Innate immune recognition of Neisseria meningitides capsular polysaccharides by macrophages can occur via both TLR2 and TLR4-MD-2 pathways.
Keywords: capsule, vaccine, macrophage, inflammation, cytokine, E5564
Abstract
CPS are major virulence factors in infections caused by Neisseria meningitidis and form the basis for meningococcal serogroup designation and protective meningococcal vaccines. CPS polymers are anchored in the meningococcal outer membrane through a 1,2-diacylglycerol moiety, but the innate immunostimulatory activity of CPS is largely unexplored. Well-established human and murine macrophage cell lines and HEK/TLR stably transfected cells were stimulated with CPS, purified from an endotoxin-deficient meningococcal serogroup B NMB-lpxA mutant. CPS induced inflammatory responses via TLR2- and TLR4-MD-2. Meningococcal CPS induced a dose-dependent release of cytokines (TNF-α, IL-6, IL-8, and CXCL10) and NO from human and murine macrophages, respectively. CPS induced IL-8 release from HEK cells stably transfected with TLR2/6, TLR2, TLR2/CD14, and TLR4/MD-2/CD14 but not HEK cells alone. mAb to TLR2 but not an isotype control antibody blocked CPS-induced IL-8 release from HEK-TLR2/6-transfected cells. A significant reduction in TNF-α and IL-8 release was seen when THP-1- and HEK-TLR4/MD-2-CD14- but not HEK-TLR2- or HEK-TLR2/6-transfected cells were stimulated with CPS in the presence of Eritoran (E5564), a lipid A antagonist that binds to MD-2, and a similar reduction in NO and TNF-α release was also seen in RAW 264.7 cells in the presence of Eritoran. CD14 and LBP enhanced CPS bioactivity, and NF-κB was, as anticipated, the major signaling pathway. Thus, these data suggest that innate immune recognition of meningococcal CPS by macrophages can occur via TLR2- and TLR4-MD-2 pathways.
Introduction
Neisseria meningitidis infections of humans can be rapidly fatal as a result of an acute inflammatory response, resulting in severe sepsis or meningitis. Meningococcal endotoxin (LOS) is a critical virulence factor that facilitates acute, proinflammatory, innate immune responses at picomolar concentrations [1]. Meningoccoccal LOS binds to MD-2 and activates the TLR4 complex, inducing cytokine/chemokine release from macrophages and monocyte-derived DCs [2, 3]. N. meningitidis CPS are also a major meningococcal virulence factor, a prerequisite for invasive disease, and form the basis of meningococcal serogroup designation and protective polysaccharide and polysaccharide-protein conjugate vaccines [4]. The most common invasive meningococcal serogroups express capsule polymers and consist of the following repeating units: serogroups A, B, C, W135, and Y [4]. CPS polymers are anchored in the meningococcal outer membrane through diacylglycerophosphate lipid anchors [5]. However, the innate immune recognition of these polymers and their role in induction of the inflammatory responses are not well understood.
CPS purified from Vibrio vulnificus and composed of a trisaccharide repeating unit (N-acetylquinovosamine, GalNAc, GalNAcA) have been found to induce the release of TNF-α in vivo and in vitro [6, 7]. Also CPS from Cryptococcus neoformans composed of glucuronoxylomannan [8] induce TLR4-mediated signaling without TNF-α release [9], whereas the helminth glycan (lacto-N-fucopentaose III) was found to induce DC maturation in a TLR4-dependent manner [10, 11]. Recently, Wang et al. reported that Bacteroides fragilis CPS, a zwitterionic tetrasaccharide repeating unit [12], stimulated innate and adaptive immunity through TLR2 [13]. Recognition of encapsulated Streptococcus suis by macrophages is TLR2-dependent, and this CPS exacerbates inflammation [14]. Further, CPS purified from Actinobacillus actinomycetemcomitans, an important pathogen causing periodontitis, induce inflammatory cytokine release from the human monocytic cell line THP-1 [15]. More recently, the immunostimulatory activity of algal polysaccharides from Chlorella pyrenoidosa was reported to induce macrophage activation via TLR4 [16]. Similarly, a polysaccharide fraction from the medicinal mushroom Polyporus umbellatus was reported to induce macrophage activation via TLR4 [17, 18].
The ability to genetically engineer a viable N. meningitidis strain with an lpxA mutant [19], which lacks LOS, provides a useful tool to dissect the role of other meningococcal molecules/ligands, such as CPS, which contribute to virulence and possibly to the severity of the inflammatory responses to meningococci. Studies using LOS-deficient meningococcal strains have suggested that non-LOS ligands cause fatal meningococcal sepsis in a mouse model via TLR4- and MyD88-dependent signaling [20–23]. However, the non-LOS ligands were not identified.
Meningococcal lpxA (NMB strain) mutants are not viable without capsule expression [19, 24]. In this study, highly purified CPS polymers from a strain NMB-lpxA mutant as well as the CPS prepared for vaccine use were used to investigate CPS innate immune recognition by host macrophages. Meningococcal CPS polymers induced inflammatory responses via TLR4-MD-2 and TLR2 in human and murine macrophage cell lines and in transfected cells.
MATERIALS AND METHODS
Reagents
RPMI-1640 medium, DMEM, FBS, penicillin/streptomycin, sodium pyruvate, and nonessential amino acids were obtained from Cellgro Mediatech (Herndon, VA, USA). Opti-MEM tissue-culture media and PMA were purchased from Gibco-BRL (Grand Island, NY, USA). Human and mouse TNF-α, IL-8, IL-6, and IP-10 ELISA kits were from R&D Systems (Minneapolis, MN, USA). Cell-based transcription factor arrays, transfection reagent, and RT-PCR arrays and reagents were from SABiosciences (Frederick, MD, USA). A dual luciferase reporter assay system was from Promega (Madison, WI, USA). RAW 264.7 and 23ScCr (TLR4-deficient) cell lines were purchased from ATCC (Manassas, VA, USA). Pam3CSK4, Basticidin, 293 HEK-TLR2/6, HEK-TLR2, and HEK-TLR4-MD-2-CD14 stably transfected cells were purchased from InvivoGen (San Diego, CA, USA). The HEK-TLR2/CD14 stably transfected cell line was provided by Dr. Evelyn Kurt-Jones (University of Massachusetts Medical Center, Worcester, MA, USA). Eritoran (E5564) [25–27] was a gift from Eisai Pharmaceuticals (Andover MA, USA). Highly purified Rhizobium LPS was a kind gift from Dr. Russell Carlson (Complex Carbohydrate Research Center, University of Georgia, Athens, GA, USA).
CPS purification
CPS were purified from an endotoxin-deficient serogroup B N. meningitidis mutant (NMB267-lpxA) and from WT meningococci of serogroups A, B, C, W135, and Y, as described previously [5, 28]. Briefly, meningococci were grown in 3 L GC broth for 16 h, and CPS were released by lysing with 10% of Cetavlon (hexadecyltrimethyl ammonium bromide), added to a final concentration of 1.0%. The precipitate and bacterial debris were collected by centrifugation (11,000 g for 15 min) and then resuspended in 50 ml distilled water. CPS-Cetavlon complexes were dissociated with 1 vol 2 M CaCl2 and by stirring for 1 h. Nucleic acids were digested with DNase and RNase, precipitated with absolute ethanol, and removed by centrifugation. CPS were precipitated by 80% ethanol (v/v), washed three times with acetone and twice with diethylether, and dried by vacuum. Contaminating proteins and phospholipids were removed with proteinase K digestion, followed by extensive dialysis against buffer (10% ethanol, 50 mM NaCl, and 5 mM Tris). Extracted CPS material was purified further by a Sephacryl 200 gel filtration column using 50 mM ammonium formate elutions, and void volume fractions were pooled and concentrated by speed vacuuming as described previously [28]. The purity of CPS preparations was demonstrated using Alcian blue and silver-staining methods [28]. Vaccine-grade CPS (MAPS) was a gift from Dr. Seshu Gudlavalleti (Center for Biologics, U.S. Food and Drug Association, Rockville, MD, USA; now at GN International Medical Corp., Omaha, NE, USA). Membrane phospholipid extraction from LOS-deficient meningococci was performed as described previously [1].
Cell cultures
THP-1 human macrophage-like cells were obtained from the ATCC and grown in RPMI 1640 with L-glutamate, supplemented with 10% FBS, 50 IU/ml penicillin, 50 μg/ml streptomycin, 1% sodium pyruvate, and 1% nonessential amino acids. Culture flasks were incubated at 37°C with humidity under 5% CO2. Murine macrophages (RAW 264.7 and 23ScCr) and HEK293 were grown in DMEM, supplemented, and incubated as noted above.
