Activation of the RhoA/Rho kinase pathway up-regulates transcriptional HGF production in response to apoptotic cells.
Keywords: macrophage, PI3K/Akt, MAPK
Abstract
Clearance of apoptotic cells by macrophages induces HGF secretion. We examined the regulatory mechanisms of HGF mRNA and protein expression in macrophages upon exposure to apoptotic cells. The interaction of RAW 264.7 macrophages with apoptotic Jurkat cells, but not with viable cells, resulted in expression of HGF mRNA and protein. Exposure of RAW 264.7 cells to apoptotic cells induced activation of RhoA, the PI3K/Akt pathway, and MAPKs, including p38 MAPK, ERK, and JNK. Down-regulation of the RhoA/Rho kinase pathway by pharmacological inhibitors or a RhoA-specific siRNA suppressed HGF mRNA and protein expression by macrophages in response to apoptotic cells through the phosphorylation of Akt and the MAPKs. Inhibition of PI3K decreased phosphorylation of Akt and the MAPKs. Inhibition of JNK, but not p38 MAPK and ERK, reduced Akt phosphorylation. The pharmacological inhibitor of PI3K and the MAPKs blocked HGF mRNA and protein expression. Other types of apoptotic cells, such as HeLa cells and murine thymocytes, could also induce HGF mRNA through the RhoA-dependent pathway. Likely, the RhoA-dependent signaling pathway was required for HGF mRNA induction in primary cells of peritoneal macrophages in response to apoptotic cells. An HGFR-blocking antibody did not alter apoptotic cell-induced activation of RhoA, Akt, and the MAPKs, as well as HGF production. Overall, the data provide evidence that activation of the RhoA/Rho kinase pathway up-regulates transcriptional HGF production in response to apoptotic cells.
Introduction
Efferocytosis, the recognition and engulfment of apoptotic cells, is a fundamental process in development, remodeling, tissue homeostasis, and immunity. The effective phagocytic clearance of apoptotic cells prior to their lysis is critical for the resolution of inflammation and inappropriate autoimmune responses by preventing the release of potentially harmful proinflammatory and immunologic contents [1–3]. Engulfment or simply recognition of apoptotic cells can also actively suppress ongoing inflammation by inducing production of anti-inflammatory mediators, such as TGF-β, IL-10, and PGE2 [1]. Furthermore, recent data indicate that efferocytosis results in the release of growth factors used for epithelium and endothelium maintenance [4, 5].
Morimoto et al. [4] reported that bronchial epithelial cells and alveolar macrophages that phagocytosed apoptotic neutrophils during bacterial pneumonia produced HGF. Furthermore, HGF was produced by murine alveolar macrophages in vitro in response to apoptotic neutrophils. Several lines of evidence underscore the importance of this response in that HGF promotes the regeneration and reconstruction of normal hepatic, renal, and lung tissue structures after tissue injury [6–8]. These in vitro and in vivo data suggest the relatively new concept that simply the interactions of apoptotic cells with the cells that recognize and engulf them play an important role in the resolution and repair process of damaged tissues. However, the signaling pathways involved in HGF production in response to apoptotic cells have not been identified.
Recognition of apoptotic cells activates a cascade of intracellular molecules in phagocytes, leading to rearrangement of the cytoskeleton, permitting the efficient engulfment of apoptotic cells [9]. Efferocytosis requires the concerted action of Rho GTPase family members, including Rho, Rac, and Cdc42 [10]. Rho GTPases are molecular switches that cycle between inactive (guanosine diphosphate-bound) and active (guanosine triphosphate-bound) configurations. Rac-1 is induced by the PS and CD91 and positively regulates efferocytosis, whereas RhoA and its downstream effector Rho kinase inhibit the process [10, 11]. However, the speed of phagosome maturation and degradation of the ingested cells is enhanced by RhoA acting on ezrin-radixin-moesin proteins through Rho kinase [12].
As RhoA is a key regulator of signaling pathways that regulate organization of the cytoskeleton, as well as gene transcription and protein synthesis [13], we focused on the role of RhoA in HGF production. The role of RhoA in HGF mRNA and protein expression has not been studied previously. Accordingly, the present study was designed to determine whether the RhoA/Rho kinse pathway was required for apoptotic cell-induced HGF gene expression and production. Additionally, downstream signaling molecules, such as the PI3K/Akt and MAPK pathways, which are known to be involved in the HGF production [14–16], were identified to be involved in the RhoA pathway.
MATERIALS AND METHODS
Reagents
Y27632, wortmanin, LY 294002, actinomycin D, and cycloheximide were purchased from Sigma Chemical Co. (St. Louis, MO, USA). SB 203580 and PD 98059 were obtained from Biomol (Plymouth Meeting, PA, USA). JNK inhibitor II was obtained from Calbiochem (San Diego, CA, USA). The gene-specific, relative RT-PCR kit was from Invitrogen (Carlsbad, CA, USA). MMLV RT was from Enzynomics (Seoul, Korea). The G-LISA™ RhoA activation assay and rC3 transferase were obtained from Cytoskeleton Inc. (Denver, CO, USA). The antibodies used in this study were antiphospho-p38 MAPK, anti-p38 MAPK, anti-phospho-ERK1/2, anti-ERK1/2, anti-phospho-JNK1/2, anti-JNK1, anti-phospho-Akt, anti-Akt, β-actin, and anti-HGF α-chain (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA); anti-HGFR and goat IgG (R&D Systems, Minneapolis, MN, USA); and anti-RhoA mAb (Cytoskeleton Inc.).
Cell line, culture, and stimulation
Murine RAW 264.7 macrophages (American Type Culture Collection, Manassas, VA, USA) were plated at 106 cells/ml and incubated overnight in DMEM (Media Tech Inc., Washington, DC, USA), supplemented with 10% FBS, 2 mM L-glutamine,100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C and 5% CO2. Before stimulation, the medium was replaced with serum-free X-vivo 10. The macrophages were stimulated with apoptotic or viable cells (3×106 cells/ml).
Experimental animals and isolation and culture of primary cells
Pathogen-free male C57BL/6 mice (Orient Bio, Sungnam, South Korea), weighing 20–22 g, were used to isolate resident peritoneal macrophages. The Animal Care Committee of the Ewha Medical Research Institute (Seoul, Korea) approved the experimental protocol.
Resident peritoneal macrophages were isolated using 5 ml ice-cold sterile HBSS to lavage the peritoneum after killing mice with CO2. Lavage fluid was centrifuged and resident peritoneal cells plated at 5 × 105 cells/well and cultured in DMEM, supplemented with 10% heat-inactivated FBS, 2 mM L-glutamine, 100 μg/ml streptomycin, and 100 U/ml penicillin in humidified 10% CO2 at 37°C. The macrophages were stimulated with apoptotic Jurkat T cells (3×106 cells/ml). Suspended peritoneal macrophages were >95% viable, as determined by trypan blue dye exclusion.
Murine thymocytes were isolated from thymi of 3- to 4-week-olds by passing thymi through a 40-μm strainer to separate individual cells.
Induction of apoptosis
Cell lines of human Jurkat T cells and HeLa epithelial cells and murine thymocytes were exposed to UV irradiation at 254 nm for 10 min and cultured in RPMI 1640 (Media Tech Inc.) for 2.5 h at 37°C and 5% CO2 before addition to macrophages. Irradiated these cells were 70–80% apoptotic by evaluation of nuclear morphology using light microscopy [17].
ELISA measurement of HGF protein
RAW 264.7 cells (2×105 cells/ml) were stimulated with apoptotic Jurkat cells (6×105cells/ml) for 24 h. Culture supernatants were collected, and HGF concentrations were measured by ELISA according to the manufacturer's instructions (R&D Systems).
Semiquantitative RT-PCR and real-time PCR
Total RNA was isolated from cultured cells using reagent solution (iNtRon Biotechnology, Seoul, Korea). The concentrations and purities of the RNA samples were evaluated by spectrophotometry. Reverse transcription was conducted for 60 min at 42°C with 2 μg total RNA using MMLV RT (Enzynomics). mRNA levels of HGF, EGF, and KGF were determined using a semiquantitative RT-PCR kit (Invitrogen). The primer sequences were used as follows: mouse-specific HGF (sense 5′-ATC CAC GAT GTT CAT GAG AG-3′ and antisense 5′-GCT GAC TGC ATT TCT CAT TC-3′), mouse-specific EGF (sense 5′-AAT AGT TAT CCA GGA TGC CC-3′ and antisense 5′-ATG CTA CCA CCC TCG ACG CA-3′), mouse-specific KGF (sense 5′-TGC CAA CTG TGC TCT ACA-3′ and antisense 5′-CCA TTT AGC TGA TGC ATA-3′), and mouse-specific β-actin (sense 5′-GAT GAC GAT ATC GCT GCG CTG-3′ and antisense 5′-GTA CGA CCA GAG GCA TAC AGG-3′). cDNA was denatured for 5 min at 95°C and amplified using a GeneAmp PCR System 2400 (PerkinElmer, Waltham, MA, USA) during 33 cycles of 95°C for 30 s, 54°C for 30 s, and 72°C for 30 s, followed by a 10-min, final extension at 72°C. Samples were visualized on 1–2% agarose gels stained with ethidium bromide. The relative amounts of HGF, EGF, or KGF, as compared with β-actin, were evaluated by densitometry.
