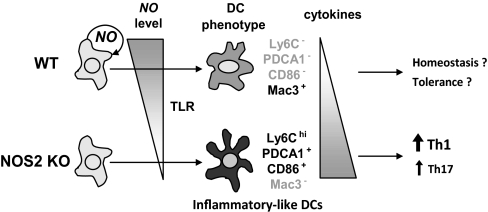
NO produced by DCs suppresses a Ly6ChiPDCA1+ DC subpopulation resembling inflammatory DCs, highly responsive to TLR stimulation and capable of initiating strong Th1 immune responses.
Keywords: NOS2 KO, TipDCs, Th17, West Nile virus
Abstract
Using NOS2 KO mice, we investigated the hypothesis that NO modulation of BM-DC contributes to the NO-mediated control of Th1 immune responses. BM-DCs from NOS2 KO mice, compared with WT BM-DCs, have enhanced survival and responsiveness to TLR agonists, develop more Ly6ChiPDCA1+ DCs that resemble inflammatory DCs and produce high levels of inflammatory cytokines. Also, compared with WT-infected mice, NOS2 KO mice infected with WNV showed enhanced expansion of a similar inflammatory Ly6ChiPDCA1+ DC subset. Furthermore, in contrast to WT DCs, OVA-loaded NOS2 KO BM-DCs promoted increased IFN-γ production by OTII CD4+ T cells in vitro and when adoptively transferred in vivo. The addition of a NO donor to NOS2 KO BM-DCs prior to OTII T cells priming in vivo was sufficient to revert Th1 immune responses to levels induced by WT BM-DCs. Thus, autocrine NO effects on maturation of inflammatory DCs and on DC programming of T cells may contribute to the protective role of NO in autoimmune diseases and infections. Regulating NO levels may be a useful tool to shape beneficial immune responses for DC-based immunotherapy.
Introduction
DCs coordinate key steps during the generation of innate and adaptive immune responses. Different DC subsets express distinct sets of PRRs that enable them to respond appropriately to pathogen-derived molecules and inflammatory signals induced following microbial infection. Understanding how DCs integrate these messages is becoming crucial to predict their ability to program adaptive immune responses when designing DC-based vaccines and immunotherapy strategies [1, 2].
Inflammation induces the expression of the NOS2/iNOS and NO production in human and murine monocytes, macrophages, and DC subsets [3–7]. NO is not only a proinflammatory cytotoxic mediator that defends hosts by inactivating and destroying infectious agents and tumor cells, it also regulates adaptive immune responses [5, 6, 8]. NOS2 KO mice, in addition to being more susceptible to several pathogens, develop stronger Th1 immune responses than WT mice [6, 9, 10]. Furthermore, NOS2 KO mice are more susceptible than WT mice to the development of autoimmune diseases such as EAE [11–13]. Exacerbated autoimmune responses in NOS2 KO mice have been mainly attributed to overexpansion and failure of autoreactive T cells to undergo apoptosis [11, 14]. Increased IL-12 production by NOS2 KO macrophages may also contribute to excessive amplification of Th1 immune responses [15]. Treatment with a NO donor inhibits IL-12p70 produced by IFN-γ/LPS-treated mouse BM-DCs [16]. In agreement with these findings, we found that NO inhibits IL-12p70 production by LPS-treated human MoDCs [17]. However, the investigation of DC regulation by NO in human DCs is limited to in vitro studies with NO donors and inhibitors and has produced disparate results. The closest equivalents of blood MoDCs that have been used for human therapies are murine BM-DCs [18–20].
It is well established that during inflammation, Ly6Chi monocytes emigrate from the BM and differentiate into DCs [21–24]. In contrast to classical murine and human DC subsets—cDCs and pDCs—Ly6Chi inflammatory DCs are not present in the steady-state but require a microbial or an inflammatory stimulus to develop [22, 25–27]. GM-CSF is also required for the in vivo generation of inflammatory DCs [25]. A CD11bhiLy6Chi population of inflammatory DCs is essential for a protective Th1 immune response against Leishmania major [24]. Interestingly, some inflammatory DCs express TNF-α and NOS2 and are termed TipDCs, which emerge during inflammatory responses and play an important role in innate immune responses against intracellular bacteria [28]. Using NOS2 KO mice, Tezuka et al. [29] found that NO-producing TipDCs in the gut are required for IgA production in the intestinal mucosa. TipDCs have been described also in human psoriasis, atherosclerosis, and carcinomas [30–32].
As murine BM-DCs share many properties with human MoDCs [18–20], we asked how NOS2 deficiency affects mouse BM-DC development and their ability to induce Th1 immune responses in vivo. We also focused initially on BM-DCs, as unlike splenic DCs, they express NOS2 and secrete high NO levels [33, 34]. We found that after exposure to TLR stimuli, NOS2 KO BM-DCs compared with WT BM-DCs strongly up-regulate a subpopulation of inflammatory-like Ly6ChiPDCA1+CD86+ DCs, expressing high levels of inflammatory cytokines. We have shown recently that WN-TX can be a potent inflammatory stimulus [35]. Strikingly, after infection with WN-TX, a similar inflammatory Ly6ChiPDCA1+CD86+ DC subset was expanded more in NOS2 KO mice than in WT mice. Furthermore, in vivo priming with OVA-loaded NOS2 KO BM-DCs, in contrast to WT BM-DCs, induced increased Th1 immune responses in OTII Tg mice. Our findings suggest that the main target responsible for the ability of NO to program T cell responses via DCs is a specific DC subset resembling Ly6Chi inflammatory DCs and thus, underscore the potentially protective role of NO acting on DCs in regulating Th1-associated pathologies.
MATERIALS AND METHODS
Mice
C57BL/6 (WT) and B6-NOS2 KO mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA; B6.129P2-Nos2tm1Lau/J, Stock No.002609). OTII TCR Tg mice were a kind gift from Keith Elkon (Department of Medicine, University of Washington, Seattle, WA, USA). Mice were housed in specific pathogen-free conditions, according to institutional guidelines, and used at 8–12 weeks of age. The University of Washington's Institutional Animal Care and Use Committee approved all animal protocols.
Generation of BM-DCs, cell culture and sorting of Ly6ChiPDCA1+ DCs
BM-DCs were generated as described [36, 37]. BM cells were prepared by flashing femurs and tibiae. Erythrocytes were lysed, and the remaining cells were seeded in 24-well plates at 1 × 106 cells/ml in RPMI 1640 supplemented with 10% FBS (R10), 20 ng/ml GM-CSF, and 10 ng/ml IL-4 (Fitzgerald Industries International, Acton, MA, USA) at 37°C in 5% CO2. Nonadherent cells were removed at Day 2, and medium was replaced with fresh medium supplemented with cytokines at Days 2 and 4 (50%). After 6–7 days, loosely adherent cells were collected; 80–99% cells were CD11c+. In some experiments, BM-DCs were seeded at 1.2–2.0 × 106 cells/2 ml in 12-well plates in R10 ± Escherichia coli LPS (Sigma Chemical Co., St. Louis, MO, USA), zymosan (Sigma Chemical Co.), or CpG (Molecular Probes, Invitrogen, Carlsbad, CA, USA), alone or with the NO donor NOR4 (100 μM; Calbiochem, San Diego, CA, USA) or the vehicle DMSO (Sigma Chemical Co.). In all experiments where NOS2 KO BM-DCs were treated with NOR4, the control samples with DMSO vehicle control gave the same results as samples without NOR4.
In some experiments, Ly6ChiPDCA1+ DCs from WT and NOS2 KO were sorted from BM-DC cultures by flow cytometry using FACSAria II (Becton Dickinson, Franklin Lakes, NJ, USA).
Splenic and LN cells and isolation of T cells
Spleens or popliteal dLNs were removed, minced into small fragments, and digested for 45 min at 37°C with liberase R1 and DNase (Roche, Indianapolis, IN, USA). The cells were washed, and erythrocytes were lysed and filtered. CD4+ T cells were isolated from spleens using a mouse CD4+ T cell enrichment kit (StemCell Technologies, Vancouver, BC, Canada).
Coculture of DCs and OTII CD4+ T cells
NOS2 KO or WT BM-DCs were pulsed for 1 h with 2.5 μM OVAp (AnaSpec, Fremont, CA, USA), washed, and cocultured in 12-well plates for 5 days with purified CD4+ OTII T cells at a ratio of 2.5:1 (1×106 T cells/well; 4×105 DCs/well) in medium only or with 0.3 μg/ml LPS. Supernatants were collected every day for 5 days, and IFN-γ and IL-17 levels were determined by ELISA.
DC priming of OTII Tg mice
To determine the priming ability of DCs, WT and NOS2 KO BM-DCs or sorted Ly6ChiPDCA1+ BM-DCs were pulsed with 2.5 μM OVAp in the presence of 0.3 μg/ml LPS ± 100 μM NOR4 for 18 h and then washed. OTII mice were injected i.p. with 1 × 106 OVAp-pulsed BM-DCs or with PBS only as a control. After 5 days, spleens were removed, and splenocytes were incubated with medium only or 2.5 μM OVAp. At the indicated times, supernatants were collected and analyzed for IFN-γ and IL-17 levels by ELISA. In some experiments, OTII mice were injected i.p. with 0.6 × 106 OVAp-pulsed Ly6ChiPDCA1+ BM-DCs from WT and NOS2 KO mice. Spleens were harvested 5 days after injection; splenocytes were cultured for 2 days in medium only or in the presence of 2.5 μM OVAp, and IFN-γ and IL-17 expression by CD4+TCRVβ5+ T cells was detected by intracellular staining.
WN-TX mouse infections
WN-TX was isolated, and virus stocks were tittered by a standard plaque assay on BHK21 cells, as described previously [35]. Working stocks of WN-TX were generated by a single round of amplification on Vero-E6 (ccl-81; American Type Culture Collection, Manassas, VA, USA) cells, and supernatants were collected, aliquoted, and stored at –80°C.
Age-matched, 6- to 10-week-old WT and NOS2 KO mice were inoculated s.c. in both rear footpads with 1000 PFU WN-TX in a 10-μl inoculum diluted in HBSS supplemented with 1% heat-inactivated FBS. Mice were monitored daily for morbidity and mortality. Alternatively, the dLNs from infected mice were harvested, and lymphocytes were prepared as described above.
