Abstract
Objectives
We sought to evaluate the accuracy and reproducibility of visual estimation of coronary artery calcium (CAC) from CT attenuation correction (CTAC) scans performed for hybrid PET/CT and SPECT/CT myocardial perfusion imaging (MPI).
Background
At the time of MPI, hybrid systems obtain a low-dose, non-ECG-gated CT scan that is used to perform attenuation correction. Utility of this CTAC scan in estimating actual CAC as measured by Agatston score (AS) on standard ECG-gated scans has not been previously studied.
Methods
492 patients from 3 centers receiving both MPI with CTAC and a standard CAC scan were studied. At each site, experienced readers blinded to AS reviewed CTAC images, visually estimating CAC on a six-level scale: classifying patients as estimated AS of 0, 1-9, 10-99, 100-300, 400-999, or ≥1000. Agreement between visually-estimated CAC (VECAC) on CTAC and AS, measured standardly and converted to the same scale, was evaluated, as was inter-reader agreement.
Results
Although CTAC images are low-dose and non-gated, a high degree of association was observed between VECAC and AS, with 63% of VECACs in the same category as the AS category and 93% within one category. Weighted kappa was 0.89 (95% confidence interval 0.88 to 0.91, p<0.0001). High weighted kappa statistics were observed for each site, scanner type, and gender. Readers reported identical scores in 65% of cases and scores within one category in 93%.
Conclusions
CAC can be visually assessed from low-dose CTAC scans with high agreement with AS. CTAC scans should be routinely assessed for VECAC.
Keywords: hybrid imaging, coronary calcium, SPECT, PET
Introduction
In recent years, PET and SPECT cameras that incorporate multi-detector row CT scanners have been introduced into clinical practice. At the time of myocardial perfusion imaging (MPI), such hybrid systems obtain a low-dose, non-ECG-gated CT scan that is used to perform attenuation correction for the radionuclide images (Figures 1 and 2). CT attenuation correction (CTAC) provides the potential to eliminate false positive perfusion defects caused by soft-tissue attenuating structures such as the diaphragm and breasts.(1).
Figure 1. Example case (Columbia) comparing non-gated, non-breath-hold CT images obtained for attenuation correction (left) with standard ECG-gated, breath-hold calcium scoring images (right).
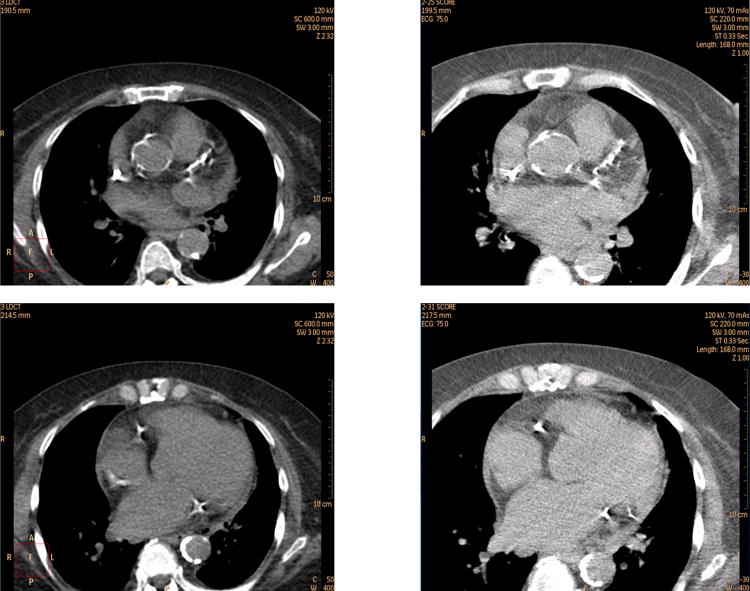
Agatston score is 2640, and was visually estimated to be ≥1000 by both readers.
Figure 2. Example case (Mid America Heart) comparing non-gated, breath-hold, very low tube current CT images obtained for attenuation correction (left) with standard ECG-gated, breath-hold calcium scoring images (right).
Agatston score is 367, and was visually estimated to be 100-399 by one reader and 400-999 by the other reader.
Many laboratories routinely perform in the same session as MPI an additional high-resolution, ECG-gated cardiac CT scan to quantitate coronary artery calcium (CAC). Calcium scoring provides supplementary information to MPI by identifying and assessing the extent of calcified plaques of the coronary arteries, even in the absence of the flow-limiting disease associated with perfusion defects. However, calcium scoring is not routinely performed with MPI and, when done, adds modestly to the total effective dose of radiation.