For real-time PCR analysis, a 2-μl aliquot of diluted cDNA (1:10) was amplified by SYBR® Green PCR master mix (Applied Biosystems, Foster City, CA, USA) to a final volume of 20 μl. A PCR reaction was performed. PCR cycles consisted of initial denaturation at 95°C for 5 min, followed by 40 cycles of 95°C for 30 s, 52°C for 30 s, and 72°C for 45 s. The primers for β-actin and HGF were used as described above. The Ct values of HGF were normalized to that of β-actin, and relative expression levels were calculated by the 2ΔΔCt method [18]. All samples were run in triplicate.
Transient cell transfection
RAW 264.7 cells were transiently transfected with 10 nM RhoA-targeting siRNA or control siRNA, premixed with 6 μg/ml Lipofectin (Invitrogen). The cells were then incubated in serum-free culture medium for 24 or 48 h before further experimentation.
RhoA activity assay
RhoA activity was measured in RAW 264.7 cell lysates using an ELISA-based RhoA activation assay Biochem Kit™ (G-LISA™, Cytoskeleton Inc.), according to the manufacturer's instructions. Briefly, cell lysates were added to the RhoA-GTP affinity plate that was coated with the Rhotekin-binding domain of RhoA for 30 min. The active GTP-bound form of RhoA was measured using indirect immunodetection, followed by a colorimetric reaction at 490 nm on a microplate spectrophotometer.
Immunoblot analysis
RAW 264.7 macrophages (106 cells/ml) were plated and incubated in serum-free medium overnight. The stimulated cells were lysed in 0.5% Trion X-100 lysis buffer and resolved on a 10% SDS-PAGE gel. Separated proteins were transferred electrophoretically onto nitrocellulose and blocked for 1 h at room temperature with Tris-buffered saline containing 3% BSA. Membranes were incubated at room temperature for 1 h with various anti-primary antibodies and probed with mouse anti-mouse, HRP-conjugated secondary antibody. Membranes were developed using the ECL system.
Statistical analysis
Data are expressed as mean ± sem. ANOVA was applied for multiple comparisons, and Tukey's post hoc test was applied where appropriate. Student's t tests were used for comparisons of two sample means. A P value of <0.05 was considered statistically significant.
RESULTS
Exposure of RAW 264.7 cells to apoptotic cells induces HGF expression
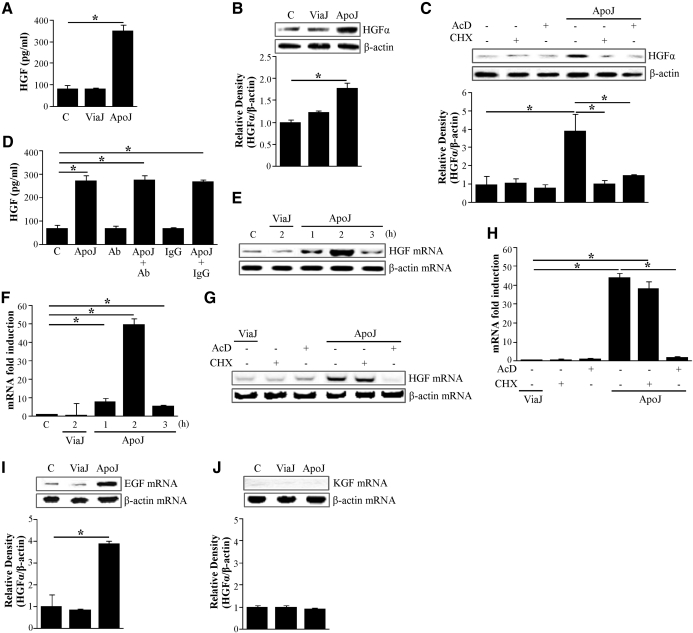
HGF secretion, as measured by ELISA, significantly increased in RAW 264.7 cells when they were exposed to apoptotic Jurkat T cells (Fig. 1A). This was not seen in resting macrophages or in those exposed to viable Jurkat cells. HGF protein was also detected by Western blot analysis using the anti-HGF α-chain antibody in lysates of cultured RAW 264.7 cells (Fig. 1B). HGF protein expression was also increased within macrophages after 24 h of exposure to apoptotic Jurkat cells but not after exposure to viable cells. To determine whether de novo protein synthesis or DNA synthesis is necessary for increased HGF expression, RAW cells were pretreated for 1 h with the DNA synthesis inhibitor actinomycin D or the protein synthesis inhibitor cycloheximide before stimulation with apoptotic cells and then extracted at 24 h. Apoptotic cell-induced HGF expression was inhibited by actinomycin D or cycloheximide, indicating that DNA synthesis and de novo protein synthesis are required (Fig. 1C).
Figure 1. Apoptotic Jurkat T cells induced HGF protein and mRNA expression by RAW 264.7 cells.
(A and B) RAW 264.7 cells were stimulated by apoptotic (ApoJ) or viable (ViaJ) Jurkat cells for 24 h. RAW 264.7 cells were pretreated with 10 μg/ml actinomycin D (AcD) or 10 μg/ml cycloheximide (CHX) for 1 h before stimulation with apoptotic Jurkat cells for 24 h (C) or 2 h (G and H). (D) RAW 264.7 cells were pretreated with 10 μg/ml anti-HGFR antibody or 10 μg/ml IgG for 1 h and then stimulated with apoptotic Jurkat cells for 24 h to detect HGF production. (A and D) HGF levels in the conditioned medium were measured by ELISA. (B and C) Western blots with anti-specific HGF α-chain antibodies were used with RAW 264.7 cell lysates. Relative values of HGF-α expression are indicated below the gel. (E and F) HGF mRNA levels of RAW 264.7 cells stimulated with apoptotic or viable Jurkat cells for 1–3 h. HGF mRNA levels were analyzed by semiquantitative RT-PCR (E and G) and/or real-time PCR (F and H). (I and J) EGF or KGF mRNA levels in RAW 264.7 cells stimulated with apoptotic or viable Jurkat cells for 2 h. mRNA levels of these growth factors were analyzed by semiquantitative RT-PCR. Values represent means ± sem of three to five separate experiments; *P < 0.05.
To evaluate the autocrine action of released HGF through c-Met, RAW 264.7 cells were treated with anti-HGFR (c-Met) antibody in the presence or absence of apoptotic cells. Fig. 1D illustrates that treatment of RAW 264.7 cells alone with the anti-HGFR antibody or control IgG did not alter HGF production. Similarly, treatment of RAW 264.7 cells with the anti-HGFR antibody or control IgG in the presence of apoptotic cells did not significantly alter HGF production, as compared with RAW 264.7 cells treated with only apoptotic cells. These data suggest that endogenous HGF does not induce HGF production through c-Met under these culture conditions.
Exposure of RAW 264.7 cells to apoptotic cells induces HGF mRNA expression
To evaluate the HGF mRNA expression, semiquantitative RT-PCR was performed using mRNA extracted from RAW 264.7 cells. Exposure to apoptotic cells induced HGF mRNA expression by RAW 264.7 cells that was detectable at 1 h, peaked at 2 h, and declined after 3 h (Fig. 1E). In addition, other apoptotic cells, which are the human HeLa epithelial cell line and primary cell type of murine thyrmocytes, were used to measure HGF mRNA. Exposure to apoptotic HeLa cells induced the same time course of HGF mRNA expression by RAW 264.7 cells as apoptotic Jurkat cells (Supplemental Fig. 1A). Apoptotic thymocyte-induced HGF mRNA expression peaked earlier at 1 h, decreased slightly at 2 h, and declined after 3 h (Supplemental Fig. 1B). These findings suggest that apoptotic cell-induced HGF mRNA expression is a global phenomenon without dependency of cell types.