FACS analyses
Cells were incubated with anti-CD16/CD32 blocking antibodies (2.4G2) for 10 min at room temperature and then stained with the corresponding antibody mixture on ice. The following mAb were purchased from eBioscience (San Diego, CA, USA) or BD Biosciences (San Jose, CA, USA), unless otherwise indicated: APC-AlexaFluor750- or FITC-, PE-, or APC-anti-CD11c (N418); FITC-, PE-, or PECy5-anti-CD86 (GL1); FITC-anti-MHCII (AF6-120.1); PE-anti-Gr1 (Ly6C/G; RB6-8C5); FITC- or PE-anti-Ly6C (AL-21); PE-anti-PDCA1 (JF05-1C2.4.1; MACS, Miltenyi Biotec, Auburn, CA, USA); PE- or FITC-anti-Mac3 (M3/84); PE-anti-CD11b (M1/70); PerCP- or APC-anti-CD8α (53-6.7); PerCP- or PE-anti-B220 (RA3-6B2); APC-anti-CD4 (RM4-5); FITC-anti-TCRVβ5 (MR9-4); Alexa Fluor 647- or PE-anti-IL-12p40 (C17.8); Alexa Fluor 647-anti-TNF-α (MP6-X722); Alexa Fluor 405-anti-NOS2 (C11; Santa Cruz Biotechnology, Santa Cruz, CA, USA); eFluor 450-anti-IFN-γ (XMG1.2); and PE-anti-IL-17 (TC11-18H10). For intracellular staining of BM-DCs, cells were stimulated for 8–14 h with the TLR stimuli with Brefeldin A (eBioscience), added for the last 5 h of stimulation. For intracellular staining of splenic CD4+TCRVβ5+ T cells from OTII mice primed with sorted Ly6ChiPDCA1+ DCs, splenocytes, after 2 days of ex vivo restimulation ± OVAp, were restimulated for 5 h with Brefeldin A (eBioscience) plus 50 ng/ml PMA/1 μM ionomycin (Sigma Chemical Co.). Cells were then stained with mAb for surface markers, fixed, and permeabilized using BD Cytofix/ Cytoperm (BD Biosciences) or 0.1% saponin in staining buffer, followed by anti-IL12p40, anti-TNF-α, and anti-NOS2 staining for BM-DCs or anti-IFN-γ and anti-IL-17 for splenic CD4+TCRVβ5+ T cells. Fluorescence acquisition was done on an LSRII FACScan analyzer (Becton Dickinson) using FACSDiva software, and data analysis was performed with FlowJo software (TreeStar Inc., Ashland, OR, USA).
Mitochondrial integrity was assayed using the dye Mitotracker Red CMXROS (Molecular Probes, Invitrogen). Cells were treated or not with LPS for 24 h, after which, they were incubated with 50 nmol/L dye for 45 min at 37°C in 5% CO2, washed with PBS, resuspended in PBS + 1% BSA, and analyzed by FACS (fluorescence 3 channel).
Cytokine detection
Concentrations of IL-12-p40, IL-12p70, IL-23, IL-6, TNF-α, IL-10, IFN-γ, and IL-17 were determined by specific ELISAs, performed in triplicate using a matched pair of cytokine-specific mAb and recombinant cytokines as standards (R&D Systems, Minneapolis, MN, USA, and eBioscience).
Statistical analysis
For in vitro experiments, the statistical significance of differences in the means ± sd of surface markers or cytokines released by cells of various groups was calculated with the two-tailed paired Student's t test. The statistical analysis for in vivo experiments with WNV infections and OTII mice was performed with two-tailed unpaired t test and Mann Whitney test, respectively. Kaplan-Meier survival curves were analyzed by the log-rank test.
Online Supplemental material
Supplemental Fig. 1 shows that BM-DCs from NOS2 KO mice have increased survival compared with WT BM-DCs. Supplemental Fig. 2 shows that TLR2 and TLR4 stimulations induce more CD86+, PDCA1+, and MHCII+ DCs in NOS2 KO than in WT GM/IL-4-DC cultures. Supplemental Fig. 3 shows that a Ly6ChiPDCA1+CD86+ DC subset is more up-regulated in NOS2 KO versus WT BM-DCs and is responsible for the major increase in IL-12/23 and TNF production. Supplemental Fig. 4 shows that NOS2 KO DCs compared with WT DCs induce CD4+ T cells to produce more IFN-γ and IL-17 in vitro and in vivo.
RESULTS
NOS2 KO DCs have enhanced survival and expression of activation markers including PDCA1 after TLR stimulation
Previous studies reported that NO induces DCs to die [38] or protects them from apoptosis [39]. NOS2 is expressed at low levels in WT BM-DCs and is up-regulated 24 h after treatment with LPS or zymosan (Supplemental Fig. 1A, and not shown). As expected, NOS2 KO BM-DCs did not express NOS2 before or after TLR stimulation (Supplemental Fig. 1A). A comparison of WT versus NOS2 KO BM-DC survival revealed that significantly less apoptotic NOS2 KO DCs were present 24 h after culture in medium, LPS, or zymosan (Supplemental Fig. 1B and C). Thus, NO produced by BM-DCs only slightly reduces DC survival.
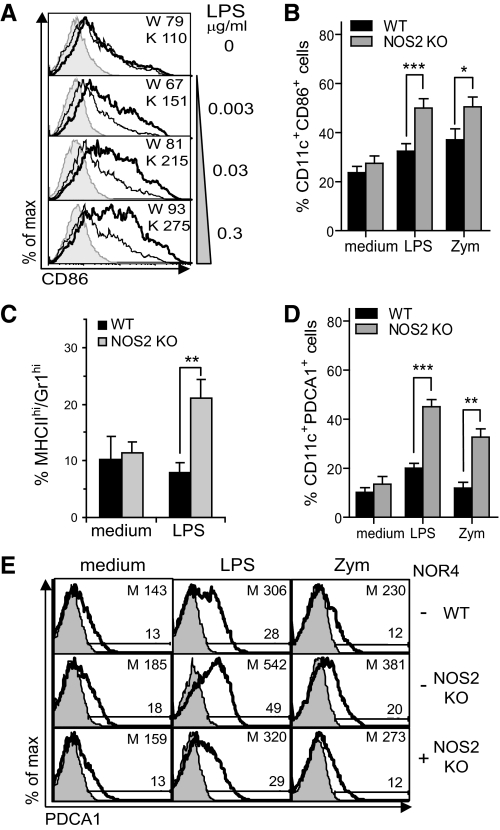
To assess if stronger Th1 immune responses in NOS2 KO mice might be, in part, a result of dysregulation of DC maturation in the absence of NO, we compared activation of NOS2 KO versus WT BM-DCs in response to TLR2, TLR4, and TLR9 agonists (Fig. 1 and Supplemental Fig. 2). A slight but not significant increase in CD86 expression was observed prior TLR stimulation in NOS2 KO DCs versus WT DCs, however, LPS induced significantly more CD86 on NOS2 KO DCs compared with WT DCs (Fig. 1A and B and Supplemental Fig. 2A and B). Similarly, zymosan and CpG-DNA induced more CD86 on NOS2 KO than WT DCs (Fig. 1B and Supplemental Fig. 2A and B; data not shown). The absence of NO in DCs also led to enhanced up-regulation of MHCII after LPS stimulation, most evident in a subpopulation of MHCIIhi cells expressing high levels of the Gr1 marker (Fig. 1C and Supplemental Fig. 2C). Ly6G and Ly6C are detected by anti-Gr1 and are expressed by granulocytes, however monocytes and some DC subsets express Ly6C but not Ly6G [27]. Therefore, the CD11c+MHCIIhiGr1hi DCs were most likely Ly6C+ DCs, which we confirmed subsequently (see below).
Figure 1. TLR stimulation induces more NOS2 KO DCs than WT DCs to develop a mature DC phenotype.
WT and NOS2 KO BM-DCs cultured for 24 h with medium, 0.3 μg/ml LPS (A–E), and 100 μg/ml zymosan (Zym; B, D, and E). Plots are gated on CD11c+ cells. (A) CD86 mean fluorescence intensity in WT (W; thin lines) versus NOS2 KO (K; thick lines) BM-DCs treated with graded doses of LPS. (B) Expansion of CD11c+CD86+ in NOS2 KO versus WT DCs. (C) Expansion of MHCIIhiGr1hi cells in NOS2 KO versus WT DCs. Graph shows mean ± sd; n = 5. (D) Expansion of CD11c+PDCA1+ in NOS2 KO versus WT DCs. (E) Representative flow cytometric analysis of PDCA1+ cells. The addition of 100 μM NOR4 to LPS NOS2 KO DC cultures restored WT phenotype. (B and D) Graphs show mean ± sem; n = 10 (LPS); n = 7 (zymosan). P values reported are from a two-tailed paired Student's t test analysis; *P < 0.05; **P < 0.01; ***P < 0.001. (A and E) Data are representative of greater than four independent experiments, and isotype controls are shown as shaded histograms. M, Mean fluorescence intensity.
To assess if the absence of NO might preferentially induce certain DC subpopulations, we further compared the surface phenotype of WT and NOS2 KO BM-DCs. We found no differences between WT and NOS2 KO BM-DCs in the expression of CD24 and CD11b before or after TLR stimulation, but BM-DCs were predominantly CD11bhiCD24low (data not shown) as reported previously [37, 40]. Surprisingly, a subpopulation of NOS2 KO or WT CD11chi/int BM-DCs expressed the marker PDCA1 (Fig. 1D and E and Supplemental Fig. 2B), initially reported to be a pDC marker. GM-CSF inhibits pDC differentiation [41], and as expected, there were no B220+ pDCs detectable in the BM-DC cultures (data not shown). Compared with WT DCs, NOS2 KO DCs expressed approximately two- to threefold more PDCA1 on CD11c+ cells after stimulation with graded doses of LPS, zymosan, or CpG-DNA (Fig. 1D and E, and data not shown).