Cellular activation
THP-1 (human macrophage-like cells) and murine RAW 264.7 (TLR4-sufficient), 23ScCr (TLR4-deficient), HEK-TLR2/6, and HEK-TLR4-MD-2-CD14 stably transfected cell lines were stimulated with meningococcal CPS polymers. Purified CPS samples were freshly dissolved in sterile H2O at 1 mg/ml stock concentration and vortexed for 2 min. Working CPS concentrations (ranging from 100 μg/ml to 50 ng/ml) were made in duplicate wells using sterile PBS by serial fold dilutions in 96-well tissue-culture plates at 50 μl final volumes. Freshly grown THP-1 cells and HEK-TLR2/6- and HEK-TLR4-MD-2-CD14-transfected cells, each adjusted to 106 cells/ml and 250 μl aliquots, were dispensed into each well at a final 250 × 103 cell density in the designated 96-well plates, which were incubated overnight at 37°C with 5% CO2 and humidity. Supernatants from stimulated cells were harvested and stored at –20°C for further use.
Cytokine profiles
Following CPS stimulation, the cytokines TNF-α, IL-6, and CXCL10 (IP-10) released from THP-1 cells and IL-8 released from HEK-TLR2-transfected cells were quantified by DuoSet ELISA (R&D Systems) as described previously [1, 29].
NO induction by murine macrophages
Freshly grown, adherent RAW 246.7 or 23ScCr (TLR4-deficient) macrophages were collected by cell scraping. Harvested cells were washed and resuspended in Dulbecco's complete media, counted, and adjusted to 106 cells/ml. Aliquots (250 μl) were then dispensed into each well at a final 250 × 103 cell density in the designated 96-well plates, prior to stimulation with purified CPS polymers as noted above. The induced RAW 264.7 or 23ScCr macrophages were incubated overnight at 37°C with 5% CO2, and supernatants were harvested and saved. NO release was quantified using the Greiss chemical method as described previously [1].
TLR2 inhibition
The human TLR2 receptor was blocked with the specific mAb clone TL2.1 from eBioscience (San Diego, CA, USA) prior to stimulation with CPS. THP-1 and HEK-TLR2/6 cells were adjusted to 1 million cells/ml, resuspended in 200 μl PBS with 10 μg anti-TLR2 antibody or isotype antibody (IgG2a from eBioscience), and incubated for 30 min at 37°C with gentle shaking. Cells were then centrifuged at 2000 rpm (500 g) for 3 min and resuspended in 1 ml RPMI 1640 or DMEM, transferred into 96-well plates, then stimulated with CPS as noted above, and incubated overnight.
TLR4-MD-2 competitive inhibition with Eritoran
Eritoran (E5564), a synthetic lipid A antagonist, binds to MD-2/TLR4 [30] and consequently, inhibits TLR4 receptor complex activation by activating ligands. THP-1 cells were counted and adjusted to 1 million cells/ml in RPMI-1640 media with 10% FBS or in serum-free conditions. RAW264 murine macrophages and human HEK-TLR4-MD-2-CD14 and HEK-TLR2/6 stably transfected cells were also counted and adjusted to 1 million cells/ml in DMEM with 10% FBS or in serum-free conditions; 250 μl was then dispensed into 96-well tissue-culture plates at 250 × 103 cells/well. Eritoran was diluted in sterile PBS at 100 μg/ml concentration, and 10 μl/well (1 μg final) was added to cells prior to the addition of CPS, ranging from 100 to 0.32 μg/ml. The plates were incubated overnight at 37°C with 5% CO2, and supernatants were harvested and saved at –20°C for further use. For time-course experiments, cells were stimulated with CPS as above, and Eritoran (1 μg final) was added together (0 min) or after 15, 30, 60, and 120 min of stimulation. For dose-response experiments, Eritoran was added at 0.5, 1, or 2.5 μg/ml prior to stimulation with CPS. Cells were then incubated overnight at 37°C with 5% CO2, and supernatants were harvested and saved at –20°C for further use. TNF-α and IP-10 (CXCL10) release was measured in THP-1 supernatants, and IL-8 release was measured in HEK cell supernatants by ELISA. NO release was measured as nitrite accumulation in RAW 264.7 supernatants by the Greiss method as described above.
Signaling pathway activity assay
To determine the signaling pathways induced upon meningococcal CPS recognition, a transcription factor array (Cignal Finder™ 10-pathway reporter arrays, SABiosciences), consisting of 10 dual-luciferase reporter assays, was used according to the manufacturer′s instructions. Each of the 10 reporter assays encodes for an inducible transcription factor-responsive firefly luciferase reporter and a constitutively expressing Renilla construct in a 40:1 ratio. Briefly, DNA reporter master mixes were prepared in SureFect transfection reagent (SABiosciences) diluted in Opti-MEM without serum or antibiotics, and 25 μl/well was dispensed into 96-well white tissue-culture plates. For reverse transfection, freshly grown cells were counted and adjusted to 1 million cells/ml in Opti-MEM without serum or antibiotics. Cells (100 μl; 100×103 cells/well) were laid over the DNA reporters in 96-well plates and incubated overnight at 37°C with 5% CO2. Transfection media were removed and replaced with 150 μl fresh DMEM, supplemented with 10% FBS, and incubated again overnight at 37°C. Cells were then induced with meningococcal CPS polymers or LOS doses for 5 h, and dual-luciferase reporter activity was determined using a dual-luciferae reporter assay system (Promega), following the manufacturer′s instructions. Induced transcription factors were reported as firefly luciferase activity normalized to Renilla luciferase activity.
Chemical inhibitors for signaling pathways
To help determine signaling pathways induced by meningococcal CPS polymers, specific chemical inhibitors were used. THP-1 cells were counted and adjusted to 1 million cells/ml, treated with 10 μM final concentration of inhibitors [SP600125 for the JNK pathway (Calbiochem, San Diego, CA, USA); SB203580 for the p38 pathway; PD98059 for the MEK pathway (Promega)] or DMSO alone, and incubated at 37°C for 30 min. Inhibitors were removed by centrifugation, and treated cells were resuspended in fresh RPMI, and then 250 μl/well was transferred into 96-well plates prior to addition of meningococcal CPS polymers in doses ranging from 10 to 0.32 μg/ml and incubated overnight at 37°C with 5% CO2. Supernatants were harvested and saved at –20°C for TNF-α and IL-6 quantification.
Adaptor protein down-regulation by plasmids expressing DN-human TRAM and TIRAP genes
Stably transfected HEK293/TLR4-MD-2-CD14 and HEK/TLR2/6 cells were seeded in 96-well plates at 3 × 105 cells/well and then transiently transfected with 0.5 μg/well the dominant negative DN-pDeNy plasmids DN-TRAM and DN-TIRAP, obtained from InvivoGen, following the manufacturer′s instructions. SuperFect transfection reagent (Qiagen, Valencia, CA, USA), diluted in Opti-MEM, was added for 3 h, and an empty pDeNy plasmid was used as a control. Fresh DMEM, supplemented as mentioned above, was added, and the cells were incubated further for 18 h. The cells were then stimulated with doses of purified CPS polymers and incubated for another 18 h, and IL-8 release in supernatant was measured by ELISA (R&D Systems).
Gene expression and real-time PCR
One million cells/ml THP-1 cells were transferred to six-well formats and then stimulated with TLR4 ligands. Unstimulated cells were used as controls for basal gene expression level. Cells will be incubated overnight at 37°C under 5% CO2. RNA was isolated using RNeasy mini kits (Qiagen). Briefly, cells were harvested in cell lysis buffer RLT (Qiagen) and passed over QiaShredder columns, and the resulting lysate was mixed in 75% ethanol and then passed over RNeasy columns, which were washed, and RNA was eluted with water and then treated with 2 units DNaseI for 1 h at 37°C in 50 μl buffer (10 mM Tris-HCl, 2.5 mM MgCl2, 0.1 mM CaCl2, pH 7.5). DNase inactivation buffer (5 μl) was added and incubated for 2 min at room temperature prior to centrifugation for 1 min. RNA was isolated from supernatants using RNeasy kit (Qiagen) and was then reverse-transcribed to cDNA by PCR. In brief, 2 μg total RNA was reverse-transcribed in a 100-μl total volume buffer at pH 8.3 [50 mM KCl, 10 mM Tris, 5.5 mM MgCl2, 0.5 mM each dNTPs, 0.125 μM random hexamer, 40 units RNase inhibitor, and 125 units MultiScribe (Applied Biosystems, Foster City, CA, USA)]. RNA mixture samples were incubated for 10 min at 25°C, 30 min at 48°C, and then 5 min at 95°C to inactivate the RT enzyme. The generated cDNA was diluted with 91 μl ddH2O to each 20 μl cDNA synthesis reaction. The experimental cocktail for real-time PCR was prepared in a sterile boat as follows: 1275 μl 2× SYBR Green PCR master mix (Applied Biosystems), 102 μl diluted cDNA, 1173 μl ddH2O. Real-time PCR was then performed using the RT2 Profiler™ PCR array (SABiosciences) in a 96-well format preloaded with the primers. Human TLR signaling pathway and human apoptosis pathway RT2 Profiler™ PCR arrays profile the expression of 84 genes related to TLR-mediated signal transduction and apoptosis pathways. In addition to primers, the array contains all positive and negative controls required for a real-time PCR procedure. To start the real-time PCR reaction, 25 μl experimental cocktail mix was added carefully to each well in the RT2 PCR array using a multi-channel pipette and then was sealed tightly with the optical adhesive film. The PCR parameters were set as follows: 2 min at 50°C, 10 min at 95°C, and 45 cycles of 95°C for 15 s, followed by 1 min at 62°C. For data analysis, the Excel-based PCR array data analysis template (downloaded from http://www.superarray.com/pcrarraydataanalysis.php) was used. Gene expression profiles were calculated automatically from threshold cycle data generated from the real-time instrument, and any comparative threshold value ≥35 will be considered negative.