Apoptotic Jurkat cell-induced HGF mRNA expression was inhibited completely by the DNA synthesis inhibitor actinomycin D but not by the protein synthesis inhibitor cycloheximide (Fig. 1I). This suggests that new protein synthesis was not required for the induction of HGF transcription. To corroborate the results obtained by semiquantitative RT-PCR, a number of samples, related to the data representing the effects of apoptotic cells on HGF mRNA expression, have been analyzed further by quantitative real-time PCR. Fig. 1F and H shows that quantitative real-time PCR analysis confirmed the mRNA data with semiquantitative RT-PCR (Fig. 1E and G, respectively). As expected, quantitative analyses revealed greater differences in the expression levels, but the general profile observed previously by conventional RT-PCR was very similar.
In addition, whether apoptotic cells induced RAW 264.7 cell mRNA expression of other key growth factors involved in epithelial cell proliferation was examined. Apoptotic cell exposure was shown to induce RAW 264.7 cell expression of EGF mRNA, but not KGF mRNA, after 2 h (Fig. 1I and J).
RhoA activation is required for apoptotic cell-induced up-regulation of HGF mRNA and protein expression
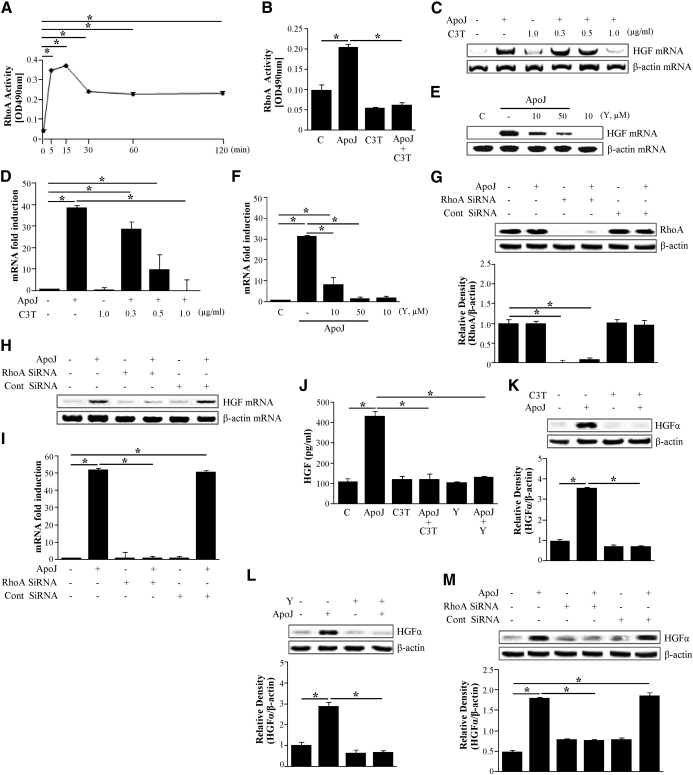
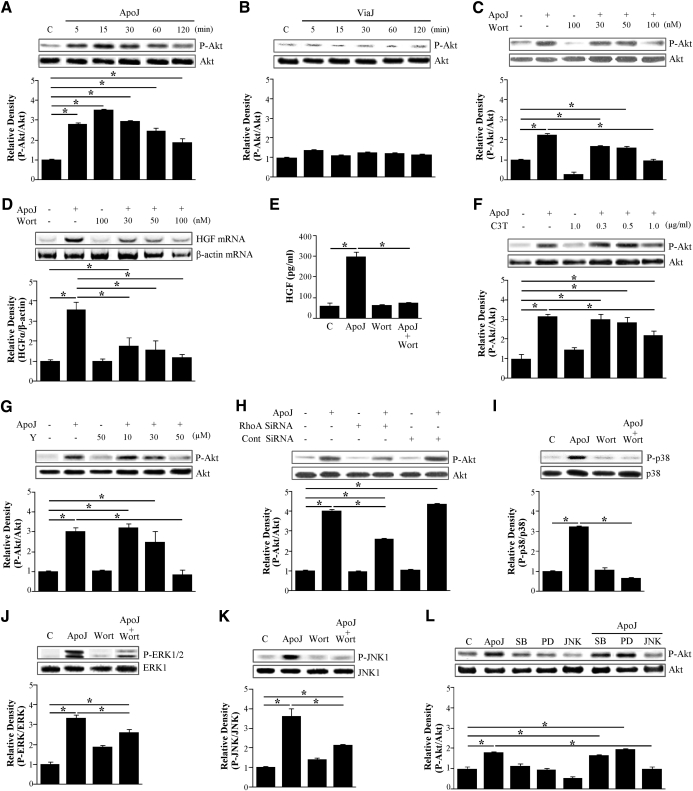
RhoA plays an important role during clearance of apoptotic cells [9–11] and in other cellular activities, such as gene transcription and protein synthesis [13]. Therefore, to determine the signaling pathways leading to HGF production by RAW 264.7 cells in response to apoptotic cells, the role of RhoA activation was evaluated. First, RhoA activity was measured by ELISA using a G-LISA™ kit after exposure of RAW 264.7 cells to apoptotic cells. RhoA activity in RAW 264.7 cells significantly increased 5 min after exposure to apoptotic cells, increased slightly further after 15 min, and then declined to two-thirds of the peak up to 2 h (Fig. 2A).
Figure 2. Activation of RhoA is required for apoptotic cell-induced HGF mRNA and protein expression.
RAW 264.7 cells were stimulated with apoptotic cells for the times indicated. (A and B) The levels of activated RhoA were determined using the RhoA activation assay Biochem Kit™ (G-LISA™). (B–F) RAW 264.7 cells were preincubated with a specific Rho inhibitor, C3 transferase (C3T; 0.3–2 μg/ml) for 20 h or a Rho kinase inhibitor Y27632 (Y; 10 and 50 μM) for 2 h before stimulation with apoptotic Jurkat cells for 2 h. (G) RhoA expression in RAW 264.7 cells transfected with RhoA siRNA or control (Cont) vehicle (siRNA-GFP) for 24 h was analyzed by Western blotting with antispecific RhoA. (H and I) RAW 264.7 cells were transfected with RhoA siRNA or control vehicle (siRNA-GFP) for 24 h and then stimulated with apoptotic Jurkat cells for 2 h. HGF mRNA levels were analyzed by semiquantitative RT-PCR (C, E, and H) and real-time PCR (D, F, and I). RAW 264.7 cells were preincubated with 1 μg/ml C3 transferase for 20 h and 50 μM Y27632 for 2 h (J–L) and transfected with RhoA siRNA or control vehicle (siRNA-GFP) for 24 h (M) and then stimulated with apoptotic Jurkat cells for 24 h. (J) HGF levels in the conditioned medium were measured by ELISA. (K–M) Western blots with anti-specific HGF α-chain were used, using the cultured cell lysates. Relative values of HGF-α expression are indicated below the gel. Values represent means ± se of three separate experiments; *P < 0.05.
The involvement of RhoA in HGF expression by RAW 264.7 cells was examined. RAW 264.7 cells were pretreated overnight with 0.3–2.0 μg/ml of the RhoA-specific inhibitor C3 transferase, which specifically inactivates Rho proteins through ADP-ribosylation. Alternately, cells were treated with 10 or 50 μM Y27632, an inhibitor of the RhoA downstream molecule Rho kinase for 2 h. Treated cells were then incubated with apoptotic Jurkat cells for 2 h. C3 transferase reduced apoptotic cell-induced HGF mRNA expression in a dose-dependent manner (Fig. 2C). C3 transferase ability to inhibit the apoptotic cell-induced RhoA activation was confirmed by finding that it inhibited the RhoA activity completely (Fig. 2B). Similarly, the Rho kinase inhibitor Y27632 also reduced apoptotic cell-induced HGF mRNA expression by RAW 264.7 cells in a dose-dependent manner (Fig. 2E).
To further examine the role of RhoA in apoptotic cell-induced HGF mRNA expression by RAW 264.7 cells, experiments were performed using a RhoA-specific siRNA. RAW 264.7 cells were transfected with RhoA-specific siRNA or negative control siRNA and cultured for 24 h or 48 h. The negative-control siRNA did not alter RhoA protein levels in cells with or without exposure to apoptotic cells. However, RhoA protein levels after 24 h were decreased completely in cells transfected with 300 pM RhoA siRNA, as illustrated by Western blot analysis (Fig. 2G). RhoA levels returned to baseline levels after 48 h of culture (data not shown). HGF mRNA expression was then measured in RhoA-specific, siRNA-transfected RAW 264.7 cells after 24 h in culture. siRNA silencing of RhoA inhibited apoptotic cell-induced HGF mRNA expression without affecting the expression of the endogenous control gene, β-actin (Fig. 2H). These data strongly suggest that recognition of apoptotic cells induces HGF mRNA expression through RhoA activation. These mRNA data with semiquantitative RT-PCR were also confirmed by further analysis with quantitative real-time PCR (Fig. 2D, F, and I). We have also examined and confirmed that HeLa epithelial cells (Supplemental Fig. 2A and B) and murine thyrmocytes (Supplemental Fig. 2C and D) induced HGF mRNA through the RhoA/Rho kinase pathway. These data overall indicated that apoptotic cell-mediated HGF mRNA induction from macrophages requires RhoA activity.
