Abstract
Prostate cancer (PCa) growth is dependent on androgens and the androgen receptor (AR), which acts by modulating gene transcription. Tetratricopeptide repeat (TPR) proteins (FKBP52, FKBP51 and Cyp40) interact with AR in PCa cells, suggesting roles in AR-mediated gene transcription and cell growth. We report here that FKBP51 and Cyp40, but not FKBP52, are significantly elevated in PCa tissues and in androgen-dependent (AD) and -independent (AI) cell lines. Overexpression of FKBP51 in AD LNCaP cells increased AR transcriptional activity in the presence and absence of androgen, whereas siRNA knockdown of FKBP51 dramatically decreased AD gene transcription and proliferation. Knockdown of Cyp40 also inhibited androgen-mediated transcription and growth in LNCaP cells. However, disruption of FKBP51 and Cyp40 in the AI C4-2 cells caused only a small reduction in proliferation, indicating that Cyp40 and FKBP51 predominantly regulate AD cell proliferation. Under knock-down conditions, the inhibitory effects of TPR ligands, CsA and FK506, on AR activity were not observed, indicating that Cyp40 and FKBP51 are the targets of CsA and FK506, respectively. Our findings demonstrate that FKBP51 and Cyp40 are positive regulators of AR that can be selectively targeted by CsA and FK506 to achieve inhibition of androgen-induced cell proliferation. These proteins and their cognate ligands thus provide new strategies in the treatment of PCa
Keywords: Transcription, prostate cancer, androgen receptor, Cyp40, FKBP51, FKBP52
Introduction
Prostate cancer (PCa) is the second leading cause of cancer death in American men (Jemal et al., 2006; Landis et al., 1999). Androgen signaling through the androgen receptor (AR), a member of the nuclear receptor superfamily (Chang et al., 1988; Mangelsdorf et al., 1995; Tsai and O'Malley, 1994), is critical for normal development of the prostate gland and the aberrant onset of PCa (Balk, 2002). Androgen ablation is frequently used in the treatment of PCa to repress AR action (Scherr et al., 2003), and this approach results in reduced expression of AR target genes and concomitant tumor regression (Amler et al., 2000; Feldman and Feldman, 2001; Mousses et al., 2001; Velasco et al., 2004). Unfortunately, PCa often recurs after androgen ablation therapy – a state referred to as androgen-independence (AI) or ablation resistance. Studies suggest that both AI and androgen-dependent (AD) tumors express AR (Chen et al., 2004), indicating that AR-regulated gene expression might play a critical role in PCa growth and progression (Haag et al., 2005; Han et al., 2005; Liao et al., 2005).
As with other steroid receptors, the AR is a modulator protein that contains an N-terminal transactivation domain, a conserved DNA-binding domain, and a C-terminal ligand binding-domain (LBD). The unliganded AR primarily exists in the cytoplasm in a complex with heat shock protein 90 (Hsp90, and tetratricopeptide repeat (TPR) proteins (FK506-binding proteins, FKBP52 and FKBP51, and cyclosporin A-binding protein, Cyp40) (Febbo et al., 2005; Heinlein and Chang, 2004; Periyasamy et al., 2007; Veldscholte et al., 1992a; Veldscholte et al., 1992b). The ability of FKBP52/FKBP51 and Cyp40 to bind the immunosuppressive drugs FK506 and cyclosporine A (CsA), respectively, has served to categorize these proteins as immunophilins. Binding of FK506 and CsA to FKBP51/FKBP52 and Cyp40, respectively, causes inhibition of the protein's peptidyl-prolyl cis-trans isomerase (PPIase) activity (Galat, 2003; Schreiber and Crabtree, 1992). A number of studies have shown contributions by FKBP52, FKBP51 and Cyp40 to protein folding, ligand binding, and nuclear localization of glucocorticoid, estrogen and progesterone receptors (Cheung-Flynn et al., 2003; Denny et al., 2000; Pratt et al., 2004; Ratajczak et al., 2003; Reynolds et al., 1999). Apart from the physical interaction of AR with Hsp90, FKBP52, FKBP51 and Cyp40, the molecular roles of these TPRs on AR action are poorly understood.
