Abstract
Alcohol abuse in schizophrenia exceeds rates in the general population and worsens illness outcomes. Neonatal ventral hippocampal lesion (NVHL) rats model multiple schizophrenia dimensions including addiction vulnerability. This study compared NVHL vs. SHAM-controls in operant alcohol seeking and consumption. NVHLs enhanced consumption of combined ethanol/sucrose solution but neither ethanol or sucrose only solutions, consistent with increased vulnerability specific to carbohydrate-laden alcohol beverages typically consumed in early stages of human alcoholism.
Keywords: Dual diagnosis, addiction, schizophrenia, mental illness, alcohol, substance use disorders
Alcohol use disorders occur at greater rates in schizophrenia patients [1] and their non-psychotic siblings [2] compared to the general population. Alcohol dependence in schizophrenia is associated with higher morbidity and mortality as well as poorer overall quality of life, medication compliance, and treatment outcome [3].
Neonatal ventral hippocampal lesions (NVHL) in rats produce a spectrum of behavioral abnormalities analogous to those seen in schizophrenia. Mimicking the positive symptoms, peri-adolescent NVHL rats are hyper-responsive to dopaminergic stimuli which are ameliorated by neuroleptics. Modeling negative and cognitive symptoms, NVHL rats also exhibit deficits in socialization, working memory, and pre-pulse inhibition. Neurobiologically, NVHL rats have reduced dendritic arborization and spine density in frontal-cortical areas as well as abnormalities in GABA and glutamate neurotransmitter systems [4].
As a model of dual diagnosis, NVHL rats express behaviors indicative of an enhanced vulnerability to addiction to several drugs of abuse. NVHLs have altered behavioral sensitization to alcohol [5], cocaine [6], and nicotine [7], as well as an enhanced addiction profile to both cocaine and methamphetamine self-administration [8, 9]. Additionally, NVHLs exhibit enhanced impulsivity, an endophenotypic marker of addiction, which is accentuated by a prior cocaine history [10].
Alcohol’s reinforcing effects are thought to be mediated, in part, by dopaminergic projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) which in turn receives modulatory glutamatergic input from the ventral hippocampus and prefrontal cortex. Both the NVHL model and schizophrenia entail similar dysfunctions within these circuits which may represent substrate mechanisms that confer addiction vulnerability [11]. The current study assessed how alcohol seeking and consumption may be altered in NVHL rats using a behavioral paradigm that has been shown to reliably separate these two distinct goal-directed behaviors [12].
To render NVHL and SHAM-control rats, male Sprague- Dawley pups that were born in house underwent either NVHL or SHAM- control surgery on post-natal day 7 based upon Lipska et al. [13]. Pups were anesthetized via hypothermia and then taped prone to a stereotaxic platform with a lateral incision made on their skull. A 26S gauge Hamilton needle was inserted bilaterally into the ventral hippocampal formation (AP −3.0 mm, ML ±3.5 mm, VD −5.0 mm from bregma) and 0.3 μL of either 10 μg/μL ibotenic acid (NVHL) or artificial cerebrospinal fluid (SHAM) was injected over 135 s. The needle was removed 3 minutes after the infusion to prevent backflow. The incision was then closed with Vetbond (3M) and pups were warmed on a heating pad before being returned to their mothers. Litters were left undisturbed until weaning (PD 21) at which time rats were double housed according to lesion status. Animal care and experiments were conducted according to the Guide for the Care and Use Laboratory Animals, and with approval by Indiana University IACUC.
Behavioral testing began in adulthood (PD 60) and occurred in operant chambers (30 × 30 × 24.5 cm) housed in sound attenuating cubicles equipped with a fan to mask extraneous noises. Each chamber contained a house light centered on the back wall, a retractable lever on one side wall and a retractable graduated cylinder tube with a rubber stopper and a stainless steel sipper spout mounted on the opposite wall. Med-Associates software (Med-Associates, East Fairfield, VT) ran on an IBM compatible PC which controlled electrical inputs and outputs of each chamber. Testing occurred Monday-Friday.
