voltage-gated calcium channel alpha1 subunit. The Journal of cell biology. 2008;181:157–170. doi: 10.1083/jcb.200712152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev H, Dimitrijevic N. Drosophila model for in vivo pharmacological analgesia research. European journal of pharmacology. 2004;491:207–208. doi: 10.1016/j.ejphar.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Melzack R. From the gate to the neuromatrix. Pain. 1999;Suppl 6:S121–126. doi: 10.1016/S0304-3959(99)00145-1. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC. The functional organization of the barrel cortex. Neuron. 2007;56:339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Pitcher GM, Yashpal K, Coderre TJ, Henry JL. Mechanisms underlying antinociception provoked by heterosegmental noxious stimulation in the rat tail-flick test. Neuroscience. 1995;65:273–281. doi: 10.1016/0306-4522(94)00477-m. [DOI] [PubMed] [Google Scholar]
- Premkumar LS. Targeting TRPV1 as an alternative approach to narcotic analgesics to treat chronic pain conditions. The AAPS journal. 2010;12:361–370. doi: 10.1208/s12248-010-9196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science (New York, NY) 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu X, Ugurbil K. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 2002;36:1195–1210. doi: 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]
- Silva AC, Lee JH, Aoki I, Koretsky AP. Manganese-enhanced magnetic resonance imaging (MEMRI): methodological and practical considerations. NMR in biomedicine. 2004;17:532–543. doi: 10.1002/nbm.945. [DOI] [PubMed] [Google Scholar]
- Sokabe T, Tsujiuchi S, Kadowaki T, Tominaga M. Drosophila painless is a Ca2+-requiring channel activated by noxious heat. J Neurosci. 2008;28:9929–9938. doi: 10.1523/JNEUROSCI.2757-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuurman N, Delbecque JP, Callaerts P, Aebi U. Ectopic overexpression of Drosophila lamin C is stage-specific lethal. Experimental cell research. 1999;248:350–357. doi: 10.1006/excr.1999.4396. [DOI] [PubMed] [Google Scholar]
- Tegeder I, Costigan M, Griffin RS, Abele A, Belfer I, Schmidt H, Ehnert C, Nejim J, Marian C, Scholz J, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nature medicine. 2006;12:1269–1277. doi: 10.1038/nm1490. [DOI] [PubMed] [Google Scholar]
- Thulborn KR, Waterton JC, Matthews PM, Radda GK. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochimica et biophysica acta. 1982;714:265–270. doi: 10.1016/0304-4165(82)90333-6. [DOI] [PubMed] [Google Scholar]
- Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Treede RD, Kenshalo DR, Gracely RH, Jones AK. The cortical representation of pain. Pain. 1999;79:105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- Venken KJ, Carlson JW, Schulze KL, Pan H, He Y, Spokony R, Wan KH, Koriabine M, de Jong PJ, White KP, et al. Versatile P[acman] BAC libraries for transgenesis studies in Drosophila melanogaster. Nature methods. 2009;6:431–434. doi: 10.1038/nmeth.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SY, Cang CL, Liu XF, Peng YQ, Ye YZ, Zhao ZQ, Guo AK. Thermal nociception in adult Drosophila: behavioral characterization and the role of the painless gene. Genes, brain, and behavior. 2006;5:602–613. doi: 10.1111/j.1601-183X.2006.00213.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.