The lack of NO production by NOS2 KO DCs appears to be responsible for their increased responsiveness, as the addition of a NO donor, NOR4, reverted CD86 and PDCA1 expression to the lower levels found on WT DCs (Fig. 1E; see also below). Thus, NO induced in DCs by TLR stimulation may act in an autocrine manner to control the expression of activation markers including PDCA1.
NOS2 KO DCs produce more inflammatory cytokines than WT DCs
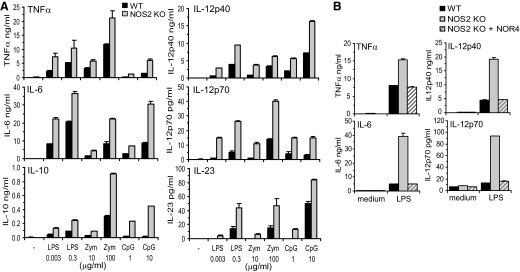
We found previously that NO regulates cytokine production by LPS-stimulated human MoDCs [17]. Thus, it was of interest to compare TLR-induced cytokine responses of WT and NOS2 KO BM-DCs. After stimulation with LPS, zymosan, or CpG, NOS2 KO DCs produced more IL-12p40, IL-12p70, IL-23, and TNF-α than WT DCs (Fig. 2A). As shown previously [42], IL-6 was up-regulated more by LPS than zymosan and IL-10 more by zymosan than LPS. However, NOS2 KO DCs released two- to threefold more of both cytokines than the WT DCs (Fig. 2A). The elevated cytokine production by NOS2 KO BM-DCs was converted back to WT levels by NOR4 (Fig. 2B). Thus, autocrine NO production by WT BM-DCs is likely to be responsible for the lower cytokine production in WT DCs.
Figure 2. NO production by DCs inhibits TLR-dependent cytokine release.
WT or NOS2 KO BM-DCs were stimulated for 24 h with medium only or the indicated doses of LPS, zymosan, or CpG-DNA (A) or 0.3 μg/ml of LPS (B), and cytokine expression was measured by ELISA. (A) Dose-dependent increase in cytokine production in NOS2 KO versus WT DCs after TLR stimulation. (B) The addition of 100 μM NOR4 to NOS2 KO DC cultures reverts cytokine release to WT levels. (A and B) Data are represented as mean ± sd and are representative of greater than three independent experiments.
A Ly6ChiPDCA1+CD86+ DC subset up-regulated in NOS2 KO DC cultures has enhanced cytokine production
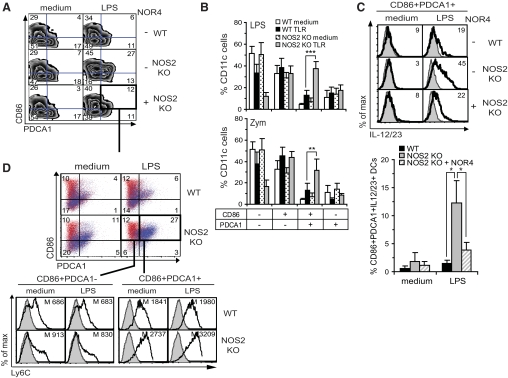
To assess if a specific DC subpopulation was responsible for increased cytokine production by NOS2 KO DCs, we compared the expression of cytokines by activated BM-DC subsets in WT versus NOS2 KO using six-color flow cytometry. Two major DC subpopulations were up-regulated upon TLR stimulation: CD86+PDCA1– DCs and CD86+PDCA1+ DCs (Fig. 3A and B). After LPS and zymosan treatment, CD86+PDCA1– DCs were up-regulated slightly in NOS2 KO and WT cultures, whereas the CD86+PDCA1+ DC subset was increased substantially more in NOS2 KO DCs versus WT DCs (Fig. 3A and B). Again, NOR4 inhibited the LPS- or zymosan-dependent up-regulation of the CD86+PDCA1+ DC subpopulation in NOS2 KO DCs (Fig. 3A and Supplemental Fig. 3A)
Figure 3. Ly6ChiPDCA1+CD86+ NOS2 KO DCs produce high levels of cytokines.
Flow cytometric analysis of WT and NOS2 KO BM-DCs cultured for 24 h (A) or 14 h (B–D) with medium, 0.3 μg/ml LPS (A–D), or 100 μg/ml zymosan (B). Plots gated on CD11c+ cells. (A) LPS stimulation induces more CD86+PDCA1+ cells in NOS2 KO versus WT DCs. (B) Enhanced CD86+PDCA1+ cells in NOS2 KO versus WT DCs. Bars show the mean ± sd; n = 8; **P < 0.01; ***P < 0.001. (C, upper panel) LPS stimulation for 24 h induces more IL-12/23 expression (thick empty histograms) in NOS2 KO than WT CD86+PDCA1+ DCs. (Lower panel) LPS (24 h) induces a greater increase in the percentage of CD86+PDCA1+IL-12/23+ DCs from NOS2 KO mice versus WT mice. Bars show the mean ± sd; n = 4; *P < 0.05. (D, upper panel) Ly6C+ DCs (blue dots) elevated in NOS2 KO versus WT LPS-treated DCs are mostly CD86+PDCA1+, and CD86+PDCA1– DCs are mainly Ly6C– (red dots). CD86+PDCA1–Ly6C+ DCs are not up-regulated after LPS treatment and are expressed to the same extent in WT and NOS2 KO cultures. The percentage of each CD86/PDCA1 DC subset expressing Ly6C is indicated in the plots. (D, lower panel) CD86+PDCA1+ DCs express more Ly6C than CD86+PDCA1– DCs, NOS2 KO DCs compared with WT DCs express higher levels of Ly6C, and Ly6C mean fluorescence intensity is indicated in the plots. (C and D) Isotype controls are shown as shaded histograms. (A and C) NOS2 KO cultures were also treated or not with 100 μM NOR4. (A, C, and D) Data are representative of greater than or equal to three independent experiments.
Interestingly, the DC subpopulation expressing CD86 and PDCA1 and up-regulated more in NOS2 KO cultures was also the DC subset that mainly increased IL-12/23 and TNF-α production after LPS or zymosan stimulation (Fig. 3C and Supplemental Fig. 3A, green, and data not shown). IL-12/23 was also up-regulated more in the NOS2 KO CD86+PDCA1– DC subset but to a lesser extent than in CD86+PDCA1+ DCs (Supplemental Fig. 3A, blue).
To assess whether the CD86+PDCA1+ DC subset was related to inflammatory DCs, we compared the expression of Ly6C on WT and NOS2 KO BM-DCs expressing PDCA1 and CD86. Most of the PDCA1+ cells before and after TLR stimulation in NOS2 KO DC cultures were Ly6Chi, a characteristic of inflammatory DCs (Supplemental Fig. 3C). Before and after TLR stimulation, most of the CD86+PDCA1+ DCs up-regulated in NOS2 KO BM-DCs expressed high levels of Ly6C, and relatively few CD86+PDCA1– DCs expressed Ly6C (Fig. 3D, blue, and Supplemental Fig. 3C). Adding NOR4 to NOS2 KO DCs partially restored Ly6C to WT levels (Supplemental Fig. 3C). As expected, Ly6ChiPDCA1+ DCs were also mainly responsible for the strong up-regulation of TNF-α and IL-12/23 in NOS2 KO DCs (Supplemental Fig. 3D, and data not shown). Together, these data suggest that a Ly6ChiPDCA1+CD86+ DC subpopulation resembling inflammatory DCs [22, 25, 27] is up-regulated in NOS2 KO BM-DC cultures and produces high levels of cytokines.
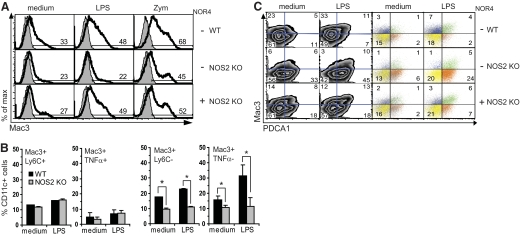
A Mac3+ DC subpopulation not expressing Ly6C, PDCA1, and CD86 is down-regulated in the absence of NO
One of the better-characterized subsets of Ly6Chi inflammatory DCs are TipDCs. Xu et al. [37] also proposed that BM-DCs are phenotypically equivalent to TipDCs. Thus, we asked whether the Ly6ChiPDCA1+ DCs enhanced in NOS2 KO cultures resembled TipDCs, which normally are CD11clow/intLy6ChiMac3+ and express NOS2 and TNF-α [26]. Mac3 was expressed on CD11clow/int BM-DCs from WT and NOS2 KO DCs. However, surprisingly, NOS2 KO DCs had fewer Mac3+ cells compared with WT DCs before or after TLR stimulation (Fig. 4A). LPS or zymosan stimulation up-regulated Mac3+ in WT DC cultures and not in NOS2 KO DC cultures unless NOR4 was added back to the cultures (Fig. 4A). This suggested that TipDC-like cells might be reduced in NOS2 KO DC cultures. However, this was not the case. Some WT Mac3+ DCs up-regulated by TLR2 and TLR4 stimulation were phenotypically equivalent to TipDCs (Ly6ChiMac3+TNF-α+NOS2+; data not shown), but frequencies of Mac3+Ly6C+ and Mac3+TNF-α+ cells were the same in WT and NOS2 KO cultures (Fig. 4B, left). Even after LPS stimulation, the Mac3+ DCs reduced in NOS2 KO cultures, unlike TipDCs, expressed neither TNF-α nor Ly6C (Fig, 4B, right) and were mainly CD86– (data not shown). Therefore, although Mac3+ cells were decreased, TipDC-like cells were not dysregulated in NOS2 KO cultures. Furthermore, these data suggest that Ly6Chi DCs expanded in NOS2 KO DC cultures, despite their high TNF-α production, were not TipDCs.
Figure 4. A Mac3+ DC subset is reduced in NOS2 KO DC cultures, but TipDCs are not dysregulated.
