Abstract
Sarcoplasmic/endoplasmic reticulum (ER) Ca2+ is the most abundant store of intracellular Ca2+, and its release is an important trigger of physiological and cell death pathways. Previous work in our laboratory revealed the importance of ER Ca2+ in toxicant-induced renal proximal tubular cell (RPTC) death. The purpose of this study was to evaluate the use of confocal microscopy and Fluo5F, a low affinity Ca2+ indicator, to directly monitor changes in RPTC ER Ca2+. Fluo5F staining reflected ER Ca2+, resolved ER structure, and showed no colocalization with tetramethyl rhodamine methyl ester (TMRM), a marker of mitochondrial membrane potential. Thapsigargin, an ER Ca2+ pump inhibitor, decreased ER fluorescence by 30% and 55% at 5 and 15 min, respectively, whereas A23187, a Ca2+ ionophore caused more rapid ER Ca2+ release (55% and 75% decrease in fluorescence at 5 and 15 min).
Carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP), a mitochondrial uncoupler, added at the end of the experiment, further decreased ER fluorescence after thapsigargin treatment, revealing that thapsigargin did not release all ER Ca2+. In contrast, FCCP did not decrease ER fluorescence after A23187 treatment, suggesting complete ER Ca2+ release. ER Ca2+ release in response to A23187 or thapsigargin resulted in a modest but significant decrease in mitochondrial membrane potential. These data provide evidence that confocal microscopy and Fluo5F are useful and effective tools for directly monitoring ER Ca2+ in intact cells.
Keywords: calcium, endoplasmic reticulum, confocal microscopy, kidney, Fluo5F
INTRODUCTION
Ca2+ acts as a universal second messenger and regulates numerous cellular functions including metabolism, motility, and transport[1]. Loss of Ca2+ homeostasis is critical to many disease processes and is a major component of cell death pathways including necrosis, apoptosis, and autophagy [2,3,4,5,6,7,8]. Sarcoplasmic/endoplasmic reticulum (ER) Ca2+ is the most abundant store of intracellular Ca2+ and its disruption often initiates the deleterious cascade of events leading to cell death and dysfunction [5,6,7,8,9,10,11].
Traditional methods of measuring of ER Ca2+ are indirect or require difficult probe loading techniques (i.e. membrane permeabilization, microinjection, or fused cell hybrids). For example, human embryonic kidney cells require 1 h of dye loading (2 μM Fluo-3 at room temperature) and extracellular Ca2+ chelation using BAPTA or EGTA, just to monitor increases in cytosolic Ca2+ as an indirect measure of ER Ca2+ stores [12]. To monitor ER Ca2+ directly, Montero and Robert et al. engineered an ER-targeted aequorin chimera, a Ca2+-sensitive photoprotein with a lower affinity for Ca2+. However, in HeLa and skeletal muscle cells, the aequorin chimera was rapidly saturated by the high Ca2+ concentrations within the ER and required a non-physiological reduction of ER Ca2+ to detect changes in Ca2+ stores [13,14].
Primary cultures of renal proximal tubular cells (RPTC) are highly aerobic, gluconeogenic, and exhibit robust transport capacity, making them ideal for the study of kidney tubular function and injury [15,16]. Previous work in our laboratory revealed the importance of ER Ca2+ in toxicant-induced kidney injury; although, the mechanism by which Ca2+ plays such a pivotal role is not completely understood [5,7,9,10,17,18].
Confocal microscopy is a useful method for the visualization and quantification of fluorophores at the subcellular level in living cells, and is compatible with most Ca2+ indicators. Chemical fluorescent (UV and visible-wavelength excitation fluorescent indicators) and bioluminescent calcium indicators (Ca2+ binding photoproteins and GFP-based Ca2+ indicators) differ in their characteristics (loading requirements, excitation/emission spectrum, permeability, compartmentalization, relative brightness, and Ca2+ affinity) and have inherent drawbacks (i.e., dye leakage, cytotoxicity, bleaching, autofluorescence, intracellular buffering, and lack of ion specificity)[19]. Fluo5F is a chemical fluorescent Ca2+ indicator with a lower affinity for Ca2+ (Kd ~2.3 μM), limited cytoxicity and bleaching, and high Ca2+ specificity, making it ideal for studying ER Ca2+. The purpose of this study was to evaluate the use of confocal microscopy and Fluo5F for directly monitoring changes in ER Ca2+ in RPTC.
