Abstract
This review addresses bone geometry and indices of skeletal strength associated with exposure to gymnastic loading during growth. A brief background characterizes artistic gymnastics as a mechanical loading model and outlines densitometric techniques, skeletal outcomes and challenges in assessment of skeletal adaptation. The literature on bone geometric adaptation to gymnastic loading is sparse and consists of results for disparate skeletal sites, maturity phases, gender compositions and assessment methods, complicating synthesis of an overriding view. Furthermore, most studies assess only females, with little information on males and adults. Nonetheless, gymnastic loading during growth appears to yield significant enlargement of total and cortical bone geometry (+10 to 30%) and elevation of trabecular density (+20%) in the forearm, yielding elevated indices of skeletal strength (+20 to +50%). Other sites exhibit more moderate geometric and densitometric adaptations (5 to 15%). Mode of adaptation appears to be site-specific; some sites demonstrate marked periosteal and endosteal expansion, whereas other sites exhibit negligible or moderate periosteal expansion coupled with endocortical contraction. Further research is necessary to address sex-, maturity- and bone tissue-specific adaptation, as well as maintenance of benefits beyond loading cessation.
Keywords: gymnastics, bone geometry, bone strength, mechanical loading, growth
Introduction
Artistic gymnastics has been studied extensively as a model of skeletal adaptation to mechanical loading. Both retrospective and prospective studies suggest that bone mineral accrual during growth is increased in gymnasts relative to non-gymnasts at most measured sites.1–7 DXA-measured skeletal parameters are elevated in female gymnasts versus non-gymnasts in childhood,1,8–10 during puberty,10–13 post-menarche and at college age.12–15 Gymnastic adaptation appears to be dose-dependent, increasing with training intensity.9,16 Furthermore, in college-age females, gymnastic exposure and withdrawal have been associated with training and de-training effects, respectively increasing and decreasing areal bone mineral density.17 Comparisons of adult former gymnasts versus non-gymnasts suggest that skeletal advantages are maintained after activity cessation and may persist in adulthood.1,18–22 However, there is limited prospective evidence linking pediatric advantages with continued benefits in adulthood, beyond training cessation. 12–13
Most gymnast versus non-gymnast comparisons use dual energy X-ray absorptiometry (DXA) to evaluate bone mineral content (BMC) and areal bone mineral density (aBMD). These DXA studies often focus on the femoral neck and lumbar spine, but there is also considerable evidence of bone adaptation in the upper extremity. 3,8–10,12–16 A subset of studies have assessed geometric adaptation underlying non-specific aBMD and BMC at appendicular sites;11,23–25 some have used pQCT to specifically assess cross-sectional bone geometry.22,26–29 Fewer studies have specifically investigated geometric adaptations at the lumbar spine and proximal femur.1,11,26,27
In contrast to weight-lifting and racquet sports, gymnastic activity applies impact loads that involve the total body mass, imparting high muscular loads and mass inertia to both upper and lower extremities.29 Gymnasts generate vertical ground reaction forces of approximately 3.5 to 10 times body weight (upper and lower extremities, respectively),30–31 rivaling lower extremity impact forces measured in other athletes.32–33 High resultant stresses in the bilateral upper extremities distinguish gymnastic from non-gymnastic loading, as most other activities preferentially load the dominant arm and generate lower stresses.
In simplified terms, gymnastic loading of the skeleton is dominated by axial compression and bending forces during tumbling, vaulting, beam and pommel horse work, while tension and torsion play a greater role during bar and ring work. In reality, gymnastic loading generates a combination of compression, tension and shear stresses. “Simple” axial compression generates bending forces in curved bones,34 resulting in compressive loads on the concave surface and tensile loads on the convex surface.35 Furthermore, compressive loads routinely generate shear stresses at a 45 degree angle to the loading axis.35 Accordingly, it is appropriate for assessment to include indices of skeletal strength related to axial compression, bending and torsion.
It is difficult to summarize the body of knowledge regarding bone geometric adaptation to gymnastic loading, as the existing literature consists of results for disparate skeletal sites, maturity phases, gender compositions and assessment methods. The majority of published studies assess only females, with a preponderance of pediatric studies, and there are few reports discussing male or adult geometric adaptation. Consequently, this review focuses on females, but includes the sole published study involving males.27 A methodological context will be provided to outline current challenges and strategies for progress in this research area.
Methodological Context
In order to evaluate skeletal adaptation to gymnastic loading, it is important to isolate loading effects from unrelated sources of skeletal variation, including normal processes of growth and maturation. Effective research should compare individuals of similar physical maturity and account for variation in age, rate of maturation and body size. There is evidence that males and females differ in skeletal growth and geometric adaptation.36–40 The sole published study evaluating males and females did not detect significant interactions between sex and gymnastic activity for bone parameters in pre-pubertal subjects, except for diaphyseal cortical thickness at the radius (50% site).27 Due to the paucity of data on male gymnasts, this review will address only female adaptations.
Gymnastic studies are observational, because randomized controlled trials evaluating “effects” of gymnastic exposure are unfeasible. To account for the limitations of observational studies, skeletal traits associated with gymnastic exposure are referred to in this review as “gymnast advantages” (relative to non-gymnasts), which may indicate higher (positive) or lower (negative) mean values. Critically, studies are presented according to maturity status at the observation, but most gymnasts initiate training in childhood or early puberty. Accordingly, most results reflect loading during early development, with or without cumulative adaptation due to pubertal gymnastic exposure. To date, no studies reflect gymnastic exposure limited to later maturity phases.
In any context, in vivo assessment of the human skeleton is challenging. At present, the most thorough, sensitive and specific methods are limited by prohibitive costs, availability, radiation doses and other factors. Both quantitative computed tomography (QCT) and magnetic resonance imaging (MRI) evaluate skeletal geometry in three dimensions and allow isolation of the vertebral bodies from the posterior elements of the spine. For evaluation of distal sites, high resolution MRI (hrMRI) and hrQCT yield extremely detailed bone geometric results, including micro-architectural parameters (trabecular spacing, number, thickness, etc.).41
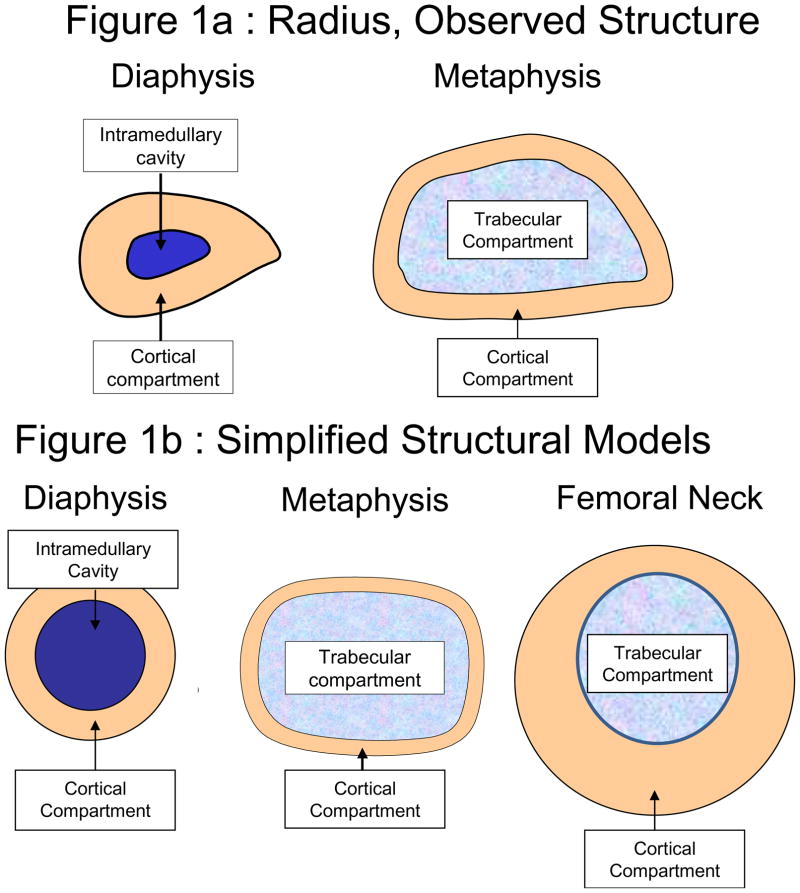
Peripheral QCT (pQCT) is a more readily available and feasible tool for assessment of cortical and trabecular cross-sectional geometry and density at distal appendicular sites (Figure 1a), with relatively low subject discomfort and radiation exposure.41 However, narrow pQCT regions of interest are sensitive to positional variation, as each 2mm slice provides a limited assessment of skeletal quality in a structure that may vary markedly between adjacent cross-sections.43,47–48 This issue is particularly influential in the growing metaphysis, where it is recommended that scans be positioned based on the physis or physeal scar to account for inter- and intra-individual structural variation.49–50 Unfortunately, determination of physeal references can be difficult and subjective, as appearance and location may vary between individuals, and from scan to scan within individuals.43
Figure 1.
Figure 1a–b: (a) A schematic representation of pQCT images is presented for the distal radius (33% diaphysis, 4% metaphysis); (b) Schematic representations of simplified geometric models for derivations of geometric and strength indices are depicted for a long bone diaphysis, a long bone metaphysis and the femoral neck. Periosteal (a.k.a. “Total” or “total bone”) circumference and cross-sectional area (CSA) can be measured at all sites, reflecting outer bone dimensions and the entire compartment within. The cortical compartment is most commonly evaluated at the femoral neck, upper and lower extremity diaphyses, and lower extremity metaphyses, yielding vBMD, CSA and thickness/width (measured to specify medial, lateral or bi-cortical versus CSA-derived mean). The marrow-filled intramedullary cavity (medullary or endocortical compartment) may be assessed for diameter, CSA and endocortical circumference. Trabecular compartment dimensions are measured in a similar manner, but trabecular volumetric BMD is an important outcome, indicating compressive properties. Trabecular vBMD results are highly analysis-dependent (affected by pQCT contour and peel mode settings).