We further examined the role of RhoA/Rho kinase for HGF protein expression in response to apoptotic cells. C3 transferase (1 μg/ml) or 50 μM Y27632 suppressed HGF protein levels in the culture medium (Fig. 2J). Furthermore, the pharmacological inhibitors and RhoA-specific siRNA also decreased apoptotic cell-induced HGF protein levels in RAW 264.7 cell lysates (Fig. 2K–M).
Activation of MAPKs up-regulates apoptotic cell-induced HGF mRNA and protein expression
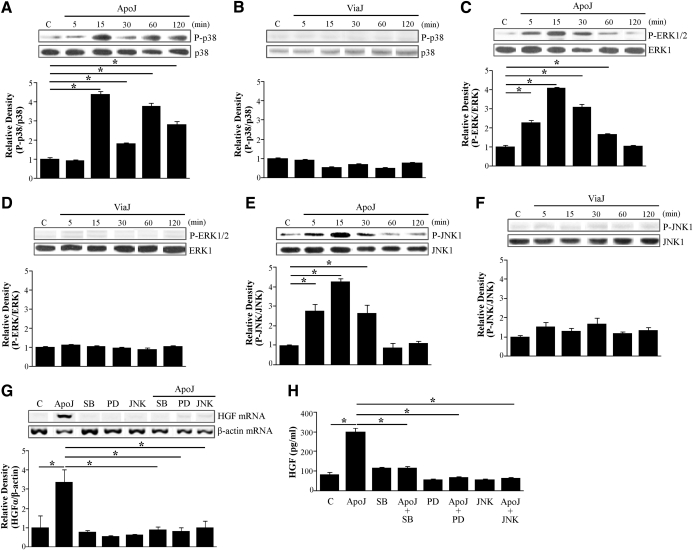
Various stimulants induce HGF mRNA expression through MAPK activation [15, 16]. Furthermore, gene expression of an angiogenic factor, cysteine-rich protein 61, was induced through RhoA and the p38 MAPK pathway in smooth muscle cells [19]. Thus, we examined the role of these MAPKs in apoptotic cell-induced up-regulation of HGF mRNA expression and protein production. When RAW 264.7 cells were stimulated with apoptotic Jurkat cells, phosphorylation of ERK1/2 and JNK1, but not p38 MAPK, was detectable as early as 5 min after exposure (Fig. 3A, C, and E). All three MAPKs peaked at 15 min postexposure. Phosphorylation of p38 MAPK declined after 15 min but increased again at 1 h postexposure. On the other hand, phosphorylation of ERK1/2 and JNK1 decreased over time and returned to resting levels at 1 and 2 h, respectively. In contrast, exposure of RAW 264.7 cells to viable Jurkat cells did not alter the levels of phosphorylation of these MAPKs up to 2 h postexposure (Fig. 3B, D, and F).
Figure 3. Roles of MAPKs in apoptotic cell-induced HGF production.
RAW 264.7 cells were stimulated with apoptotic (A, C, and E) or viable Jurkat cells (B, D, and F) for the times indicated. Total cell lysates were immunoblotted for phospho (P)-p38 MAPK/p38 MAPK (p38), phospho-ERK/ERK, or phospho-JNK/JNK, respectively. Relative values for phosphorylated MAPK versus unphosphorylated MAPK, respectively, are indicated below the gel. RAW 264.7 cells were preincubated with inhibitors of p38 MAPK, ERK, or JNK [10 μM SB 203580 (SB), 30 μM PD 98059 (PD), or 30 μM JNK inhibitor II, respectively] for 1 h and then stimulated with apoptotic Jurkat cells for 2 h to detect HGF mRNA expression (G) and for 24 h to detect secreted HGF (H). (G) HGF mRNA levels were analyzed by semiquantitative RT-PCR and normalized to β-actin mRNA levels. (H) Secreted HGF levels were measured by ELISA. Values represent means ± se of three separate experiments; *P < 0.05.
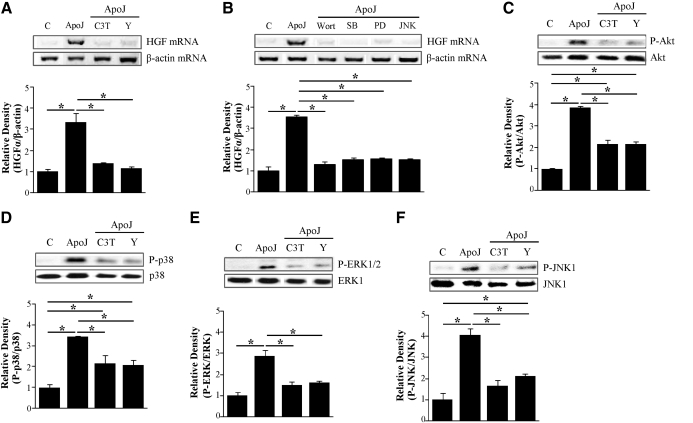
To further confirm the role of these kinases in HGF induction, RAW 264.7 cells were pretreated with SB 203580, a specific p38 MAPK inhibitor, PD 98059, a specific MEK-1 inhibitor, or the JNK inhibitor II (30 μM) for 1 h. Following treatment with the inhibitors, cells were incubated with apoptotic Jurkat cells for 2 h for mRNA analyses or 24 h for secreted HGF protein. SB 203580 (10 μM), 30 μM PD 98059, or 30 μM JNK inhibitor II, at which concentrations were shown previously to be effective and without cell toxicity [20, 21], reduced the levels of apoptotic cell-induced HGF mRNA expression (Fig. 3G) and secreted HGF protein (Fig. 3H). These data suggest that activation of these MAPKs is required for transcriptional up-regulation of HGF production.
MAPK activation is downstream of the RhoA/Rho kinase pathway
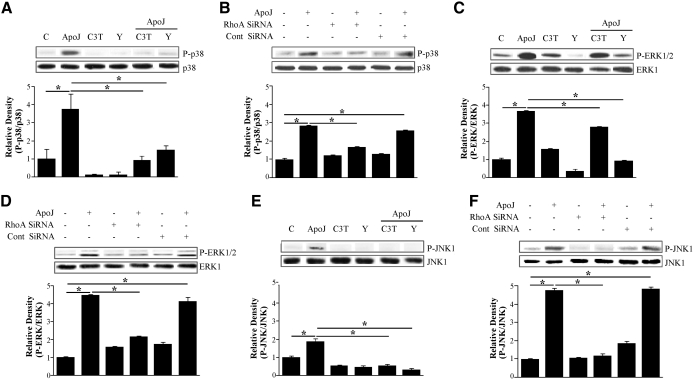
The RhoA/Rho kinase-dependent p38 MAPK localizes into the nucleus [22]. As described above, activation of RhoA and three MAPKs—p38 MAPK, ERK, and JNK—is required for apoptotic cell-induced HGF mRNA and protein production. Thus, we examined whether the RhoA/Rho kinase pathway acts as an upstream regulator of these MAPKs. Pharmacological inhibitors of RhoA/Rho kinase, C3 transferase (2 μg/ml), and Y27632 (50 μM; Fig. 4A, C, and E) and RhoA-specific siRNA (Fig. 4B, D, and F) reduced apoptotic cell-induced phosphorylation of p38 MAPK, ERK1/2, and JNK1. These data suggest that RhoA activation occurs upstream of MAPK phosphorylation.
Figure 4. MAPK activation is downstream of RhoA/Rho kinase.
(A, C, and E) RAW 264.7 cells were pretreated with 2 μg/ml C3 transferase for 20 h or 50 μM Y27632 for 2 h and then stimulated with apoptotic Jurkat cells for 15 min. Total cell lysates were immunoblotted for phospho-p38 MAPK, phospho-ERK, or phospho-JNK. (B, D, and F) RAW 264.7 cells were transfected with RhoA siRNA or control vehicle (siRNA-GFP) for 24 h and then stimulated with apoptotic Jurkat cells for 15 min. Relative values for phosphorylated MAPKs versus unphosphorylated MAPKs are indicated below the gel. Values represent means ± se of three separate experiments; *P < 0.05.