Visual comparison of non-gated CTAC scans with ECG-gated scans performed in the same patients for calcium scoring suggests that much of the information relating to the extent of CAC is available from the low radiation dose, non-gated CTAC scan (Figures 1 and 2). However, the non-gated scan is subject to blurring of coronary arteries, which may change CT number in some voxels, which can affect calcium scores. Moreover, lack of ECG-gating has potential to introduce gaps and/or duplications in the image data set due to cardiac motion. The purpose of this study was to evaluate the accuracy of visual estimation of CAC from CTAC scans, in comparison to “gold-standard” Agatston score (AS) measurement on standard ECG-gated CT scans, as well as to assess the reproducibility of this visual estimation.
Methods
Study design
492 patients from 3 centers performing hybrid PET/CT or SPECT/CT were studied. Institutional review board approval or waiver was obtained at each site. Each patient had received both an MPI study with CTAC and a separate CAC scan performed on the same day. Each CTAC scan was visually assessed by two independent readers at each site and graded on a six point scale, to estimate the extent of CAC. AS was determined for each CAC scan and reclassified on the same scale. Agreement between visually-estimated coronary artery calcium (VECAC) scores and Agatston scores was determined using weighted kappa statistics and percentage agreements, as was inter-reader reproducibility of VECAC scores.
PET/CT and SPECT/CT scanning
At Columbia University Medical Center (116 women, 81 men), each patient was scanned using a Philips Precedence 16P SPECT/CT scanner. CTAC scans were performed free breathing without ECG-gating, in spiral mode with pitch 0.94, collimation 16×1.5 mm, scan length 14 mm, tube voltage 120 kVp, and effective mAs 50, adjusted by the technologist according to patient habitus. Typical dose-length-product was 75 mGy·cm, corresponding to estimated effective dose of 1.3 mSv.(2) CAC scans were performed using an end-inspiratory breath hold with ECG-gating, axial mode, collimation 8×3.0 mm, scan length 14 cm, tube voltage 120 kVp, and effective mAs 70, on rare occasions increased by the technologist to reflect patient body habitus. Typical dose-length-product was 58 mGy·cm, corresponding to estimated effective dose of 1.0 mSv.
At Mid America Heart (111 women, 75 men), each patient was scanned using a Siemens Biograph 16 PET/CT scanner. Post-stress CTAC scans were performed using a light end-expiratory breath hold without ECG-gating, spiral mode with pitch 2, collimation 16×0.75 mm, tube voltage 120 kVp, effective mAs 9, and typical dose-length-product 15 mGy·cm, corresponding to estimated effective dose 0.3 mSv. CAC scans were performed using an extended craniocaudal scan also used for rest attenuation correction. This used an end-expiratory breath hold with ECG-gating, spiral mode with pitch 0.28, collimation 16×1.5 mm, tube voltage 120 kVp, effective mAs ∼220 mAs adjusted by the technologist to reflect patient habitus, and typical dose-length-product 278 mGy·cm, corresponding to estimated effective dose 4.7 mSv.
At Cedars-Sinai (50 women, 59 men), each patient was scanned using a Siemens Biograph 64 PET/CT scanner. CTAC scans were performed free breathing without ECG-gating, spiral mode with pitch 1.5, collimation 24×1.2 mm, tube voltage 120 kVp, effective mAs 11, and typical dose-length product 16 mGy·cm, corresponding to estimated effective dose 0.3 mSv. CAC scans were performed using an end-inspiratory breath hold with prospective ECG-gating, collimation 30×0.6 mm, tube voltage 120 kVp, effective mAs 150, and typical dose-length-product 173 mGy·cm, corresponding to estimated effective dose 2.9 mSv.
Analysis of CT Scans
At each site, AS was determined using standard methodology on a dedicated workstation. At each site, two experienced readers blinded to the AS independently reviewed CTAC images and visually estimated CAC on a six level scale, classifying patients as having estimated AS of 0, 1-9, 10-99, 100-300, 400-999, or ≥1000. In addition, a single reader from the Mid America Heart and Cedars-Sinai sites each blindedly visually estimated CAC on 99 scans from Columbia University Medical Center. For the first 20 cases, readers were given post hoc feedback as to the correct classification based on the AS. Each reader also visually evaluated each CTAC scan for the presence or absence of calcium in each of the major coronary arteries (left main, left anterior descending (LAD), circumflex, and right coronary artery).