Statistical analysis
Mean values ± sd and P values (Student′s t test) of at least four independent determinations were calculated with Microsoft Excel software.
RESULTS
Bioactivity of meningococcal CPS
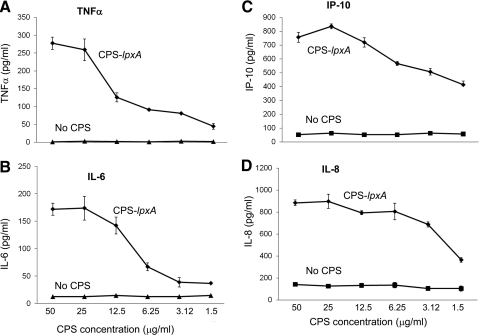
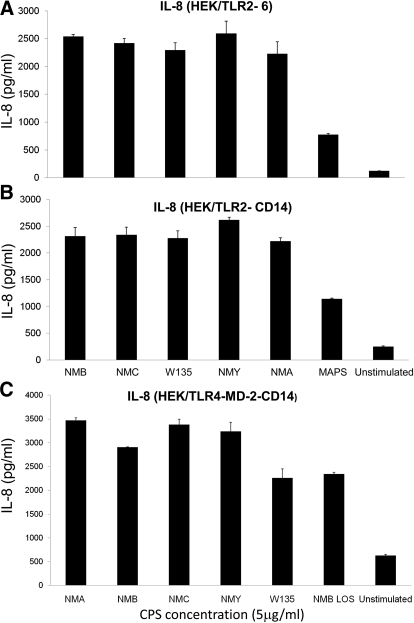
Established human and murine macrophage cell lines and stably transfected HEK293 cells were exposed to serogroup B meningococcal CPS polymers purified from a LOS-deficient lpxA mutant. Serogroup B CPS-lpxA polymers induced TNF-α, IL-6, IL-8, and CXCL10/IP-10 release from THP-1 cells (Fig. 1). Serogroup B CPS-lpxA polymers also induced TNF-α, NO, IP-10, and MIP-2 from RAW264.7 murine macrophages (Supplemental Fig. 1) in a dose-dependent manner. These results suggested that meningococcal CPS polymers devoid of endotoxin contamination are biologically active and induced proinflammatory cytokines and NO release in a dose-dependent manner. In support of this conclusion, meningococcal CPS polymers purified from WT meningococci expressing CPS A, B, C, W135, and Y induced a dose-dependent IL-8 release from HEK293 cells stably transfected with TLR2/6, TLR2-CD14, TLR4-MD-2-CD14 (Fig. 2A–C, respectively), and TLR2 (data not shown), but not HEK293 cells alone. Further, meningococcal CPS polymers from these serogroups induced NO release from RAW264.7 (TLR4-sufficient) and ScCr (TLR4-deficient) murine macrophages (Supplemental Fig. 2A and B). Monosaccharides alone (e.g., GlcNac, ManNAc, and sialic acid) did not activate macrophages, even at high concentrations. CPS polymers purified for commercial vaccine use from N. meningitidis serogroup A, designated MAPS, also induced proinflammatory responses in human and murine macrophages and in stably transfected HEK293 cells (Supplemental Fig. 2A and Fig. 2A and B).
Figure 1. Meningococcal serogroup B CPS polymers purified from a LOS-deficient (lpxA) mutant (CPS-lpxA) induced inflammatory cytokine (TNF-α and IL-6) and chemokine (IP-10 and IL-8) release from human macrophages.
THP-1 cells (250×103/well) in 96-well plates were induced with serogroup B CPS-lpxA polymers (50–1.56 μg/ml) overnight. Cytokine and chemokine release was quantified by ELISA. (A) TNF-α; (B) IL-6; (C) IP-10 (CXCL10); and (D) IL-8 release. Unstimulated macrophages incubated simultaneously were used as the control. Error bars represent sd from the mean of quadruplicate readouts. The results are representative of four independent experiments.
Figure 2. Meningococcal CPS from strains causing invasive disease induce IL-8 release via TLR2 and TLR4-MD-2.
Stably transfected HEK293 cells seeded in a 96-well plate at 250 × 103/well with (A) TLR2/6, (B) TLR2-CD14, or (C) TLR4-MD-2-CD14 were induced with a 5-μg dose of meningococcal CPS polymers from serogroups A (NMA), B (NMB), C (NMC), Y (NMY), and W135. MAPS is the vaccine-grade meningococcal serotype A CPS polymer used. Meningococcal LOS (NMB LOS) was used at 1 ng/ml (∼0.56 pmole) as a control. Unstimulated macrophages incubated simultaneously were also used as a control. IL-8 release was measured by ELISA. Error bars represent sd from the mean of quadruplicate readouts. The results are representative of three independent experiments.
TLR2- and TLR4-MD-2-mediated recognition of meningococcal CPS
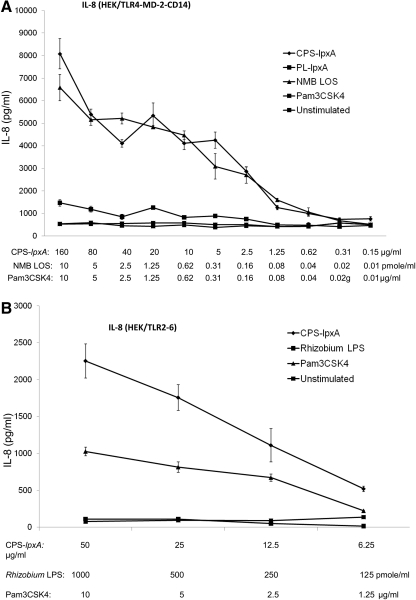
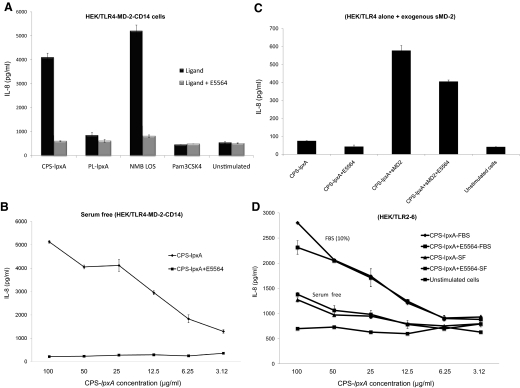
Meningococcal CPS induced cytokine and chemokine responses in TLR4-sufficent and TLR4-deficient macrophages and in HEK/TLR4-MD-2-CD14-, TLR2/6-, and TLR2-transfected cells. No response was detected in HEK293 cells alone, which suggested a TLR-specific-mediated recognition. The role of the TLR4-MD-2 complex and TLR2 in recognizing meningococcal CPS polymers was investigated further. Serogroup B CPS-lpxA polymers and meningococcal LOS, but not the synthetic TLR2 ligand Pam3CSK4 or meningococcal membrane phospholipids extracted from the lpxA mutant, induced a dose-dependent IL-8 release from HEK/TLR4-MD-2-CD14-transfected cells (Fig. 3A). In contrast, meningococcal CPS-lpxA polymers and Pam3CSK4, but not meningococcal LOS or Rhizobium LPS, induced IL-8 release from HEK/TLR2/6-transfected cells (Fig. 3B).
Figure 3. Meningococcal CPS polymers activate TLR2- and TLR4-MD-2-mediated IL-8 responses.
Dose-dependent IL-8 release from stably transfected HEK293 cells induced with meningococcal serogroup B CPS-lpxA polymers overnight. (A) HEK/TLR4-MD-2-CD14 stably transfected cells were induced with serogroup B CPS-lpxA or a membrane phospholipid extract designated PL-lpxA from the same strain. NMB LOS and Pam3CSK4 were used as controls. (B) HEK/TLR2/6 stably transfected cells were stimulated with CPS-lpxA polymers. Rhizobium LPS and Pam3CSK4 were used as controls. IL-8 release was measured by the ELISA method. Error bars represent sd from the mean of quadruplicate readouts. The results are representative of three independent determinations.