MATERIALS AND METHODS
Materials
Female New Zealand White rabbits (1.5–2.0 kg) were purchased from Myrtle’s Rabbitry (Thompson Station, TN). Tetramethyl rhodamine methyl ester (TMRM) and Fluo-5F-AM were purchased from Molecular Probes, Invitrogen (Carlsbad, CA). All other chemical and materials were obtained from Sigma Chemical (St. Louis, MO).
Isolation of Rabbit RPTC and Culture Conditions
Rabbit RPTC were isolated using the iron oxide perfusion method and grown to confluence in 35-mm tissue culture dishes under improved conditions as previously described [15,16]. The culture medium was a 1:1 mixture of Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium (without glucose, phenol red, or sodium pyruvate) supplemented with 15 mM HEPES buffer, 2.5 mM L-glutamine, 1 μM pyridoxine HCl, 15 mM sodium bicarbonate, and 6 mM lactate. Hydrocortisone (50 nM), selenium (5 ng/ml), human transferrin (5 μg/ml), bovine insulin (10 nM), and L-ascorbic acid-2-phosphate (50 μM) were added daily to fresh culture medium.
RPTC Loading
RPTC were loaded with 2 μM Fluo-5F-AM (Fluo5F) and 100 nM TMRM for 20 min at 37° C, washed twice with 37° C phosphate buffered saline, and media was replaced. Then, 1 μM Fluo5F and 40 nM TMRM were added to the media to maintain dye equilibrium and incubated for 30 min at 37° C prior to imaging. The TMRM loading protocol was modified from Lemasters and Ramshesh [20].
Microscopic Imaging and Analysis
RPTC were imaged on a Leica Microsystems, TCS SP2 AOBS laser scanning confocal microscope (LSCM) using standardized pinhole, gain, and black level settings. A 63×0.9 NA water-immersion objective was used in an upright microscope configuration. All images were acquired at 8-bit resolution and at 1024×1024 with a line averaging of two.
RPTC were monitored for 10 min prior to treatment to establish baseline. Then, RPTC were treated and monitored an additional 15 min prior to Carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP, 1 μM) addition to depolarize mitochondria. Images were acquired in 1-min intervals during the course of experiments. To quantify data, images were analyzed using Adobe Photoshop. Mean intensities of images in the green and red channel after background subtraction were interpreted as a quantitative measure of ER Ca2+ and mitochondrial membrane potential, respectively. Values are graphed as the percent mean intensity of each subsequent image vs. mean intensity of the image taken at time zero. Images comprise of 7–10 cells.
Statistical Analysis
RPTC isolated from one animal represents one experiment (N=1). Data are presented as means ± SE, and multiple means were compared using Student-Newman-Keuls test at each time point. Means with values p<0.05 were considered statistically significant.
RESULTS AND DISCUSSION
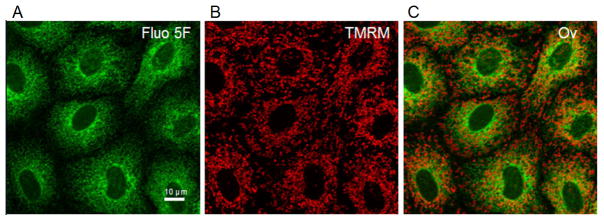
To investigate the use of confocal microscopy to monitor ER Ca2+ dynamics in RPTC, Fluo5F fluorescence was monitored as described above (Fig. 1a). Fluo5F fluorescence revealed a reticular pattern around nuclei void of staining. The peripheral granularity of staining and scarcity of staining at the cell margins provided evidence that the probe is not fluorescing in the cytosol. To confirm that Fluo5F was not in the mitochondria, RPTC were loaded with tetramethyl rhodamine methyl ester (TMRM), a marker of polarized mitochondria. TMRM staining (Fig. 1b) had a punctuate/tubular pattern unlike Fluo5F staining with no co-localization (Fig. 1c), confirming that Fluo5F does not reflect mitochondrial Ca2+, the second most significant storage/buffering compartment for intracellular Ca2+[4].
Figure 1. Morphology of Renal Proximal Tubule Cells (RPTC) co-loaded with Fluo5F and TMRM using confocal microscopy.