Since systematically missing a proximal LAD lesion may have important clinical implications, a subsequent analysis was performed in one center (Columbia) of cases with a discrepancy between visual assessment of LAD calcium on CTAC images, by either reader, and its measurement (0 or >0) on the CAC images, to determine the location in the vessel of the erroneous visual estimation. This was performed by a single reader who visually inspected the LAD of discrepant cases and classified calcium as being present or absent in the proximal, mid, and/or distal vessel. Analysis was performed on separate days for the CTAC and CAC images, with images being presented in random order on each day and the reader blinded to classifications from previous reading sessions.
Effect on Interpretation of Borderline MPI Scans
One use of the AS in hybrid imaging can be to provide an additional source of information to assist in interpretation of borderline MPI scans. For example, for a borderline, nonextensive perfusion defect, some readers will call the MPI study “probably normal” in the absence of coronary calcium, but report the perfusion defect in the presence of coronary calcium. Thus, inaccuracy of VECAC in estimating the AS could potentially lead not only to inaccurate characterization of coronary calcium, but also to inaccurate characterization of myocardial perfusion. To assess the effect of VECAC on MPI interpretation in such borderline scans, in one center (Columbia), we reassessed MPI in all scans which had been read as having a rest or stress perfusion defect in 1-3 segments of the AHA 17-segment model, none of which were described as a “severe” defect. MPI scans were read independently by each reader on 3 separate days, presented in random order on each occasion. On the first day, each reader read the MPI scan, using the 17-segment model. On the second day, each reader was presented with the MPI images, their original interpretation of these images, and the CAC scan images, and asked to reinterpret the MPI images in this context. The third reading day was analogous to the second day, except that CTAC scan images were presented rather than CAC scan images.
Statistical Analysis
Agreement between VECAC on the CTAC scan and AS, measured on the standard CAC scan and converted to the same six-level scale, was determined using quadratic-weighted kappa statistics, with 95% confidence intervals (CIs) estimated using bootstrap with 1000 replications. Agreement between readers in VECAC was similarly assessed using quadratic weighted kappa statistics. Vessel-based analysis was performed by evaluating the proportion of segments with correct identification of calcium on the CTAC scan and associated standard kappa scores. Statistical analysis was performed using Stata 10.1 (StataCorp, College Station, TX).
Results
Agreement between VECAC and Agatston score
A high degree of association was observed between VECAC and AS, with 63% of VECAC scores in exactly the same category as the AS category and 93% varying by no more than one category (Table 1). Only one significant outlier occurred, for a patient with marked LAD calcification close to the pulmonary artery, resulting in an AS of 1082. This was misidentified by one reader as pulmonary arterial calcification and thus visually classified as having no coronary calcification. Overall weighted kappa was 0.89 (p<0.0001). High weighted kappa statistics were observed for each site (Table 2), for women and men (0.89 and 0.90, respectively), and for both low-dose CTAC scans performed on a Philips SPECT/CT scanner (0.90, 95% CI 0.87-0.93) and even lower-dose CTAC scans performed on a Siemens PET/CT scanner (0.88, 95% CI 0.85-0.89).
Table 1.
Agreement between VECAC and Agatston Score in 492 patients with 2 readers per patient.
| Visually Estimated Calcium Score (VECAC) | ||||||
|---|---|---|---|---|---|---|
| Agatston Score | 0 | 1-9 | 10-99 | 100-399 | 400-999 | 1000+ |
| 0 | 323 | 14 | 11 | 2 | 0 | 0 |
| 1-9 | 46 | 8 | 8 | 0 | 0 | 0 |
| 10-99 | 37 | 13 | 100 | 24 | 2 | 0 |
| 100-399 | 5 | 4 | 57 | 73 | 33 | 2 |
| 400-999 | 0 | 0 | 1 | 44 | 62 | 19 |
| 1000+ | 1 | 0 | 1 | 5 | 33 | 56 |
Table 2.