HGF mRNA and protein expression in response to apoptotic cells are regulated by RhoA/Rho kinase, PI3K/Akt, and MAPKs
Like Rho GTPases, PI3K is involved in apoptotic cell phagocytosis [11]. As described above, RhoA/Rho kinase, p38 MAPK, ERK, and JNK are important in the signaling pathway, leading to transcriptional up-regulation of HGF production (Figs. 2–4). Thus, we examined how PI3K/Akt contributes to apoptotic cell-induced mRNA and protein expression and how it is involved in the RhoA/Rho kinase pathway. Exposure of RAW 264.7 cells to apoptotic Jurkat cells resulted in increased phosphorylation of Akt after 5 min, with peak phosphorylation at 15 min (Fig. 5A). Akt phosphorylation declined after the 15-min peak. As hypothesized, the levels of phosphorylated Akt did not change after exposure to viable cells (Fig. 5B).
Figure 5. Role of PI3K and Akt in apoptotic cell-induced HGF expression.
RAW 264.7 cells were stimulated with apoptotic (A) or viable Jurkat cells (B) for the times indicated. (C) RAW 264.7 cells were pretreated with the PI3K inhibitor wortmannin (Wort) at the indicated concentrations for 1 h and then stimulated with apoptotic Jurkat cells for 15 min. RAW 264.7 cells were pretreated with the PI3K inhibitor and then stimulated with apoptotic Jurkat cells for 2 h to detect HGF mRNA expression (D) or for 24 h to detect secreted HGF (E). (D) HGF mRNA levels were analyzed using semiquantitative RT-PCR and normalized to β-actin mRNA levels. (E) HGF levels in the conditioned medium were measured by ELISA. RAW 264.7 cells were pretreated with C3 transferase for 20 h (F) or a Rho kinase inhibitor Y27632 for 1 h (G) and were transfected with RhoA siRNA or control vehicle (siRNA-GFP) for 24 h (H). (I–L) RAW 264.7 cells were pretreated with the PI3K inhibitor (100 nM wortmannin) or with inhibitors of p38 MAPK, ERK, or JNK (10 μM SB 203580, 30 μM PD 98059, or 30 μM JNK inhibitor II, respectively) for 1 h. RAW cells were then stimulated with apoptotic Jurkat cells for 15 min to detect phosphorylation of MAPKs or Akt. Total cell lysates were immunoblotted for phospho-Akt/Akt, phospho-p38 MAPK/p38 MAPK, phospho-ERK/ERK, or phospho-JNK/JNK. Relative values for phosphorylated kinase versus unphosphorylated kinase are indicated below the gel. Values represent means ± se of three separate experiments; *P < 0.05.
To confirm the involvement of PI3K in apoptotic cell-induced Akt phosphorylation, RAW 264.7 cells were pretreated with the PI3K inhibitor wortmannin (30–100 nM) for 1 h prior to stimulation with apoptotic cells. The inhibitor reduced Akt phosphorylation in a dose-dependent manner (Fig. 5C). Moreover, apoptotic cell-induced increases in expression of HGF mRNA (Fig. 5D) and secreted HGF protein (Fig. 5E) were inhibited. These data suggest that the PI3K/Akt pathway is involved in transcriptional regulation of HGF production.
The Rho kinase inhibitors C3 transferase (0.3–1.0 μg/ml) and Y27632 (10–50 μM) also inhibited apoptotic cell-induced phosphorylation of Akt in a dose-dependent manner (Fig. 5F and G). As expected, phosphorylation of Akt was inhibited by RhoA-specific siRNA (Fig. 5H). Therefore, the RhoA/Rho kinase pathway likely acts upstream of PI3K/Akt during transcriptional regulation of HGF in response to apoptotic cells.
We then examined whether the PI3K/Akt pathway acts upstream of the MAPKs in response to apoptotic cells. The PI3K inhibitor wortmannin (100 nM) inhibited apoptotic cell-induced phosphorylation of p38 MAPK, ERK1/2, and JNK1 (Fig. 5I–K). However, 10 μM SB 203580 and 30 μM PD 98059 did not alter apoptotic cell-induced phosphorylation of Akt, and 30 μM JNK inhibitor II decreased Akt phosphorylation levels (Fig. 5L). These data indicate that the PI3K/Akt pathway acts upstream of all three MAPKs, but only JNK regulates Akt phosphorylation. Akt and JNK signaling appears to cross-talk upon apoptotic cell stimulation. However, the functional role of this cross-talk in the induction of HGF production remains unclear. Collectively, these findings suggest that apoptotic cells induce mRNA expression and production of HGF through activation of RhoA/Rho kinase, PI3K/AKT, and MAPKs.
RhoA-dependent signaling is required for apoptotic cell-induced up-regulation of HGF mRNA expression in murine peritoneal macrophages
In addition to RAW 264.7 murine macrophages, to determine RhoA-dependent signaling in primary cell models, resident peritoneal macrophages were isolated by lavage from naive mice and then incubated with apoptotic Jurkat T cells. Likely, apoptotic cell stimulation resulted in an increase in HGF mRNA expression (Fig. 6A). However, inhibitors of the RhoA/Rho kinase pathway, C3 transferase (1 μg/ml), and Y27632 (50 μM), the PI3K inhibitor wortmannin (100 nM), and the MAPK inhibitors SB 203580 (10 μM), PD 98059 (30 μM), or JNK inhibitor II (30 μM) suppressed HGF mRNA expression (Fig. 6A and B). Furthermore, the inhibition of the RhoA/Rho kinase pathway decreased phosphorylation of Akt and MAPKs, including p38 MAPK, ERK1/2, and JNK1 (Fig. 6C–F). These findings indicate that RhoA-dependent signaling is required for HGF mRNA induction in primary cells of peritoneal macrophages upon exposure to apoptotic cells, as seen in the RAW 264.7 cells.
Figure 6. RhoA-dependent signaling is required for HGF mRNA induction in murine peritoneal macrophages upon exposure to apoptotic cells.
(A and B) Peritoneal macrophages were pretreated with C3 transferase (1 μg/ml) for 20 h and a Rho kinase inhibitor Y27632 (50 μM), PI3K inhibitor (100 nM wortmannin), or inhibitors of p38 MAPK, ERK, or JNK (10 μM SB 203580, 30 μM PD 98059, or 30 μM JNK inhibitor II, respectively) for 1 h and then stimulated with apoptotic Jurkat cells for 2 h. HGF mRNA levels were analyzed using semiquantitative RT-PCR and normalized to β-actin mRNA levels. (C–F) Peritoneal macrophages were pretreated with C3 transferase (1 μg/ml) for 20 h and a Rho kinase inhibitor Y27632 (50 μM) and then stimulated with apoptotic Jurkat cells for 15 min to detect phosphorylation of Akt or MAPKs. Total cell lysates were immunoblotted for phospho-Akt/Akt, phospho-p38 MAPK/p38 MAPK, phospho-ERK/ERK, or phospho-JNK/JNK. Relative values for phosphorylated kinase versus unphosphorylated kinase are indicated below the gel. Values represent means ± se of three separate experiments; *P < 0.05.
Anti-HGFR antibody did not affect HGF mRNA expression in response to apoptotic cells
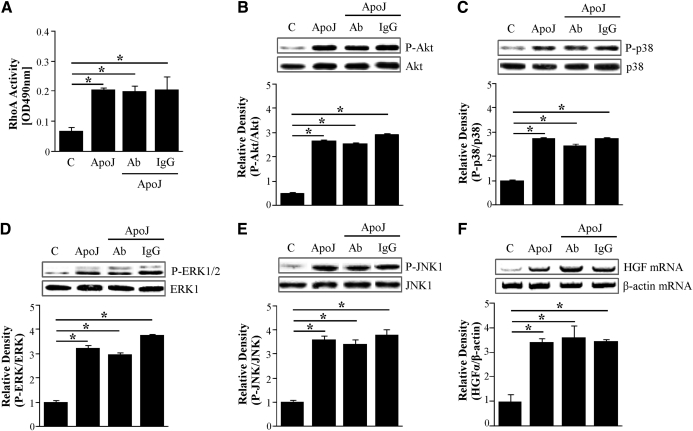
As described above, HGF produced by RAW 264.7 cells exposed to apoptotic cells did not function in an autocrine manner. Thus, we examined the effect of endogenous HGF on the HGFR, c-Met, signaling pathway related to HGF production through its receptor (c-Met) in this in vitro setting. As hypothesized, 10 μg/ml anti-HGFR antibody did not alter the apoptotic cell-induced RhoA activity and phosphorylation of Akt or activation of MAPKs (Fig. 7A–E). Moreover, apoptotic cell-induced HGF mRNA expression was not inhibited by this antibody (Fig. 7F). Therefore, this HGF induction is not dependent on the HGFR.