In contrast, because DXA scans sample a broader length of bone, positional variation is less influential.43 However, standard DXA output represents a 2-dimensional mean over the region of interest. Thus, higher BMC and aBMD values may indicate denser and/or larger bone and do not delineate tissue-specific geometry or density.10,43 In addition, fan beam magnification may affect scan results, although new generation DXA scanners have attempted to alleviate magnification error. In particular, supine lateral lumbar spine scans improve assessment of vertebral body BMAD and geometry. Nonetheless, magnification error and integrated spinal anatomy still pose problems in anteroposterior (AP) fan beam DXA studies of the proximal femur and spine that compare bodies of disparate or changing size.44–46 In contrast, at the distal radius, DXA-derived bone geometry and strength indices51 correlate well with pQCT-measures (strongest agreement for cortical CSA, Z/SSI, IBS: r=0.96, 0.92, 0.90; 33% total CSA, r=0.93, p<0.0001)43 (Table 1, Figure 1a and 1b); these derivations may provide a useful compromise between “non-specific” DXA and overly-specific pQCT output, with favorable subject safety and comfort.43
Table 1. Skeletal Strength Indices.
Methodology and basis for commonly reported skeletal strength indices are presented. Preferred sites and model assumptions are noted.
| Index | Description | Assesses | Method, Basis | Preferred Sites | 2D or 3D | Bone Mineral Distribution/Shape Model Assumptions |
|---|---|---|---|---|---|---|
| Z (mm3) | section modulus (non-density-weighted) index based solely on geometric model, no account for vBMD |
bending/torsion | DXA-derivation, based on AP bone width, BMC25,43,51 | Diaphysis | 2D | Circular model, cortical width based on uniform cortical vBMD |
| pQCT 42 | Diaphysis | 3D | pQCT-measured shape | |||
| DXA hip structural analysis, based on AP bone width & AP BMC distribution profile53 | Femoral Neck | 2D | Circular model, partial distribution assessment 60/40= cort/trab ratio | |||
| Femoral Diaphysis | 2D | Circular model, assumed symmetry 100/0= cort/trab ratio | ||||
| Polar SSI (mm3) | polar strength-strain index (density-weighted section modulus) accounts for vBMD distribution | bending/torsion | pQCT, measured geometry & voxel-specific vBMD42 | Diaphysis | 3D | pQCT-measured shape & vBMD distribution |
| CSMI (I, mm4) | cross-sectional moment of inertia index based on bone geometry, no account for vBMD |
bending/torsion | DXA hip structural analysis, based on AP bone width & AP BMC distribution profile53 | Femoral Neck | 2D | Circular Model, partial distribution assessment 60/40= cort/trab ratio |
| Femoral Diaphysis | 2D | Circular model, assumed symmetry 100/0= cort/trab ratio | ||||
| pQCT42 | Diaphysis | 3D | pQCT-measured bone dimensions | |||
| EI (Nm*mm4) | cross-sectional bending stiffness24 (Young’s elastic modulus * CSMI) (E * I) material/geometric index | bending | MRTA (mechanical response tissue analysis)24 | Diaphysis | __ __ |
_____ |
| IBS or BSIc (g2/cm4) | index of structural strength in axial compression51 or bone strength index (compressive)48 considers bone size AND density |
compression | DXA-derivation25,43,51, based on aBMD or AP width & BMC | Metaphysis | 2D | Elliptical model, assumes uniform vBMD & simplified shape |
| pQCT, calculated from CSA & total vBMD29 | 3D | pQCT-measured shape & vBMD distribution |
2D= 2-dimensional; 3D= 3-dimensional; DXA= dual energy X-ray absorptiometry; AP= anteroposterior; BMC= bone mineral content; pQCT= peripheral quantitative computed tomography; vBMD = volumetric bone mineral density; cort= cortical bone; trab= trabecular bone; CSA= cross-sectional area.
Numerous skeletal strength indices have been utilized in studies of gymnastics and other loading modalities (Table 1). As noted previously, gymnastic loading generates bending, torsional and compressive loads. It is customary to evaluate bending/torsional strength at the diaphysis and axial compressive strength at the metaphysis; all three are relevant at the proximal femur. For the lumbar spine, vertebral body compressive strength assessment is most appropriate.52 Reports of section modulus (Z) are common, including non-density-weighted11,25,43 and density-weighted indices.27–29 Polar strength-strain index (SSI) is a volumetric density-weighted section modulus, reported as standard pQCT output for radial and tibial diaphyses.42 In contrast, non-density-weighted Z is approximated for the femoral neck and shaft using Beck’s DXA hip structural analysis, incorporating observed cortical asymmetry for the femoral neck11,53 (Table 1, Figure 1b). Radial diaphysis Z may be derived using similar simplified geometric models (Figure 1b, Table 1).25,43,51 At metaphyseal sites, an index of structural strength in axial compression may be calculated (IBS or BSIc, Table 1).25,29,43,51 At present, generalization of findings is challenging due to inconsistent reporting of indices of skeletal strength..
Adaptations to Gymnastic Loading
Distal Appendicular Sites (Figures 2a–d)
Figure 2.
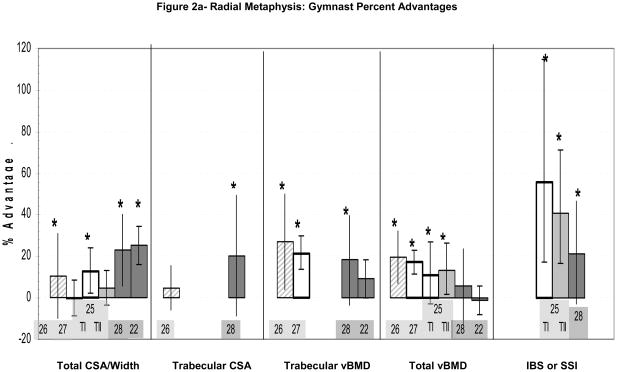
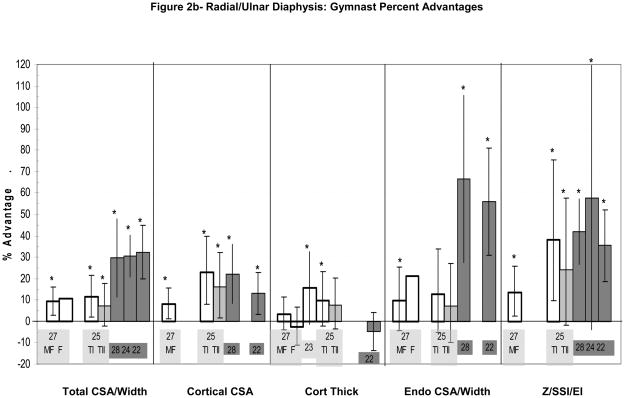
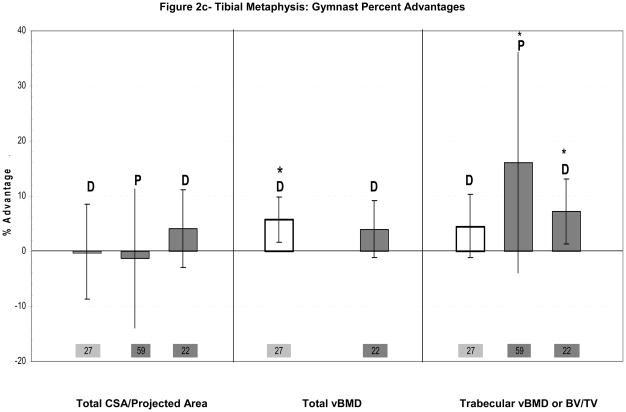
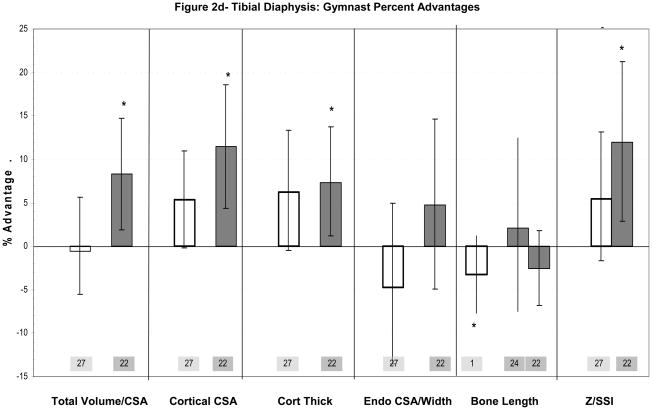
Figure 2a–d: Percent advantages for gymnasts versus non-gymnasts are presented for comparable bone sites and outcomes, as follows: (a) Radial Metaphysis; (b) Radial/ulnar Diaphysis; (c) Tibial Metaphysis, (d) Tibial Diaphysis. Error bars indicate 95% Confidence Intervals for the gymnast percentage advantage. Error bars with horizontal borders represent confidence intervals as reported in the source publication, whereas unbordered error bars indicate confidence intervals derived from reported standard deviations or standard errors of the means. Columns are color-coded by maturity phase: white= Tanner I; white/light gray stripe= Tanner I/II mix; light gray= Tanner II; dark gray= post-menarcheal/adult. Study reference numbers are presented for each column. Asterisks indicate reported statistically significant differences or large effect sizes (Cohen’s d). Where proximal and distal site locations yield contrasting results, P= proximal; D= distal. Tibial EI percentage advantage is not presented graphically, because it is not feasible (mid-tibia EI gymnast advantage=228%).
As previously noted, gymnastic maneuvers apply loads to both upper extremities that rival lower extremity ground reaction forces during jumping.30–33 Because most other activities concentrate upper extremity loading on the dominant arm and do not involve total body mass impacts, the non-dominant forearm provides an excellent site for evaluating skeletal adaptation to gymnastic loading. As the distal radius bears the majority of axial compressive loads applied to the hand,54–55 it may serve as a particularly valuable indicator of gymnastic loading. In addition, the radius includes both cortical (diaphyseal) and trabecular (metaphyseal) regions of interest for bone tissue-specific assessment.25,41,43,48 In the lower extremity, the tibia provides a similar opportunity for bone tissue-specific evaluation of adaptation to high magnitude forces (ten times body weight).30–31 However, as tibial loading occurs with most sports and activities of daily life, gymnast vs. non-gymnast contrasts may be less dramatic at this site than in the forearm. Finally, the peripheral nature of both the radius and tibia enables quantification of bone compartment densities and geometry using pQCT.41,43,48
Forearm Adaptations Observed in Childhood and Early Puberty (Tanner I, Tanner II)(Table 2a)
Table 2a. Gymnastic Loading Studies: Pre-puberty/Early Puberty.