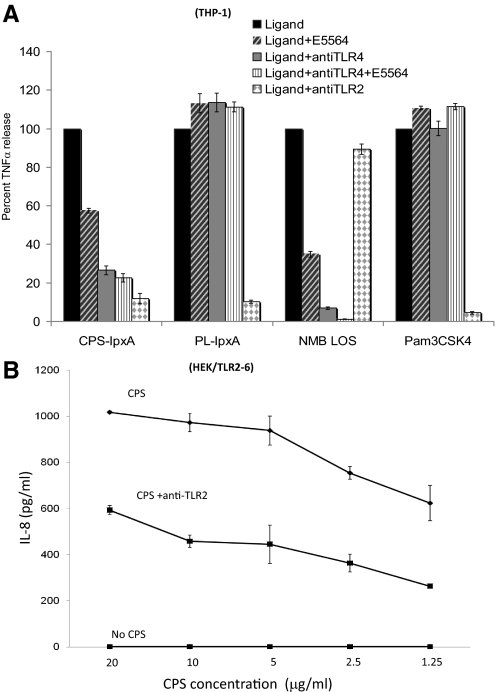
To confirm the role of TLR2 in recognizing meningococcal CPS polymers, THP-1 cells that express TLR4 and TLR2 receptors were blocked with anti-TLR2 or anti-TLR4 antibodies prior to stimulation with CPS. A significant reduction in TNF-α (Fig. 4A) and IL-6 release (data not shown) was seen in TLR2 blocked cells induced with CPS-lpxA or the phospholipid fraction PL-lpxA or Pam3CSK4, but not with the isotype control antibody blocked cells or unblocked THP-1 cells. In contrast, TNF-α release was inhibited in THP-1 blocked with anti-TLR4 antibody prior to stimulation with meningococcal LOS or CPS-lpxA but not with the PL-lpxA fraction or Pam3CSK4 control (Fig. 4A). A similar reduction of IL-8 release from HEK/TLR2/6 stably transfected cells was seen when cells were blocked with the anti-TLR2 antibody (Fig. 4B). The data demonstrated that meningococcal CPS polymers are biologically active ligands that induced inflammatory responses in macrophages via TLR2 and TLR4 receptors.
Figure 4. Blocking of TLR2 and TLR4 with antibodies reduced meningococcal CPS polymer-induced cellular activation.
(A) TNF-α release from THP-1 cells blocked with TLR2 and TLR4 antibodies for 30 min prior to the addition of serogroup B CPS-lpxA (50 μg/ml), the phospholipid (sham control) extraction PL-lpxA (50 μg/ml), NMB LOS (1 ng/ml), or Pam3CSK4 (5 μg/ml) ± E5564 (1 μg/ml), the synthetic TLR4-MD-2 inhibitor, and incubated overnight. (B) IL-8 release from HEK-TLR2/6 stably transfected cells stimulated with CPS in the presence or absence of anti-TLR2 blocking antibody. TNF-α and IL-8 release was measured by ELISA. The results are representative of two independent experiments.
MD-2 is required for meningococcal CPS recognition via TLR4
To further define TLR4-mediated meningococcal CPS inflammatory responses, the synthetic lipid A antagonist E5564 (designated Eritoran), which binds MD-2 [30], was used in a competitive inhibition assay. E5564 (1 μg/ml) inhibited meningococcal serogroup B CPS-lpxA polymer, and LOS induced IL-8 release from HEK/TLR4-MD-2-CD14-transfected cells (Fig. 5A). In serum-free conditions, E5564 (0.5 μg/ml) completely abolished IL-8 release from HEK/TLR4-MD-2-CD14 cells (Fig. 5B), even when exogenous rCD14 and LBP were added (data not shown). CPS-lpxA polymers did not activate HEK293 cells transfected with TLR4 alone without MD-2 and failed to induce IL-8 release (Fig. 5C). However, IL-8 release was restored by the addition of exogenous, sMD-2 and was reduced significantly in the presence of E5564 (Fig. 5C). In contrast, E5564 did not inhibit meningococcal CPS-lpxA polymer-induced IL-8 release from HEK/TLR2/6-transfected cells in serum-free conditions or in the presence of 10% FBS (Fig. 5D). Thus, E5564 exerted competitive inhibitory effects only in TLR4-sufficient macrophages and TLR4-transfected cells. The inhibitory effect of E5564 was not species-specific, as E5564 (1 μg/ml) significantly inhibited murine TNF-α and NO release from RAW264 cells stimulated with meningococcal CPS-lpxA polymers (Supplemental Fig. 3A and B). Moreover, E5564 did not inhibit IL-8 release from HEK/TLR2 cells, even at a high dose (Supplemental Fig. 3C). In contrast, E5564 significantly reduced IL-6 release from human THP-1 cells induced by meningococcal CPS polymers purified from serogroup A and from the WT serogroup B (Supplemental Fig. 3D). Similarly, E5564 inhibited TNF-α, IL-8, and NO release from human THP-1 cells and RAW264 cells induced with meningococcal serogroups A and B (data not shown). Thus, the results indicated that MD-2 is required for TLR4-mediated meningococcal CPS inflammatory responses but not for activation via TLR2 or TLR2/6. The strong, competitive, inhibitory effect of E5564, a well-documented MD-2 ligand, suggested that meningococcal CPS polymers may directly bind to MD-2.
Figure 5. MD-2 is required for meningococcal CPS recognition via TLR4.
(A) HEK/TLR4-MD-2-CD14 stably transfected cells were induced with serogroup B CPS-lpxA, with or without 1 μg/ml E5564, a synthetic lipid A antagonist that binds MD-2, in 10% FBS. NMB LOS was used as the control. (B) HEK/TLR4-MD-2-CD14 stably transfected cells were induced with serogroup B CPS-lpxA, with or without 0.5 μg/ml E5564 in serum-free conditions. (C) HEK/TLR4 stably transfected cells alone induced with serogroup B CPS-lpxA, with or without exogenous sMD and sCD14 or 1 μg/ml E5564. (D) HEK/TLR2/6 stably transfected cells induced with CPS-lpxA in serum-free and 10% FBS conditions in the presence or absence of 1 μg/ml E5564. The results are representative of two independent experiments.
CD14 and LBP enhance meningococcal CPS bioactivity
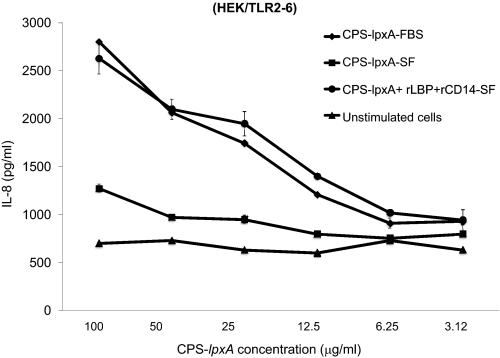
The role of CD14 and LBP in enhancing cellular responses and recognition of meningococcal CPS was investigated using human rCD14 and LBP. Stably transfected HEK/TLR2/6 cells were stimulated with CPS-lpxA doses in serum-free condition or in 10% FBS, where exogenous rCD14 and rLBP were added at 20 ng/ml final concentration. IL-8 release was enhanced significantly in the presence of exogenous rCD14 and rLBP in serum-free and 10% FBS conditions (Fig. 6). Similar results were seen in HEK/TLR4-MD-2-CD14 and THP-1 cells (data not shown). The data suggest that CD14 and LBP enhance signaling by transferring meningococcal CPS polymers to the sensing PRRs TLR2 and TLR4 and possibly others, similar to their role in transferring lipopeptides and endotoxin.
Figure 6. CD14 and LBP enhanced meningococcal CPS bioactivity.
Dose-dependent IL-8 release from HEK/TLR2–6 stably transfected cells induced with serogroup B CPS-lpxA in serum-free (SF) or 10% FBS conditions, with or without exogenous rCD14 or rLBP added at 20 ng/ml. The results are representative of two independent experiments.
Inflammatory signaling pathways induced by meningococcal CPS polymers
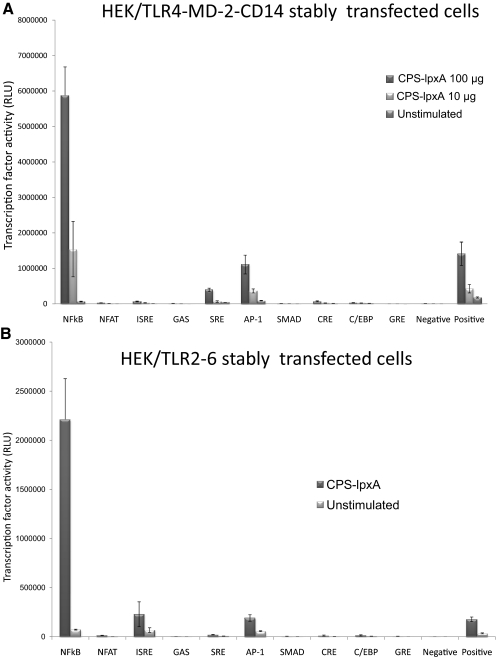
To determine the signaling pathways induced upon TLR2 and TLR4-MD-2 activation by meningococcal CPS polymers, a 10-pathway reporter array was used. The array included the following pathway/transcription factor reporter assays: NF-κB/NF-κB; PKC/Ca++/NFAT; type 1 IFNs/ISRE; IFN-γ/GAS; MAPK-ERK/SRE; MAPK-JNK/AP-1; TGF-β/SMAD; cAMP-PKA/CRE; C-EBP/C-EBP; and glucocorticoid receptor/GRE. Stably transfected HEK/TLR2/6 and HEK/TLR4-MD-2-CD14 cells were used in transient reverse transfection with the 10-pathway reporter array prior to stimulation with meningococcal serogroup B CPS polymers (100 μg and 10 μg doses) for 5 h. NF-κB was found to be the major signaling pathway activated and was highly induced in HEK/TLR4-MD-2-CD14 in a dose-dependent manner (Fig. 7A) and in HEK-TLR2/6 cells (Fig. 7B). Other pathways, such as type 1 IFN/ISRE and MAPK-JNK/AP-1, were also induced but less than the NF-κB pathway. Further, IL-8 release from stimulated cells above was measured, and the results showed that cells across the array were stimulated equally (data not shown).