Recently, new evidence for TPR control of AR function during reproductive organ development has come to light. Our laboratory (Yong et al., 2007) and others (Cheung-Flynn et al., 2005) have shown that male mice with targeted ablation of FKBP52 are infertile due to a developmental defect of the penis termed hypospadias – a condition prevalent in newborn boys and thought to arise from androgen insensitivity (Batch et al., 1992; Nakao et al., 1992). Complete prostate dysgenesis was also noted in FKBP52KO mice. We have shown overexpression of FKBP52, FKBP51 and Cyp40 in PCa cell lines and that the TPR ligands, FK506 and CsA, inhibited androgen-induced cell growth and gene transcription in PCa cells through inhibition of hormone binding and nuclear localization of the AR (Periyasamy et al., 2007). Others have shown FKBP51 overexpression in PCa tissues compared to noncancerous controls (Tomlins et al., 2007; Velasco et al., 2004; Zhu et al., 2001). Moreover, reports exist showing that, like the PSA and KLK2 genes, FKBP51 is a highly-sensitive AR-regulated gene (Amler et al., 2000; Magee et al., 2006; Vanaja et al., 2002), whose up-regulation serves to increase AR activity (Febbo et al., 2005). Based on these observations, we hypothesized that FKBP52, FKBP51 and Cyp40 may serve as potential activators of AR and that their up-regulation might increase AR activity in PCa cells.
In this report, we show that expression of FKBP51 and Cyp40, but not FKBP52, was elevated in PCa tissues and cell lines. Using knockdown and over-expression techniques, we also show that FKBP51 and Cyp40 are required for optimal AR activity in AD PCa cells, and that each TPR serves as the principal target for the inhibitory effects of FK506 and CsA on AR. Lastly, we show that both FKBP51 and Cyp40 principally control AD, rather than AI, growth of prostate cells. These findings are the first of their kind and provide potential new targets in the treatment of PCa.
Results
Over-expression of Cyp40 and FKBP51 in human PCa specimens and cell lines
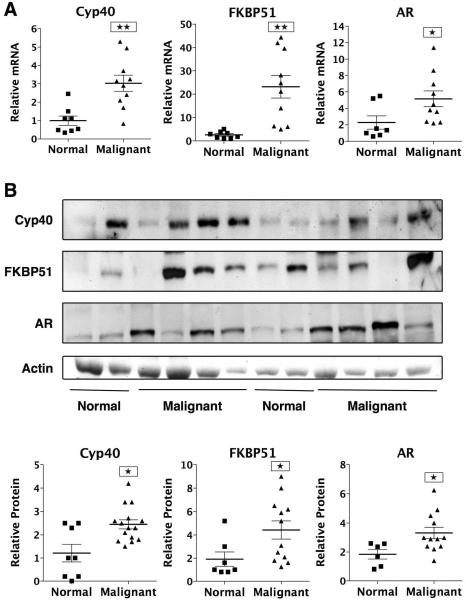
Previously, high levels of Cyp40, FKBP51 and FKBP52 were found in the LNCaP, PC-3 and DU145 PCa cell lines (Periyasamy et al. 2007). To further validate involvement by these TPRs in PCa, we measured the mRNA and protein expression of Cyp40, FKBP51 and FKBP52 in PCa specimens. Normal and PCa tissues samples were purchased from the Cooperative Human Tissue Network (CHTN). Analysis by real-time RT-PCR (Fig. 1A) and Western blotting (Fig.1B and Supplementary Fig. S1B) revealed significant upregulation of Cyp40, FKBP51 and AR in most of the PCa samples. Interestingly, FKBP52 mRNA and protein levels were unaltered when comparing normal prostate tissues to PCa samples (data not shown). This suggests that FKBP51 and Cyp40, but not FKBP52, might play a critical role in PCa growth and progression. Although the FKBP51 results are consistent with prior reports showing high FKBP51 expression in primary and recurrent PCa (Amler et al. 2000; Mousses et al. 2001; Tomlins et al. 2007; Velasco et al. 2004), these are the first observations showing over-expression of Cyp40 in cancerous prostate tissues.
Figure 1.