This table summarizes cohort composition, skeletal assessment methods, sites and outcomes for published studies, examining geometric and strength indices associated with gymnastic loading.
| Study | Cohort | Scan | 2D/3D | Site | Total Outcomes | Compartmental Outcomes | ||
|---|---|---|---|---|---|---|---|---|
| Bass1 1998 | TI Female GYM n= 45 (age μ= 10.4 yrs) NON n= 35 (age μ= 9.3 yrs) |
DXA | 2D | Lumbar Spine (L2–4) | Derived Volume BMAD |
-- | ||
| Femur Shaft (mid) | AP Width BMAD |
Bicortical thickness Endocortical diameter |
||||||
| Dyson26 1997 | TI/TII Female GYM n=16 (age μ= 9.8 yrs) NON n= 16 (age μ= 9.9 yrs) |
pQCT | 3D | 6% Distal Radius (articular reference) | Total CSA Total vBMD |
Trabecular and Cortical CSA (% Total CSA) Trabecular vBMD |
||
| DXA | 2D | Femoral Neck Lumbar Spine Total Body Trochanter |
aBMD BMAD |
|||||
| Ward27 2005 | TI (age 5–12yrs) Male & Female (M+F unless noted) GYM Male n=17 (age μ= 9.4 yrs) Female n= 27 (age μ=8.7 yrs) NON Male n=20 (age μ= 8.9 yrs) Female n=22 (age μ= 8.6 yrs) |
pQCT | 3D | 4% Distal Radius (physeal reference) | Total CSA Total vBMD |
Trabecular vBMD | ||
| 50% Distal Radius (physeal reference) | Total CSA Muscle CSA SSI |
Cortical CSA Endocortical CSA Cortical width (M, F, M+F) |
Cortical BMC Cortical vBMD |
|||||
| 10mm Distal Tibia (physeal reference) | Total CSA Total vBMD |
Trabecular vBMD | ||||||
| 65% Proximal Tibia (physeal reference?) | Total CSA Muscle CSA (M, F, M+F) SSI |
Cortical CSA Endocortical CSA Mean Cortical width |
Cortical BMC Cortical vBMD |
|||||
| DXA | 2D | Lumbar Spine | Area BMAD |
BMC aBMD |
-- | |||
| Total Body | Area | BMC aBMD |
-- | |||||
| Dowthwaite 200725 | TI vs TII Female TI, TII GYM n=12, 16 (age μ= 10.0, 11.4yrs) NON n=10, 18 (age μ= 10.4, 11.0yrs) |
DXA | 2D | Ultradistal Radius | AP Width BMAD Derived IBS |
BMC aBMD |
-- | |
| 1/3 Distal Radius | AP Width BMAD Derived Z |
BMC aBMD |
Derived cortical width Derived cortical CSA Derived endocortical diameter |
|||||
| Study | Cohort | Method | 2D/3D | Site | Total Outcomes | Compartmental Outcomes | ||
| Laing9 2005 | LONGITUDINAL STUDY: TI Girls (4–8 yrs, +2yrs) GYM n= 65 (baseline μ=6.0 yrs) NON n= 78 (baseline μ=6.3 yrs) |
DXA Semi-Annual (0,6,12,18,24 months) |
2D | Total Body | Projected Area BMC aBMD |
-- | ||
| Lumbar Spine | ||||||||
| Total Hip | ||||||||
| Forearm | ||||||||
| Nanyan23 2005 | TI Girls GYM/judo n=17 (age μ= 10.6yrs) NON n=32 (age μ= 9.8 yrs) |
Digital radiogram | 2D | 1/3 Radius | -- | Medial & Lateral Cortical widths | ||
| DXA | 2D | 1/3 Radius | BMC aBMD (forearm only) lean mass |
-- | ||||
| Forearm | ||||||||
TI= Tanner Stage I, TII= Tanner Stage II, TV= Tanner Stage V, GYM= gymnasts, NON= non-gymnasts, μ= mean, yrs= years, DXA= dual energy X-ray absorptiometry, pQCT= peripheral quantitative computed tomography, 2D= 2-dimensional, 3D= 3-dimensional, AP= antero-posterior, BMAD= bone mineral apparent density, CSA= cross-sectional area, vBMD= volumetric bone mineral density, aBMD= areal bone mineral density, SSI= strength-strain index, BMC= bone mineral content, M= male, F= female, M+F= pooled sample of males and females, Area= bone projected area, IBS= index of structural strength in axial compression, Z= section modulus
At the radial metaphysis, significant enlargement of periosteal dimensions (10–12% advantage)25–26 and significant elevation of total vBMD (11–20% advantage)25–26,27 and trabecular vBMD (21–27% advantage)26,27 have been reported in immature gymnasts relative to non-gymnasts (Figure 2a). These advantages in bone geometry and density appear to confer significant benefits in indices of skeletal strength (41–56% IBS advantage)(Figure 2a).25 One study reported a significant advantage in metaphyseal cortical vBMD;26 however, this result may be unreliable due to the probable influence of partial volume effects at this site.48
At the radial diaphysis (Figure 2b), studies have reported significant enlargement of periosteal dimensions (7–12%),25,27 and cortical CSA (8–23%)25,27 in Tanner I/II gymnasts relative to non-gymnasts. In contrast, cortical thickness results are mixed, with reports of both significant gymnast advantages (+10 to +16%, DXA-derivation, digital radiogram)25,23 and no thickness differential (−2.7%, ns, pQCT)27. Gymnast advantages in diaphyseal bone geometry confer advantages in indices of skeletal strength (14% SSI, 24–38% Z).27,25 Associated advantages in muscle CSA (Ward et al.)27 and forearm lean mass (Nanyan et al.),23 suggest a link between muscular and skeletal parameters at the radial diaphysis.
Laing et al. (2005) presented longitudinal evidence of geometric adaptation to pre-pubertal gymnastic loading at the forearm, but did not present the magnitude of gymnast advantages. They assessed skeletal changes semi-annually over two years, following initiation of training in gymnasts, compared to non-gymnasts.9 Groups were initially matched for age, but gymnasts were significantly shorter, lighter, leaner and had lower unadjusted BMC and bone area at all measured sites (baseline and Year 2).9 Nonetheless, compared to non-gymnasts, gymnasts exhibited larger two-year increases in forearm bone area. Furthermore, forearm area increased more in high- than low-level gymnasts (distinguished by frequency and difficulty of maneuvers), suggesting that bone geometry varies with loading dose (p<0.01).9 It is possible that forearm area advantages were a function of increasing bone length (not reported), as area was not adjusted for this variable. In addition, bone growth velocity is not expected to decrease until after menarche, but decreasing velocity was artificially imposed upon all subject growth curves. The effect of this procedure on data interpretation is uncertain. Nonetheless, gymnastics initiation was associated with accelerated growth in forearm area, indicating skeletal adaptation to gymnastic loading that is not attributable to pre-existing characteristics.
Upper Extremity Adaptations Observed during Peri-menarcheal Growth (Table 2b)
Table 2b. Gymnastic Loading Studies: Mixed maturity phases, Post-menarche and Adulthood.
This table summarizes cohort composition, skeletal assessment methods, sites and outcomes for published studies, examining geometric and strength indices associated with gymnastic loading.
| Study | Cohort | Scan | 2D/3D | Site | Total Outcomes | Compartmental Outcomes | |
|---|---|---|---|---|---|---|---|
| Faulkner11 2003 | TI-TV Girls GYM n= 30 (age μ= 11.7 yrs) NON n= 30 (age μ= 11.5 yrs) |
DXA HSA | 2D | Femoral Narrow Neck | BMC AP width Derived CSMI Derived Z |
aBMD | Derived cortical CSA Derived endocortical diameter |
| Proximal Femur Shaft | BMC AP width Derived CSMI Derived Z Derived Z/bone length |
aBMD | Derived cortical CSA Derived endocortical diameter |
||||
| Lehtonen-Veromaa59,60 2000 (a & b) | TI-TV Girls (9–16 yrs baseline, +1yr) GYM n= 66 NON n= 60 |
DXA | 2D | Lumbar Spine Femoral Neck |
BMC (adjusted for Area) aBMD Area (discussed) |
||
| Laing6 2002 | Peri-/Pubertal Girls (baseline range 8–13 yrs) (baseline μ=10.7yrs+ 3) GYM n= 7 NON n=10 |
DXA | 2D | Total Body Lumbar Spine Femoral Neck Total Hip Trochanter Forearm (2 yr report) |
aBMD BMC Area |
||
| Scerpella13 In press | Peri-menarcheal Girls (~1.6yr pre to ~1.6yr post) GYM n= 9 (pre age μ= 12.0yrs) (post age μ= 15.1yrs) EX n= 8 (pre age μ= 11.8yrs) (post age μ= 15.1yrs) NON n=13 (pre age μ= 11.6yrs) (post age μ= 14.7yrs) |
DXA | 2D | Forearm | aBMD BMC Area |
-- | |
| Dowthwaite28 2007 | Post-menarcheal Girls 4% (33%) GYM n= 20 (17) (age μ= 16.7 yrs) NON n= 14 (13) (age μ= 16.3 yrs) |
pQCT | 3D | 4% Distal Radius (Physeal Reference) | Total CSA Total vBMD SSI (IBS, unpublished) |
Trabecular CSA Trabecular vBMD |
|
| 33% Distal Radius | Total CSA SSI |
Cortical CSA Endocortical CSA |
|||||
| Dowthwaite29 2009 | Post-menarcheal Girls (Gyn Age 2.3–4.7 yrs) 4% & DXA (33%) GYM n= 19 (17) (age μ= 16.7 yrs) NON n= 14 (13) (age μ= 16.2 yrs) |
pQCT | 3D | 4% Distal Radius (Physeal Reference) | Total CSA IBS |
||
| 33% Distal Radius | SSI | Cortical CSA | |||||
| DXA | 2D | Total Arm | BMC | ||||
| Liang24 2005 | Post-menarcheal Women GYM n= 8 (age μ= 20 yrs) NON n= 16 (age μ= 22 yrs) |
SPA | 2D | ~15% Distal Ulna | AP width | ||
| MRTA | --- | 50% (Mid) Ulna 50% (Mid) Tibia |
EI | ||||
| Modlesky57 2008 | Post-menarcheal Women GYM n= 8 (age μ= 19.9 yrs) (menarche age μ= 15.0yrs) NON n= 8 (age μ= 21.1 yrs) (menarche age μ=12.8yrs) |
hrMRI | 3D | Proximal tibia | Trabecular BV/TV Trabecular number Trabecular thickness Trabecular separation |
||
| DXA | 2D | Proximal tibia Total Body Non-dominant arm Non-dominant leg |
BMC aBMD Area |
||||
| Eser22 In press | Post-menarcheal Women EX-GYM n=30 (age μ= 23.0 yrs; 18–36) (Post-quit μ= 6.1 yrs; 3–18) (menarche age μ= 14.7yrs) NON n= 30 (age μ= 25.1 yrs; 18–44) (menarche age μ= 11.8yrs) |
pQCT | 3D | 4% Distal Radius 4% Distal Femur 4% Distal Tibia (Articular References) |
BMC Total CSA Total vBMD Trabecular vBMD |
||
| 66% Distal Radius 25% Distal Humerus 66% Distal Tibia 25% Distal Femur |
Total CSA Cortical CSA Endocortical CSA SSI |
BMC Cortical vBMD Cortical Wall Thickness |
|||||
TI= Tanner Stage I, TII= Tanner Stage II, TV= Tanner Stage V, GYM= gymnasts, NON= non-gymnasts, μ= mean, yrs= years, DXA= dual energy X-ray absorptiometry, pQCT= peripheral quantitative computed tomography, 2D= 2-dimensional, 3D= 3-dimensional, AP= antero-posterior, BMAD= bone mineral apparent density, CSA= cross-sectional area, vBMD= volumetric bone mineral density, aBMD= areal bone mineral density, SSI= strength-strain index, BMC= bone mineral content, M= male, F= female, M+F= pooled sample of males and females, Area= bone projected area, IBS= index of structural strength in axial compression, Z= section modulus, CSMI= cross-sectional moment of inertia, pre= pre-menarche measurement, post= post-menarche measurement, SPA= single photon absorptiometry, MRTA= mechanical response tissue analysis
Two groups have evaluated DXA forearm area in gymnasts and non-gymnasts during puberty. Laing et al (2002, different cohort from Laing 2005) noted a 7.5% non-significant gymnast advantage in radius area; unfortunately, forearm area was not evaluated longitudinally in this 3-year study.6 In a different longitudinal analysis, our group compared forearm bone parameters in gymnasts, ex-gymnasts and non-gymnasts over 2–3 years of peri-menarcheal growth. Repeated measures ANOVA evaluated output from forearm DXA scans performed approximately one year pre-menarche and two years post-menarche, entering post-menarcheal gynecological age, height and lean mass as covariates.12–13 Extremely large effect sizes (Cohen’s d) indicated meaningful advantages in forearm area for gymnasts over non-gymnasts at both time points (10–16%).12–13 Advantages were maintained by near parallel rates of peri-menarcheal growth for gymnasts and non-gymnasts, suggesting that geometric benefits were accrued during earlier growth and maintained during peri-menarcheal loading. Similarly, comparisons of ex-gymnasts (who quit gymnastics before menarche) vs. non-gymnasts suggest that geometric benefits of gymnastic loading were maintained for at least two years after training cessation (+7% to +10%). Furthermore, these results indicate that childhood gymnastic loading is associated with periosteal expansion at the forearm, with maintenance of this benefit beyond menarche.