All rats were initially trained on a continuous reinforcement schedule (i.e., a single lever press resulted in a 30 s sipper tube presentation containing 10% sucrose solution) until reliable responding was obtained. Rats were then randomly assigned to one of two groups: ethanol or sucrose. For the ethanol group, a sucrose fading procedure, in which sucrose concentration is decreased while ethanol concentration is concurrently increased, occurred until the final concentration of the solution was 10% ethanol (10E). The sucrose group underwent a similar fading procedure (without the introduction of ethanol) until the final concentration of the solution was 2% sucrose (2S). For both groups, the sipper tube presentation was reduced to 15 s during the first week and the response requirement was gradually increased to a fixed-ratio 4 requirement during the fading procedure. After 3 weeks of training, the schedule of reinforcement was changed so that completion of the response requirement yielded access to the sipper tube for one 20 minute presentation at which point consummatory data were recorded. The lever press response requirement was gradually increased to 12 required responses for 20 minute access to the sipper tube. After 12 reinforced response requirement sessions with the 10E or 2S solution, a single extinction session was administered. This session lasted for 20 minutes during which lever presses were recorded, but with no scheduled consequence and was used as a measure of seeking behavior. Rats in the ethanol group then underwent an additional 12 response requirement sessions with a 2S/10E solution under the same criteria as with the 10E solution, followed by an extinction session.
Upon completion of the behavioral testing, rats were decapitated and their brains were rapidly removed, flash frozen in isopentane, and stored at −80 °C until sectioning. 40 μm sections were sliced every 400 μm from −3.3 to −5.8 mm relative to bregma and stained with cresyl violet to visualize and assess lesion damage. Animals with damage extending beyond the ventral hippocampus or unilateral damage were excluded from the study. Only animals with histologically verified lesions (NVHL) or absence of damage (SHAM) were included in the study (Figure 1). One SHAM rat was excluded from analysis for failing to consistently complete the response requirement resulting in a final group size for the ethanol group of SHAM n=9, NVHL n=7 and in the sucrose group SHAM n=7, NVHL n=7.
Figure 1.
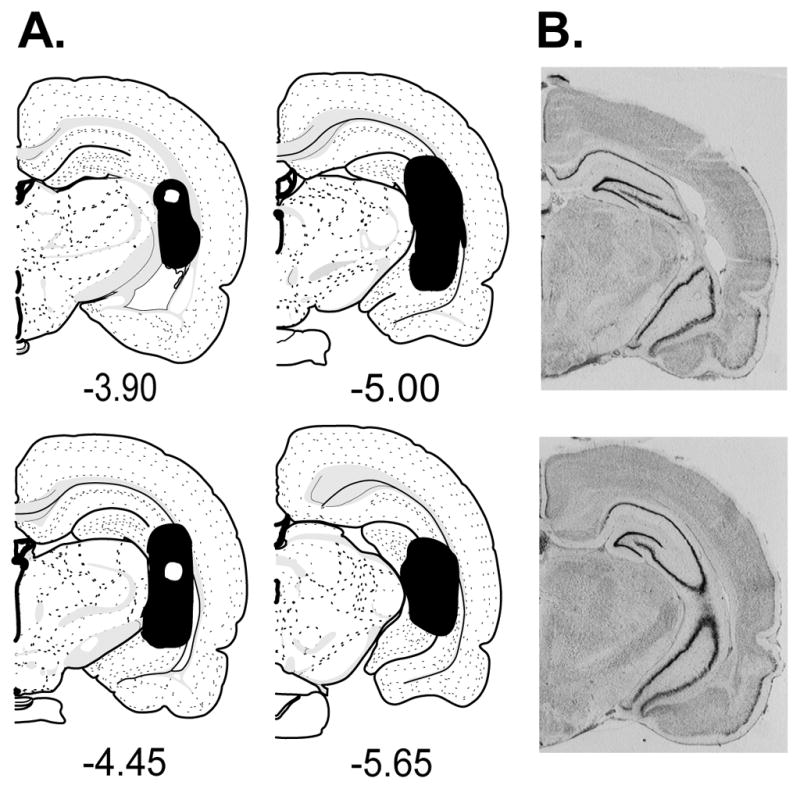
A. Greatest (solid black) and least (white within the black area) extent of damage allowed for rats included in the study. B. Typical example of lesion (top) and SHAM (bottom) histology.