Individual reader agreement between visually estimated calcium score (VECAC) and Agatston Score (AS).
| All Sites | Columbia | Mid America Heart | Cedars-Sinai | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Typical effective mAs (CTAC) | 50 | 9 | 11 | ||||||||
| CTAC breathing | Free-Breathing | End-Expiratory | Free-Breathing | ||||||||
| Typical effective mAs (AS) | 70 | 220 | 150 | ||||||||
| % of cases with AS of 0 | 53 | 26 | 20 | ||||||||
| Overall | Reader 1 | Reader 2 | Overall | Reader 1 | Reader 2 | Overall | Reader 1 | Reader 2 | Overall | ||
| Weighted kappa | 0.89 | 0.92 | 0.89 | 0.90 | 0.9 | 0.89 | 0.89 | 0.84 | 0.85 | 0.84 | |
| Weighted kappa (lower limit of 95% CI) | 0.88 | 0.89 | 0.82 | 0.87 | 0.86 | 0.86 | 0.87 | 0.77 | 0.78 | 0.80 | |
| Weighted kappa (upper limit of 95% CI) | 0.91 | 0.95 | 0.93 | 0.93 | 0.93 | 0.91 | 0.91 | 0.9 | 0.9 | 0.88 | |
| % of Cases with correct VECAC | 63 | 73 | 71 | 72 | 61 | 52 | 56 | 61 | 59 | 60 | |
| % of Cases within 1 category | 93 | 95 | 94 | 94 | 94 | 93 | 94 | 90 | 87 | 89 | |
Abbreviations: CTAC denotes CT attenuation scan, from which VECAC is estimated.
Statistics for each reader are summarized in Table 2, which reveals minimal variability between readers and sites in measures of agreement. When reviewing the same set of images from Columbia, all four readers obtained virtually identical diagnostic performance (Table 3), suggesting no significant intrinsic differences in classificatory ability between readers, which could serve as a confounder for comparisons between sites.
Table 3.
Individual reader agreement between visually estimated calcium score (VECAC) and Agatston Score for 100 cases from Columbia University Medical Center
| Columbia Reader 1 |
Columbia Reader 2 |
Mid America Reader 1 |
Cedars-Sinai Reader 2 |
|
|---|---|---|---|---|
| Weighted kappa | 0.93 | 0.87 | 0.91 | 0.92 |
| Weighted kappa (lower limit of 95% CI) | 0.89 | 0.77 | 0.85 | 0.89 |
| Weighted kappa (upper limit of 95% CI) | 0.96 | 0.94 | 0.95 | 0.96 |
| % of Cases with correct VECAC | 73 | 72 | 74 | 71 |
| % of Cases within 1 category | 97 | 93 | 94 | 95 |
Images obtained using typical effective mAs of 50 mAs and free-breathing. CI denotes confidence interval.
For each AS range, the most common VECAC score was its corresponding score on the six level scale, and vice versa, with the exception of AS between 1 and 9. Of the 31 patients with AS 1-9, each for whom VECAC was estimated by two readers, VECAC was estimated as 0 in 46 cases, 1-9 in 8 cases, and 10-99 in 8 cases. Of the 39 cases in which VECAC was estimated as 1-9, the actual AS was 0 in 14 cases, 1-9 in 8 cases, 10-99 in 13 cases, and 100-399 (maximum 207) in 4 cases.
Vessel-based analysis
Agreement between the CTAC scan and the calcium scoring scan in identifying coronary artery calcium was good for all vessels. For the left main, LAD, circumflex, and right coronary arteries, percent agreement was 78.9, 89.6, 84.6, and 83.5%, respectively. Corresponding kappas were 0.41, 0.79, 0.67, and 0.65, respectively. A discrepancy for one or both readers in the presence of LAD calcium between CAC and CTAC scans was only observed in 20 of the 197 cases. On subsequent localization analysis, in only 3 of these cases was proximal LAD calcium missed on the CTAC scan and observed on the CAC scan, however in 2 of these, left main calcium had been observed on the CTAC scan by one reader. This suggests that VECAC does not systematically miss proximal disease.
Interobserver reproducibility
A high degree of interobserver reproducibility in VECAC scores was observed with readers reporting identical scores in 65% of cases and scores within one category of each other in 93% of cases (Table 4). Weighted kappa was 0.90 (95% confidence interval 0.87-0.92, p<0.0001). High weighted kappas were observed for each site (0.90, 0.88, and 0.90), for women and men (0.89 and 0.90, respectively), and for both low-dose CTAC scans performed on a Philips SPECT/CT scanner (0.90, 95% CI 0.85-0.94) and lower-dose CTAC scans performed on a Siemens PET/CT scanner (0.89, 95% CI 0.85-0.91).
Table 4.