Upper Extremity Adaptations Observed after Menarche (Table 2b)
Several studies have evaluated post-menarcheal skeletal characteristics, comparing girls exposed to gymnastic loading during growth (gymnasts and/or ex-gymnasts) versus non-gymnasts.22,24,28–29 Gymnast populations differed slightly, as Liang evaluated current gymnasts (adult),24 our group evaluated a mixture of current and ex-gymnasts (late-adolescent),28–29 and Eser studied a broad age range of ex-gymnasts who ceased elite level gymnastics 3–18 years prior22. In the forearm, diaphyseal measurements were made using pQCT at the 66% site by Eser and the 33% site by our group; both protocols assessed the 4% metaphysis.22,28–29 Eser et al. also assessed diaphyseal properties at the 25% distal humerus; Eser’s analyses were not adjusted for gynecological age or body size at any site, despite significant group differences in age at menarche and wide age variation.22 Our group used ANCOVA to adjust for gynecological age (years post-menarche) and height at both sites.28 Liang assessed distal ulna width and mid-ulna bending stiffness with Mechanical Response Tissue Analysis (MRTA), adjusting bending stiffness for body weight.24 As these studies were performed in post-menarcheal females, advantages may not be attributed to loading during a specific phase of development; loading benefits may have accumulated over multiple maturational phases.
At the 4% radial metaphysis (Figure 2a), ex-gymnasts and gymnasts exhibited large advantages in periosteal geometry22,28 and skeletal strength indices,28 with evidence of elevated trabecular vBMD22,28. Our work also demonstrated gymnast advantages in trabecular CSA (+20%, large effect size) and cortical CSA (+27%, large effect size), indicating global expansion of skeletal geometry.28 Neither study provided strong evidence for a total vBMD gymnast advantage,22,28 suggesting that geometric expansion may limit vBMD. Evidence for a trabecular vBMD advantage was strong in the younger cohort of active and ex-gymnasts (+18%, large effect size),28 whereas the older cohort of elite ex-gymnasts demonstrated only a strong trend (+9%, p=0.056)22. Comparison of these results may suggest greater long-term retention of geometric adaptations than vBMD advantages. On the whole, results from both cohorts suggest that gymnast metaphyses adapt via a combination of moderate global enlargement of bone geometry (periosteal, cortical and trabecular compartments) coupled with moderate elevation of trabecular density.
At forearm diaphyseal sites, gymnasts demonstrated large, significant advantages for periosteal geometry (~30%) and skeletal strength indices (36–58%) (Figure 2b).22,24,28 Of particular note, endocortical CSA was highly variable and, on average, more than 50% higher among gymnasts and ex-gymnasts versus non-gymnasts.22,28 Cortical CSA advantages were larger and more pronounced in late adolescent current/ex-gymnasts (33% site)28 than in older ex-gymnasts (66% site)22. In contrast, gymnast advantages in cortical thickness22 and cortical vBMD22,28 have not been detected at the radial diaphysis. In fact, cortical vBMD was lower in gymnasts than non-gymnasts at the 66% radius (−2.6%, p<0.05).22 Overall, radial diaphyseal skeletal strength appears to be a function of geometric adaptation (coupled periosteal and endocortical compartment expansion).
Eser et al. also assessed the 25% distal humerus, where the pattern of diaphyseal adaptation differed from that observed at the radius. In contrast to the radius,22,28 humerus endocortical CSA was not larger in gymnasts vs. non-gymnasts (+3.9%, ns)22. In the absence of endocortical expansion, moderate periosteal enlargement (+20%, p<0.05) yielded significant advantages in humeral cortical thickness and CSA (+15%, +24%, respectively).22 As a result, in gymnasts, humeral advantages in theoretical skeletal strength (+38%, p<0.05) were more closely proportioned to BMC advantages (+24%, p<0.05). Differences in humeral cortical vBMD were not detected (−0.01%, ns).22
Upper Extremity Muscle-Bone Relationship
Strong relationships between upper extremity muscular parameters and skeletal indices have been reported in gymnasts and non-gymnasts of all age groups,22,23,27,29 indicating an influential muscle-bone unit. Thirty to sixty percent of variation in bone outcomes was explained by muscular parameters in two studies evaluating post-menarcheal ex/gymnasts and non-gymnasts.22,29 However, both reports also suggested that “non-muscular” aspects of gymnastic loading may play an independent role in skeletal adaptation. In one study, gymnasts exhibited higher BMC than was expected from muscle CSA22; in the other study, after accounting for gynecological age, height and either muscle CSA or arm FFM, gymnastic exposure still explained 25–61% of the variation in bone outcomes.29 Further evidence of potential “non-muscular” aspects of gymnastic loading is provided by Liang et al., who reported no correlation between ulnar bending strength and indices of muscular strength.24
In a site-specific example of the muscle-bone unit, Nanyan et al. reported cortical ring asymmetry at the distal radius.23 They hypothesized that the action of muscle attachments on the ulnar aspect of the radius may generate significant gymnast advantages in ulnar side cortical thickness, but not radial side thickness.23 Even in the absence of muscular loading, radial asymmetry may be stimulated by compressive loads applied at the hand which are transmitted along the ulnar aspect of the radius via the interosseous ligament55, suggesting a potential mode of non-muscular adaptation at this site. Both types of stimuli may be influential in skeletal adaptation to gymnastic loading.
Upper Extremity Adaptations- Summary
Upper extremity adaptations to gymnastic loading are site-specific. The radial diaphysis appears to adapt via expansion of the periosteal, cortical and endocortical (intramedullary) compartments, with no advantage in cortical vBMD.22,27,28 This geometric expansion produces large advantages in skeletal strength (~15–40%) which are underestimated by gross indices of bone mass (aBMD, BMC).24–25,29 At the radial diaphysis, distribution of the cortical ring over a wider area limits or even prevents cortical width advantages in both pre- and post-menarcheal gymnasts. In contrast, at the distal humerus diaphysis, the cortex is expanded primarily through periosteal apposition, with little endocortical expansion, increasing both cortical CSA and thickness. Endocortical CSA appears to be highly variable at all maturity levels, suggesting that strategies of endocortical expansion versus contraction may be genetically determined mechanisms for bone strength adaptation.
Although no published reports delineate skeletal changes across maturation, available studies at the radial diaphysis represent a range of maturity phases. Inter-study comparisons suggest maturity-specific adaptation at this site. Childhood adaptation yields diaphyseal advantages of approximately 10% for periosteal CSA and 20% for cortical CSA. During peri-menarcheal growth, continued gymnastic loading may accelerate endocortical resorption and periosteal expansion, as advantages in periosteal and endocortical dimensions appear greater in post-menarcheal gymnasts than in their pre-menarcheal counterparts (approximately 30% vs. 10%, periosteal; 60% vs. 10%, endocortical).12,13,22,24–25,27–28 In ex-gymnasts, upper extremity advantages in bone geometry and indices of skeletal strength appear to be maintained long-term, at least two years after activity cessation and into adulthood.12,13,22
Gymnast adaptations at the radial metaphysis are closely tied to gross indices of bone mass, as this site exhibits advantages in both skeletal geometry and density. Periosteal and trabecular CSA are larger in post-menarcheal gymnasts, yielding greater geometric advantages than have been observed in childhood and early puberty (~20% vs. 5–10%) (Figure 2a). In contrast, post-menarcheal gymnast advantages in metaphyseal vBMD (total and trabecular) and indices of bone strength are not greater than Tanner I/II gymnast advantages. This phenomenon may be a consequence of geometric expansion. In the face of limited mineral resources, geometric expansion would occur at the expense of vBMD, limiting relative gains in axial compressive strength. Although calcium supplementation to augment mineral resources might be expected to yield further increases in vBMD and indices of skeletal strength, the results of Ward et al. suggest that calcium supplementation does not enhance bone accrual in pre- and early pubertal gymnasts; this has not been studied in later maturity phases.56
Tibial Adaptations Observed in Childhood (Tanner I, pre-puberty)(Table 2a)
Ward’s evaluation of pre-pubertal gymnastic adaptation included assessment of the tibia (Figure 2c,d). Gymnast advantages at this lower extremity site were less notable than radius advantages.27 At the tibial distal metaphysis and proximal diaphysis, there was no significant indication of loading-related periosteal expansion. However, total metaphyseal vBMD was significantly higher in gymnasts than non-gymnasts (+5.7%), with a trend toward higher metaphyseal trabecular vBMD (+4.5%, p=0.11)(Figure 2c).27 Strong trends were also exhibited toward increased diaphyseal cortical CSA, cortical thickness and SSI in gymnasts versus non-gymnasts (Figure 2d, p<0.15),27 apparently via reduced endocortical resorption or increased endocortical apposition. Female gymnasts exhibited no advantage in muscle CSA at the 66% tibia (−1.3%, p=0.73).27 It should be noted that non-gymnasts in this study were quite active, averaging over 6 hours per week of organized physical activity;27 less active non-gymnasts might have provided higher tibial loading contrast for detection of group differences.