Figure 7. Inflammatory signaling transcription factors are induced by meningococcal CPS-lpxA polymers.
(A) Inducible transcription factors dual luciferase reporters (see Materials and Methods) were transfected into HEK/TLR4-MD-2-CD14 stably transfected cells and then stimulated with serogroup B meningococcal CPS-lpxA polymers (100 and 10 μg doses) for 5 h. Inducible transcription factor firefly luciferase activity was normalized to the constitutively expressed Renilla luciferase reporter. (B) Inducible transcription factor dual luciferase reporters transfected into HEK/TLR2/6 stably transfected cells and then stimulated with serogroup B meningococcal CPS-lpxA (100 μg doses) for 5 h. Inducible transcription factor firefly luciferase activity was normalized to constitutively expressing the Renilla luciferase reporter. Negative control is transfected with the noninducible firefly luciferase reporter. Positive control is a mixture of constitutively expressing GFP and the firefly luciferase construct. This experiment is representative of two independent determinations. RLU, Relative light unit.
To verify the signaling pathway induced by meningococcal CPS polymers, THP-1 cells were exposed to chemical inhibitors specific for MAPKs p38 (SB203580), JNK (SP600125), and MEK1/2 (PD98059) pathways prior to CPS stimulation. Compared with THP-1 cells in DMSO, all three inhibitors resulted in a dramatic reduction in TNF-α release upon stimulation after induction with CPS polymers (Supplemental Fig. 4).
TIRAP and TRAM adaptor proteins are involved in meningococcal CPS-induced signaling
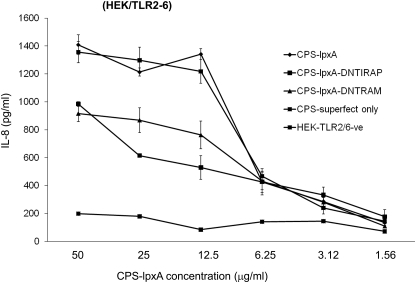
Upon TLR-ligand ligation, the membrane-tethered adaptor proteins TIRAP and TRAM bridge TLR cytoplasmic TIR domains to MyD88 and TRIF adaptor proteins, respectively, to initiate inflammatory signaling pathways [31]. Sacre et al. [32] have shown that TRAM is an adaptor protein for LPS and LTA signaling via TLR4 and TLR2/6, respectively. TRAM dependence was observed in primary human cells, synovial fibroblasts, HUVECs, and murine embryonic fibroblasts from TRAM-deficient cells or TRAM-sufficient cells transfected with DN-TRAM plasmid [32]. To determine the role of TRAM and TIRAP adaptor proteins in the proinflammatory cytokine induction by meningococcal CPS polymers, the DN-TIRAP and DN-TRAM constructs were used to block the MyD88- and TRIF-dependent signaling pathways in THP-1 cells and HEK/TLR2/6 cells by transient transfection prior to stimulation with meningococcal CPS-lpxA polymers. Significant reduction in IL-8 release was seen in HEK/TLR2/6 cells transfected with DN-TIRAP or DN-TRAM constructs (Fig. 8). Similar reduction in TNF-α and IL-8 release from THP-1 transfected with DN-TIRAP and DN-TRAM was seen (data not shown). The data suggest that inflammatory signaling pathways induced by meningococcal CPS polymers via TLR2 and TLR4-MD-2 used the TIRAP and TRAM adaptor proteins. Further, the inflammatory signaling induced by meningococcal CPS was confirmed by the real-time PCR method using the RT2 Profiler™ PCR array (see Materials and Methods). Differential gene induction profiles were observed in human macrophage-like THP-1 cells induced by CPS-lpxA (10 μg/ml) compared with meningococcal LOS (2 pmole/ml∼4 ng/ml). Although THP-1 cells were induced with >1000-fold of meningococcal CPS-lpxA compared with LOS, differential gene induction was demonstrated (Supplemental Fig. 5). In contrast, Rhizobium LPS, a well-known TLR4 antagonist [33], failed to induce inflammatory responses in THP-1 cells. Taken together, the data provided additional evidence that CPS-lpxA polymers are immune-stimulatory and devoid of any other TLR4 ligands.
Figure 8. TIRAP and TRAM adaptor proteins are required for meningococcal CPS-induced IL-8 release.
HEK/TLR2/6 stably transfected cells were transiently transfected with 0.5 μg DN-TIRAP or DN-TRAM constructs. An empty vector was used as a control in the presence of CPS-lpxA, and tranfection buffer designated CPS-superfect only. Unstimulated cells HEK/TLR2/6-ve (negative) were used as a control for DN-construct but without CPS-lpxA.
DISCUSSION
Meningococcal CPS form the basis for serogroup designation and highly protective meningococcal vaccines [4]. Meningococcal CPS is also a key meningococcal virulence factor essential for invasive disease, providing antiphagocytic properties and allowing the organism to evade human complement and bactericidal killing [4]. If and how the host innate immune system, mainly macrophages, senses or recognizes these different α-glucan polymers remain largely unknown. I found evidence that structurally distinct meningococcal CPS polymers were recognized via TLR2 and TLR4-MD-2 and induced proinflammatory cytokine and chemokine release from human and murine macrophages. Monosaccharides alone, such as GlcNac, ManNAc, and sialic acid, did not activate macrophages, even at high doses, indicating that CPS polymerization and structure were key in macrophage activation.
Meningococcal LOS is a potent inducer of innate inflammation and cytokine storm during meningococcemia or meningococcal meningitis [1, 29]. Circulating levels as low as 1 ng/ml are correlated with septic shock and death [34]. However, the construction of LOS-deficient meningococcal mutants by inactivation of lpxA [19] provided evidence that LOS-deficient meningococci induce innate-inflammatory responses [24, 35–38]. Plant et al. [21] reported that MyD88-deficient mice are resistant to i.p. challenge with the LOS-deficient lpxA mutant and concluded that non-LOS ligands induce MyD88-dependent meningococcal sepsis. They also demonstrated that TLR4-deficient mice are resistant to challenge with the LOS-deficient meningococcal lpxA mutant, again suggesting that non-LOS ligands induced TLR4-mediated sepsis [20]. Further, using an experimental porcine model, Hellerud et al. [23] reported that the LOS-deficient meningococcal mutant induced cardiovascular and hematologic changes quite similar to those caused by the LOS-sufficient strain, suggesting the contribution of a non-LOS ligand to the endotoxic activity of N. meningitidis. Of note, LOS-deficient meningococcal lpxA mutants created in serogroup B strain H44/76 are not viable without the expression of CPS [19, 39]. The construction of a nonencapsulated meningococcal LOS-deficient mutant in the NMB strain was also not successful, although Bos and Tommassen [40] reported the viability of a capsule- and LOS-deficient meningococcal mutant constructed in the nonencapsulated strain HB-1, derived from meningococcal H44/76 strain. Meningococcal CPS polymers appear to be one non-LOS ligand that induces TLR4-MyD88. Other non-LOS ligands, such as the porin protein PorB, induce TLR2- but not TLR4-mediated inflammatory responses [41]. In contrast to LOS, which induces TLR4-MD-2 activation at picomolar concentrations, CPS concentrations ≥1 μg/ml are required. The results suggest that meningococcal LOS has approximately 1000-fold more bioactivity compared with meningococcal CPS. In several studies comparing LOS-deficient and WT meningococci, 108 CFUs were required for the LOS-deficient lpxA mutant to demonstrate significant cytokine release [36–38]. Following an extensive TLR literature search, I noticed that all described synthetic and natural TLR ligands (e.g., Pam3CSK4, CpG, poly-inosinic:polycytidylic acid, LTA, flagellin, porin A, plant-derived carbohydrate polymers, and fungal-derived and parasitic ligands) were used at microgram concentration and not at picomolar or nanogram concentrations, like LOS or LPS, which translate to an ∼1000-fold higher dose. To my knowledge, LOS or LPS is the only TLR ligand used at nanogram or picomolar concentration that elicited a detectable cellular response.