Agreement between readers in 493 patients.
| Reader 2 | ||||||
|---|---|---|---|---|---|---|
| Reader 1 | 0 | 1-9 | 10-99 | 100-399 | 400-999 | 1000+ |
| 0 | 183 | 12 | 13 | 1 | 0 | 0 |
| 1-9 | 5 | 3 | 7 | 0 | 0 | 0 |
| 10-99 | 12 | 7 | 48 | 26 | 4 | 0 |
| 100-399 | 2 | 2 | 13 | 31 | 27 | 0 |
| 400-999 | 1 | 0 | 0 | 15 | 29 | 18 |
| 1000+ | 0 | 0 | 0 | 0 | 7 | 26 |
Effect on Interpretation of Borderline MPI Scans
Borderline MPI imaging was observed in 19 cases. In none of these did use of the CTAC scan rather than the CAC scan result in a difference in MPI interpretation for both readers. In 3 cases, use of CTAC rather than CAC resulted in a difference in MPI interpretation for one reader. In one case, for Reader 1, an apical defect on stress-only MPI was read as normal when read with the CAC scan, but still read as an apical defect when the CTAC scan was substituted; Reader 2 read this scan as “normal” in all 3 instances. In the second case, Reader 1 called a mild, fixed inferoapical defect which was unchanged with CAC or CTAC images; Reader 2 called the defect “normal” on standard MPI and MPI with CTAC, but called the fixed inferoapical defect when reading MPI with CAC images. In the third case, Reader 1 called a reversible anteroapical defect in the presence of breast attenuation which was unchanged with CAC or CTAC images; Reader 2 called the scan “normal” on standard MPI, “probably normal” on MPI with CTAC, but called the reversible defect on MPI with CAC. Thus, the effect of CTAC rather than CAC images on interpretation of borderline MPI appears to be small, with any discrepancies not consistent between readers.
Discussion
Implications
The findings of this study have several important implications. Firstly, coronary calcium can be assessed from a low-dose CTAC scan. While the classification of CAC categories was not identical for the two methods, the 93% agreement within one category suggests that much of the diagnostic and prognostic information provided by calcium scoring is available from the scan that is already performed for attenuation correction in patients undergoing hybrid PET/CT or SPECT/CT MPI. This may in some cases obviate the need for a separate calcium scoring scan, with its attendant modest addition to the total radiation burden of the study.
A second important implication of this study is that it suggests the possibility that standard calcium scoring may be performed using lower radiation dose protocols than have been conventionally used. A recent paper evaluating radiation dose from coronary artery calcium scoring found that effective doses from standard scanning protocols used in the MESA and CARDIA studies, the International Consortium on Standardization in Cardiac CT(3), and three university medical centers, ranged from a low of 0.8 mSv to a high of 10.5 mSv in one CARDIA site.(4) Effective dose associated with the CTAC scan protocol used in two of the three sites here was 0.3 mSv, markedly lower than the effective doses used in the corresponding CAC scan protocols (4.7 and 2.9 mSv), despite the greater scan length. Certainly, very low-dose CAC protocols would require further validation prior to adoption for clinical use. Even so, Mid America Heart has already adjusted its standard clinical protocol to lower radiation dose from calcium scoring. While cancer risks associated with calcium scoring are extremely low, with estimates for a typical protocol ranging between 0.003% and 0.028% depending upon patient age and gender, screening approximately 50 million individuals with calcium scoring as has been proposed in the SHAPE guidelines(5) could result in an estimated 5,600 cancers(4), based on the assumptions of the BEIR VII report.(6) Validation of very-low-dose calcium scoring protocols would favorably affect the risk-benefit balance of such a screening strategy.
Benefits of CTAC
Performance of a CTAC scan now potentially adds three pieces of diagnostic information to an MPI examination: attenuation correction, extra-coronary findings, and coronary artery calcium classification. Due to the significant amount of attenuation, attenuation correction is regarded as essential for PET MPI, and is routinely employed by all laboratories performing this test. For SPECT MPI, it provides the possibility to eliminate false positive perfusion defects(1), thereby improving test specificity and positive predictive value and avoiding unnecessary downstream testing including invasive angiography. Reflecting this, a multi-societal position statement maintains that attenuation correction “will improve image quality, interpretive certainty, and diagnostic accuracy.”(7) Several studies have now shown the high frequency of relevant extra-cardiac findings in CT scans performed for cardiac evaluation(8) or for attenuation correction.(9) While such findings are incidental in some patients, in others they are causal. Thus, CTAC offers the potential to identify non-cardiac causes (e.g. hiatal hernia, aortic dissection, emphysema, and pulmonary edema) of symptomatology, such as chest pain or dyspnea, for which patients present for MPI. As demonstrated here, CTAC also adds the ability to semi-quantitatively assess coronary artery calcium.