Over-expression of Cyp40 and FKBP51 in PCa specimens. (a) Total RNA was isolated from 8 normal and 10 malignant human prostate tissues, followed by real-time PCR using Cyp40-, FKBP51- and AR-specific primers and normalization to ribosomal 18S. RNA levels were expressed as relative to normal prostate tissue #1. *P<0.05, ** P<0.01. (b) Whole cell extracts from normal and malignant prostate specimens were analyzed by Western blotting for expression of AR, FKBP51, Cyp40 and actin. Quantitation was performed by densitometric scanning of the bands in 8 normal and 15 malignant prostate specimens and normalization to actin. *P<0.05. Western blot of remaining samples are seen in Fig. S1b.
We next measured protein expression levels of Cyp40, FKBP51 and AR in several PCa cell lines (Fig. 2A). Normal prostate epithelial cells (PrECs) were used as controls and compared to AD (LNCaP, LAPC-4, VCaP and DuCaP) and AI (C4-2, C-81 and CWR22R) PCa cell lines. All PCa cell lines expressed the AR, albeit to different degrees, and in a manner that did not correlate with androgen growth sensitivity. As expected, the AR protein was not detected in normal PrECs (Periyasamy et al., 2007). With the exception of VCaP cells, high levels of Cyp40 and FKBP51 were found in all PCa cell lines compared to normal PrECs, providing further evidence that FKBP51 and Cyp40, rather than FKBP52 and other immunophilins, are the actual mediators of the inhibitory effects of FK506 and CsA on PCa cell growth. The data of Fig. 2 also showed higher FKBP51 and Cyp40 levels in AI PCa cell lines compared to AD cells, suggesting that each TPR may be involved in PCa progression to androgen independence. In the work that follows, we will therefore test whether FKBP51 and Cyp40 are the targets of immunophilin drug action, and whether these proteins contribute to androgen-dependent or androgen-independent growth of PCa cells, or both.
Figure 2.
Over-expression of Cyp40, FKBP51 PCa cell lines. (a) WCEs from normal prostate epithelial cells (PrEC), androgen-dependent (AD) (LNCaP, LAPC-4, VCaP and DuCaP) and- independent (AI) (C4-2, C-81 and CWR22R) human PCa cell lines were prepared and analyzed by Western blot for expression of the AR, FKBP51, Cyp40 and actin, respectively, as described in Materials and Methods. (b) Densitometric scanning of the bands corresponding to protein expression of Cyp40, FKBP51 and the AR in PrEC, AD and AI human prostate cell lines. Cyp40, FKBP51 and AR protein levels were expressed as relative expression levels, taking the values obtained from LNCaP cells as 1. Results are representative of two independent experiments. AD vs PrEC, **P values ranged from <0.01 to <.001. AI vs AD, ##P<0.01.
Inhibition of AR activity by FK506 and CsA is mediated by FKBP51 and Cyp40
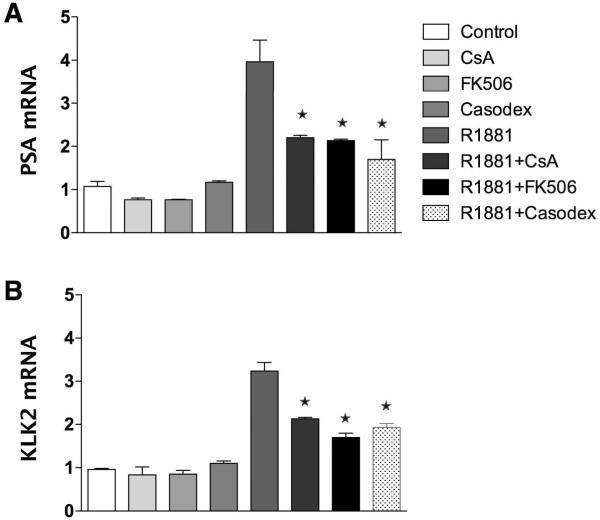
In prior work, we showed inhibition of LNCaP cell proliferation by FK506 and CsA that correlated with reduced hormone-induced AR activity as measured by reporter gene assays (Periyasamy et al. 2007). To confirm that these results were not artifacts of reporter gene assays, expression of the androgen-regulated PSA (Fig. 3A) and KLK2 (Fig. 3B) genes was measured by real-time RT-PCR in LNCaP cells. The results show elevated PSA and KLK2 expression levels in response to R1881 that was effectively inhibited by the AR antagonist, casodex (bicalutamide). More importantly, both FK506 and CsA decreased expression of PSA and KLK2 in response to R1881.