Flow cytometric analysis of WT and NOS2 KO BM-DCs cultured for 14 h with medium, 0.3 μg/ml LPS (A–C), or 100 μg/ml zymosan (A). Plots are gated on CD11c+ cells. (A) Reduced percentages (gates) of Mac3+ cells in NOS2 KO versus WT DCs. NOR4 (100 μM), added to NOS2 KO cultures, reverts the phenotype to WT DCs. Isotype controls are shown as shaded histograms. (B) Mac3+TNF-α+ and Mac3+Ly6C+ (left), in contrast to Mac3+TNF-α– and Mac3+Ly6C– cells (right), were not reduced in NOS2 KO versus WT DCs. Bars show the mean values; n = 3; *P < 0.05. (C) Mac3 versus PDCA1 expression (left panel) and IL-12/23 expressed by the Mac3 and PDCA1 DC subsets (right panel). Colored dots and percentage in the plots represent IL-12/23-expressing cells from each DC subset assessed by intracellular staining: blue, Mac3+PDCA1–; green, Mac3+PDCA1+; orange, Mac3–PDCA1+; yellow, Mac3–PDCA1–. (A and C) Data are representative of six and three independent experiments, respectively.
We analyzed further whether a fraction of cytokine-producing PDCA1+ DCs enhanced in NOS2 KO cultures was Mac3+. Only a small PDCA1+Mac3+ DC subset was slightly increased and released cytokines upon LPS stimulation but was expressed similarly in WT and NOS2 KO DCs, thus confirming that PDCA1+ DCs enhanced in NOS2 KO cultures were not TipDCs (Fig. 4C, left). WT cultures had more PDCA1–Mac3+ DCs, and NOS2 KO cultures expressed more PDCA1+Mac3– DCs (Fig. 4C, left). This difference was enhanced further by TLR4 stimulation, and NOR4 added to NOS2 KO DCs partially restored the Mac3+ WT phenotype (Fig. 4A and C). Furthermore, in contrast to WT cultures, PDCA1+Mac3– DCs were mainly responsible for the increased IL-12/23 production in NOS2 KO DCs (Fig. 4C, right).
Taken together, these data suggest that NO produced by WT DCs reciprocally regulates the maturation of Ly6ChiPDCA1+Mac3– DC versus Ly6C–PDCA1–Mac3+ DC subpopulations. NO enables the development of a DC subpopulation with a more macrophage phenotype (Mac3+) and lower responsiveness to several TLR stimuli, while restraining the activation of a Ly6ChiPDCA1+CD86+ DC subset that releases large amounts of cytokines in response TLR2, TLR4, and TLR9 stimulation.
NOS2 KO Ly6ChiPDCA1+ DCs induce increased IFN-γ production by antigen-specific CD4+ T cells in vivo
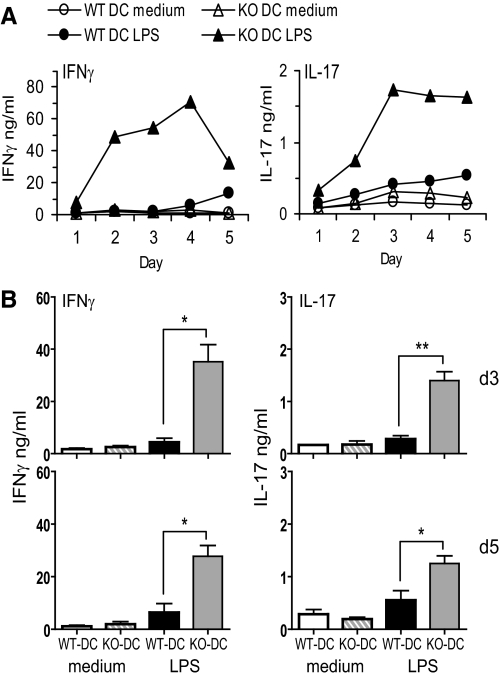
We next asked if activated NOS2 KO BM-DCs induced stronger Th1 immune responses than WT BM-DCs. We first tested whether anti-CD3-stimulated WT CD4+ T cells cocultured with NOS2 KO DCs produced more IFN-γ and/or IL-17 than those cocultured with WT DCs. Activated NOS2 KO DCs induced significantly more IFN-γ and IL-17 expression by WT CD4+ T cells than WT DCs (Supplemental Fig. 4A and B). NOS2 KO BM-DCs loaded with OVAp also induced significantly more IFN-γ and IL-17 production by OVA-specific OTII Tg CD4+ T cells than WT BM-DCs (Fig. 5A and B).
Figure 5. NOS2 KO BM-DCs induce more IFN-γ and IL-17 production by antigen-specific CD4+ T cells than WT BM-DCs in vitro.
(A and B) OVAp-loaded WT or NOS2 KO BM-DCs were cocultured for 5 days with OTII CD4+ T cells in medium only or LPS. IFN-γ and IL-17 production was measured by ELISA. (A) Time-course of IFN-γ and IL-17 release by OTII CD4+ T cells. Data are representative of three independent experiments. (B) IFN-γ and IL-17 released at Day 3 (upper panels) and Day 5 (lower panels). Data are means ± sd; n = 3; *P < 0.05; **P < 0.01.
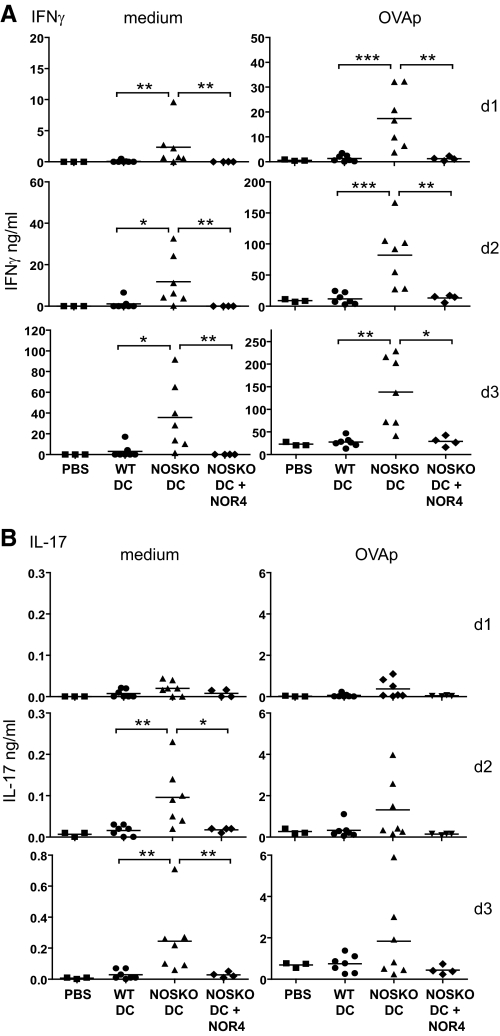
We also examined whether NOS2 KO DCs were better at priming OVA-specific OTII T cells for Th1 immune responses in vivo. After 18 h prestimulation with OVAp plus LPS, WT or NOS2 KO BM-DCs were injected i.p. into NOS2+ OTII Tg mice; 5 days later, spleen cells were harvested, restimulated for 1–3 days in vitro in the absence or presence of OVAp, and assessed for IFN-γ and IL-17 production (Fig. 6). Splenocytes from OTII mice injected with antigen-pulsed WT DCs did not significantly increase IFN-γ (Fig. 6A) or IL-17 production (Fig. 6B) compared with spleen cells from control mice. In contrast, injection of OVA-loaded NOS2 KO DCs significantly increased the ability of the OTII spleen cells to produce IFN-γ, with or without OVAp restimulation compared with WT DCs or PBS controls (Fig. 6A). The results for IL-17 production were slightly different: only after 2–3 days of culture without OVAp, splenocytes from OTII mice injected with antigen-pulsed NOS2 KO DCs produced significantly more IL-17 than splenocytes from OTII mice injected with antigen-pulsed WT DCs (Fig. 6B). The results were similar when IFN-γ and IL-17 production was determined on a per-cell basis (Supplemental Fig. 4C).
Figure 6. NOS2 KO DCs, in contrast to WT DCs, prime OVA-specific OTII T cells for strong Th1 immune responses in vivo.
(A and B) BM-DCs from WT or NOS2 KO mice were treated for 18 h with OVAp plus LPS, in the absence or presence of 100 μM NOR4 for NOS2 KO DCs, and then injected i.p. into OTII TCR Tg mice. Control mice were injected with PBS only. Spleens were harvested from mice 5 days after injection, and splenocytes cultured for 3 days in medium only or in the presence of OVAp and IFN-γ (A) and IL-17 (B) expression were measured by ELISA. Lines represent the mean values of each group; n = 7 for WT and NOS2 KO DCs; n = 4 for NOS2 KO plus NOR4 DCs; n = 3 for PBS; *P < 0.05; **P < 0.01; ***P < 0.001.
Importantly, NOS2 KO DCs treated with NOR4, in addition to OVAp and LPS prior to injection into OTII Tg mice, gave similar results as Tg mice inoculated with WT BM-DCs (Fig. 6). In other words, the presence of NO during NOS2 KO DC antigen loading and activation, but not during T cell priming, was sufficient to restore the low level of IFN-γ and IL-17 produced by in vivo priming with WT DCs; this suggests that the induction of OTII Th1 immune responses by NOS2 KO BM-DCs was mainly a result of the absence of autocrine effects of NO on DCs.
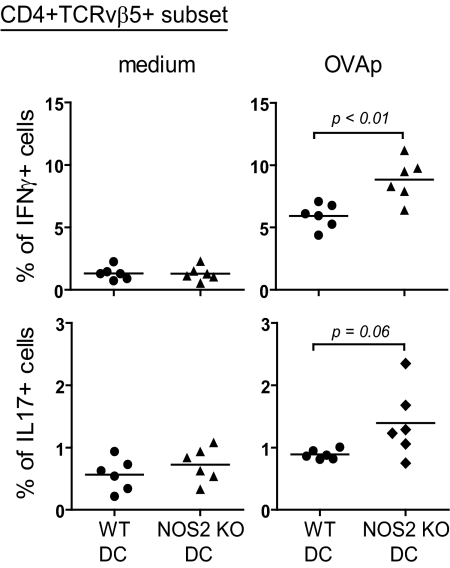
The increased ability of NOS2 KO versus WT BM-DCs to prime Th1 immune responses (Fig. 6) could be a result of the increased numbers of Ly6ChiPDCA1+ DCs in NOS2 KO DC cultures (Fig. 3) and/or qualitative differences between WT and NOS2 KO Ly6ChiPDCA1+ DCs. To investigate whether Ly6ChiPDCA1+ BM-DCs from NOS2 KO mice were qualitatively different, we sorted Ly6ChiPDCA1+ DCs from both genotypes, pulsed them with OVAp plus LPS, and tested their ability to induce IFN-γ and IL-17 production from antigen-specific CD4 T cells after adoptive transfer into OTII mice. Antigen-pulsed Ly6ChiPDCA1+ NOS2 KO DCs were more potent than the same number of Ly6ChiPDCA1+ WT DCs in inducing IFN-γ production by OTII T cells (Fig. 7). Thus, NO inhibits not only the frequency of Ly6ChiPDCA1+ DCs but also their ability to induce Th1 immune responses.