Given these three additional pieces of diagnostic information resulting from a CTAC scan added to an MPI examination, we propose that the time has arrived for consideration of its reimbursement by the Centers for Medicare and Medicaid Services and third-party payers. Payment generally is deemed reasonable when a procedure incurs extra expense and work, when it is widely used in different settings, and when it has been shown to convey clinical benefit. CTAC scanning improves quality of patient care by i) potentially improving diagnostic performance of MPI, ii) decreasing healthcare costs and morbidity from unnecessary follow-up testing, iii) clarifying etiology of presenting symptoms in some patients, and iv) supplementing perfusion imaging with an assessment of the presence and extent of coronary artery disease, which can affect clinical decision making in terms of treatment with anti-platelet and lipid-lowering agents in patients without known flow-limiting coronary disease.(10) A complete CTAC study, with documentation of the effect of attenuation correction on perfusion defects, pertinent extra-coronary findings, and a VECAC score, merits consideration for an additional billing code.
CTAC protocol differences between sites
CTAC scan protocols used in this study differed somewhat between sites. Differences in technique included the use of an end-expiratory breath-hold at Mid America Heart versus free breathing at other sites, and the use of a higher tube current at Columbia. Free breathing can result in gaps in the CT data set due to movement of the heart cranially with expiration, resulting in missed segments of coronary calcium, while lowering tube current results in increased image noise. Nevertheless, there were only minor differences in diagnostic performance between sites, with overlapping 95% CIs for weighted kappa between any pair of sites or readers.
The foremost purpose of the CTAC scan is to provide accurate attenuation correction, and protocol selection must be aimed at ensuring this. Myocardial perfusion images are acquired over an acquisition lasting several minutes, during which the patient is freely breathing, motivating many sites and vendors to prefer a free-breathing CT scan for attenuation correction. While limited data suggest that an end-expiratory CT scan may be as effective for attenuation correction as a free-breathing scan,(11,12) this issue requires further study, and the optimal breathing protocol for CTAC remains uncertain.(13) Even if end-expiratory CT scanning is preferred, in practice, many patients are unable to effectively follow instructions to reliably perform an end-expiratory scan.
It also remains unclear what tube current is necessary to ensure optimal CTAC. Columbia, using the vendor's suggested protocol, typically employed effective mAs of 50, while the other sites used ∼10 mAs, resulting in appreciably noisier images (Figure 2). Nevertheless, the experience is that these noisier images are generally adequate for PET attenuation correction. The spatial resolution of the CT scan is much higher than that of Rb-82 PET or Tc-99m SPECT, resulting in image noise being decreased by averaging CT numbers in the larger voxels used for nuclear imaging. PET/CT data obtained from N-13 ammonia assessment of myocardial blood flow evaluation(14) and F-18 fluorodeoxyglucose metabolic imaging(15), as well as phantom work(16), suggest that very-low-dose CTAC is indeed adequate, however further validation for myocardial perfusion would be desirable.
Limitations
Our study has a few limitations. The six-level system used typically resulted in accurate classifications, with the exception of patients classified with VECAC of 1-9, who usually had AS of either 0 or 10-99, with similar representation in each group (14 vs. 13 cases). While a grading system with fewer categories could be considered, by grouping a VECAC of 1-9 together with either VECAC of 0 or VECAC of 10-99, each of these approaches would have limitations in terms of risk stratification.(17)
A lower kappa value was noted for the left main than for other coronary arteries. The left main can be difficult to distinguish from the proximal LAD or circumflex, especially on low-dose and non-breathhold scans.
While we observed generally good inter-reader reproducibility, all 6 readers were highly experienced in reading standard CAC scores prior to initiation of the current study. The subjective assessment involved in assigning a VECAC score offers the potential for more variability among less experienced readers. A possible solution to this limitation would be a true quantitative measure of coronary artery calcium on the CTAC scans, obtained by marking regions of potential interest identified with coronary calcification and performing some calculation based on these regions. Use of a CT number threshold of 130 Hounsfield units, as in the Agatston score(18), would result in under-identification of some areas of coronary calcification. Due to CTAC scan technique, and especially free-breathing which may blur coronary arteries and thereby result in decreased CT numbers, in our experience some regions with mean CT numbers of even 80 Hounsfield units appear consistent with coronary artery calcium. This differs from the findings of Wu et al, who observed that a standard Agatston method could be reliably used on 0.9 mSv CT scans performed for lung cancer screening.(19) For CTAC scans, any threshold chosen and calculation performed would likely need to vary depending on scan technique. While this is a potentially important area for future study, our results suggest that the semi-quantitative assessment provided by VECAC scoring provides outstanding agreement with AS while generally requiring little time for assessment.