To assess differences between groups, one way ANOVAs with lesion status as the independent variable and total solution intake or lever presses during the extinction session as the dependent variable was used. Differences across sessions were analyzed using a repeated measures ANOVA with lesion status and session as independent variables. Where significant interactions occurred (lesion × session), post-hoc testing using polynomial contrasts was used to discern significant time-dependent linear trends in the data.
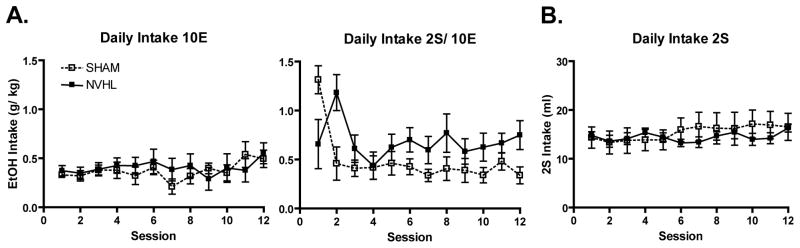
In the ethanol group, intake of the 10E solution was variable across sessions [F(1,11)=1.863, p<0.05] with no differences observed between NVHL and SHAM rats (Figure 2A, left panel). Both SHAM and NVHL animals had relatively low levels of 10E consumption which may be due to the strain of rat used (Sprague Dawley) as this strain has been shown to have low rates of ethanol consumption [14]. The subsequent intake of the 2S/10E solution (Figure 2A, right panel) also changed across sessions [F(1,11)=3.872, p<0.001] which was differentially affected by the lesion (session × lesion: [F(1,11)=3.849, p<0.001]) with a marginal main effect of lesion status [F(1, 14)=4.041, p=0.064].
Figure 2.
A. Daily intake for the ethanol group of the 10E (left) and 2S/10E (right) solution. B. Daily intake for the sucrose group of the 2S solution.
Upon switching from the 10E to a 2S/10E solution, consumption changed across the two sessions [F(1, 14)=9.240, p<0.01] which was differentially affected by the lesion [session × lesion: F(1, 14)=5.501, p<0.05]. SHAM animals had a sharp increase in consumption followed by a rapid decline and then stable, low levels of consumption. Conversely, NVHLs had similar consumption on the first 2S/10E session compared to the prior 10E session, followed by a marked increase in 2S/10E consumption on the second session, with a slight decline which stabilized and remained elevated when compared to SHAMs. Post-hoc analysis revealed a significant linear trend in SHAMs [F(1, 8)=33.273, p<0.001] which was not present in NVHLs indicating that SHAMs have an overall reduction in consumption across sessions whereas NVHL rats showed no such trend.
In rats consuming sucrose-only solution (Figure 2B), there was no lesion main effect to increase consumption. Intake of the 2S solution did change across sessions [F(1,11)=2.588, p<0.01] and a session × lesion interaction [F(1, 11)=2.418, p<0.01] occurred. Post-hoc analysis revealed a significant linear trend in SHAMs [F(1, 6)=6.523, p<0.05] that did not occur in NVHLs, associated with a trend for the SHAM rats to escalate their consumption whereas NVHLs had similar levels of intake across sessions. These data suggest that lesion-based differences in 2S/10E consumption could not simply be an artifact of lesion-based differences in sucrose consumption.