Figure 7. In vivo priming with sorted Ly6ChiPDCA1+ BM-DCs from NOS2 KO mice induces increased IFN-γ expression by OVA-specific OTII T cells.
Ly6ChiPDCA1+ BM-DCs from WT or NOS2 KO mice were sorted and treated for 18 h with OVAp plus LPS and then injected i.p. into OTII mice. Five days after injection, splenocytes were cultured for 2 days ± OVAp and restimulated for 5 h with Brefeldin A/PMA/ionomycin. IFN-γ and IL-17 were detected by intracellular staining. Plots are gated on CD4+TCRVβ5+ cells. Lines represent mean values for each genotype; n = 6.
Together, these data suggest that in vivo priming of OTII T cells with NOS2 KO DCs compared with WT DCs increases the ability of T cells to produce IFN-γ and IL-17, even without antigen restimulation; the increased frequency and function of Ly6ChiPDCA1+ DCs in NOS2 KO BM-DCs play major roles in skewing toward Th1 immune responses; BM-DCs are the main source of NO that inhibits Th1 differentiation in vivo; and the autocrine effect of NO on DCs plays a major role in suppressing Th1 immune responses.
A Ly6ChiPDCA1+ DC subset is expanded more in NOS2 KO than WT mice after WNV infection
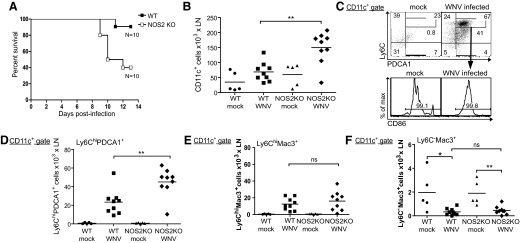
We found recently that after footpad infection with a pathogenic WNV (WN-TX), DC numbers expand in dLNs, particularly Ly6C+ DCs [35]. To test if NO regulates Ly6C+ inflammatory DCs in vivo, we infected WT and NOS2 KO mice with WN-TX. NOS2 KO mice were highly susceptible to WN-TX infection, and half of the mice die within 10 days postinfection (Fig. 8A). The susceptibility of NOS2 KO mice to WN-TX was associated with dysregulated DCs: after infection with WN-TX, more CD11c+ DCs were present in the dLNs of NOS2 KO than WT mice (Fig. 8B). We also analyzed the expression of PDCA1, CD86, and Ly6C in the CD11c+ population. Consistent with previous studies [43], only CD11c+B220+ pDCs expressed PDCA1 under resting conditions, and there was no difference in the number of pDCs in WT and NOS2 KO mice (data not shown). However, after WN-TX infection, DCs expressing PDCA1 and Ly6C were increased substantially in WT and NOS2 KO mice; these were not pDCs, as they were B220– (Fig. 8C and D). The Ly6C+PDCA1+ DCs were CD86+ and strongly expressed Ly6C before or after infection (Fig. 8C and D). These results are consistent with our in vitro data that TLR-induced PDCA1+CD86+ BM-DCs are Ly6Chi and support the hypothesis that activated Ly6ChiPDCA1+CD86+ DCs may correspond to inflammatory DCs. Furthermore, these Ly6ChiPDCA1+CD86+ DCs, like previously characterized inflammatory DCs, were also CD11b+ and CD8α– (data not shown).
Figure 8. After WN-TX infection, more Ly6ChiPDCA1+ DCs expand in NOS2 KO than in WT mice.
(A) Survival rate of WT and NOS2 KO mice infected in the footpad with 103 PFU WN-TX. Mortality was recorded daily, and surviving mice were monitored for 14 days. Differences in survival ratio were assessed for significance using the log-rank test; P = 0.0120; n = 10. (B) Total numbers of CD11c+ cells in dLNs of WT and NOS2 KO mice 24 h after WN-TX infection (WNV). (C) Gating strategy for Ly6ChiPDCA1+ DCs in dLNs of WT or NOS2 KO mice 24 h after WN-TX infection. Ly6ChiPDCA1+ DCs are also CD86+. (D–F) Total Ly6ChiPDCA1+ DCs (D), Ly6ChiMac3+ (E), and Ly6C–Mac3+ (F) in dLNs of WT or NOS2 KO mice 24 h after WN-TX infection. (C–F) Plots are gated on CD11c+B220– cells. (B and D–F) Lines represent mean values for each group; n = 9; *P < 0.05; **P < 0.01.
Although the Ly6ChiPDCA1+ DCs expanded in dLNs of both infected WT and NOS2 KO mice, the NOS2 KO mice had more Ly6ChiPDCA1+ inflammatory DCs in dLNs than WT mice (Fig. 8D). The levels of Ly6ChiMac3+ TipDC-like cells were increased in dLNs from WN-TX-infected WT or NOS2 KO mice, but in agreement with our in vitro results, there was not a significant difference between the two genotypes (Fig. 8E). This suggests that the dysregulated expansion in NOS2 KO mice was restricted to the Ly6ChiPDCA1+ DC subset. Interestingly, a small Ly6C–Mac3+ DC subset was down-regulated in dLNs from WT and NOS2 KO mice after WN-TX infection (Fig. 8F). Thus, following a potent inflammatory stimulus via WN-TX infection, unlike the inflammatory Ly6ChiPDCA1+ DCs that were recruited to the dLNs, Ly6C–Mac3+ DCs were reduced.
DISCUSSION
We have found that NO regulates the development of a DC subset that resembles inflammatory DCs [22, 25–27] and affects the quality of T cell responses. Enhanced Th1 immune responses in NOS2 KO mice have been attributed to reduced, NO-induced T cell apoptosis [11, 14]. Studies using NO donors or inhibitors reported that NO induces [38, 44] or protects [39] DCs from apoptosis. Thus, it was important to assess how lack of NOS2 affects DC survival. The slightly increased survival of NOS2 KO BM-DCs compared with WT BM-DCs suggests that DC apoptosis induced by autocrine NO production plays only a minor role in altering immune responses.
Huang et al. [15] suggested that increased IL-12 production by NOS2 KO macrophages contributes to the excessive Th1 immune responses in NOS2 KO mice after infection with L. major. Consistent with the possibility that DCs may also play a role, we found that the lack of NOS2 substantially affects the ability of BM-DCs to respond to TLR2, TLR4, and TLR9 stimulation. Our findings are in agreement with previous studies reporting that several TLR agonists can induce NO production by BM-DCs [45–48]. A number of recent studies have shown that some adjuvants [7, 49], as well as pathological conditions [3, 30–32, 50], may induce human DCs to produce NO. NOS2 expression and NO production have also been reported in human MoDCs [4, 7]. The use of NO donors and inhibitors in studies addressing the effect of NO on human and mouse DCs led to disparate results. We and others [17, 51, 52] have shown that NO can have distinct effects on DC maturation, IL-12p70, and other cytokines released by human MoDCs. In mice, NO has been shown to contribute to BM-DC maturation [45], inhibit IL-12p40/p70 release from BM-DCs [16] and macrophages [15], and up-regulate TNF-α in macrophages. Xiong et al. [16] showed an increase in IL-12p40 mRNA and protein in total splenocytes from NOS2 KO versus WT mice after stimulation with a combination of LPS plus IFN-γ but did not analyze purified DC populations or BM-DCs from NOS2 KO mice.
For a more definitive approach, we examined how the lack of NOS2 by BM-DCs affects their ability to release a wide range of cytokines. In contrast to NOS2 KO macrophages, BM-DCs lacking NOS2 up-regulated several inflammatory cytokines, including TNF-α, IL-6, IL-12p70, and IL-23. The substantial enhancement in cytokine production on a per-cell basis by NOS2 KO DCs ruled out that the increased cytokine levels were a result of increased DC survival. One possibility was that all NOS2 KO DCs have an intrinsic ability to produce more cytokines. However, unlike BM-DCs, NOS2 KO and WT BM-derived-DCs differentiated with FL-DCs produce similar levels of inflammatory cytokines (unpublished data). Thus, the lack of NO production by NOS2 KO BM-DCs seems to selectively affect DC subpopulations specialized in the production of high levels of inflammatory cytokines.
A DC subset resembling inflammatory Ly6ChiGr1hiCD11bhiCD24lo DCs [22, 25–27] was substantially expanded in NOS2 KO BM-DC cultures and in vivo after WN-TX infection. Ly6Chi inflammatory DCs differentiate from monocytes and are recruited to LNs after certain infections [22–24, 28]. It is unclear what role this DC subset might play in resistance or susceptibility to WN-TX infection. On the one hand, several studies, also using NOS2 KO mice, have implicated NOS2-expressing, inflammatory DCs in the control of disease progression in several infection models. Serbina et al. [28] found that the lack of TiP-DCs in CCR2 KO mice impairs innate immune responses against Listeria monocytogenes. Muraille's group showed that NOS2+ inflammatory DCs are the main cell type infected in L. major and Brucella melitensis and are critical for the resolution of the infections [53, 54]. Our observation that NOS2 KO mice lacking NOS2+ inflammatory DCs are more susceptible to WN-TX is consistent with these studies.