Extensive efforts were made to eliminate other TLR ligands in the CPS preparations used in this study, as well as by using cells expressing defined TLRs. In addition to the endotoxin-deficient background, CPS preparations were subjected to proteinase K digestion, DNase, and RNase treatment; ultracentrifugation and sephacryl gel filtration; and extensive dialysis with a 30-KD size cut-off value (see Materials and Methods), which removed small molecules but left the larger CPS polymers. CPS extraction from the endotoxin-deficient meningococci was prepared in parallel with a phospholipid extraction (PL-lpxA) from the same GC broth-grown bacteria. In contrast to the CPS-lpxA preparation, which strongly induced IL-8 release from stably transfected HEK/TLR4-MD-2-CD14 cells, the PL-lpxA preparation did not induce IL-8 release. Some activity of the PL-lpxA preparation was observed in HEK/TLR2 cells at high concentrations but was unlikely to account for the bioactivity of the CPS preparations. Steeghs et al. [39] extensively analyzed the outer membrane composition of the LOS-deficient mutant compared with WT and reported a major change in phospholipid composition of the meningococcal lpxA mutant cell envelop. They found that the absence of LOS led to a shift in phosphatidylethanolamine and phosphatidylglycerol species containing shorter and saturated fatty acyl chains [39].
In support of the finding that carbohydrate polymers induce TLR4-mediated responses, a recent study demonstrated that the exopolysaccharide extracted from the plant-pathogenic bacterium Xanhomonas campestri was recognized by TLR4 when used at 100 μg/ml concentration [42]. Other studies demonstrated that glycans derived from algae [16], helminth [11], and macrofungi [43] induce TLR4-mediated responses in the absence of endotoxin. Very recently, polysaccharide extraction from the herbal Chinese medicinal mushroom P. umbellatus, in addition to its anti-tumor activity, was reported to induce macrophage activation via TLR4 [17, 18]. Moreover, live N. meningitidis, Streptococcus pneumonia, and Haemophilus influenze were reported to induce inflammatory response via TLR2, -4, and -9 [44]. S. pneumoniae vaccine-based CPS polymers were found to be conducive through TLR4 [45]. C. neoformans CPS polymers were also reported to induce TLR4 [8, 9]. These studies lend strong support to this report that carbohydrate polymers induce TLR4-mediated responses. Other studies [13, 14] have shown that CPS from B. fragilis and S. suis stimulate via TLR2. An earlier study had shown that the immune-stimulatory activity of carbohydrate-based glucan polymers depends on MW, linkage, and charge [46]. Recently, meningococcal CPS (unconjugated vaccine grade) from serogroup A was found to possess immunostimulatory activity and induced a higher titer of IgG to an antigen when used as an adjuvant, indicating that carbohydrate polymers can induce innate immunity [47]. Meningococcal CPS polymers are anionic as a result of sialic acid and phosphomannose contents. We reported recently [48] that the host defense cationic peptide LL-37 interacted with meningococcal CPS and inhibited inflammatory mediator release from macrophages. In that report, we demonstrated the physical interaction between the negatively charged meningococcal polymer CPS-lpxA and the cationic peptide LL-37. This study provides strong support to the current report that meningococcal CPS polymers are bioactive and induce inflammatory activity via TLR2 and TLR4-MD-2.
MD-2 was required for meningococcal CPS-induced TLR4 activation but not for TLR2 activation [49–52]. The requirement for MD-2 was confirmed by the competitive inhibition studies with E5564 (Eritoran), suggesting the direct interaction of CPS with MD-2. In support, pulmonary SP-A is reported to interact with MD-2 and alter LPS signaling [53], and another pulmonary SP-D binds MD-2 through the carbohydrate recognition domain in a Ca++-dependent manner [54]. SP-A and SP-D modulate inflammatory innate immune responses and contribute to pathogen clearance [55–58]. Thus, meningococcal CPS may bind directly to MD-2, compete with SP-D and other innate immune coreceptors, and modulate human host responses.
Recently, Kocabas et al. [59] reported that MCPS inhibit LOS-induced cellular activation via TLR4 by binding to CD14 and LBP. This report differs from my findings that meningococcal CPS polymers of serogoups A, B, C, W135, and Y induce TLR4-MD-2- and TLR2-mediated responses in a dose-dependent manner. The authors showed that MCPS failed to induce TNF-α and IL-6 in THP-1 cells, but the encapsulated as well as the isogenic, nonencapsulated, heat-killed meningococcal FAM18C strains induced large amounts of proinflammatory cytokines in vivo and in vitro at 108 CFU [59]. An inhibitory effect of MCPS on LOS-induced activity was abolished when LOS was allowed to bind to sMD-2; however, when MCPS and LOS were incubated together with sMD-2, the inhibitory effect was exerted. Polymer structure may be a key feature that explains differences in the studies. Polymer preparation may influence biological activity, possibly by changing the polymer size, degree of polymerization, conformation, or lipid anchors [60]. In support, zymosan, a yeast-derived β-glucan polymer, lost its biological activity to induce the NF-κB construct via TLR2 when boiled with alkali [61]. Further, meningococcal polysaccharide vaccine induced inflammatory cytokine release from DCs in a TLR4-dependent manner (Dr. Bali Pulendran, personal communication, Emory Vaccine Center, Atlanta, GA, USA, unpublished results). However, the pathophysiologic relevance of meningococcal CPS immunostimulatory activity in meningococcal disease is not well understood. During invasive meningococcal infection, bacterial membrane blebs containing CPS, membrane proteins, phospholipids, and LPS are shed in large amounts. The capsule is essential for virulence; hence, invasive meningococci express a thick capsule. Therefore, the contribution of purified CPS to meningococcal sepsis will be investigated further using a whole blood assay method described by Zollinger and colleagues [62].
In summary, I found that meningococcal CPS polymers induce NF-κB pathway inflammatory responses in human and murine macrophages. Meningococcal CPS polymers were recognized by TLR2 and TLR4-MD-2 receptors in human and murine macrophages. However, the involvement of other PRRs, such as C-type lectins in meningococcal CPS polymer recognition, remains a possibility. The role of meningococcal CPS polymer conformation, length (number of repeating units), total negative charges, hydrophobicity, hydrophilicity, and lipidation in exerting biological activity is also under investigation. Understanding how meningococcal CPS polymers are recognized by host macrophages may help explain the severe inflammation of meningococcal disease and aid in the design of better meningococcal vaccines.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by a grant to S.M.Z. from Georgia Research Alliance and by National Institutes of Health grants R01 AI033517 and AI140247 to David S. Stephens. I thank Xiao-Liu Zhou and Larry Martin for meningococcal CPS extractions and purification. I thank Lane Pucko for administrative assistance. I am grateful to Dr. Seshu Gudlavalleti for providing the vaccine-grade CPS MAPS. The author gratefully acknowledges Dr. David S. Stephens, without whose generous support this work could not have been accomplished. I thank Dr. Stephens for critically reviewing the manuscript.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- ATCC
- American Type Culture Collection
- CPS
- capsular polysaccharides
- CPS-lpxA
- capsular polysaccharides purified from lpxA mutant
- ddH2O
- double-distilled H2O
- DN
- dominant-negative
- GalNAc
- N-acetylgalactosamine
- GAS
- γ-activated site
- GlcNac
- N-acetyl-D-glucosamine
- GRE
- glucocorticoid response element
- HEK
- human embryonic kidney
- IP-10
- IFN-inducible protein 10
- ISRE
- IFN-stimulated response element
- LBP
- LPS-binding protein
- LOS
- lipooligosaccharides
- LTA
- lipoteichoic acid
- ManNAc
- N-acetylmannosamine
- MAPS
- meningococcal group A polysaccharide
- MCPS
- meningococcal serogroup C capsular polysaccharide polymers
- MD-2
- myeloid differentiation protein 2
- NMB
- Neisseria meningitidis
- Pam3CSK4
- palmitoyl-3-cysteine-serine-lysine-4
- PL-lpxA
- phospholipid extracted from lpxA mutant
- s
- soluble
- serogroup A
- (α1→6)-N-acetyl-D-mannosamine-1-phosphate
- serogroup B
- (α 2→8)-N-acetylneuraminic acid
- serogroup C
- (α 2→9)-N-acetylneuraminic acid
- serogroup W135
- 6-D-Gal (α1→4)-N-acetylneuraminic acid (α 2→6)
- serogroup Y
- 6-D-Glc (α1→4)-N-acetylneuraminic acid (α 2→6)
- SP-A
- surfactant protein A
- SRE
- serum-response element
- TIRAP
- Toll-IL-1R domain-containing adaptor protein
- TRAM
- Toll-IL-1R domain-containing adaptor molecule 2
- TRIF
- Toll/IL-1R domain-containing adaptor-inducing IFN-β
AUTHORSHIP
S.M.Z. conceived of, designed, and performed the experiments, analyzed the data, and wrote the manuscript.