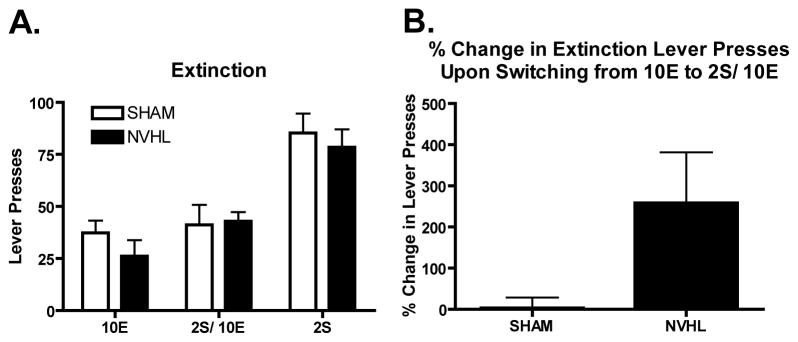
Lever pressing in NVHL vs SHAM rats during the extinction sessions according to prior reinforcer is shown in Figure 3A. A simple ANOVA for each solution showed no significant differences between NVHL and SHAMs. Since the same rats underwent extinction after exposure to both 10E and 2S/10E solutions, a repeated measures (lesion × extinction session) analysis was also performed on these data revealing a difference between extinction sessions [F(1,14)=4.578, p=0.05] indicating greater overall reward seeking for the 2S/10E solution compared to the 10E solution that was not qualified by a lesion main effect or lesion × session interaction. However, suggesting that the lesion rats predominantly carried this trend as a percent increase of extinction responding in the 2S/10E session compared to the baseline 10E session (calculated per rat as ((2S/10E – 10E)/(10E))*100%), NVHL rats showed greater percent increases in their extinction lever pressing from the 10E to the 2S/10E solution compared to SHAMs [F(1,15)=5.260, p<0.05] (Figure 2B). This suggests that NVHL’s motivation to obtain reward was augmented by the addition of sucrose to the ethanol solution to a greater extent than occurred in SHAMs. Also note that the extinction sessions were conducted following each of the 12 sessions of intake testing. Therefore, the 10E extinction test followed self-administration in the NVHLs of only about 0.4 g/kg ethanol in 20 minutes, while the 2S/10E test followed intake of 0.7 g/kg.
Figure 3.
A. Lever presses during the extinction sessions. B. Percent increase in lever presses for the ethanol group upon switching from the 10E to the 2S/10E solution
The major finding of this study is that alterations in ethanol consumption associated with sugar content are augmented in the NVHL model of schizophrenia. During the initial fading procedure when sucrose was removed from the ethanol solution, consumption decreased significantly across training sessions [F(4, 56)=23.197, p<0.001] from 1.14 ± 0.09 g/kg and 1.09 ± 0.19 g/kg of the 10S/10E solution to 0.35 ± .05 g/kg and 0.30 ± 0.02 g/kg of the 10E solution for SHAM and NVHLs respectively. Outbred rats generally tend to avoid alcohol at concentrations above 6% [15] however, the sucrose fading procedure used in this paradigm has been shown to reliably initiate drinking of a 10% alcohol solution in rats, representing an alcohol concentration comparable to human consumption [16]. The reduction in consumption with the 10E solution may have resulted, in part, from the aversive gustatory property of alcohol. When sucrose was reintroduced to the ethanol solution, it may have been comparatively more palatable thereby producing overall increased consumption. It was at this point where lesion based differences in consumption emerged; NVHL rats showed a sustained elevation in 2S/10E consumption across sessions that did not occur in SHAMs indicated by a strong trend for a main effect of lesion in the 2S/10E sessions, while no such main effect was observed with the 10E solution. The addition of sucrose has been shown to increase alcohol consumption in rats [17], and the 2S/10E (compared to the 10E solution) more closely models the carbohydrate content of alcoholic drinks typically consumed by humans.
Why NVHL-based differences in ethanol consumption emerge best with a carbohydrate/ethanol content that resembles human alcohol consumption is not clear. Seeking and consumption of a sucrose natural reward in NVHL rats has previously been shown to differ from SHAMs depending on the experimental testing conditions. Le Pen noted a decreased preference for a saccharin solution [18] while others have observed an increase in sucrose consumption [19] and enhanced acquisition of sucrose reward seeking [8]. The reinforcing effects of both natural and drug rewards are mediated within cortico-striatal-limbic circuits [20]. Neurodevelopmental abnormalities involving these systems in NVHLs may be particularly sensitive to the reinforcing effects of ethanol when sucrose is present, as suggested by our findings. While the euphoric effects of ethanol may be a subjective correlate of the positive reinforcing effects of ethanol [21], the euphoric effects of ethanol have been shown to be enhanced in schizophrenia when patients are given a carbohydrate/ethanol solution [22].
In summary, these findings suggest that NVHLs have enhanced consumption and motivation to seek an ethanol/sucrose solution when compared to SHAM rats which may be indicative of an enhanced susceptibility to alcohol addiction particularly with respect to modeling the early stages of alcohol use in humans when higher carbohydrate-content alcoholic beverages are commonly consumed. These observations are consistent with previous findings using cocaine and methamphetamine reinforcers [8, 9], suggesting a generalized addiction vulnerability. Further investigations are needed to understand the mechanisms underlying NVHL-related changes in the permissive effect of sugar content on ethanol consumption and how this might fit into a more generalized addiction vulnerability.