On the other hand, some studies of WNV and influenza virus, as well as Trypanosoma brucei infections, suggest that dysregulated recruitment of inflammatory DCs may be more detrimental than beneficial [35, 55–57]. Similarly to NOS2 KO mice, IFN-β promoter stimulator-1 KO mice are highly susceptible to WN-TX infection and have an increased expansion of Ly6Chi inflammatory DCs in dLNs after infection, which correlates with uncontrolled inflammation and immunopathogenesis [35]. Getts et al. [58] showed that CCL2-dependent, inflammatory Ly6Chi monocyte migration is critical for the increase in CNS microglia during WN-TX infection and may play a pathogenic role in WN-TX encephalitis. Our data raise the additional possibility that NOS2 may regulate the inflammatory DC phenotype and the quality of adaptive immune responses. Thus, although it is likely that the lack of the protective effect of NO on innate immunity plays a role in the increased mortality of NOS2 KO mice to WNV infections, it will be interesting to determine whether the aberrant expansion of inflammatory DCs contributes to the progression of the disease. Studies are in progress to explore this possibility and further characterize the immunopathogenesis to WN-TX in NOS2 KO versus WT mice. As NOS2 KO mice are more susceptible to EAE and flaviviral encephalitis [11, 13, 59], both involving neurological inflammation [35, 60, 61], an interesting possibility is that the expansion of Ly6Chi inflammatory DCs in NOS2 KO mice plays an important role in pathologies involving CNS damage.
The Ly6Chi inflammatory-like DCs expanded in NOS2 KO mice after TLR treatment in vitro or after WNV infection in vivo expressed PDCA1/BST-2/CD317. PDCA1 was used initially as a marker for CD11clo pDCs, however T cells, B cells, and splenic cDCs express BST-2 after activation [43]. We found that PDCA1/BST-2 is also expressed on activated BM-DCs and in particular, on a CD11chiCD11bhiLy6ChiPDCA1+CD8 6+ subset that is a robust producer of cytokines and expanded in NOS2 KO DCs. In support of our finding of PDCA1/BST-2 being associated to myeloid Ly6Chi inflammatory-like DCs, a recent study found that Toxoplasma gondii preferentially infects a DC subset that expresses not only the pDC markers Gr-1/Ly6C and PDCA1 but also CD11chi and CD11bhi [62]. BST-2 may play a role in sorting membrane and secreted proteins [43]; this function could be related to the ability of DCs expressing BST-2 to produce high amounts of cytokines.
Xu et al. [37] proposed that BM-DCs, unlike FL-DCs, are phenotypically equivalent to inflammatory TipDCs, as they are mostly CD24loCD11bhi, produce TNF-α and NO, and express Mac3. Thus, it was possible that the inflammatory-like Ly6ChiPDCA1+ DCs expanded in NOS2 KO cultures were related to TipDCs. However, TLR stimulation up-regulated Mac3+ DCs in WT but not NOS2 KO cultures, and Ly6ChiMac3+ TipDC-like cells were not dysregulated in NOS2 KO DC cultures or in vivo after WNV infection. Furthermore, the inflammatory-like Ly6ChiPDCA1+Mac3– DCs released IL-12/23 in addition to TNF-α in response to TLR stimuli. Tam and Wick [63] found that after Listeria infection, in addition to TipDCs, another DC subset was recruited to LNs and spleens. This DC subset, in contrast to TipDCs, which contributed to the initial innate immune response, produced TNF-α and IL-12 and was triggered by WT Listeria but not by a bacteria strain incapable of inducing adaptive immune responses. Similarly, BM-DCs from NOS2 KO mice with up-regulated, inflammatory-like Ly6ChiPDCA1+ DCs, in contrast to DCs from WT mice, induced potent, antigen-specific Th1 immune responses in vitro or in vivo when adoptively transferred into NOS2+ OTII mice. These findings suggest that the BM-derived Ly6ChiPDCA1+ DC subpopulation identified here may be related to inflammatory DCs involved in Th1 immune responses [24], unlike TipDCs, which are mainly linked to innate immunity [28]. Furthermore, the increased ability of NOS2 KO BM-DCs to push toward Th1 immune responses appears to be not only a result of the increased frequency of this DC subset but also because Ly6ChiPDCA1+ NOS2 KO DCs compared with WT counterparts are more potent inducers of IFN-γ from OTII T cells in vivo. Thus, DC-derived NO in WT mice may be important to prevent the overproduction and overactivation of this inflammatory-like DC subset and thus, keep Th1 immune responses under control.
Interestingly, CD11b+ myeloid DCs resembling inflammatory DCs are essential for the development of autoimmune disease involving Th1 and Th17 immune responses in human and mice [50, 64, 65], and NOS2 KO mice are more susceptible to some of these autoimmune conditions [11–13]. In agreement with these studies, we found that splenocytes from OTII mice injected with NOS2 KO DCs, in addition to IFN-γ, without antigen restimulation, also produced more IL-17 than spleen cells from mice injected with WT DCs.
The inability of mature, OVA-loaded WT BM-DCs to efficiently up-regulate IFN-γ or IL-17 production from OTII T cells in vitro and in vivo is consistent with previous observations that autoantigen-pulsed BM-DCs but not splenic DCs induce T cell hyporesponsiveness and tolerance in transplantation and autoimmune models [18, 66–70]. Human and mouse BM-derived myeloid cells, in particular, CD11b+Gr1+ cells that play a major role in tumor-induced tolerance [8], suppress T cell responses through a NO-dependent mechanism [30, 71, 72]. We found that in NOS2+ OTII recipients, the absence of NOS2 only in BM-DC-priming T cells was sufficient to facilitate antigen-specific Th1 immune responses. This is the first in vivo evidence using NOS2 KO mice that BM-DCs may be the main source of NO that inhibits Th1 differentiation and possibly autoimmune responses [66, 73]. Stromal cells or IFN-γ induce NO-producing, regulatory DCs that can suppress T cell proliferation and that may also promote regulatory T cells [74–77]. Although a direct effect of NO produced by DCs on T cells [14] cannot be excluded, two findings strongly support the possibility that autocrine regulation of DCs by NO plays a major role in programming Th immune responses. First, similar numbers of antigen-specific OTII CD4+ splenic T cells were found after adoptive transfer with WT or NOS2 KO DCs. Second and most importantly, the presence of NO only during antigen loading and DC activation was sufficient to revert the potent, OVA-specific Th1 immune response induced by NOS2 KO DCs to levels induced by WT DCs. The NOS2 KO DCs pretreated with NO in vitro cannot produce NO in vivo; this suggests that the alteration of T cell responses that we detected is a result of NO acting on DCs and not on T cells.
In summary, NO produced by DCs suppresses the development of inflammatory-like Ly6ChiPDCA1+CD86+Mac3– DCs, highly responsive to TLR stimulation and capable of priming Th1 immune responses, and promotes development of a more macrophage-like, DC-expressing Mac3 (Ly6C–PDCA1–CD86–Mac3 +), less responsive to TLR signaling and unable to prime for Th1 immune responses (Fig. 9). Further studies are needed to assess whether these DC subpopulations represent different stages of activation or two separate DC subsets. Nevertheless, NO appears to play a role in programming adaptive immune responses through the regulation of inflammatory-like DC subpopulations. Given the implication of NO-producing DCs in several pathologies, from cancer to transplant rejection and autoimmune diseases [8, 30–32], these findings underscore the possible use of regulating NO production by specific DC subsets and/or DC exposure to NO for cell-based immunogenic and tolerogenic immunotherapies [2, 18, 19].
Figure 9. Schematic model of how NO may affect DC phenotype.
NO produced by DCs acts in an autocrine manner to regulate the expression of two distinct DC subpopulations: NO promotes the development of Ly6C–PDCA1–CD86–Mac3 + DCs, which are less responsive to TLR signals and produce low levels of cytokines, and inhibits the expansion of Ly6ChiPDCA1+CD86+Mac3– inflammatory DCs, which are highly responsive to TLR stimulation and produce high levels of cytokines.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported principally by National Institutes of Health grants AI44257 (E.A.C.) and AI83019 (M.G., Jr.) and in part by AI52203 (E.A.C.) and AI57568 (M.G. Jr.) and the State of Washington (M.G. Jr.). We thank Sabrina Richards for helpful comments.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- APC
- allophycocyanin
- BM-DCs
- GM-CSF and IL-4-derived bone marrow DCs
- BST-2
- bone marrow stromal cell antigen 2
- cDC
- conventional DC
- dLN
- draining LN
- EAE
- experimental autoimmune encephalitis
- FL-DCs
- fms-like tyrosine kinase 3 ligand DCs
- Mac3
- macrophage antigen 3
- MHCII
- MHC class II
- MoDC
- monocyte-derived DC
- NOS2 KO
- NOS2 knockout
- OVAp
- OVA peptide 323–339
- pDC
- plasmacytoid DC
- Tg
- transgenic
- TipDC
- TNF-α and NOS2-producing DC
- WN-TX
- West Nile virus isolate TX 2002-HC
- WNV
- West Nile virus
AUTHORSHIP
Each author contributed substantially to the work. D.G. designed and performed research, collected, analyzed, and interpreted data, performed statistical analysis, and wrote the manuscript. C.L. performed research and analyzed and interpreted data. M.S.S. designed and performed research, collected, analyzed, and interpreted data, and performed statistical analysis. K.E.D. performed research and analyzed and interpreted data. D.Y.M. designed and performed research. M.G. Jr. contributed vital reagents and analytical tools and interpreted data. E.A.C. designed research, interpreted data, contributed to manuscript writing, and also contributed with vital reagents and analytical tools.
DISCLOSURE
The authors have no conflicting financial interests.