Figure 3.
Inhibition of AR activity by FK506 and Cyp40 is mediated by FKBP51 and Cyp40. LNCaP cells were treated with R1881 (1.0 nM) in the presence and absence of CsA (5.0 μM) or FK506 (10.0 μM) or Casodex (10.0 μM) for 20 h. Total RNA was isolated and converted into cDNA. Real-time RT- PCR was done using specific primers for PSA (a) and KLK2 (b). PSA and KLK2 mRNA levels were normalized with the values obtained from 18S expression. PSA and KLK2 mRNA levels are expressed as relative expression values, taking the value obtained from cells treated with vehicle (control) as 1. Results are means ± SEM of two independent experiments run in triplicate. *P<0.05 for ligand treated vs R1881.
In the prior work, we also showed that FK506 inhibition of AR activity was not mediated by the classical immunophilins, such as FKBP12, but our results did not discriminate between FKBP51 and FKBP52. Here, this question was addressed by down-regulating expression of FKBP51 with short-interfering RNA (siRNA). Fig. 4A shows effective knock-down of FKBP51 expression in LNCaP cells while leaving levels of FKBP52 unchanged. We then treated control-siRNA and FKBP51-siRNA LNCaP cells with FK506. FK506 had no effect on expression levels of FKBP51 and AR under basal conditions, but did inhibit the ability of R1881 to increase FKBP51 expression in control cells (Fig. 4B). Interestingly, both FK506 and knock-down of FKBP51 had no effect on R1881 induction of the AR protein. With respect to AR activity at PSA-Luc, FK506 caused an approximate 50% reduction, as expected, in control cells (Fig. 4C). However, FKBP51 knock-down alone also caused a ~50% reduction and this activity was not further reduced by treatment with FK506. These results demonstrate that FKBP51, but not FKBP52, is the target of FK506 action on AR.
Figure 4.
Down-regulation of FKBP51 inhibits AR activity. LNCaP cells were transiently transfected with FKBP51 siRNA or non-target control siRNA. Ninety-six hours later, the WCEs were prepared, and expression of FKBP51, FKBP52 and actin (control) was measured by Western blotting (a). LNCaP cells were transiently transfected with FKBP51 siRNA or non-target control siRNA, and the PSA-Luc promoter reporter construct along with a β-gal plasmid. Forty-eight hours later, cells were treated with or without R1881, FK506, FK506 and R1881 for 24 h. Lysates were prepared and analyzed for the AR and FKBP51 expression by Western blotting (b) and for AR reporter gene activity (c). Results are means ± SEM of two independent experiments. *P<0.05 and **P<0.01 vs R1881 control siRNA.
To address the direct role of Cyp40 in AR function, siRNA was also used to down-regulate Cyp40 in LNCaP (Fig. 5). Cells transfected with Cyp40-siRNA showed a large reduction of the Cyp40 protein, with no change in the related cyclophilin Cyp18 or AR protein (data not shown). The latter result suggests that Cyp40, like FKBP51, is not essential for AR expression and stability. In control-siRNA cells, CsA treatment caused inhibition of AR activity, as expected. In response to Cyp40 down-regulation, a large reduction of androgen-induced PSA promoter activity was seen, with no further inhibitory effect of CsA. These results identify Cyp40 as the target of CsA action on AR.
Figure 5.
Down-regulation of Cyp40 inhibits AR activity. LNCaP cells were transiently transfected with Cyp40 siRNA or non-target control siRNA. Ninety-six hours later, the WCEs were prepared, and expression of Cyp40 was measured by Western blotting (a). LNCaP cells were transfected with Cyp40 siRNA or control siRNA, and the PSA-Luc reporter construct along with a β-gal plasmid. Forty-eight hours later, cells were treated with or without R1881, CsA, CsA and R1881 for 24 h. Lysates were prepared and analyzed AR reporter gene activity (b). Results are means ± SEM of two independent experiments. *P<0.05 vs R1881 control siRNA.