Acknowledgments
This research was supported by NIAAA-1RC2AA019366-01 and NIDA-K08-DA019850.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. Jama. 1990;264:2511–8. [PubMed] [Google Scholar]
- 2.Smith MJ, Barch DM, Wolf TJ, Mamah D, Csernansky JG. Elevated rates of substance use disorders in non-psychotic siblings of individuals with schizophrenia. Schizophr Res. 2008;106:294–9. doi: 10.1016/j.schres.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drake RE, Osher FC, Wallach MA. Alcohol use and abuse in schizophrenia. A prospective community study. J Nerv Ment Dis. 1989;177:408–14. doi: 10.1097/00005053-198907000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conroy SK, Rodd Z, Chambers RA. Ethanol sensitization in a neurodevelopmental lesion model of schizophrenia in rats. Pharmacology, biochemistry, and behavior. 2007;86:386–94. doi: 10.1016/j.pbb.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers RA, Taylor JR. Animal modeling dual diagnosis schizophrenia: sensitization to cocaine in rats with neonatal ventral hippocampal lesions. Biol Psychiatry. 2004;56:308–16. doi: 10.1016/j.biopsych.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Berg SA, Chambers RA. Accentuated behavioral sensitization to nicotine in the neonatal ventral hippocampal lesion model of schizophrenia. Neuropharmacology. 2008;54:1201–7. doi: 10.1016/j.neuropharm.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27:889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brady AM, McCallum SE, Glick SD, O’Donnell P. Enhanced methamphetamine self-administration in a neurodevelopmental rat model of schizophrenia. Psychopharmacology. 2008;200:205–15. doi: 10.1007/s00213-008-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers RA, Jones RM, Brown S, Taylor JR. Natural reward-related learning in rats with neonatal ventral hippocampal lesions and prior cocaine exposure. Psychopharmacology. 2005;179:470–8. doi: 10.1007/s00213-004-2042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: rodent models. Int Rev Neurobiol. 2003;54:107–43. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- 13.Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- 14.Martinetti MP, Lowery EG, Vona SR, Wichnick AM, Adler RA, Finch DG. Limited-access consumption of ascending ethanol concentrations in alcohol-preferring, nonpreferring, and Sprague-Dawley rats. Alcoholism, clinical and experimental research. 2006;30:836–43. doi: 10.1111/j.1530-0277.2006.00098.x. [DOI] [PubMed] [Google Scholar]
- 15.Richter CP, Campbell KH. Alcohol Taste Thresholds and Concentrations of Solution Preferred by Rats. Science. 1940;91:507–8. doi: 10.1126/science.91.2369.507. [DOI] [PubMed] [Google Scholar]
- 16.Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcoholism, clinical and experimental research. 1986;10:436–42. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- 17.Czachowski CL, Samson HH, Denning CE. Blood ethanol concentrations in rats drinking sucrose/ethanol solutions. Alcohol Clin Exp Res. 1999;23:1331–5. [PubMed] [Google Scholar]
- 18.Le Pen G, Gaudet L, Mortas P, Mory R, Moreau JL. Deficits in reward sensitivity in a neurodevelopmental rat model of schizophrenia. Psychopharmacology. 2002;161:434–41. doi: 10.1007/s00213-002-1092-4. [DOI] [PubMed] [Google Scholar]
- 19.Macedo CE, Sandner G, Angst MJ, Guiberteau T. Rewarded associative and instrumental conditioning after neonatal ventral hippocampus lesions in rats. Brain Res. 2008;1215:190–9. doi: 10.1016/j.brainres.2008.03.069. [DOI] [PubMed] [Google Scholar]
- 20.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–76. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Koob GF. Alcoholism: allostasis and beyond. Alcoholism, clinical and experimental research. 2003;27:232–43. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- 22.D’Souza DC, Gil RB, Madonick S, Perry EB, Forselius-Bielen K, Braley G, et al. Enhanced sensitivity to the euphoric effects of alcohol in schizophrenia. Neuropsychopharmacology. 2006;31:2767–75. doi: 10.1038/sj.npp.1301207. [DOI] [PubMed] [Google Scholar]