REFERENCES
- 1. Joffre O., Nolte M. A., Sporri R., Reis e Sousa C. (2009) Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 227, 234–247 [DOI] [PubMed] [Google Scholar]
- 2. Steinman R. M., Banchereau J. (2007) Taking dendritic cells into medicine. Nature 449, 419–426 [DOI] [PubMed] [Google Scholar]
- 3. Díaz N. L., Arvelaez F. A., Zerpa O., Tapia F. J. (2006) Inducible nitric oxide synthase and cytokine pattern in lesions of patients with American cutaneous leishmaniasis. Clin. Exp. Dermatol. 31, 114–117 [DOI] [PubMed] [Google Scholar]
- 4. Fernández-Ruiz V., Lopez-Moratalla N., Gonzalez A. (2005) Production of nitric oxide and self-nitration of proteins during monocyte differentiation to dendritic cells. J. Physiol. Biochem. 61, 517–525 [DOI] [PubMed] [Google Scholar]
- 5. Guzik T. J., Korbut R., Adamek-Guzik T. (2003) Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 54, 469–487 [PubMed] [Google Scholar]
- 6. Bogdan C. (2001) Nitric oxide and the immune response. Nat. Immunol. 2, 907–916 [DOI] [PubMed] [Google Scholar]
- 7. Bagley K. C., Abdelwahab S. F., Tuskan R. G., Lewis G. K. (2006) Cholera toxin indirectly activates human monocyte-derived dendritic cells in vitro through the production of soluble factors, including prostaglandin E(2) and nitric oxide. Clin. Vaccine Immunol. 13, 106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marigo I., Dolcetti L., Serafini P., Zanovello P., Bronte V. (2008) Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol. Rev. 222, 162–179 [DOI] [PubMed] [Google Scholar]
- 9. MacMicking J. D., Nathan C., Hom G., Chartrain N., Fletcher D. S., Trumbauer M., Stevens K., Xie Q. W., Sokol K., Hutchinson N., et al. (1995) Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81, 641–650 [DOI] [PubMed] [Google Scholar]
- 10. Wei X. Q., Charles I. G., Smith A., Ure J., Feng G. J., Huang F. P., Xu D., Muller W., Moncada S., Liew F. Y. (1995) Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375, 408–411 [DOI] [PubMed] [Google Scholar]
- 11. Sahrbacher U. C., Lechner F., Eugster H. P., Frei K., Lassmann H., Fontana A. (1998) Mice with an inactivation of the inducible nitric oxide synthase gene are susceptible to experimental autoimmune encephalomyelitis. Eur. J. Immunol. 28, 1332–1338 [DOI] [PubMed] [Google Scholar]
- 12. Kolb H., Kolb-Bachofen V. (1998) Nitric oxide in autoimmune disease: cytotoxic or regulatory mediator? Immunol. Today 19, 556–561 [DOI] [PubMed] [Google Scholar]
- 13. Fenyk-Melody J. E., Garrison A. E., Brunnert S. R., Weidner J. R., Shen F., Shelton B. A., Mudgett J. S. (1998) Experimental autoimmune encephalomyelitis is exacerbated in mice lacking the NOS2 gene. J. Immunol. 160, 2940–2946 [PubMed] [Google Scholar]
- 14. Dalton D. K., Wittmer S. (2005) Nitric-oxide-dependent and independent mechanisms of protection from CNS inflammation during Th1-mediated autoimmunity: evidence from EAE in iNOS KO mice. J. Neuroimmunol. 160, 110–121 [DOI] [PubMed] [Google Scholar]
- 15. Huang F. P., Niedbala W., Wei X. Q., Xu D., Feng G. J., Robinson J. H., Lam C., Liew F. Y. (1998) Nitric oxide regulates Th1 cell development through the inhibition of IL-12 synthesis by macrophages. Eur. J. Immunol. 28, 4062–4070 [DOI] [PubMed] [Google Scholar]
- 16. Xiong H., Zhu C., Li F., Hegazi R., He K., Babyatsky M., Bauer A. J., Plevy S. E. (2004) Inhibition of interleukin-12 p40 transcription and NF-κB activation by nitric oxide in murine macrophages and dendritic cells. J. Biol. Chem. 279, 10776–10783 [DOI] [PubMed] [Google Scholar]
- 17. Giordano D., Magaletti D. M., Clark E. A. (2006) Nitric oxide and cGMP protein kinase (cGK) regulate dendritic-cell migration toward the lymph-node-directing chemokine CCL19. Blood 107, 1537–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O′Neill D. W., Bhardwaj N. (2007) Exploiting dendritic cells for active immunotherapy of cancer and chronic infections. Mol. Biotechnol. 36, 131–141 [DOI] [PubMed] [Google Scholar]
- 19. Van Duivenvoorde L. M., van Mierlo G. J., Boonman Z. F., Toes R. E. (2006) Dendritic cells: vehicles for tolerance induction and prevention of autoimmune diseases. Immunobiology 211, 627–632 [DOI] [PubMed] [Google Scholar]
- 20. Palucka A. K., Ueno H., Fay J. W., Banchereau J. (2007) Taming cancer by inducing immunity via dendritic cells. Immunol. Rev. 220, 129–150 [DOI] [PubMed] [Google Scholar]
- 21. Randolph G. J., Inaba K., Robbiani D. F., Steinman R. M., Muller W. A. (1999) Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11, 753–761 [DOI] [PubMed] [Google Scholar]
- 22. Domínguez P. M., Ardavin C. (2010) Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol. Rev. 234, 90–104 [DOI] [PubMed] [Google Scholar]
- 23. Serbina N. V., Pamer E. G. (2006) Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317 [DOI] [PubMed] [Google Scholar]
- 24. León B., Lopez-Bravo M., Ardavin C. (2007) Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 26, 519–531 [DOI] [PubMed] [Google Scholar]
- 25. Shortman K., Naik S. H. (2007) Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 7, 19–30 [DOI] [PubMed] [Google Scholar]
- 26. Serbina N. V., Jia T., Hohl T. M., Pamer E. G. (2008) Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 26, 421–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. León B., Ardavin C. (2008) Monocyte-derived dendritic cells in innate and adaptive immunity. Immunol. Cell Biol. 86, 320–324 [DOI] [PubMed] [Google Scholar]
- 28. Serbina N. V., Salazar-Mather T. P., Biron C. A., Kuziel W. A., Pamer E. G. (2003) TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19, 59–70 [DOI] [PubMed] [Google Scholar]
- 29. Tezuka H., Abe Y., Iwata M., Takeuchi H., Ishikawa H., Matsushita M., Shiohara T., Akira S., Ohteki T. (2007) Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature 448, 929–933 [DOI] [PubMed] [Google Scholar]
- 30. Bluth M. J., Zaba L. C., Moussai D., Suarez-Farinas M., Kaporis H., Fan L., Pierson K. C., White T. R., Pitts-Kiefer A., Fuentes-Duculan J., Guttman-Yassky E., Krueger J. G., Lowes M. A., Carucci J. A. (2009) Myeloid dendritic cells from human cutaneous squamous cell carcinoma are poor stimulators of T-cell proliferation. J. Invest. Dermatol. 129, 2451–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bacci S., Pieri L., Buccoliero A. M., Bonelli A., Taddei G., Romagnoli P. (2008) Smooth muscle cells, dendritic cells and mast cells are sources of TNFα and nitric oxide in human carotid artery atherosclerosis. Thromb. Res. 122, 657–667 [DOI] [PubMed] [Google Scholar]
- 32. Lowes M. A., Chamian F., Abello M. V., Fuentes-Duculan J., Lin S. L., Nussbaum R., Novitskaya I., Carbonaro H., Cardinale I., Kikuchi T., Gilleaudeau P., Sullivan-Whalen M., Wittkowski K. M., Papp K., Garovoy M., Dummer W., Steinman R. M., Krueger J. G. (2005) Increase in TNF-α and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc. Natl. Acad. Sci. USA 102, 19057–19062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoffman R. A., Mahidhara R. S., Wolf-Johnston A. S., Lu L., Thomson A. W., Simmons R. L. (2002) Differential modulation of CD4 and CD8 T-cell proliferation by induction of nitric oxide synthesis in antigen presenting cells. Transplantation 74, 836–845 [DOI] [PubMed] [Google Scholar]
- 34. Powell T. J., Jenkins C. D., Hattori R., MacPherson G. G. (2003) Rat bone marrow-derived dendritic cells, but not ex vivo dendritic cells, secrete nitric oxide and can inhibit T-cell proliferation. Immunology 109, 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suthar M. S., Ma D. Y., Thomas S., Lund J. M., Zhang N., Daffis S., Rudensky A. Y., Bevan M. J., Clark E. A., Kaja M. K., Diamond M. S., Gale M., Jr. (2010) IPS-1 is essential for the control of West Nile virus infection and immunity. PLoS Pathog. 6, e1000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R. M. (1992) Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176, 1693–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu Y., Zhan Y., Lew A. M., Naik S. H., Kershaw M. H. (2007) Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J. Immunol. 179, 7577–7584 [DOI] [PubMed] [Google Scholar]
- 38. Lu L., Bonham C. A., Chambers F. G., Watkins S. C., Hoffman R. A., Simmons R. L., Thomson A. W. (1996) Induction of nitric oxide synthase in mouse dendritic cells by IFN-γ, endotoxin, and interaction with allogeneic T cells: nitric oxide production is associated with dendritic cell apoptosis. J. Immunol. 157, 3577–3586 [PubMed] [Google Scholar]
- 39. Falcone S., Perrotta C., De Palma C., Pisconti A., Sciorati C., Capobianco A., Rovere-Querini P., Manfredi A. A., Clementi E. (2004) Activation of acid sphingomyelinase and its inhibition by the nitric oxide/cyclic guanosine 3′,5′-monophosphate pathway: key events in Escherichia coli-elicited apoptosis of dendritic cells. J. Immunol. 173, 4452–4463 [DOI] [PubMed] [Google Scholar]
- 40. Naik S. H., Proietto A. I., Wilson N. S., Dakic A., Schnorrer P., Fuchsberger M., Lahoud M. H., O′Keeffe M., Shao Q. X., Chen W. F., Villadangos J. A., Shortman K., Wu L. (2005) Cutting edge: generation of splenic CD8+ and CD8– dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J. Immunol. 174, 6592–6597 [DOI] [PubMed] [Google Scholar]
- 41. Gilliet M., Boonstra A., Paturel C., Antonenko S., Xu X. L., Trinchieri G., O′Garra A., Liu Y. J. (2002) The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 195, 953–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dillon S., Agrawal S., Banerjee K., Letterio J., Denning T. L., Oswald-Richter K., Kasprowicz D. J., Kellar K., Pare J., van Dyke T., Ziegler S., Unutmaz D., Pulendran B. (2006) Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Invest. 116, 916–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blasius A. L., Giurisato E., Cella M., Schreiber R. D., Shaw A. S., Colonna M. (2006) Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 177, 3260–3265 [DOI] [PubMed] [Google Scholar]