Figure 7. Anti-HGFR antibody does not alter apoptotic cell-induced activation of RhoA, PI3K, and MAPKs and HGF mRNA expression.
RAW 264.7 cells were pretreated with 10 μg/ml anti-HGFR antibody or 10 μg/ml IgG for 1 h and then stimulated with apoptotic Jurkat cells for 15 min (A–E) or 2 h (F). (A) The levels of RhoA activity were determined using the RhoA activation assay Biochem Kit™. (B–E) Total cell lysates were immunoblotted for phospho-Akt/Akt, phospho-p38 MAPK/p38 MAPK, phospho-ERK/ERK, or phospho-JNK/JNK. Relative values for phosphorylated kinase versus unphosphorylated kinase are indicated below the gel. (F) HGF mRNA levels were analyzed using semiquantitative RT-PCR and normalized to β-actin mRNA levels. Values represent means ± se of three separate experiments; *P < 0.05.
DISCUSSION
Recently, it was proposed that recognition alone of apoptotic cells induces replenishment stimuli by responding cells, such as macrophages or neighboring intact tissue cells, in addition to inducing anti-inflammatory signals [23]. Phagocytosis of apoptotic cells by epithelial cells leads to gene expression and secretion of growth and survival factors, including VEGF [5]. These secreted factors enhance the proliferation of pulmonary microvascular endothelial cells and epithelial wound closure. Similarly, apoptotic cell clearance in vivo and in vitro enhances HGF production by murine alveolar macrophages [4]. The importance of this response is suggested by the potential effects of HGF on epithelial cell proliferation and tissue repair. HGF also possesses a potent, antifibrotic ability, preventing the initiation and progression of tissue fibrosis, and inhibits TGF-β expression in a wide variety of animal models [24–27]. Thus, it is conceivable that the apoptotic cell recognition system itself may have a mechanism for the down-regulation of TGF-β action, the prime contributor to tissue fibrosis, through HGF expression. However, the molecular mechanism regulating apoptotic cell-induced HGF production is unknown. In the present study, the signaling pathway involved in transcriptional up-regulation of HGF production in response to apoptotic Jurkat T cells was characterized.
RAW 264.7 cells increased secreted and intracellular HGF production in response to apoptotic cells. This increase in HGF expression requires not only translation but also transcription. Recently, endogenous HGF has been reported to stimulate its specific receptor, c-Met, by acting in an autocrine manner [28–30]. We evaluated the possibility of the autocrine feedback loops for the HGF production using anti-c-Met antibody. HGF, which was induced by apoptotic cells, did not provide positive autostimulation under these culture conditions.
RAW 264.7 cells exposed to apoptotic cells increased levels of HGF mRNA, for which new protein synthesis was not required. This observation was also shown when RAW cells were exposed to other apoptotic cells, such as HeLa cells and murine thymocytes, indicating a global phenomenon. HGF mRNA levels peaked at 1 or 2 h after exposure to these variable types of apoptotic cells. A nonapoptotic cell stimulant LPS, or mAb217, an IgM mAb, mimicking exactly the effects of PS presented on apoptotic cells upon engulfment [31], also induced HGF mRNA in RAW 264.7 cells after 1 or 2 h of exposure (data not shown). Consistently, increased HGF production in vivo was also found in murine alveolar macrophages after instillation of apoptotic Jurkat T cells into the lungs of bleomycin-treated mice (data not shown). However, viable Jurkat T cells did not induce HGF mRNA and protein expression when compared with apoptotic cells, suggesting specificity for HGF induction for apoptotic cell recognition systems. Presumably, PS on the surface of apoptotic cells provides the signals leading to induction of HGF in macrophages such as anti-inflammatoy mediators—TGF-β1, IL-4, and IL-10 [32–34]. Treatment with apoptotic cells also increased RAW 264.7 cellular levels of epithelial growth factor EGF but not KGF mRNA. These data suggest that the expression of growth factors by responding cells, stimulated by interactions with apoptotic cells, may be induced selectively. It is important to note that the key anti-inflammatory factor, TGF-β, is also induced by apoptotic cells [2, 32]. TGF-β often acts as an antiproliferative agent. However, how a balance between these molecules is maintained during homeostatic conditions or is altered during pathophysiologic conditions, such as tissue damage or inflammation, remains unclear.
Surprisingly, RhoA activity increased rapidly and substantially by 5 min, continued to increase lightly 15 min, and maintained two-thirds of the peak activity up to 2 h post-apoptotic cell exposure. This observation is independent of phagocytic activity, and therefore, it is not a consequence of the rate of uptake of the apoptotic cells. The uptake rate in macrophages correlates to the length of exposure to apoptotic Jurkat cells, up to 90 min [12]. Pharmacological inhibition of RhoA and Rho kinase, as well as siRNA-mediated knockdown of RhoA reduced apoptotic cell-induced HGF mRNA and protein expression. These findings suggested that the RhoA/Rho kinase pathway is involved in transcriptional regulation of HGF. Furthermore, these findings were independent of the type of apoptotic cells. In contrast, Xiao and colleagues [32] demonstrated that RhoA plays a role in the regulation of apoptotic cell-induced TGF-β production at a translational step but not at a transcriptional step in 3T3TβRII cells with truncated TGF-βRII. Thus, further study is needed under the same conditions to determine the specific functions of the RhoA signaling pathway for the production of different growth factors, such as TGF-β.
In addition to RhoA, we evaluated the progression of activation of three MAPKs—p38 MAPK, ERK, and JNK—for 2 h in RAW 264.7 cells after apoptotic cell stimulation. Phosphorylation of all three MAPKs increased rapidly in RAW 264.7 cells in response to apoptotic cells and peaked after 15 min. Each individual MAPK inhibitor suppressed apoptotic cell-induced HGF expression almost completely at the mRNA and protein level. Activation of MAPKs occurs in the cytoplasm, but to exert many of their actions, they must translocate into the nucleus to interact with transcription factors [33]. MAPKs have cis-acting regulatory elements in the mouse-HGF promoter region, which respond to various transcription factors, including Stat-3, specificity protein-1, C/EBP-β and -δ, and activating protein 1 [34–39]. Thus, it is possible that apoptotic cell-induced HGF mRNA expression is mediated through activation of these transcription factors via MAPK signaling.
A large and growing body of evidence suggests that the PI3K/Akt pathway can regulate many cellular responses, including proliferation, cytoskeletal rearrangements, angiogenesis, and cell migration [40–42]. The PI3K/Akt pathway can be activated by growth factors, cytokines, and insulin [43]. Indeed, PI3K, including vacuolar sorting protein-34 (class III PI3KVPS34), is required during apoptotic cell phagocytosis [11, 44] and phagosome maturation in macrophages [44]. In the present study, we found that phosphorylation of Akt was increased substantially in RAW 264.7 cells in response to apoptotic cells after 5 min and peaked after 15 min. This activation of Akt was inhibited by pretreatment with a selective inhibitor of PI3K in a dose-dependent manner (1–100 nM). Furthermore, this inhibitor suppressed apoptotic cell-induced HGF mRNA and protein expression, suggesting the involvement of the PI3K/Akt pathway. A dose response for wortmannin indicates that class I PI3Ks, but not all classes, are involved in HGF transcription, as class I + II + III PI3Ks are all inhibited at and above 500 nM [44].
Collectively, our findings suggest that RhoA, three MAPKs, and the PI3K/Akt pathway are involved in apoptotic cell-induced transcriptional up-regulation of HGF. The RhoA/Rho kinase pathway has been shown to be upstream of MAPK, which leads to the development of osteogenesis [45] and cardiomyocyte hypertrophy [46]. On the other hand, RhoA acts as an upstream regulator of the PI3K/Akt pathway for preventing cardiomyocyte death [47] and for promoting cell-cycle progression and proliferation of vascular endothelial cells [48]. Similarly, RhoA is a key mediator of the PI3K/Akt and MAPK signaling pathways, which lead to HGF mRNA and protein expression. This was demonstrated by pharmacological and siRNA-mediated inhibition of the RhoA/Rho kinase pathway, which suppressed phosphorylation of all three MAPKs and Akt significantly. Furthermore, we evaluated the cross-talk between the downstream kinases, PI3K/Akt, and the MAPK pathways using selective inhibitors. The PI3K inhibitor suppressed apoptotic cell-induced phosphorylation of all three MAPKs, whereas the inhibitors of p38 MAPK and MEK, excluding the JNK inhibitor, did not affect Akt phosphorylation. These data suggest that p38 MAPK and ERK act downstream of PI3K/Akt but that there is cross-talk between JNK and Akt during the response to apoptotic cells. Because JNK inhibitor II (SP 600125) has relatively low specificity [49], a highly specific JNK inhibitor [JNK inhibitor I (L)-form/transactivator of transcription-JNK-interacting protein] was also examined here to confirm the role of JNK. Similar to the findings with SP 600125, this inhibitor significantly suppressed in apoptotic cell-induced HGF mRNA expression and production and phosphorylation of Akt (data not shown).