Tibial Adaptations Observed after Menarche (Table 2b)
Tibial Metaphysis (Figure 2c)
Two published studies evaluated the tibial metaphyses in adult gymnasts and ex-gymnasts.22,57 One study used hrMRI and DXA to evaluate the proximal metaphysis57 and the other used pQCT to evaluate the distal metaphysis22. Neither study detected a significant geometric advantage (projected area or CSA). However, both identified gymnast advantages in trabecular vBMD (+7%, trabecular vBMD, distal; +16.1%, trabecular bone volume for compartment volume (BV/TV), proximal).22,57 At the proximal metaphysis, this architectural advantage appeared to be a function of higher trabecular number and lower trabecular separation (+7.8% and −13.7%, respectively, p<0.05), with some evidence of thicker trabeculae (+6.9%, NS).57 These studies suggest that adaptation to gymnastic loading at the tibial metaphyses occurs via enhanced trabecular structure rather than enlarged periosteal dimensions. In both studies, menarche occurred earlier in non-gymnasts, indicating lower physical maturity among gymnasts; statistical adjustment for physical maturity might have improved detection of gymnast advantages in bone geometry. Even so, reported gymnast advantages imply that mechanical loading trumps the negative influence of muted estrogen exposure upon trabecular structure. Ex-gymnasts appear to maintain significant long-term benefits in trabecular structure,22 but the observation of greater percent advantages in current gymnasts57 than in ex-gymnasts22 suggests some deterioration in trabecular tissue after loading cessation.
Tibial Diaphysis (Figure 2d)
Tibial diaphyseal assessments were included in Eser’s post-menarcheal pQCT study discussed above (Table 2b).22 Significant ex-gymnast tibial advantages ranged from +7% to +12%.22 The general pattern of tibial diaphyseal adaptation appears similar to that of the humerus; limited periosteal advantages with no significant advantage in cortical vBMD (−0.5%, ns) or endocortical CSA (+5%, ns) yield increased cortical thickness, cortical CSA and diaphyseal strength.22
Liang et al. measured bending stiffness (EI) at the tibial mid-shaft in adult female gymnasts, swimmers and non-athletes using MRTA.24 The reported gymnast advantage for this unusual index of mid-tibial material and bending strength (+228%)24 markedly exceeds advantages observed at the tibia in other studies (different indices, +4% to +16%)22,27,57 and reported advantages at all other sites (+60% or less). It is likely that this disparity is a function of the unique nature of the index itself.
Tibial Adaptations-Summary
On the whole, gymnast advantages are lower at the tibia than at the radius (<10% vs. 10–60%). High background loading of the lower extremities may reduce gymnast/non-gymnast loading differentials, limiting detection of tibial adaptations. In childhood, loading appears to enhance tibial axial compressive strength via increased trabecular and total volumetric density, whereas significant advantages in periosteal dimensions have not been identified. Adult gymnasts and ex-gymnasts provide stronger evidence of tibial adaptation, with elevated indices of diaphyseal bending stiffness and strength, complemented by enhanced metaphyseal trabecular structure and density. In adult gymnasts, non-significant childhood patterns are amplified; cortical thickening is emphasized over periosteal and endocortical expansion, yielding significant advantages in cortical dimensions and indices of skeletal strength in proportion with BMC advantages. However, in contrast to childhood trends of endocortical contraction (−4%, ns), trends in adult ex-gymnasts suggest endocortical expansion during puberty (+4%, ns). Overall, continued high level loading from childhood through puberty may be necessary for development of significant advantages at the tibia.
Spine and Femur (Figures 3a–c, Tables 2a–b)
Figure 3.
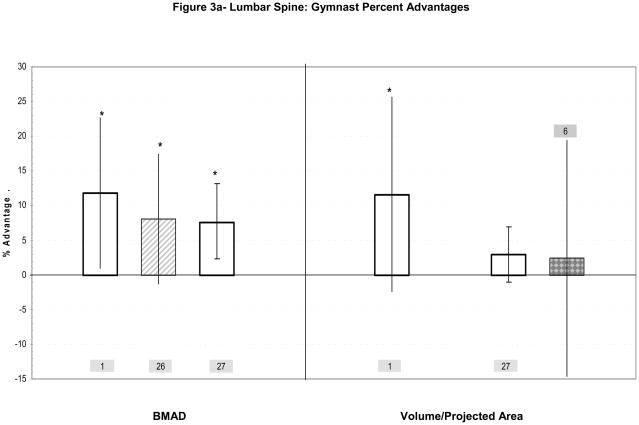
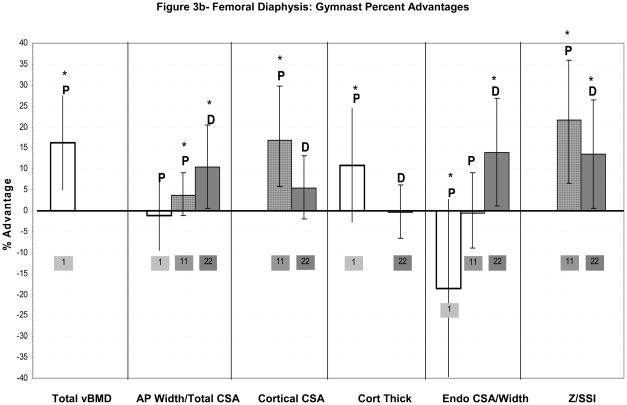
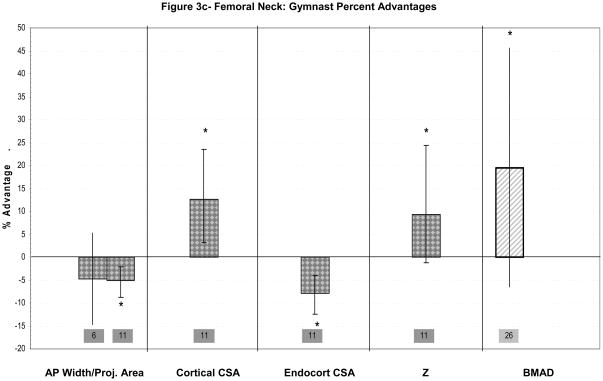
Figure 3a–c: Percent advantages for gymnasts versus non-gymnasts are presented for comparable bone sites and outcomes, as follows: (a) Lumbar spine; (b) Femoral diaphysis; (c) Femoral neck. Error bars indicate 95% Confidence Intervals for the gymnast percentage advantage. Error bars with horizontal borders represent confidence intervals as reported in the source publication, whereas unbordered error bars indicate confidence intervals derived from reported standard deviations or standard errors of the means. Columns are color-coded by maturity phase: white= Tanner I; white/light gray stripe= Tanner I/II mix; gray diamonds= Tanner I-V; dark gray= post-menarcheal/adult. Study reference numbers are presented for each column. Asterisks indicate reported statistically significant differences. Where proximal and distal site locations yield contrasting results, P= proximal; D= distal.
The lumbar spine and femur serve major weight-bearing roles during daily life activities. Because the lumbar spine and proximal femur contribute strongly to rates of osteoporotic fracture and resultant morbidity and mortality, loading-related enhancement of these sites during childhood and adolescent growth may be particularly important. Compared to non-athletes, several groups of non-gymnast athletes have demonstrated increased densitometric and geometric parameters at these locations.14,58 To date, most reports have focused on aBMD or BMAD; evaluations of geometric adaptation at these sites are quite sparse.
Lumbar Spine Adaptations Observed in Childhood and Early Puberty (Tanner I, Tanner II)
Bass et al. compared bone-age matched pre-pubertal gymnasts vs. non-gymnasts (Table 2a; Figure 3a).1 Gymnast advantages were demonstrated for spine aBMD, lumbar spine vertebral volumes and lumbar spine BMAD (p<0.05).1 Vertebral diameter was not specifically reported, but greater lumbar vertebral volume combined with shorter sitting height suggests expanded lumbar vertebral diameter in gymnasts. Over 12 months of follow-up, sitting height and vertebral volume increased less in gymnasts than non-gymnasts, but bone mass increased more, yielding a significant increase in vertebral BMAD in gymnasts, not non-gymnasts.1 As lumbar width was not reported at follow-up, geometric growth could not be assessed. Adjustment for body size differences may have yielded greater gymnast advantages, as gymnasts exhibited lower mean height, sitting height and fat mass (p<0.05).1
Lumbar spine advantages in pre-pubertal gymnasts are corroborated by two other studies (Figure 3a). Dyson et al. detected significant lumbar spine aBMD and BMAD advantages in female gymnasts.26 Ward et al. reported a non-significant elevation in lumbar spine projected area (+2.9% p=0.15) and significant advantages in BMC, aBMD and BMAD in pre-pubertal male and female gymnasts vs. non-gymnasts.27 Although further study is necessary to detail loading-related adaptations at the lumbar spine, these studies suggest a role for adaptation in both volumetric density and geometry,1,26,27 theoretically increasing vertebral resistance to axial compression.