RPTC were co-loaded with Fluo5F (2 μM) and TMRM (100 nM) as described in the materials and methods. Green fluorescence of Fluo5F and red fluorescence of TMRM were imaged by laser scanning confocal microscopy. The white bar represents 10 μm. Fluo5F fluorescence (A) represents endoplasmic reticulum (ER) Ca2+, TMRM fluorescence (B) represents polarized mitochondria, and there was no co-localization (C).
We investigated the use of Fluo5F to monitor real-time changes in RPTC ER Ca2+. TMRM was used to measure mitochondrial membrane potential. In control cells, both Fluo5F and TMRM fluorescence intensity, morphology, and resolution were maintained over time (Fig. 2). Carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP), a mitochondrial uncoupler, was added at the end of each experiment to cause mitochondrial depolarization and indirectly ER Ca2+ release, due to ATP depletion and cessation of sarcoplasmic/ER Ca2+ ATPase (SERCA) activity.
Figure 2. ER Ca2+ release RPTC exposed to A23187 and thapsigargin.
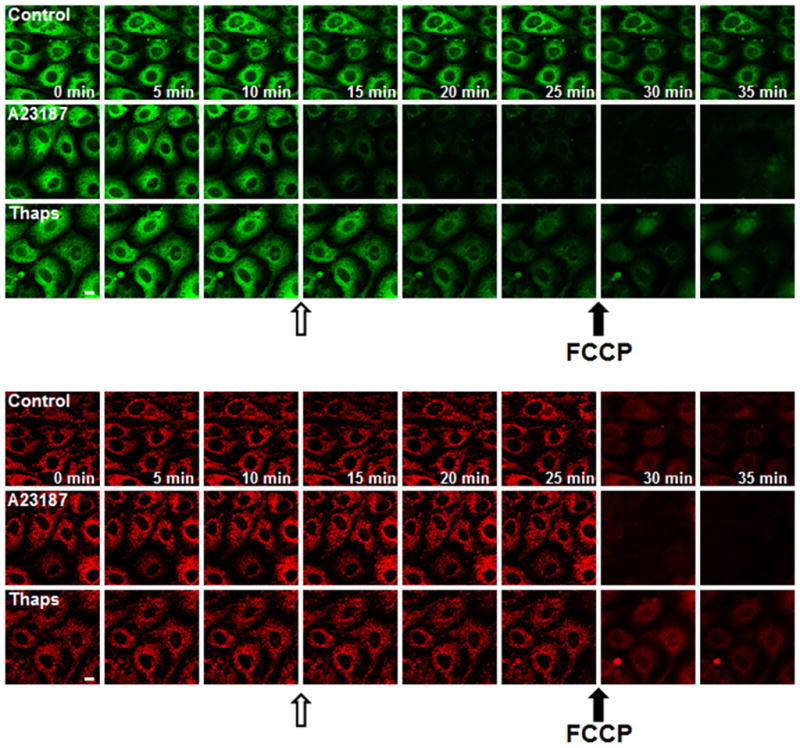
RPTC were loaded with Fluo5F and TMRM (see in Fig. 1), monitored for 10 min to establish baseline, exposed to diluent (DMSO, Control), A23187 (10 μM), or thapsigargin (10 μM) for 15 min, and then exposed to FCCP (1 μM), a mitochondrial uncoupler. Open arrows represent time of A23187 or thapsigargin treatment and closed arrows represent the addition of FCCP. Green fluorescence (A) and red fluorescence (B) represents ER Ca2+ and mitochondrial polarization, respectively. Note the more rapid ER Ca2+ release (A) in response to A23187 exposure compared to thapsigargin and the rapid loss of mitochondrial polarization (B) in response to FCCP.