Another limitation related to reproducibility is that we did not assess interscan reproducibility for VECAC or AS. Since patients were already obtaining two CT scans of the chest, one for CTAC and one for AS, we did not consider it justifiable in terms of radiation burden to perform two additional scans to assess interscan reproducibility. Nevertheless, it is known that repetition of calcium scoring is associated with a small difference in AS between scans. With a 16-slice scanner, Horiguchi et al observed mean interscan difference in AS of 7% with low motion artifact and 19% with high motion artifact,(20) while with a 64-slice scanner Matsuura et al observed mean interscan difference in diastole of 8%.(21) In utilizing a 6-level VECAC score rather than a precise quantification of calcium, our goal was to stratify patients into broad categories corresponding to levels of cardiovascular risk, rather than to precisely estimate AS. Nevertheless, the effect of interscan variability of AS on the estimation of AS by VECAC requires further study.
Another potential limitation relates to the generalizability of our findings. Each site in our study used a hybrid scanner with at least 16 slices for CT. Systems with fewer slices may be more susceptible to motion artifacts, especially with free breathing.(22) Although standard multi-detector row scanners with ≥4 slices and gantry rotation time ≤0.5 seconds are generally regarded as adequate for calcium scoring, the agreement of VECAC with AS for scanners with <16 slices has not been studied. Some SPECT/CT scanners only allow tube currents considerably lower than those used in this study (e.g. maximum 2.5 mA), increased tube voltage, slow rotation speed, and decreased spatial resolution.(23) This combination of scan parameters will likely result in decreased accuracy of VECAC. Thus, estimation of VECAC from CTAC images on such scanners should not be performed in the absence of independent validation of its diagnostic performance.
Conclusion
In summary, coronary artery calcium can be visually estimated from a CTAC scan with outstanding agreement with Agatston score and a high degree of interobserver reproducibility. CTAC scanning should be routinely used when available, and the associated images inspected. This approach supplements the diagnostic information from myocardial perfusion imaging by correcting perfusion defects caused by soft-tissue attenuation, demonstrating extra-coronary causes of chest pain and dyspnea, and characterizing coronary artery calcium.
Acknowledgments
Financial Support: Dr. Einstein is supported in part by an NIH K12 institutional career development award (5 KL2 RR024157-04).
Abbreviations
- CAC
Coronary artery calcium
- CT
Computed tomography
- CTAC
CT attenuation correction
- PET
Positron emission tomography
- SPECT
Single photon emission computed tomography
- MPI
Myocardial perfusion imaging
- ECG
Electrocardiogram
- AS
Agatston score
- VECAC
Visually estimated CAC
- LAD
Left anterior descending
- CI
Confidence interval
Footnotes
Presented in part at ACC.09, March 30, 2009, and at ICNC9, May 12, 2009.
Relationship with Industry: No industry funding was received for this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garcia EV. SPECT attenuation correction: an essential tool to realize nuclear cardiology's manifest destiny. J Nucl Cardiol. 2007;14:16–24. doi: 10.1016/j.nuclcard.2006.12.144. [DOI] [PubMed] [Google Scholar]
- 2.Bongartz G, Golding SJ, Jurik AG, et al. European Guidelines on Quality Criteria for Computed Tomography. The European Commission's Study Group on Development of Quality Criteria for Computed Tomography. 2000 [Google Scholar]
- 3.McCollough CH, Ulzheimer S, Halliburton SS, Shanneik K, White RD, Kalender WA. Coronary artery calcium: a multi-institutional, multimanufacturer international standard for quantification at cardiac CT. Radiology. 2007;243:527–38. doi: 10.1148/radiol.2432050808. [DOI] [PubMed] [Google Scholar]