Lumbar Spine Adaptations Observed during Puberty (Tanner I-V)
Two groups evaluated lumbar spine adaptation to gymnastic activity during puberty.6,59–60 In a cross-sectional study, one group used DXA to compare gymnasts and non-athletes at different stages of physical maturity, reporting only qualitative differences.59 Significant gymnast advantages were observed for lumbar spine aBMD and BMC adjusted for area (statistical adjustment, distinct from aBMD), but not projected area.59 This group also performed one-year longitudinal aBMD analyses, adjusting for age, height, Tanner stage, baseline aBMD, and 1-yr height and weight growth rates; no significant differences were detected for aBMD accrual or growth in projected area.60 The second group evaluated bone area changes in adolescent gymnasts and non-gymnasts over a three-year period.6 Subjects were matched for age and body size, exhibiting similar pubertal status and maturation rates (mean skeletal age, Tanner stage). Bone area increased at parallel rates in gymnasts and non-gymnasts for the total body and lumbar spine, with no significant advantage in unadjusted lumbar spine bone area at “baseline”(Figure 3a).6 The limited evidence provided by these studies suggests elevated vBMD accrual but no enhancement of bone geometric growth at the lumbar spine during peri-pubertal gymnastic loading.
Femoral Adaptations Observed in Childhood and Early Puberty (Tanner I, Tanner II)
There are only two studies evaluating gymnastic adaptation at the femur during these maturity phases. Neither study reports a gymnast advantage in pre-pubertal periosteal dimensions at the femur. At the femoral neck, Dyson et al. reported a significant gymnast advantage in femoral neck BMAD (Tanner I/II females)(Figure 3c), but did not report geometric indices.26 Bass et al. evaluated bone geometry at the mid-femur diaphysis (Figure 3b).1 Gymnasts exhibited lower mean endocortical diameter and higher mean bi-cortical width, yielding increased femoral shaft vBMD compared to non-gymnasts (p<0.05)(Figure 3b).1 Mid-shaft femoral periosteal diameters were not significantly different.1 As femoral shaft analyses were not adjusted for differences in height, weight or bone length, it is unclear whether gymnasts’ periosteal and endosteal dimensions were low or high for body size. Bass concluded that decreased endosteal resorption or increased endocortical apposition produced cortical thickening in response to gymnastic loading.1 Leg aBMD and 1-year growth rate in leg aBMD were significantly higher in gymnasts than non-gymnasts, suggesting a loading response.1 Bone compartment dimensions were not compared at follow-up, so geometric and vBMD growth cannot be assessed.
Proximal Femur Adaptation Observed during Puberty (Tanner I-V)
Two groups evaluated standard DXA parameters at the proximal femur in pubertal females.6,59–60 In their cross-sectional study, Lehtonen-Veromaa identified significant gymnast advantages for femoral neck aBMD and for femoral neck BMC adjusted for area, but not for area.59 In their longitudinal analysis, aBMD accrual at the femoral neck and greater trochanter was significantly higher for gymnasts than non-athletes over one year of growth.60 No significant differences were observed for growth in proximal femur projected area.60 Similarly, over three years, Laing et al. (2002) reported parallel rates of increase in bone area for gymnasts and non-gymnasts at the femoral neck and total hip.6 Furthermore, at these sites and the greater trochanter, gymnasts exhibited no significant advantages in unadjusted bone area at “baseline” (Figure 3c).6 Results from these analyses suggest minimal geometric adaptation to continued gymnastic loading at the proximal femur during pubertal development.
Faulkner et al. applied DXA hip structural analysis to evaluate bone geometric and theoretical strength parameters at the femoral narrow neck and proximal shaft (2 cm distal to lesser trochanter).11 At the narrow neck (Figure 3c), gymnasts exhibited significantly lower sub-periosteal width and endosteal diameter than non-gymnasts, but higher cortical CSA, BMC and Z (p<0.05) (Figure 3a).11 At the proximal femoral shaft (Figure 3b), gymnasts demonstrated significantly higher bone geometric and strength indices, except for endosteal diameter (Figure 3b). Although the authors reported equal numbers of pre-pubertal girls in each activity group, non-gymnasts were overrepresented in advanced pubertal stages (Tanner pubic stages IV/V: non-gym=40% vs. gym=17%).11 Thus, between-group maturational variability may have confounded analyses, underestimating gymnasts’ geometric and densitometric advantages; this effect may have been partially mitigated by adjustment for height and weight. On the whole, gymnasts’ proximal femur cortical bone geometry and indices of skeletal strength were enhanced for their physical maturity and body size, highlighting the association between cortical thickening and mechanical loading.
Distal Femur Adaptations Observed in Adults (Post-menarcheal Ex-gymnasts)(Figure 3b)
In their evaluation of adult ex-gymnasts at the 4% distal femoral metaphysis, Eser et al. reported non-significant trends toward ex-gymnast advantages in BMC (+7%, p=0.07), total vBMD (+4%, p=0.08) and trabecular vBMD (+5%, p=0.07), with little evidence of periosteal expansion in response to loading (total CSA, +2%, ns). The absence of significant ex-gymnast advantages at the distal femur metaphysis may indicate loss of benefit after activity cessation or insufficient contrast between gymnastic loading and non-gymnast background loading.
At the 25% distal femoral diaphysis, geometric adaptation dominated, echoing radial diaphyseal patterns and contrasting with femoral metaphyseal patterns. Despite a strong trend toward lower cortical vBMD (−1%, p<0.06) and no advantage in cortical thickness (−0.3%, ns), ex-gymnasts demonstrated significant advantages in total CSA (+10%) and endocortical CSA (+14%), yielding elevated diaphyseal bending strength (SSI, +14%, p<0.05). Compared to the radial shaft, distal femoral diaphysis cortical CSA advantages were muted (+6%, p<0.20) and geometric and strength advantages were more proportional to the BMC advantage (+11%, p<0.05). On the whole, endocortical and periosteal expansion appear to be the predominant modes of gymnastic adaptation at the distal femoral diaphysis.
Lumbar Spine and Femur Summary
In summary, results are limited and disparate for lumbar spine and femur geometric adaptation. Lumbar spine loading appears to increase vertebral volumetric density in children and adolescent girls (+8 to +12%); limited evidence suggests that axial compressive strength may also be increased through vertebral body geometric expansion (2–12%). Proximal femur adaptation includes cortical thickening, with increases in BMAD (15–20%) and aBMD. These cortical thickness/CSA advantages (12–15%) appear to result from endocortical contraction, with little or no enlargement of proximal femur periosteal dimensions. In contrast, distal femur diaphyseal adaptation may rely primarily upon periosteal expansion with low endocortical resorption to maximize advantages in skeletal bending strength (10–20%). Discrepancies in observed patterns of skeletal adaptation at the proximal femur in immature active gymnasts1,11 versus the distal femur in adult ex-gymnasts22 may indicate site-specific variation, maturity-specific variation or deterioration of benefits after gymnastic cessation.
At the femur and lumbar spine, significant gymnast advantages are fewer and of lower magnitude (absolute value=4% to 24%) than upper extremity advantages (absolute value=7% to 58%). This observation supports the view that upper extremity sites provide greater sensitivity to evaluate loading adaptation. Use of improved methodology (supine lateral lumbar spine DXA, DXA HSA, MRI, QCT) is necessary to delineate tissue-specific adaptations to loading of the vertebral bodies and femur. Additional longitudinal studies with well-matched non-gymnast “controls” and statistical adjustment for body size will generate more definitive information regarding geometric adaptation to gymnastic loading during growth.
Conclusion
Skeletal adaptation to gymnastic loading during growth appears to be sex-, maturity-, site- and bone-tissue specific, with upper extremities providing the most sensitive regions of interest for skeletal evaluation. Furthermore, there is accumulating evidence of benefit persistence after activity cessation and into adulthood.
Detailed evaluations of the tibia and forearm support the concept that skeletal adaptations to gymnastic loading are bone tissue- and site-specific. Metaphyseal sites are comprised of a large proportion of cancellous bone and adapt predominantly by increasing bone volume per trabecular compartment volume (~10% greater vBMD), likely through increased trabecular number and size. Gymnast advantages in periosteal dimensions are not significant at the proximal or distal tibial metaphyses. However, at the distal radial metaphysis, moderate geometric expansion (10–20%) contributes to increased skeletal resistance to bending, torsion and axial compression in gymnasts. Similarly, concomitant geometric expansion and metaphyseal vBMD elevation have been observed in racquet sport players and weight-lifters,61–63 although adaptations are generally more striking in gymnasts.
In contrast to metaphyseal sites, adaptation to gymnastic loading of the radial and tibial diaphyses is dominated by geometric enlargement. In gymnasts, expanded cortical, endocortical and periosteal dimensions (10–20%, 10–60%, 10–30%, respectively) yield greater indices of skeletal strength (20–60%) at these predominantly cortical sites. These gymnast advantages parallel those associated with other loading modalities, where enlarged cortical CSA improves bending and torsional strength,61–62,64–67 particularly when expansion occurs around a widened intramedullary cavity.65,67–69 In gymnasts, comparisons of different regions of interest within radial, humeral, tibial and femoral diaphyses suggest that the mode of diaphyseal adaptation (endocortical expansion vs. contraction) may be a function of skeletal site, varying from bone to bone and within a single bone. High variability in diaphyseal endocortical dimensions also suggests the potential for genetic influence. Finally, site-specificity in adaptive patterns for periosteal CSA, endocortical CSA, cortical CSA and cortical thickness may generate variable observations in diaphyseal total vBMD, highlighting the importance of reporting these geometric outcomes.
Limited evidence for lumbar spine adaptation to axial compressive loading includes potential expansion in vertebral width (2–12%) and observed increases and advantages in vBMD (~10%). Similarly, at the proximal femur (neck and shaft), gymnast advantages in both cortical bone geometry (12–15%) and total vBMD (15–20%) have been identified, theoretically improving resistance to bending (10–20%) and axial compression (not assessed). Gymnast advantages in proximal femoral cortical dimensions likely result from endocortical contraction, with limited evidence of femoral shaft periosteal expansion during puberty and no reports of femoral neck periosteal expansion at any maturity level. This enlargement of proximal femoral cortical dimensions corroborates loading adaptations observed in runners and high- and odd-impact athletes.64,58 In contrast, at the distal femoral shaft, simultaneous periosteal and endocortical compartment expansion, without enlargement of cortical CSA or thickness, appears to yield improvements in skeletal strength that persist after activity cessation.
Although awareness and understanding of geometric responses to skeletal loading have increased dramatically in recent years, much additional work remains. Further research should elucidate skeletal loading dose-response curves and the sex- and maturity-dependence of skeletal adaptation, detailing the micro- and macro-architectural characteristics of anatomical sites with varied tissue compositions. These research goals will be accomplished through application of modern techniques to evaluate bone geometric adaptation across childhood and adolescence, evaluating maintenance of benefits into adulthood.