REFERENCES
- 1. Zughaier S. M., Tzeng Y. L., Zimmer S. M., Datta A., Carlson R. W., Stephens D. S. (2004) Neisseria meningitidis lipooligosaccharide structure-dependent activation of the macrophage CD14/Toll-like receptor 4 pathway. Infect. Immun. 72, 371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zimmer S. M., Zughaier S. M., Tzeng Y. L., Stephens D. S. (2007) Human MD-2 discrimination of meningococcal lipid A structures and activation of TLR4. Glycobiology 17, 847–856 [DOI] [PubMed] [Google Scholar]
- 3. Zughaier S., Agrawal S., Stephens D. S., Pulendran B. (2006) Hexa-acylation and KDO(2)-glycosylation determine the specific immunostimulatory activity of Neisseria meningitidis lipid A for human monocyte derived dendritic cells. Vaccine 24, 1291–1297 [DOI] [PubMed] [Google Scholar]
- 4. Stephens D. S., Greenwood B., Brandtzaeg P. (2007) Epidemic meningitis, meningococcemia, and Neisseria meningitidis. Lancet 369, 2196–2210 [DOI] [PubMed] [Google Scholar]
- 5. Tzeng Y. L., Datta A. K., Strole C. A., Lobritz M. A., Carlson R. W., Stephens D. S. (2005) Translocation and surface expression of lipidated serogroup B capsular polysaccharide in Neisseria meningitidis. Infect. Immun. 73, 1491–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Powell J. L., Wright A. C., Wasserman S. S., Hone D. M., Morris J. G., Jr. (1997) Release of tumor necrosis factor α in response to Vibrio vulnificus capsular polysaccharide in in vivo and in vitro models. Infect. Immun. 65, 3713–3718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bush C. A., Patel P., Gunawardena S., Powell J., Joseph A., Johnson J. A., Morris J. G. (1997) Classification of Vibrio vulnificus strains by the carbohydrate composition of their capsular polysaccharides. Anal. Biochem. 250, 186–195 [DOI] [PubMed] [Google Scholar]
- 8. Monari C., Pericolini E., Bistoni G., Casadevall A., Kozel T. R., Vecchiarelli A. (2005) Cryptococcus neoformans capsular glucuronoxylomannan induces expression of fas ligand in macrophages. J. Immunol. 174, 3461–3468 [DOI] [PubMed] [Google Scholar]
- 9. Shoham S., Huang C., Chen J. M., Golenbock D. T., Levitz S. M. (2001) Toll-like receptor 4 mediates intracellular signaling without TNF-α release in response to Cryptococcus neoformans polysaccharide capsule. J. Immunol. 166, 4620–4626 [DOI] [PubMed] [Google Scholar]
- 10. Harn D. A., McDonald J., Atochina O., Da′dara A. A. (2009) Modulation of host immune responses by helminth glycans. Immunol. Rev. 230, 247–257 [DOI] [PubMed] [Google Scholar]
- 11. Thomas P. G., Carter M. R., Atochina O., Da′Dara A. A., Piskorska D., McGuire E., Harn D. A. (2003) Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism. J. Immunol. 171, 5837–5841 [DOI] [PubMed] [Google Scholar]
- 12. Tzianabos A., Wang J. Y., Kasper D. L. (2003) Biological chemistry of immunomodulation by zwitterionic polysaccharides. Carbohydr. Res. 338, 2531–2538 [DOI] [PubMed] [Google Scholar]
- 13. Wang Q., McLoughlin R. M., Cobb B. A., Charrel-Dennis M., Zaleski K. J., Golenbock D., Tzianabos A. O., Kasper D. L. (2006) A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J. Exp. Med. 203, 2853–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Graveline R., Segura M., Radzioch D., Gottschalk M. (2007) TLR2-dependent recognition of Streptococcus suis is modulated by the presence of capsular polysaccharide which modifies macrophage responsiveness. Int. Immunol. 19, 375–389 [DOI] [PubMed] [Google Scholar]
- 15. Iwata T., Mitani A., Ishihara Y., Tanaka S., Yamamoto G., Kikuchi T., Naganawa T., Matsumura Y., Suga T., Koide M., Sobue T., Suzuki T., Noguchi T. (2005) Actinobacillus actinomycetemcomitans Y4 capsular polysaccharide induces IL-1β mRNA expression through the JNK pathway in differentiated THP-1 cells. Clin. Exp. Immunol. 141, 261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsu H. Y., Jeyashoke N., Yeh C. H., Song Y. J., Hua K. F., Chao L. K. (2010) Immunostimulatory bioactivity of algal polysaccharides from Chlorella pyrenoidosa activates macrophages via Toll-like receptor 4. J. Agric. Food Chem. 58, 927–936 [DOI] [PubMed] [Google Scholar]
- 17. Li X., Xu W., Chen J. (2010) Polysaccharide purified from Polyporus umbellatus (Per) Fr induces the activation and maturation of murine bone-derived dendritic cells via Toll-like receptor 4. Cell. Immunol. 265, 50–56 [DOI] [PubMed] [Google Scholar]
- 18. Li X., Xu W. (2010) TLR4-mediated activation of macrophages by the polysaccharide fraction from Polyporus umbellatus(pers.) Fries. J. Ethnopharmacol., Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 19. Steeghs L., den Hartog R., den Boer A., Zomer B., Roholl P., van der Ley P. (1998) Meningitis bacterium is viable without endotoxin. Nature 392, 449–450 [DOI] [PubMed] [Google Scholar]
- 20. Plant L., Wan H., Jonsson A. B. (2007) Non-lipooligosaccharide-mediated signaling via Toll-like receptor 4 causes fatal meningococcal sepsis in a mouse model. Cell. Microbiol. 9, 657–669 [DOI] [PubMed] [Google Scholar]
- 21. Plant L., Wan H., Jonsson A. B. (2006) MyD88-dependent signaling affects the development of meningococcal sepsis by nonlipooligosaccharide ligands. Infect. Immun. 74, 3538–3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zarantonelli M. L., Huerre M., Taha M. K., Alonso J. M. (2006) Differential role of lipooligosaccharide of Neisseria meningitidis in virulence and inflammatory response during respiratory infection in mice. Infect. Immun. 74, 5506–5512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hellerud B. C., Nielsen E. W., Thorgersen E. B., Lindstad J. K., Pharo A., Tonnessen T. I., Castellheim A., Mollnes T. E., Brandtzaeg P. (2010) Dissecting the effects of lipopolysaccharides from nonlipopolysaccharide molecules in experimental porcine meningococcal sepsis. Crit. Care Med. 38, 1467–1474 [DOI] [PubMed] [Google Scholar]
- 24. Van der Ley P., Steeghs L. (2003) Lessons from an LPS-deficient Neisseria meningitidis mutant. J. Endotoxin Res. 9, 124–128 [DOI] [PubMed] [Google Scholar]
- 25. Czeslick E., Struppert A., Simm A., Sablotzki A. (2006) E5564 (Eritoran) inhibits lipopolysaccharide-induced cytokine production in human blood monocytes. Inflamm. Res. 55, 511–515 [DOI] [PubMed] [Google Scholar]
- 26. Rossignol D. P., Wasan K. M., Choo E., Yau E., Wong N., Rose J., Moran J., Lynn M. (2004) Safety, pharmacokinetics, pharmacodynamics, and plasma lipoprotein distribution of Eritoran (E5564) during continuous intravenous infusion into healthy volunteers. Antimicrob. Agents Chemother. 48, 3233–3240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rossignol D. P., Lynn M. (2002) Antagonism of in vivo and ex vivo response to endotoxin by E5564, a synthetic lipid A analogue. J. Endotoxin Res. 8, 483–488 [DOI] [PubMed] [Google Scholar]
- 28. Gudlavalleti S. K., Datta A. K., Tzeng Y. L., Noble C., Carlson R. W., Stephens D. S. (2004) The Neisseria meningitidis serogroup A capsular polysaccharide O-3 and O-4 acetyltransferase. J. Biol. Chem. 279, 42765–42773 [DOI] [PubMed] [Google Scholar]
- 29. Zughaier S. M., Zimmer S. M., Datta A., Carlson R. W., Stephens D. S. (2005) Differential induction of the Toll-like receptor 4-MyD88-dependent and -independent signaling pathways by endotoxins. Infect. Immun. 73, 2940–2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim H. M., Park B. S., Kim J. I., Kim S. E., Lee J., Oh S. C., Enkhbayar P., Matsushima N., Lee H., Yoo O. J., Lee J. O. (2007) Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 130, 906–917 [DOI] [PubMed] [Google Scholar]
- 31. Kumar H., Kawai T., Akira S. (2009) Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 388, 621–625 [DOI] [PubMed] [Google Scholar]