- 4.Kim KP, Einstein AJ, Berrington de Gonzalez A. Coronary artery calcification screening: estimated radiation dose and cancer risk. Arch Intern Med. 2009;169:1188–94. doi: 10.1001/archinternmed.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naghavi M, Falk E, Hecht HS, et al. From vulnerable plaque to vulnerable patient--Part III: Executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Am J Cardiol. 2006;98:2H–15H. doi: 10.1016/j.amjcard.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. Health Risks From Expsure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington: The National Academies Press; 2006. [PubMed] [Google Scholar]
- 7.Heller GV, Links J, Bateman TM, et al. American Society of Nuclear Cardiology and Society of Nuclear Medicine joint position statement: attenuation correction of myocardial perfusion SPECT scintigraphy. J Nucl Cardiol. 2004;11:229–30. doi: 10.1016/j.nuclcard.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Horton KM, Post WS, Blumenthal RS, Fishman EK. Prevalence of significant noncardiac findings on electron-beam computed tomography coronary artery calcium screening examinations. Circulation. 2002;106:532–4. doi: 10.1161/01.cir.0000027136.56615.de. [DOI] [PubMed] [Google Scholar]
- 9.Osman MM, Cohade C, Fishman EK, Wahl RL. Clinically significant incidental findings on the unenhanced CT portion of PET/CT studies: frequency in 250 patients. J Nucl Med. 2005;46:1352–5. [PubMed] [Google Scholar]
- 10.Thompson RC, McGhie AI, Moser KW, et al. Clinical utility of coronary calcium scoring after nonischemic myocardial perfusion imaging. J Nucl Cardiol. 2005;12:392–400. doi: 10.1016/j.nuclcard.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Dey S, Kritzman JN, Ficaro EP, Corbett JR. Comparison of end-expiratory breath hold and shallow breathing protocols for CT based attenuation correcgtion of 99Tc-sestamibi myocardial perfusion images using multi-slice SPECT-CT. J Nucl Cardiol. 2005;12:S122. [Google Scholar]
- 12.Gilman MD, Fischman AJ, Krishnasetty V, Halpern EF, Aquino SL. Optimal CT breathing protocol for combined thoracic PET/CT. AJR Am J Roentgenol. 2006;187:1357–60. doi: 10.2214/AJR.05.1427. [DOI] [PubMed] [Google Scholar]
- 13.Slomka PJ, Le Meunier L, Hayes SW, et al. Comparison of myocardial perfusion 82Rb PET performed with CT- and transmission CT-based attenuation correction. J Nucl Med. 2008;49:1992–8. doi: 10.2967/jnumed.108.056580. [DOI] [PubMed] [Google Scholar]
- 14.Koepfli P, Hany TF, Wyss CA, et al. CT attenuation correction for myocardial perfusion quantification using a PET/CT hybrid scanner. J Nucl Med. 2004;45:537–42. [PubMed] [Google Scholar]
- 15.Souvatzoglou M, Bengel F, Busch R, et al. Attenuation correction in cardiac PET/CT with three different CT protocols: a comparison with conventional PET. Eur J Nucl Med Mol Imaging. 2007;34:1991–2000. doi: 10.1007/s00259-007-0492-4. [DOI] [PubMed] [Google Scholar]
- 16.Wu TH, Chu TC, Huang YH, et al. A positron emission tomography/computed tomography (PET/CT) acquisition protocol for CT radiation dose optimization. Nucl Med Commun. 2005;26:323–30. doi: 10.1097/00006231-200504000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. J Am Coll Cardiol Img. 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 19.Wu MT, Yang P, Huang YL, et al. Coronary arterial calcification on low-dose ungated MDCT for lung cancer screening: concordance study with dedicated cardiac CT. AJR Am J Roentgenol. 2008;190:923–8. doi: 10.2214/AJR.07.2974. [DOI] [PubMed] [Google Scholar]
- 20.Horiguchi J, Fukuda H, Yamamoto H, et al. The impact of motion artifacts on the reproducibility of repeated coronary artery calcium measurements. Eur Radiol. 2007;17:81–6. doi: 10.1007/s00330-006-0278-2. [DOI] [PubMed] [Google Scholar]
- 21.Matsuura N, Horiguchi J, Yamamoto H, et al. Optimal cardiac phase for coronary artery calcium scoring on single-source 64-MDCT scanner: least interscan variability and least motion artifacts. AJR Am J Roentgenol. 2008;190:1561–8. doi: 10.2214/AJR.07.3120. [DOI] [PubMed] [Google Scholar]
- 22.Shyn PB. Protocol considerations for thoracic positron emission tomography-computed tomography. Semin Ultrasound CT MR. 2008;29:242–50. doi: 10.1053/j.sult.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Hamann M, Aldridge M, Dickson J, Endozo R, Lozhkin K, Hutton BF. Evaluation of a low-dose/slow-rotating SPECT-CT system. Phys Med Biol. 2008;53:2495–508. doi: 10.1088/0031-9155/53/10/003. [DOI] [PubMed] [Google Scholar]