Acknowledgments
We are grateful to Dr. Frank Rauch for his invitation to present this review. We would like to thank Dr. Kate Ward, as well as Professors C.J. Blimkie and R.A. Faulkner for their prompt responses to our queries and for their intellectual generosity, assisting our efforts to interpret their work. Funding for this work was provided by the National Institute of Arthritis, Musculoskeletal and Skin Diseases.
References
- 1.Bass S, Pearce G, Bradney M, Hendrich E, Delmas PD, Harding A, Seeman E. Exercise before puberty may confer residual benefits in bone density in adulthood: Studies in active prepubertal and retired female gymnasts. J Bone Miner Res. 1998;13(3):500–7. doi: 10.1359/jbmr.1998.13.3.500. [DOI] [PubMed] [Google Scholar]
- 2.Cadogan J, Blumsohn A, Barker ME, Eastell R. A longitudinal study of bone gain in pubertal girls: Anthropometric and biochemical correlates. J Bone Miner Res. 1998;13:1602–1612. doi: 10.1359/jbmr.1998.13.10.1602. [DOI] [PubMed] [Google Scholar]
- 3.Courteix D, Lespessailles E, Jaffre C, Obert P, Benhamou CL. Bone mineral acquisition and somatic development in highly trained girl gymnasts. Acta Paediatr. 1999;88:803–808. doi: 10.1080/08035259950168694. [DOI] [PubMed] [Google Scholar]
- 4.Nickols-Richardson SM, O’Connor PJ, Shapses SA, Lewis RD. Longitudinal bone mineral density changes in female child artistic gymnasts. J Bone Miner Res. 1999;14(6):994–1002. doi: 10.1359/jbmr.1999.14.6.994. [DOI] [PubMed] [Google Scholar]
- 5.Gero N, Cole J, Kanaley J, van der Meulen M, Scerpella TA. Increased bone accrual in premenarcheal gymnasts: A longitudinal study. Ped Ex Sci. 2005;17:43–45. [Google Scholar]
- 6.Laing EM, Massoni JA, Nickols-Richardson SM, Modlesky CM, O’Connor PJ, Lewis RD. A prospective study of bone mass and body composition in female adolescent gymnasts. J Pediatr. 2002;141:211–216. doi: 10.1067/mpd.2002.126599. [DOI] [PubMed] [Google Scholar]
- 7.Nurmi-Lawton JA, Baxter-Jones AD, Mirwald RL, Bishop JA, Taylor P, Cooper C, New SA. Evidence of sustained skeletal benefits from impact-loading exercise in young females: A 3-year longitudinal study. J Bone Miner Res. 2004;19(2):314–22. doi: 10.1359/JBMR.0301222. [DOI] [PubMed] [Google Scholar]
- 8.Courteix D, Lespessailles E, Loiseau-Peres S, Obert P, Ferry B, Benhamou C-L. Lean tissue mass is a better predictor of bone mineral content and density than body weight in prepubertal girls. Rev Rhum (Engl Ed) 1998;65(5):328–336. [PubMed] [Google Scholar]
- 9.Laing EM, Wilson AR, Modlesky CM, O’Connor PJ, Hall DB, Lewis RD. Initial years of recreational artistic gymnastics training improves lumbar spine bone mineral accrual in 4- to 8- year-old females. J Bone Miner Res. 2005;20(3):509–19. doi: 10.1359/JBMR.041127. [DOI] [PubMed] [Google Scholar]
- 10.Dowthwaite JN, DiStefano JG, Ploutz-Snyder RJ, Kanaley JA, Scerpella TA. Maturity and activity-related differences in bone mineral density: Tanner I vs. II and gymnasts vs. non-gymnasts. Bone. 2006;39(4):895–900. doi: 10.1016/j.bone.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Faulkner RA, Forwood MR, Beck TJ, Mafukidze JC, Russell K, Wallace W. Strength indices of the proximal femur and shaft in prepubertal female gymnasts. Med Sci Sports Exerc. 2003;35(3):513–518. doi: 10.1249/01.MSS.0000053724.33480.8B. [DOI] [PubMed] [Google Scholar]
- 12.Dowthwaite JN, Ploutz-Snyder RJ, Scerpella TA. Skeletal benefits of pre-menarcheal gymnastic activity are retained after activity cessation: A preliminary longitudinal comparison. Bone. 2007;40(6, Suppl 1):S40. doi: 10.1123/pes.22.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scerpella TA, Dowthwaite JN, Gero NM, Kanaley JA, Ploutz-Snyder RJ. Skeletal Benefits of Pre-menarcheal Gymnastics Are Retained After Activity Cessation. Ped Ex Sci. doi: 10.1123/pes.22.1.21. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fehling PC, Alekel L, Clasey J, Rector A, Stillman RJ. A comparison of bone mineral densities among female athletes in impact loading and active loading sports. Bone. 1995;17(3):205–210. doi: 10.1016/8756-3282(95)00171-9. [DOI] [PubMed] [Google Scholar]
- 15.Taaffe DR, Snow-Harter C, Connolly DA, Robinson TL, Brown MD, Marcus R. Differential effects of swimming versus weight-bearing activity on bone mineral status of eumenorrheic athletes. J Bone Miner Res. 1995;10(4):586–593. doi: 10.1002/jbmr.5650100411. [DOI] [PubMed] [Google Scholar]
- 16.Scerpella TA, Davenport M, Morganti CM, Kanaley JA, Johnson LM. Dose-related association of impact activity and bone mineral density in pre-pubertal girls. Calcif Tissue Int. 2003;72:24–31. doi: 10.1007/s00223-001-1131-x. [DOI] [PubMed] [Google Scholar]
- 17.Snow CM, Williams DP, LaRiviere J, Fuchs RK, Robinson TL. Bone gains and losses follow seasonal training and detraining in gymnasts. Calcif Tissue Int. 2001;69:7–12. doi: 10.1007/s00223-001-0014-5. [DOI] [PubMed] [Google Scholar]
- 18.Kirchner EM, Lewis RD, O’Connor P. Effect of past gymnastics participation on adult bone mass. J Appl Physiol. 1996;80:226–32. doi: 10.1152/jappl.1996.80.1.226. [DOI] [PubMed] [Google Scholar]
- 19.Kudlac J, Nichols DL, Sanborn CF, DiMarco NM. Impact of detraining on bone loss in former collegiate female gymnasts. Calcif Tissue Int. 2004;75:482–487. doi: 10.1007/s00223-004-0228-4. [DOI] [PubMed] [Google Scholar]
- 20.Zanker CL, Osborne C, Cooke CB, Oldroyd B, Truscott JG. Bone density, body composition and menstrual history of sedentary female former gymnasts, aged 20–32 years. Osteoporos Int. 2004;15:145–154. doi: 10.1007/s00198-003-1524-y. [DOI] [PubMed] [Google Scholar]
- 21.Pollock NK, Laing EM, Modlesky CM, O’Connor PJ, Lewis RD. Former college artistic gymnasts maintain higher BMD: a nine-year follow-up. Osteoporos Int. 2006;17:1691–1697. doi: 10.1007/s00198-006-0181-3. [DOI] [PubMed] [Google Scholar]
- 22.Eser P, Hill B, Ducher G, Bass SL. Skeletal benefits after long-term retirement in former elite female gymnasts. J Bone Miner Res. 2009 doi: 10.1359/jbmr.090521. in press. [DOI] [PubMed] [Google Scholar]
- 23.Nanyan P, Prouteau S, Jaffre C, Benhamou L, Courteix D. Thicker radial cortex in physically active prepubertal girls compared to controls. Int J Sports Med. 2005;26:110–115. doi: 10.1055/s-2004-817859. [DOI] [PubMed] [Google Scholar]
- 24.Liang MTC, Arnaud SB, Steele CR, Hatch P, Moreno A. Ulnar and tibial bending stiffness as an index of bone strength in synchronized swimmers and gymnasts. Eur J Appl Physiol. 2005;94:400–407. doi: 10.1007/s00421-005-1351-2. [DOI] [PubMed] [Google Scholar]
- 25.Dowthwaite JN, Flowers PPE, Spadaro JA, Scerpella TA. Bone geometry, density and strength indices of the distal radius reflect loading via childhood gymnastic activity. J Clin Densitom. 2007;10:65–75. doi: 10.1016/j.jocd.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dyson K, Blimkie CJR, Davison KS, Webber CE, Adachi JD. Gymnastic training and bone density in pre-adolescent females. Med Sci Sports Exerc. 1997;29(4):443–450. doi: 10.1097/00005768-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Ward KA, Roberts SA, Adams JE, Mughal MZ. Bone geometry and density in the skeleton of pre-pubertal gymnasts and school children. Bone. 2005;36:1012–1018. doi: 10.1016/j.bone.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Dowthwaite JN, Flowers PPE, Spadaro JA, Hickman RM, Scerpella TA. Artistic gymnastics during growth is linked to high post-menarcheal bone geometry and strength indices: Preliminary pQCT results. Bone. 2007;40(6, Suppl 1):S40–S41. [Google Scholar]
- 29.Dowthwaite JN, Kanaley JA, Spadaro JA, Hickman RM, Scerpella TA. Muscle indices do not fully account for enhanced upper extremity bone mass and strength in gymnasts. J Musculoskelet Neuronal Interact. 2009;9(1):2–14. [PubMed] [Google Scholar]
- 30.Daly RM, Rich PA, Klein R, Bass S. Effects of high-impact exercise on ultrasonic and biochemical indices of skeletal status: A prospective study in young male gymnasts. J Bone Miner Res. 1999;14(7):1222–1230. doi: 10.1359/jbmr.1999.14.7.1222. [DOI] [PubMed] [Google Scholar]
- 31.Bradshaw EJ, Le Rossignol P. Anthropometric and biomechanical field measures of floor and vault ability in 8 to 14 year old talent-selected gymnasts. Sports Biomechanics. 2004;3(2):249–62. doi: 10.1080/14763140408522844. [DOI] [PubMed] [Google Scholar]
- 32.Simpson KJ, Kanter L. Jump distance of dance landings influencing internal joint forces: I. axial forces Med Sci Sports Exerc. 1997;29(7):916–27. doi: 10.1097/00005768-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Harman EA, Rosenstein MT, Frykman PN, Rosenstein RM. The effects of arms and countermovement on vertical jumping. Med Sci Sports Exerc. 1990;22(6):825–33. doi: 10.1249/00005768-199012000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Warden SJ, Hurst JA, Sanders MS, Turner CH, Burr DB, Li J. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J Bone Miner Res. 2005;20:809–816. doi: 10.1359/JBMR.041222. [DOI] [PubMed] [Google Scholar]