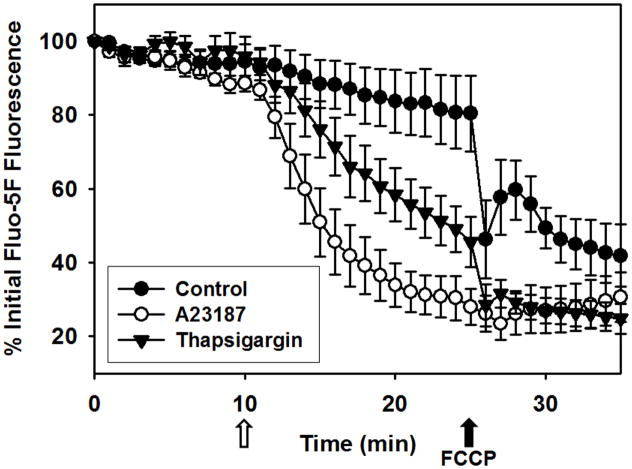
We then explored the effects of two pharmacological agents known to alter ER Ca2+. RPTC were treated with the SERCA inhibitor, thapsigargin, and the Ca2+ ionophore, A23187. Thapsigargin decreased ER Ca2+ over several min (Fig. 2A), which was more indicative of ER Ca2+ leakage than receptor-dependent ER Ca2+ release that is characterized by DAG, ryanodine, and IP3 receptor sensitive stores [1,21,22]. In contrast, A23187 caused a more rapid release of ER Ca2+, an anticipated finding due to its ionophore activity.
Temporal and incremental changes in ER Ca2+ release and mitochondrial membrane potential were quantified (Fig. 3). A23187 decreased fluorescence by 50% and 70% at 5 and 15 min after treatment, respectively, and FCCP addition did not decrease fluorescence further. Thapsigargin decreased fluorescence by 25% and 55% at 5 and 15 min after treatment, respectively. In contrast to A23187, FCCP addition further decreased fluorescence after thapsigargin, providing evidence that thapsigargin did not completely release all ER Ca2+ stores under these conditions.
Figure 3. Quantification of A23187- and thapsigargin- induced ER Ca2+ release in RPTC.
RPTC were co-loaded with Fluo5F and TMRM, treated as described in fig. 2, and images acquired as described in Materials and Methods. Values are mean intensities of images in the green channel ± SE and loss of intensity represents ER Ca2+ release. The open arrow represents exposure to A23187, thapsigargin, or diluent control and the closed arrow represents the addition of FCCP. Note the more rapid ER Ca2+ release after A23187 exposure compared to thapsigargin and an additional loss of fluorescence with FCCP following thapsigargin exposure.
The advantage of this approach is that ER Ca2+ stores are measured directly, allowing observation of spatiotemporal differences between A23187- and thapsigargin-induced ER Ca2+ release that would otherwise be masked by measuring cytosolic free Ca2+. Interestingly, Jiang et al. reported a higher rate and magnitude of cytosolic Ca2+ increase in response to A23187 over thasigargin using Fluo-3, but this approach required chelation of extracellular Ca2+ prior to treatment [12].
The effect of ER Ca2+ release on mitochondrial polarization was monitored using TMRM (Fig. 4). A23187- and thapsigargin-induced ER Ca2+ release decreased TMRM fluorescence by approximately 10%. In contrast, FCCP, the mitochondrial uncoupler added at the end of each experiment, decreased TMRM fluorescence by 60%, reflecting complete mitochondrial depolarization. Together, these data reveal that ER Ca2+ release produced by A23187 or thapsigargin partially decreases mitochondrial polarization and is consistent with previous reports [23].
Figure 4. Quantification of A23187- and thapsigargin-induced mitochondrial depolarization in RPTC.
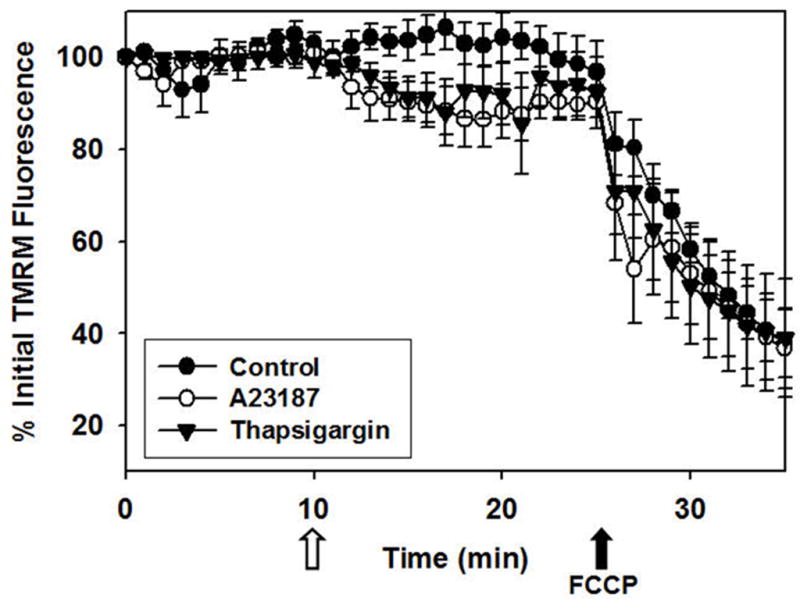
See above for experimental details. Values are mean intensities of images in the red channel ± SE and loss of intensity represents mitochondrial depolarization. The open arrow represents exposure to A23187, thapsigargin, or diluent control and the closed arrow represents the addition of FCCP. Note the decrease in mitochondrial polarization following A23187 or thapsigargin exposure compared with control and the rapid loss of mitochondrial polarization following FCCP exposure in all groups.