A series of our experiments emphasized the particular importance of the RhoA/Rho kinase/PI3K/Akt/MAPKs, including p38 MAPKs, ERK, and JNK, an axis that is required for the up-regulation of HGF mRNA and protein in RAW 264.7 cells in response to apoptotic cells. Importantly, the RhoA-mediated signaling leading to HGF mRNA was also confirmed in primary cells of murine peritoneal macrophages.
HGF induces activation of RhoA, MEK, and PI3K signal pathways through binding with its receptor, c-Met, which then interacts with downstream target proteins, such as Grb2, Src, PI3K, PLCgamma, and Src homology 2-containing tyrosine phosphatase 2 [50–53]. Dong et al. [54] reported that HGF-induced activation of MEK and PI3K signaling pathways contributed to expression of proangiogenic cytokines, such as IL-8 and VEGF, in a tumor cell line. In the present study, we evaluated this possibility using anti-c-Met antibody. The levels of activation of RhoA, Akt, and the MAPKs, as well as HGF mRNA expression, in response to apoptotic cells were not affected by this antibody under these culture conditions, suggesting incompetency of endogenous HGF signaling.
In summary, our findings provide novel evidence for a signaling mechanism in which the RhoA/Rho kinase-dependent pathway up-regulates transcription of HGF in response to apoptotic cells. This signaling pathway could be critical to sort out the contribution of the various apoptotic cell-recognizing receptors and bridge molecules involved in its production. The RhoA-mediated signaling pathway may be a novel target for enhancing the potential effects of apoptotic cells on epithelial regeneration and tissue replenishment after tissue injury.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a National Research Foundation (NRF) grant funded by the Korean government (MEST; 2008-0061522 and 2010-0029353) and by National Institutes of Health grant HL81151.
The online version of this manuscript, found at www.jleukbio.org, includes supplemental information.
- Ct
- cycling threshold
- HGF
- hepatocyte growth factor
- KGF
- keratinocyte growth factor
- PS
- phosphatidylserine
- siRNA
- small interfering RNA
AUTHORSHIP
H-J.P. and Y-H.C. designed and performed research. Y-H.C.,Y.J.C., and J.L.K. analyzed and interpreted data. J.L.K. designed and wrote the manuscript. P.M.H. contributed important intellectual input and the supervision for the study.
REFERENCES
- 1. Fadok V. A., Bratton D. I., Konowal A., Freed P. W., Westcott J. Y., Henson P. M. (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 101, 890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huynh M. L., Fadok V. A., Henson P. M. (2002) Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J. Clin. Invest. 109, 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freire-de-Lima C. G., Xiao Y. Q., Gardai S. J., Bratton D. L., Schiemann W. P., Henson P. M. (2006) Apoptotic cells, through transforming growth factor-β, coordinately induced anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J. Biol. Chem. 281, 38376–38384 [DOI] [PubMed] [Google Scholar]
- 4. Morimoto K., Amano H., Sonoda F., Baba M., Senba M., Yoshimine H., Yamamoto H., Ii T., Oishi K., Nagatake T. (2001) Alveolar macrophages that phagocytose apoptotic neutrophils produce hepatocyte growth factor during bacterial pneumonia in mice. Am. J. Respir. Cell Mol. Biol. 24, 608–615 [DOI] [PubMed] [Google Scholar]
- 5. Golpon H. A., Fadok V. A., Taraseviciene-Stewart L., Scerbavicius R., Sauer C., Welte T., Henson P. M., Voelkel N. F. (2004) Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J. 18, 1716–1718 [DOI] [PubMed] [Google Scholar]
- 6. Matsuda Y., Morimoto K., Ichida T., Nakamura T. (1995) Hepatocyte growth factor suppresses the onset of the liver cirrhosis and abrogates lethal hepatic dysfunction in rats. J. Biochem. 118, 643–649 [DOI] [PubMed] [Google Scholar]
- 7. Kawaida K., Matsumoto K., Shimazu H., Nakamura T. (1994) Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc. Natl. Acad. Sci. USA 91, 4357–4361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mason R. J., Leslie C. C., McCormik-Shannon K., Deterding R. R., Nakamura T., Rubin J. S., Shannon J. M. (1994) Hepatocyte growth factor is a growth factor for rat alveolar type II cells. Am. J. Respir. Cell Mol. Biol. 11, 561–567 [DOI] [PubMed] [Google Scholar]
- 9. Nakaya M., Tanaka M., Okabe Y., Hanayama R., Nagata S. (2006) Opposite effects of Rho family GTPases on engulfment of apoptotic cells by macrophages. J. Biol. Chem. 281, 8836–8842 [DOI] [PubMed] [Google Scholar]
- 10. Tosello-Trampont A. C., Nakada-Tsukui K., Ravichandran K. S. (2003) Engulfment of apoptotic cells is negatively regulated by Rho-mediated signaling. J. Biol. Chem. 278, 49911–49919 [DOI] [PubMed] [Google Scholar]
- 11. Leverrier Y., Ridley A. J. (2001) Requirement for Rho GTPases and PI 3-kinases during apoptotic cell phagocytosis by macrophages. Curr. Biol. 11, 195–199 [DOI] [PubMed] [Google Scholar]
- 12. Erwig L. P., Mcphillips K. A., Wynes M. W., Ivetic A., Ridley A. J., Henson P. M. (2006) Differential regulation of phagosome maturation in macrophages and dendritic cells mediated by Rho GTPases and ezrin-radixin-moesin (ERM) proteins. Proc. Natl. Acad. Sci. USA 103, 12825–12830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mackay D. J., Hall A. (1998) RhoGTPases. J. Biol. Chem. 273, 20685–20688 [DOI] [PubMed] [Google Scholar]
- 14. Skrtic S., Wallenius K., Gressner A. M., Jansson J. O. (1999) Insulin-like growth factor signaling pathways in rat hepatic stellate cells: importance for deoxyribonucleic acid synthesis and hepatocyte growth factor production. Endocrinology 140, 5729–5735 [DOI] [PubMed] [Google Scholar]
- 15. Takami Y., Takahiro M., Yamamoto I., Gohda E. (2005) Synergistic induction of hepatocyte growth factor in human skin fibroblasts by the inflammatory cytokines interleukin-1 and interferon-γ. Biochem. Biophys. Res. Commun. 327, 212–217 [DOI] [PubMed] [Google Scholar]
- 16. Motoki T., Sugiura Y., Matsumoto Y., Tsuji T., Kubota S., Takigawa M., Gohda E. (2008) Induction of hepatocyte growth factor expression by maleic acid in human fibroblasts through MAPK activation. J. Cell. Biochem. 104, 1465–1476 [DOI] [PubMed] [Google Scholar]
- 17. Hoffmann P. R., deCathelineau A. M., Ogden C. A., Leverrier Y., Bratton D. L., Daleke D. L., Ridley A. J., Fadok V. A., Henson P. M. (2001) Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J. Cell Biol. 155, 649–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(–Δ ΔC(T))method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 19. Han J. S., Macarak E., Rosenbloom J., Chung K. C., Chaqour B. (2003) Regulation of cyr61/CCN1 gene expression through RhoAGTPase and p38 MAPK signaling pathways. Eur. J. Biochem. 270, 3408–3421 [DOI] [PubMed] [Google Scholar]
- 20. Xiao Y. Q., Malcom K., Worthen G. S., Gardai S., Schiemann W. P., Fadok V. A., Bratton D. L., Henson P. M. (2002) Cross-talk between ERK transforming growth factor-β. J. Biol. Chem. 277, 14884–14893 [DOI] [PubMed] [Google Scholar]
- 21. Xiao Y. Q., Minami K., Mue S., Ohuchi K. (1998) Pharmacological analysis of protein kinases responsible for chemotaxis of rat peritoneal neutrophils. Eur. J. Pharmacol. 360, 195–204 [DOI] [PubMed] [Google Scholar]
- 22. Zeidan A., Javadov S., Chakrabarti S., Karmazyn M. (2008) Leptin-induced cardiomyocyte hypertrophy involves selective caveolae and RhoA/ROCK-dependent p38 MAPK translocation to nuclei. Cardiovasc. Res. 77, 64–72 [DOI] [PubMed] [Google Scholar]
- 23. Henson P. M. (2003) Possible roles for apoptosis and apoptotic cell recognition in inflammation and fibrosis. Am. J. Respir. Cell Mol. Biol. 29, S70–S76 [PubMed] [Google Scholar]