- 35.Turner CH, Burr DB. Basic Biomechanical Measurements of Bone: A Tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 36.Magarey AM, Boulton TJC, Chatterton BE, Schultz C, Nordin BEC, Cockington RA. Bone growth from 11 to 17 years: relationship to growth, gender and changes with pubertal status including timing of menarche. Acta Paediatr. 1999:139–46. doi: 10.1080/08035259950170286. [DOI] [PubMed] [Google Scholar]
- 37.Kontulainen SA, Macdonald HM, Khan KM, McKay H. Examining bone surfaces across puberty: A 20-month pQCT trial. J Bone Miner Res. 2005;20(7):1202–1207. doi: 10.1359/JBMR.050214. [DOI] [PubMed] [Google Scholar]
- 38.Macdonald HM, Kontulainen SA, MacKelvie-O’Brien KJ, Petit MA, Janssen P, Khan KM, McKay HM. Maturity- and sex-related changes in tibial bone geometry, strength and bone-muscle strength indices during growth: A 20 month pQCT study. Bone. 2005;36:1003–1011. doi: 10.1016/j.bone.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Macdonald H, Kontulainen S, Petit M, Janssen P, McKay H. Bone strength and its determinants in pre- and early pubertal boys and girls. Bone. 2006;39:598–608. doi: 10.1016/j.bone.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 40.Sundberg M, Gardsell P, Johnell O, Karlsson MK, Ornstein E, Sandstedt B, Sernbo I. Physical activity increases bone size in prepubertal boys and bone mass in prepubertal girls: A combined cross-sectional and 3-year longitudinal study. Calcif Tissue Int. 2002;71(5):406–415. doi: 10.1007/s00223-001-1105-z. [DOI] [PubMed] [Google Scholar]
- 41.Wehrli FW. Structural and functional assessment of trabecular and cortical bone by micro magnetic resonance imaging. J Magn Reson Imaging. 2007;25:390–409. doi: 10.1002/jmri.20807. [DOI] [PubMed] [Google Scholar]
- 42.Schoenau E, Neu CM, Rauch F, Manz F. The development of bone strength at the proximal radius during childhood and adolescence. J Clin Endocrinol Metab. 2001;86(2):613–618. doi: 10.1210/jcem.86.2.7186. [DOI] [PubMed] [Google Scholar]
- 43.Dowthwaite JN, Hickman RM, Kanaley JA, Ploutz-Snyder RJ, Spadaro JA, Scerpella TA. Distal radius strength: A comparison of DXA-derived vs. pQCT-measured parameters in adolescent females. J Clin Densitom. 2009;12(1):42–53. doi: 10.1016/j.jocd.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Pocock NA, Noakes KA, Majerovic Y, Griffiths MR. Magnification error of femoral geometry using fan beam densitometers. Calcif Tissue Int. 1997;60:8–10. doi: 10.1007/s002239900177. [DOI] [PubMed] [Google Scholar]
- 45.Cole JH, Scerpella TA, van der Meulen MCH. Fan-beam densitometry of the growing skeleton: Are we measuring what we think we are? J Clin Densitom. 2005;8:57–64. doi: 10.1385/jcd:8:1:057. [DOI] [PubMed] [Google Scholar]
- 46.Cole JH, Dowthwaite JN, Scerpella TA, van der Meulen MCH. Correcting Fan-Beam Magnification in Clinical Densitometry Scans of Growing Subjects. J Clin Densitom. 2009;12(3):322–329. doi: 10.1016/j.jocd.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee DC, Gilsanz V, Wren TAL. Limitations of peripheral quantitative computed tomography metaphyseal bone density measurements. J Clin Endocrinol Metab. 2007;92(11):4248–4253. doi: 10.1210/jc.2007-0126. [DOI] [PubMed] [Google Scholar]
- 48.Zemel B, Bass S, Binkley T, Ducher G, Macdonald H, McKay H, Moyeur-Mileur L, Shepherd J, Specker B, Ward K, Hans D. Peripheral quantitative tomography in children and adolescents: The 2007 ISCD pediatric official positions. J Clin Densitom. 2008;11(1):59–74. doi: 10.1016/j.jocd.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Rauch F, Neu C, Manz F, Schönau E. The development of metaphyseal cortex-implications for distal radius fractures during growth. J Bone Miner Res. 2001;16:1547–55. doi: 10.1359/jbmr.2001.16.8.1547. [DOI] [PubMed] [Google Scholar]
- 50.Rauch F, Schonau E. Peripheral quantitative computed tomography of the distal radius in young subjects- new reference data and interpretation of results. J Musculoskelet Neuronal Interact. 2005;5(2):119–126. [PubMed] [Google Scholar]
- 51.Sievänen H, Kannus P, Nieminen V, Heinonen A, Oja P, Vuori I. Estimation of various mechanical characteristics of human bones using dual energy x-ray absorptiometry: methodology and precision. Bone. 1996;18:17S–27S. doi: 10.1016/8756-3282(95)00376-2. [DOI] [PubMed] [Google Scholar]
- 52.Carter DR, Bouxsein ML, Marcus R. New approaches to interpreting projected bone densitometry data. J Bone Miner Res. 1992;7(2):137–145. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 53.Beck TJ. Program Copyright 2000. Johns Hopkins University, School of Medicine; Hip Structural Analysis (HSA) Program (BMD and Structural Geometry Methodology) [Google Scholar]
- 54.Palmer AK, Werner FW. Biomechanics of the distal radioulnar joint. Clin Orthop. 1984;187:26–35. [PubMed] [Google Scholar]
- 55.Pfaeffle HJ, Fischer KJ, Manson TT, Tomaino MM, Woo SLY, Herndon JH. Role of the forearm interosseous ligament: Is it more than just longitudinal load transfer? J Hand Surg [Am] 2000;25(4):683–68. doi: 10.1053/jhsu.2000.9416. [DOI] [PubMed] [Google Scholar]
- 56.Ward KA, Roberts SA, Adams JE, Lanham-New S, Mughal MZ. Calcium supplementation and weight bearing physical activity- Do they have a combined effect on the bone density of prepubertal children? Bone. 2007;41:496–504. doi: 10.1016/j.bone.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 57.Modlesky CM, Majumdar S, Dudley GA. Trabecular bone microarchitecture in female collegiate gymnasts. Osteoporosis Int. 2008;19:1011–1018. doi: 10.1007/s00198-007-0522-x. [DOI] [PubMed] [Google Scholar]
- 58.Nikander R, Sievanen H, Heinonen A, Kannus P. Femoral neck structure in adult female athletes subjected to different loading modalities. J Bone Miner Res. 2005;20(3):520–528. doi: 10.1359/JBMR.041119. [DOI] [PubMed] [Google Scholar]
- 59.Lehtonen-Veromaa M, Mottonen T, Svedstrom E, Hakola P, Heinonen OJ, Viikari J. Physical activity and bone mineral acquisition in peripubertal girls. Scand J Med Sci Sports. 2000;10:236–243. doi: 10.1034/j.1600-0838.2000.010004236.x. [DOI] [PubMed] [Google Scholar]
- 60.Lehtonen-Veromaa M, Mottonen T, Irjala K, Nuotio I, Leino A, Viikari J. A 1-Year prospective study on the relationship between physical activity, markers of bone metabolism, and bone acquisition in peripubertal girls. J Clin Endocrinol Metab. 2000;85(10):3726–3732. doi: 10.1210/jcem.85.10.6889. [DOI] [PubMed] [Google Scholar]
- 61.Heinonen A, Sievänen H, Kannus P, Oja P, Vuori I. Site specific skeletal response to long-term weight-training seems to be attributable to principal loading modality: A pQCT study of female weightlifters. Calcif Tissue Int. 2002;70:469–474. doi: 10.1007/s00223-001-1019-9. [DOI] [PubMed] [Google Scholar]
- 62.Kontulainen S, Sievänen H, Kannus P, Pasanen M, Vuori I. Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: A peripheral quantitative computed tomography study between young and old starters and controls. J Bone Miner Res. 2003;18(2):352–9. doi: 10.1359/jbmr.2003.18.2.352. [DOI] [PubMed] [Google Scholar]
- 63.Haapasalo H, Kontulainen S, Sievanen H, Kannus P, Jarvinen M, Vuori I. Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: A peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone. 2000;27(3):351–57. doi: 10.1016/s8756-3282(00)00331-8. [DOI] [PubMed] [Google Scholar]
- 64.Duncan CS, Blimkie CJR, Kemp A, Higgs W, Cowell CT, Woodhead H, Briody JN, Howman-Giles R. Mid-femur geometry and biomechanical properties in 15- to 18-yr old female athletes. Med Sci Sports Exerc. 2002;34(4):673–81. doi: 10.1097/00005768-200204000-00018. [DOI] [PubMed] [Google Scholar]
- 65.Ducher G, Courteix D, Même S, Magni C, Viala JF, Benhamou CL. Bone geometry in response to long-term tennis playing and its relationship with muscle volume: A quantitative magnetic resonance imaging study in tennis players. Bone. 2005;37(4):457–66. doi: 10.1016/j.bone.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 66.Wang QJ, Suominen H, Nicholson PHF, Zou LC, Alen M, Koistinen A, Cheng S. Influence of physical activity and maturation status on bone mass and geometry in early pubertal girls. Scand J Med Sci Sports. 2005;15:100–106. doi: 10.1111/j.1600-0838.2004.00402.x. [DOI] [PubMed] [Google Scholar]
- 67.Forwood MR, Baxter-Jones AD, Beck TJ, Mirwald RL, Howard A, Bailey DA. Physical activity and strength of the femoral neck during the adolescent growth spurt: A longitudinal analysis. Bone. 2006;38:576–583. doi: 10.1016/j.bone.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 68.Nikander R, Sievanen H, Uusi-Rasi K, Heinonen A, Kannus P. Loading modalities and bone structures at nonweight-bearing upper extremity and weight-bearing lower extremity: A pQCT study of adult females. Bone. 2006;39:886–94. doi: 10.1016/j.bone.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 69.Liu L, Maruno R, Mashimo T, Sanka K, Higuchi T, Hayashi K, Shirasaki Y, Mukai N, Saitoh S, Tokuyama K. Effects of physical training on cortical bone at midtibia assessed by peripheral QCT. J Appl Physiol. 2003;95:219–224. doi: 10.1152/japplphysiol.01055.2002. [DOI] [PubMed] [Google Scholar]