In conclusion, confocal microscopy and Fluo5F are valuable tools for visualization of spatiotemporal dynamics of ER Ca2+ release in complex cellular systems in real time and will aid in our understanding of cellular dynamics and death.
RESEARCH HIGHLIGHTS.
A new method of monitoring ER Ca2+ directly in intact cells
Confocal microscopy and low affinity Ca2+ indicator, Fluo5F, reveals ER Ca2+ morphology
Fluo5F fluorescence shows no co-localization with mitochondria or cytosol
Stimulation of ER Ca2+ release reveal spatiotemporal differences in ER Ca2+ release dynamics
Acknowledgments
This work was supported by an NIH fellowship (F30 ES015964) to AE and NIH grant (DK-062028) to RGS, and by the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs. Animal facilities were funded by NIH grant C06 RR-015455 and Cell and Molecular Imaging (CMI) facility was supported, in part, by grant P30 CA138313 to the Hollings Cancer Center, Medical University of South Carolina. The authors also thank Dr. Venkat Ramshesh and Dr. John Lemasters (Medical University of South Carolina) for their advice and constructive discussions during the execution of these experiments.
Abbreviations
- ER
endoplasmic reticulum
- EGTA
ethylene glycol tetraacetic acid
- RPTC
renal proximal tubular cell
- TMRM
tetramethyl rhodamine methyl ester
- Fluo5F
Fluo-5F-AM
- LSCM
laser scanning confocal microscopy
- FCCP
Carbonylcyanide-p-trifluoromethoxyphenylhydrazone
- SERCA
sarcoplamic/endoplasmic reticulum ATPase
- DAG
diacylglycerol
- IP3
inositol triphosphate
- DMSO
dimethyl sulfoxide
Footnotes
Disclaimer. The contents of this manuscript do not represent the views of the Department of Veteran Affairs or the United States Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berridge MJ. Inositol trisphosphate and calcium signaling. Ann N Y Acad Sci. 1995;766:31–43. doi: 10.1111/j.1749-6632.1995.tb26646.x. [DOI] [PubMed] [Google Scholar]
- 2.Cummings BS, Kinsey GR, Bolchoz LJ, Schnellmann RG. Identification of caspase-independent apoptosis in epithelial and cancer cells. J Pharmacol Exp Ther. 2004;310:126–134. doi: 10.1124/jpet.104.065862. [DOI] [PubMed] [Google Scholar]
- 3.Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 4.Lemasters JJ, Qian T, Elmore SP, Trost LC, Nishimura Y, Herman B, Bradham CA, Brenner DA, Nieminen AL. Confocal microscopy of the mitochondrial permeability transition in necrotic cell killing, apoptosis and autophagy. Biofactors. 1998;8:283–285. doi: 10.1002/biof.5520080316. [DOI] [PubMed] [Google Scholar]
- 5.Schnellmann RG. Intracellular calcium chelators and oxidant-induced renal proximal tubule cell death. J Biochem Toxicol. 1991;6:299–303. doi: 10.1002/jbt.2570060410. [DOI] [PubMed] [Google Scholar]
- 6.Waters SL, Sarang SS, Wang KK, Schnellmann RG. Calpains mediate calcium and chloride influx during the late phase of cell injury. J Pharmacol Exp Ther. 1997;283:1177–1184. [PubMed] [Google Scholar]
- 7.Waters SL, Wong JK, Schnellmann RG. Depletion of endoplasmic reticulum calcium stores protects against hypoxia- and mitochondrial inhibitor-induced cellular injury and death. Biochem Biophys Res Commun. 1997;240:57–60. doi: 10.1006/bbrc.1997.7606. [DOI] [PubMed] [Google Scholar]