- 44. Stanford A., Chen Y., Zhang X. R., Hoffman R., Zamora R., Ford H. R. (2001) Nitric oxide mediates dendritic cell apoptosis by downregulating inhibitors of apoptosis proteins and upregulating effector caspase activity. Surgery 130, 326–332 [DOI] [PubMed] [Google Scholar]
- 45. Wong S. H., Santambrogio L., Strominger J. L. (2004) Caspases and nitric oxide broadly regulate dendritic cell maturation and surface expression of class II MHC proteins. Proc. Natl. Acad. Sci. USA 101, 17783–17788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nishioka Y., Wen H., Mitani K., Robbins P. D., Lotze M. T., Sone S., Tahara H. (2003) Differential effects of IL-12 on the generation of alloreactive CTL mediated by murine and human dendritic cells: a critical role for nitric oxide. J. Leukoc. Biol. 73, 621–629 [DOI] [PubMed] [Google Scholar]
- 47. Wang Q., McLoughlin R. M., Cobb B. A., Charrel-Dennis M., Zaleski K. J., Golenbock D., Tzianabos A. O., Kasper D. L. (2006) A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J. Exp. Med. 203, 2853–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ivanov S., Dragoi A. M., Wang X., Dallacosta C., Louten J., Musco G., Sitia G., Yap G. S., Wan Y., Biron C. A., Bianchi M. E., Wang H., Chu W. M. (2007) A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood 110, 1970–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pérez O., Bracho G., Lastre M., Mora N., del Campo J., Gil D., Zayas C., Acevedo R., Gonzalez D., Lopez J. A., Taboada C., Turtle C., Solis R. L. (2004) Novel adjuvant based on a proteoliposome-derived cochleate structure containing native lipopolysaccharide as a pathogen-associated molecular pattern. Immunol. Cell Biol. 82, 603–610 [DOI] [PubMed] [Google Scholar]
- 50. Haider A. S., Lowes M. A., Suarez-Farinas M., Zaba L. C., Cardinale I., Khatcherian A., Novitskaya I., Wittkowski K. M., Krueger J. G. (2008) Identification of cellular pathways of ″type 1,″ Th17 T cells, and TNF- and inducible nitric oxide synthase-producing dendritic cells in autoimmune inflammation through pharmacogenomic study of cyclosporine A in psoriasis. J. Immunol. 180, 1913–1920 [DOI] [PubMed] [Google Scholar]
- 51. Corinti S., Pastore S., Mascia F., Girolomoni G. (2003) Regulatory role of nitric oxide on monocyte-derived dendritic cell functions. J. Interferon Cytokine Res. 23, 423–431 [DOI] [PubMed] [Google Scholar]
- 52. Paolucci C., Burastero S. E., Rovere-Querini P., De Palma C., Falcone S., Perrotta C., Capobianco A., Manfredi A. A., Clementi E. (2003) Synergism of nitric oxide and maturation signals on human dendritic cells occurs through a cyclic GMP-dependent pathway. J. Leukoc. Biol. 73, 253–262 [DOI] [PubMed] [Google Scholar]
- 53. Copin R., De Baetselier P., Carlier Y., Letesson J. J., Muraille E. (2007) MyD88-dependent activation of B220-CD11b+LY-6C+ dendritic cells during Brucella melitensis infection. J. Immunol. 178, 5182–5191 [DOI] [PubMed] [Google Scholar]
- 54. De Trez C., Magez S., Akira S., Ryffel B., Carlier Y., Muraille E. (2009) iNOS-producing inflammatory dendritic cells constitute the major infected cell type during the chronic Leishmania major infection phase of C57BL/6 resistant mice. PLoS Pathog. 5, e1000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lin K. L., Suzuki Y., Nakano H., Ramsburg E., Gunn M. D. (2008) CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J. Immunol. 180, 2562–2572 [DOI] [PubMed] [Google Scholar]
- 56. Aldridge J. R., Jr., Moseley C. E., Boltz D. A., Negovetich N. J., Reynolds C., Franks J., Brown S. A., Doherty P. C., Webster R. G., Thomas P. G. (2009) TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc. Natl. Acad. Sci. USA 106, 5306–5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bosschaerts T., Guilliams M., Stijlemans B., Morias Y., Engel D., Tacke F., Herin M., De Baetselier P., Beschin A. (2010) Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-γ and MyD88 signaling. PLoS Pathog. 6, e1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Getts D. R., Terry R. L., Getts M. T., Muller M., Rana S., Shrestha B., Radford J., Van Rooijen N., Campbell I. L., King N. J. (2008) Ly6c+ ″inflammatory monocytes″ are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J. Exp. Med. 205, 2319–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lobigs M., Mullbacher A., Wang Y., Pavy M., Lee E. (2003) Role of type I and type II interferon responses in recovery from infection with an encephalitic flavivirus. J. Gen. Virol. 84, 567–572 [DOI] [PubMed] [Google Scholar]
- 60. Samuel M. A., Diamond M. S. (2006) Pathogenesis of West Nile virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J. Virol. 80, 9349–9360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Willenborg D. O., Staykova M., Fordham S., O′Brien N., Linares D. (2007) The contribution of nitric oxide and interferon γ to the regulation of the neuro-inflammation in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 191, 16–25 [DOI] [PubMed] [Google Scholar]
- 62. Bierly A. L., Shufesky W. J., Sukhumavasi W., Morelli A. E., Denkers E. Y. (2008) Dendritic cells expressing plasmacytoid marker PDCA-1 are Trojan horses during Toxoplasma gondii infection. J. Immunol. 181, 8485–8491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tam M. A., Wick M. J. (2006) Differential expansion, activation and effector functions of conventional and plasmacytoid dendritic cells in mouse tissues transiently infected with Listeria monocytogenes. Cell. Microbiol. 8, 1172–1187 [DOI] [PubMed] [Google Scholar]
- 64. Dogan R. N., Elhofy A., Karpus W. J. (2008) Production of CCL2 by central nervous system cells regulates development of murine experimental autoimmune encephalomyelitis through the recruitment of TNF- and iNOS-expressing macrophages and myeloid dendritic cells. J. Immunol. 180, 7376–7384 [DOI] [PubMed] [Google Scholar]
- 65. Bailey S. L., Schreiner B., McMahon E. J., Miller S. D. (2007) CNS myeloid DCs presenting endogenous myelin peptides ″preferentially″ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat. Immunol. 8, 172–180 [DOI] [PubMed] [Google Scholar]
- 66. Huang Y. M., Yang J. S., Xu L. Y., Link H., Xiao B. G. (2000) Autoantigen-pulsed dendritic cells induce tolerance to experimental allergic encephalomyelitis (EAE) in Lewis rats. Clin. Exp. Immunol. 122, 437–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xiao B. G., Duan R. S., Link H., Huang Y. M. (2003) Induction of peripheral tolerance to experimental autoimmune myasthenia gravis by acetylcholine receptor-pulsed dendritic cells. Cell. Immunol. 223, 63–69 [DOI] [PubMed] [Google Scholar]
- 68. Salazar L., Aravena O., Abello P., Escobar A., Contreras-Levicoy J., Rojas-Colonelli N., Catalan D., Aguirre A., Zuniga R., Pesce B., Gonzalez C., Cepeda R., Cuchacovich M., Molina M. C., Salazar-Onfray F., Delgado M., Toes R. E., Aguillon J. C. (2008) Modulation of established murine collagen-induced arthritis by a single inoculation of short-term lipopolysaccharide-stimulated dendritic cells. Ann. Rheum. Dis. 67, 1235–1241 [DOI] [PubMed] [Google Scholar]
- 69. Yamazaki S., Inaba K., Tarbell K. V., Steinman R. M. (2006) Dendritic cells expand antigen-specific Foxp3+ CD25+ CD4+ regulatory T cells including suppressors of alloreactivity. Immunol. Rev. 212, 314–329 [DOI] [PubMed] [Google Scholar]
- 70. Taieb A., Breitinger J. J., Unadkat J. V., Shufesky W. J., Morelli A. E., Thomson A. W., Lee W. P., Feili-Hariri M. (2007) Intrinsic ability of GM+IL-4 but not Flt3L-induced rat dendritic cells to promote allogeneic T cell hyporesponsiveness. Clin. Immunol. 123, 176–189 [DOI] [PubMed] [Google Scholar]
- 71. Mazzoni A., Bronte V., Visintin A., Spitzer J. H., Apolloni E., Serafini P., Zanovello P., Segal D. M. (2002) Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 168, 689–695 [DOI] [PubMed] [Google Scholar]
- 72. Rössner S., Voigtlander C., Wiethe C., Hanig J., Seifarth C., Lutz M. B. (2005) Myeloid dendritic cell precursors generated from bone marrow suppress T cell responses via cell contact and nitric oxide production in vitro. Eur. J. Immunol. 35, 3533–3544 [DOI] [PubMed] [Google Scholar]
- 73. Xu L., Huang Y., Yang J., Van Der Meide P. H., Levi M., Wahren B., Link H., Xiao B. (1999) Dendritic cell-derived nitric oxide is involved in IL-4-induced suppression of experimental allergic encephalomyelitis (EAE) in Lewis rats. Clin. Exp. Immunol. 118, 115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wood K. J., Sawitzki B. (2006) Interferon γ: a crucial role in the function of induced regulatory T cells in vivo. Trends Immunol. 27, 183–187 [DOI] [PubMed] [Google Scholar]
- 75. Zhang M., Tang H., Guo Z., An H., Zhu X., Song W., Guo J., Huang X., Chen T., Wang J., Cao X. (2004) Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat. Immunol. 5, 1124–1133 [DOI] [PubMed] [Google Scholar]
- 76. Feng G., Gao W., Strom T. B., Oukka M., Francis R. S., Wood K. J., Bushell A. (2008) Exogenous IFN-γ ex vivo shapes the alloreactive T-cell repertoire by inhibition of Th17 responses and generation of functional Foxp3+ regulatory T cells. Eur. J. Immunol. 38, 2512–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tang H., Guo Z., Zhang M., Wang J., Chen G., Cao X. (2006) Endothelial stroma programs hematopoietic stem cells to differentiate into regulatory dendritic cells through IL-10. Blood 108, 1189–1197 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.