- 32. Sacre S. M., Lundberg A. M., Andreakos E., Taylor C., Feldmann M., Foxwell B. M. (2007) Selective use of TRAM in lipopolysaccharide (LPS) and lipoteichoic acid (LTA) induced NF-κB activation and cytokine production in primary human cells: TRAM is an adaptor for LPS and LTA signaling. J. Immunol. 178, 2148–2154 [DOI] [PubMed] [Google Scholar]
- 33. Forsberg L. S., Carlson R. W. (1998) The structures of the lipopolysaccharides from Rhizobium etli strains CE358 and CE359. The complete structure of the core region of R. etli lipopolysaccharides. J. Biol. Chem. 273, 2747–2757 [DOI] [PubMed] [Google Scholar]
- 34. Brandtzaeg P., Ovsteboo R., Kierulf P. (1992) Compartmentalization of lipopolysaccharide production correlates with clinical presentation in meningococcal disease. J. Infect. Dis. 166, 650–652 [DOI] [PubMed] [Google Scholar]
- 35. Bonnah R. A., Hoelter J., Steeghs L., Enns C. A., So M., Muckenthaler M. U. (2005) Lipooligosaccharide-independent alteration of cellular homeostasis in Neisseria meningitidis-infected epithelial cells. Cell. Microbiol. 7, 869–885 [DOI] [PubMed] [Google Scholar]
- 36. Pridmore A. C., Wyllie D. H., Abdillahi F., Steeghs L., van der Ley P., Dower S. K., Read R. C. (2001) A lipopolysaccharide-deficient mutant of Neisseria meningitidis elicits attenuated cytokine release by human macrophages and signals via Toll-like receptor (TLR) 2 but not via TLR4/MD2. J. Infect. Dis. 183, 89–96 [DOI] [PubMed] [Google Scholar]
- 37. Sprong T., Stikkelbroeck N., van der Ley P., Steeghs L., van Alphen L., Klein N., Netea M. G., van der Meer J. W., van Deuren M. (2001) Contributions of Neisseria meningitidis LPS and non-LPS to proinflammatory cytokine response. J. Leukoc. Biol. 70, 283–288 [PubMed] [Google Scholar]
- 38. Ovstebø R., Olstad O. K., Brusletto B., Moller A. S., Aase A., Haug K. B., Brandtzaeg P., Kierulf P. (2008) Identification of genes particularly sensitive to lipopolysaccharide (LPS) in human monocytes induced by wild-type versus LPS-deficient Neisseria meningitidis strains. Infect. Immun. 76, 2685–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steeghs L., de Cock H., Evers E., Zomer B., Tommassen J., van der Ley P. (2001) Outer membrane composition of a lipopolysaccharide-deficient Neisseria meningitidis mutant. EMBO J. 20, 6937–6945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bos M. P., Tommassen J. (2005) Viability of a capsule- and lipopolysaccharide-deficient mutant of Neisseria meningitidis. Infect. Immun. 73, 6194–6197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Singleton T. E., Massari P., Wetzler L. M. (2005) Neisserial porin-induced dendritic cell activation is MyD88 and TLR2 dependent. J. Immunol. 174, 3545–3550 [DOI] [PubMed] [Google Scholar]
- 42. Takeuchi A., Kamiryou Y., Yamada H., Eto M., Shibata K., Haruna K., Naito S., Yoshikai Y. (2009) Oral administration of xanthan gum enhances antitumor activity through Toll-like receptor 4. Int. Immunopharmacol. 9, 1562–1567 [DOI] [PubMed] [Google Scholar]
- 43. Li W., Liu M., Lai S., Xu C., Lu F., Xiao X., Bao Y. (2010) Immunomodulatory effects of polysaccharopeptide (PSP) in human PBMC through regulation of TRAF6/TLR immunosignal-transduction pathways. Immunopharmacol. Immunotoxicol. 32, 576–584 [DOI] [PubMed] [Google Scholar]
- 44. Mogensen T. H., Paludan S. R., Kilian M., Ostergaard L. (2006) Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J. Leukoc. Biol. 80, 267–277 [DOI] [PubMed] [Google Scholar]
- 45. Cohen N., Stolarsky-Bennun M., Amir-Kroll H., Margalit R., Nussbaum G., Cohen-Sfady M., Pevsner-Fischer M., Fridkin M., Bercovier H., Eisenbach L., Jung S., Cohen I. R. (2008) Pneumococcal capsular polysaccharide is immunogenic when present on the surface of macrophages and dendritic cells: TLR4 signaling induced by a conjugate vaccine or by lipopolysaccharide is conducive. J. Immunol. 180, 2409–2418 [DOI] [PubMed] [Google Scholar]
- 46. Cleary J. A., Kelly G. E., Husband A. J. (1999) The effect of molecular weight and β-1,6-linkages on priming of macrophage function in mice by (1,3)-β-D-glucan. Immunol. Cell Biol. 77, 395–403 [DOI] [PubMed] [Google Scholar]
- 47. Menéndez T., Carmenate T., Cruz-Leal Y., Coizeau E., Caballero E., Bello D., Guirola M., Alvarez A., Guillen G. (2010) Purified capsular polysaccharide of Neisseria meningitidis serogroup A as immune potentiator for antibody production. Curr. Microbiol. 60, 79–84 [DOI] [PubMed] [Google Scholar]
- 48. Zughaier S., Svoboda P., Pohl J., Stephens D. S., Shafer W. M. (2010) The host defense peptide LL-37 interacts with Neisseria meningitidis capsular polysaccharides and inhibits inflammatory mediators release. PLoS ONE 5, e13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Park B. S., Song D. H., Kim H. M., Choi B. S., Lee H., Lee J. O. (2009) The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458, 1191–1195 [DOI] [PubMed] [Google Scholar]
- 50. Zimmer S. M., Liu J., Clayton J. L., Stephens D. S., Snyder J. P. (2008) Paclitaxel binding to human and murine MD-2. J. Biol. Chem. 283, 27916–27926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brodsky I., Medzhitov R. (2007) Two modes of ligand recognition by TLRs. Cell 130, 979–981 [DOI] [PubMed] [Google Scholar]
- 52. Ohto U., Fukase K., Miyake K., Satow Y. (2007) Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science 316, 1632–1634 [DOI] [PubMed] [Google Scholar]
- 53. Yamada C., Sano H., Shimizu T., Mitsuzawa H., Nishitani C., Himi T., Kuroki Y. (2006) Surfactant protein A directly interacts with TLR4 and MD-2 and regulates inflammatory cellular response. Importance of supratrimeric oligomerization. J. Biol. Chem. 281, 21771–21780 [DOI] [PubMed] [Google Scholar]
- 54. Nie X., Nishitani C., Yamazoe M., Ariki S., Takahashi M., Shimizu T., Mitsuzawa H., Sawada K., Smith K., Crouch E., Nagae H., Takahashi H., Kuroki Y. (2008) Pulmonary surfactant protein D binds MD-2 through the carbohydrate recognition domain. Biochemistry 47, 12878–12885 [DOI] [PubMed] [Google Scholar]
- 55. Haagsman H. P., Hogenkamp A., van Eijk M., Veldhuizen E. J. (2008) Surfactant collectins and innate immunity. Neonatology 93, 288–294 [DOI] [PubMed] [Google Scholar]
- 56. Sorensen G. L., Husby S., Holmskov U. (2007) Surfactant protein A and surfactant protein D variation in pulmonary disease. Immunobiology 212, 381–416 [DOI] [PubMed] [Google Scholar]
- 57. Takahashi H., Sano H., Chiba H., Kuroki Y. (2006) Pulmonary surfactant proteins A and D: innate immune functions and biomarkers for lung diseases. Curr. Pharm. Des. 12, 589–598 [DOI] [PubMed] [Google Scholar]
- 58. Kishore U., Greenhough T. J., Waters P., Shrive A. K., Ghai R., Kamran M. F., Bernal A. L., Reid K. B., Madan T., Chakraborty T. (2006) Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol. Immunol. 43, 1293–1315 [DOI] [PubMed] [Google Scholar]
- 59. Kocabas C., Katsenelson N., Kanswal S., Kennedy M. N., Cui X., Blake M. S., Segal D. M., Akkoyunlu M. (2007) Neisseria meningitidis type C capsular polysaccharide inhibits lipooligosaccharide-induced cell activation by binding to CD14. Cell. Microbiol. 9, 1297–1310 [DOI] [PubMed] [Google Scholar]
- 60. Bardotti A., Averani G., Berti F., Berti S., Galli C., Giannini S., Fabbri B., Proietti D., Ravenscroft N., Ricci S. (2005) Size determination of bacterial capsular oligosaccharides used to prepare conjugate vaccines against Neisseria meningitidis groups Y and W135. Vaccine 23, 1887–1899 [DOI] [PubMed] [Google Scholar]
- 61. Gantner B. N., Simmons R. M., Canavera S. J., Akira S., Underhill D. M. (2003) Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197, 1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stoddard M. B., Pinto V., Keiser P. B., Zollinger W. (2010) Evaluation of a whole-blood cytokine release assay for use in measuring endotoxin activity of group B Neisseria meningitidis vaccines made from lipid A acylation mutants. Clin. Vaccine Immunol. 17, 98–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.