- 8.Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Van Vleet T, Schnellmann RG. The role of calpain in oncotic cell death. Annu Rev Pharmacol Toxicol. 2004;44:349–370. doi: 10.1146/annurev.pharmtox.44.101802.121804. [DOI] [PubMed] [Google Scholar]
- 10.Harriman JF, Liu XL, Aleo MD, Machaca K, Schnellmann RG. Endoplasmic reticulum Ca(2+) signaling and calpains mediate renal cell death. Cell Death Differ. 2002;9:734–741. doi: 10.1038/sj.cdd.4401029. [DOI] [PubMed] [Google Scholar]
- 11.Muruganandan S, Cribb AE. Calpain-induced endoplasmic reticulum stress and cell death following cytotoxic damage to renal cells. Toxicol Sci. 2006;94:118–128. doi: 10.1093/toxsci/kfl084. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y, Cypess AM, Muse ED, Wu CR, Unson CG, Merrifield RB, Sakmar TP. Glucagon receptor activates extracellular signal-regulated protein kinase 1/2 via cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 2001;98:10102–10107. doi: 10.1073/pnas.131200398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robert V, De Giorgi F, Massimino ML, Cantini M, Pozzan T. Direct monitoring of the calcium concentration in the sarcoplasmic and endoplasmic reticulum of skeletal muscle myotubes. J Biol Chem. 1998;273:30372–30378. doi: 10.1074/jbc.273.46.30372. [DOI] [PubMed] [Google Scholar]
- 14.Montero M, Brini M, Marsault R, Alvarez J, Sitia R, Pozzan T, Rizzuto R. Monitoring dynamic changes in free Ca2+ concentration in the endoplasmic reticulum of intact cells. EMBO J. 1995;14:5467–5475. doi: 10.1002/j.1460-2075.1995.tb00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nowak G, Schnellmann RG. Improved culture conditions stimulate gluconeogenesis in primary cultures of renal proximal tubule cells. Am J Physiol. 1995;268:C1053–1061. doi: 10.1152/ajpcell.1995.268.4.C1053. [DOI] [PubMed] [Google Scholar]
- 16.Nowak G, Schnellmann RG. L-ascorbic acid regulates growth and metabolism of renal cells: improvements in cell culture. Am J Physiol. 1996;271:C2072–2080. doi: 10.1152/ajpcell.1996.271.6.C2072. [DOI] [PubMed] [Google Scholar]
- 17.Cummings BS, McHowat J, Schnellmann RG. Role of an endoplasmic reticulum Ca2+-independent phospholipase A2 in cisplatin-induced renal cell apoptosis. J Pharmacol Exp Ther. 2004;308:921–928. doi: 10.1124/jpet.103.060541. [DOI] [PubMed] [Google Scholar]
- 18.Cummings BS, McHowat J, Schnellmann RG. Role of an endoplasmic reticulum Ca(2+)-independent phospholipase A(2) in oxidant-induced renal cell death. Am J Physiol Renal Physiol. 2002;283:F492–498. doi: 10.1152/ajprenal.00022.2002. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi A, Camacho P, Lechleiter JD, Herman B. Measurement of intracellular calcium. Physiol Rev. 1999;79:1089–1125. doi: 10.1152/physrev.1999.79.4.1089. [DOI] [PubMed] [Google Scholar]
- 20.Lemasters JJ, Ramshesh VK. Imaging of mitochondrial polarization and depolarization with cationic fluorophores. Methods Cell Biol. 2007;80:283–295. doi: 10.1016/S0091-679X(06)80014-2. [DOI] [PubMed] [Google Scholar]
- 21.Bian JH, Ghosh TK, Wang JC, Gill DL. Identification of intracellular calcium pools. Selective modification by thapsigargin. J Biol Chem. 1991;266:8801–8806. [PubMed] [Google Scholar]
- 22.Hirose K, Kadowaki S, Tanabe M, Takeshima H, Iino M. Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science. 1999;284:1527–1530. doi: 10.1126/science.284.5419.1527. [DOI] [PubMed] [Google Scholar]
- 23.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]