- 24. Liu Y. (2002) Hepatocyte growth factor and the kidney. Curr. Opin. Nephrol. Hypertens. 11, 23–30 [DOI] [PubMed] [Google Scholar]
- 25. Mizuno S., Nakamura T. (2004) Suppression of chronic glomerular injuries and TGF-β1 production by HGF in attenuation of murine diabetic nephropathy. Am. J. Physiol. Renal Physiol. 286, F134–F143 [DOI] [PubMed] [Google Scholar]
- 26. Yang J., Dai C., Liu Y. (2003) Hepatocyte growth factor suppresses renal interstitial myofibroblast activation and intercepts Smad signal transduction. Am. J. Pathol. 163, 621–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xia J. L., Dai C., Michalopoulos G. K., Liu Y. (2006) Hepatocyte growth factor attenuates liver fibrosis induced by bile duct ligation. Am. J. Pathol. 168, 1500–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nayeri F., Xu J., Abdiu A., Nayeri T., Aili D., Liedberg B., Carlsson U. (2006) Autocrine production of biologically active hepatocyte growth factor (HGF) by injured human skin. J. Dermatol. Sci. 43, 49–56 [DOI] [PubMed] [Google Scholar]
- 29. Mildner M., Mlitz V., Gruber F., Wojta J., Tschachler E. (2007) Hepatocyte growth factor establishes autocrine and paracrine feedback loops for the protection of skin cells after UV irradiation. J. Invest. Dermatol. 127, 2637–2644 [DOI] [PubMed] [Google Scholar]
- 30. Onimaru Y., Tsukasaki K., Murata K., Imaizumi Y., Choi Y. L., Hasegawa H., Sugahara K., Yamada Y., Hayashi T., Nakashima M., Taguchi T., Mano H., Kamihira S., Tomonaga M. (2008) Autocrine and/or paracrine growth of aggressive ATLL cells caused by HGF and c-Met. Int. J. Oncol. 33, 697–703 [PubMed] [Google Scholar]
- 31. Fadok V. A., Bratton D. L., Rose D. M., Pearson A., Ezekewitz R. A., Henson P. M. (2000) A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405, 85–90 [DOI] [PubMed] [Google Scholar]
- 32. Xiao Y. Q., Freire-de-Lima C. G., Schiemann W. P., Bratton D. L., Vandivier R. W., Henson P. M. (2008) Transcriptional and translational regulation of TGF-β production in response to apoptotic cells. J. Immunol. 181, 3575–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Price M. A., Cruzalegui F. H., Treisman R. (1996) The p38 and ERK MAP kinase pathways cooperate to activate ternary complex factors and c-fos transcription in response to UV light. EMBO J. 15, 6552–6563 [PMC free article] [PubMed] [Google Scholar]
- 34. Jiang J. G., Zarnegar R. (1997) A novel transcriptional regulatory region within the core promoter of the hepatocyte growth factor gene is responsible for its inducibility by cytokines via the C/EBP family of transcription factors. Mol. Cell. Biol. 17, 5758–5770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang J. G., Chen Q., Bell A., Zarnegar R. (1997) Transcriptional regulation of the hepatocyte growth factor (HGF) gene by the Sp family of transcription factors. Oncogene 14, 3039–3049 [DOI] [PubMed] [Google Scholar]
- 36. Wojcik E. J., Sharifpoor S., Miller N. A., Wright T. G., Watering R., Tremblay E. A., Swan K., Mueller C. R., Elliott B. E. (2006) A novel activating function of c-Src and Stat3 on HGF transcription in mammary carcinoma cells. Oncogene 25, 2773–2784 [DOI] [PubMed] [Google Scholar]
- 37. Wierenga A. T., Vogelzang I., Eggen B. J., Vellenga E. (2003) Erythropoietin-induced serine 727 phosphorylation of SAT3 in erythroid cells is mediated by a MEK-ERK-, and MSK1-dependent pathway. Exp. Hematol. 31, 398–405 [DOI] [PubMed] [Google Scholar]
- 38. Larsen L., Størling J., Darville M., Eizirik D. L., Bonny C., Billestrup N., Mandrup-Poulsen T. (2005) Extracellular signal-regulated kinase is essential for interleukin-1-induced and nuclear factor κB-mediated gene expression in insulin-producing INS-1E cells. Diabetologia 48, 2582–2590 [DOI] [PubMed] [Google Scholar]
- 39. Marcinkowska E., Garay E., Gocek E., Chrobak A., Wang X., Studzinski G. P. (2006) Regulation of C/EBPβ isoforms by MAPK pathways in HL60 cells induced to differentiate by 1,25-dihydroxy vitamin D3. Exp. Cell Res. 312, 2054–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brazil D. P., Park J., Hemmings B. A. (2002) PKB binding proteins. Getting in on the Akt. Cell 111, 293–303 [DOI] [PubMed] [Google Scholar]
- 41. Higaki M., Shimokado K. (1999) Phosphatidylinositol 3-kinase is required for growth factor-induced amino acid uptake by vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 19, 2127–2132 [DOI] [PubMed] [Google Scholar]
- 42. Hixon M. L., Muro-Cacho C., Wagner M. W., Obejero-Paz C., Millie E., Fujio Y., Kureishi Y., Hassold T., Walsh K., Gualberto A. (2000) Akt1/PKB upregulation leads to vascular smooth muscle cell hypertrophy and polyploidization. J. Clin. Invest. 106, 1011–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dunbar J. C., Hu Y., Lu H. (1997) Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes 46, 2040–2043 [DOI] [PubMed] [Google Scholar]
- 44. Kinchen J. M., Doukoumetzidis K., Almendinger J., Stergiou L., Tosello-Trampont A., Sifri C. D., Hengartner M. O., Ravichandran K. S. (2008) A pathway for phagosome maturation during engulfment of apoptotic cells. Nat. Cell Biol. 10, 556–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khatiwala C. B., Kim P. D., Peyton S. R., Putnam A. J. (2009) ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK. J. Bone Miner. Res. 24, 886–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reuveny M., Heller H., Bengal E. (2004) RhoA controls myoblast survival by inducing the phosphatidylinositol 3-kinase-Akt signaling pathways. FEBS Lett. 569, 129–134 [DOI] [PubMed] [Google Scholar]
- 47. Del Re D. P., Miyamoto S., Brown J. H. (2008) Focal adhesion kinase as a RhoA-activable signaling scaffold mediating Akt activation and cardiomyocyte protection. J. Biol. Chem. 283, 35622–35629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Coupel S., Leboeuf F., Boulday G., Soulillou J. P., Charreau B. (2004) RhoA activation mediates phosphatidylinositol 3-kinase-dependent proliferation of human vascular endothelial cells: an alloimmune mechanism of chronic allograft nephropathy. J. Am. Soc. Nephrol. 15, 2429–2439 [DOI] [PubMed] [Google Scholar]
- 49. Yeh M. C., Mukaro V., Hii C. S., Ferrante A. (2010) Regulation of neutrophil-mediated killing of Staphylococcus aureus and chemotaxis by c-jun NH2 terminal kinase. J. Leukoc. Biol. 87, 925–932 [DOI] [PubMed] [Google Scholar]
- 50. Dohda T., Nakamura Y., Kamihira M., Iijima S. (2004) Functional role of RhoA in growth regulation of primary hepatocytes. J. Biochem. 135, 631–637 [DOI] [PubMed] [Google Scholar]
- 51. Ponzetto C., Bardelli A., Zhen Z., Maina F., dalla Zonca P., Giordano S., Graziani A., Panayotou G., Comoglio P. M. (1994) A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 77, 261–271 [DOI] [PubMed] [Google Scholar]
- 52. Fixman E. D., Naujokas M. A., Rodrigues G. A., Moran M. F., Park M. (1995) Efficient cell transformation by the Tpr-Met oncoprotein is dependent upon tyrosine 489 in the carboxy-terminus. Oncogene 10, 237–249 [PubMed] [Google Scholar]
- 53. Fournier T. M., Kamikura D., Teng K., Park M. (1996) Branching tubulogenesis but not scatter of Madin-Darby canine kidney cells requires a functional Grb2 binding site in the Met receptor tyrosine kinase. J. Biol. Chem. 271, 22211–22217 [DOI] [PubMed] [Google Scholar]
- 54. Dong G., Chen Z., Li Z. Y., Yeh N. T., Bancroft C. C., Waes C. V. (2001) Hepatocyte growth factor/scatter factor-induced activation of MEK and PI3K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma. Cancer Res. 61, 5911–5918 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.