FKBP51 and Cyp40 preferentially control androgen-dependent proliferation of PCa cells
The results of Fig. 2 showed over-expression of Cyp40 and FKBP51 in several AD PCa cell lines and even higher levels of each TPR in AI cells. The latter result suggested a possible role for the proteins in the AD to AI functional transition of PCa cells. To test this hypothesis, we compared AI C4-2 to AD LNCaP cells, as each expressed approximately equal amounts of AR, yet had differences with respect to FKBP51 (Fig. 2). To confirm androgen sensitivity, C4-2 and LNCaP growth was measured in response to increasing concentrations of R1881. LNCaP cells showed a strong stimulatory effect by R1881 (200% at 1 nM), while C4-2 cells showed a small but reproducible growth response to hormone (data not shown). These findings are consistent with previously reported observations in LNCaP and C4-2 cells (Dehm and Tindall, 2006). However, C4-2 cells showed higher growth rates than LNCaP cells under hormone-free conditions (data not shown) – a key feature of androgen-independent PCa cells (Igawa et al., 2002; Thalmann et al., 2000). To assess the contribution of the AR to the growth properties of C4-2 and LNCaP cells, AR activity at the PSA-Luc reporter gene was measured (Fig. 6A). As expected, LNCaP cells showed low basal activity by hormone-free AR and a strong response to ligand (~14,000%). In contrast, the defining feature of C4-2 cells was basal AR activity that was much higher (~2,500%) than LNCaP cells (100%). Because of its high basal activity, C4-2 cells showed only a 10-fold induction in response to R1881, while LNCaP cells had a 130-fold induction of AR activity. These results demonstrate that, compared to LNCaP cells, the AR of C4-2 cells has high constitutive activity and overall higher hormone-induced activity, but a lower degree of responsiveness to androgens.
Figure 6.
FKBP51 over-expression increases basal and hormone-induced AR activity. (a) LNCaP and C4-2 cells were transiently transfected with a PSA-Luc and β-gal constructs, followed by treatment with R1881 (1.0 nM) for 20 h and assay for luciferase activity normalized to β-gal. Parentheses shows fold increase relative to hormone-free cells. Results are means ± SEM of three to four independent experiments. **P<0.01, C4-2 vs LNCaP. (b, c, d) LNCaP cells were transfected with Flag-FKBP51 or control plasmid (mock), followed by treatment with or without R1881 for 20 h. (b) AR, Flag-FKBP51 and endogenous FKBP51 protein were measured by Western blotting with (c) quantization relative to actin and setting mock AR expression without R1881 as 1. *P<0.05, mock AR and endogenous FKBP51 in presence and absence of androgen. #P<0.05, Flag-FKBP51 in presence and absence of androgen. (d) PSA-luc activity was normalized to actin and expressed as percent of mock control. Parentheses show fold increase in response to hormone. Results are means ± SEM of three independent experiments. **P<0.01, Flag-FKBP51 vs Mock.
To test the possibility that the high levels of FKBP51 in C4-2 cells contribute to their high basal AR activity, we overexpressed Flag-tagged FKBP51 in LNCaP cells. As expected, hormone treatment increased expression of the AR and endogenous FKBP51 in both non-transfected (mock) and Flag-FKBP51 transfected LNCaP cells (Fig. 6B&C). Overexpression of Flag-FKBP51 had no effect on AR protein levels, once again indicating that FKBP51 is not involved in expression and stability of the AR. To our surprise, levels of Flag-FKBP51 were further enhanced by R1881 treatment, even though the Flag-FKBP51 plasmid is not under the control of the FKBP51 promoter. In mock-transfected LNCaP cells, the low basal reporter activity was increased by ~5–6-fold in response to hormone (Fig. 6D). In FKBP51-transfected LNCaP cells, basal AR activity was ~2–3-fold higher than mock cells, and increased by only 3-fold in response to hormone. However, like C4-2 cells, the overall hormone-induced AR activity was higher than controls. These results substantiate the notion that FKBP51 functions as a positive regulator of the AR, serving to increase both the basal and overall activities of AR. The results also indicate that FKBP51 overexpression can cause the AR of AD LNCaP cells to mimic AR found in the AI C4-2 cells by increasing the constitutive and hormone-induced activities of the AR.
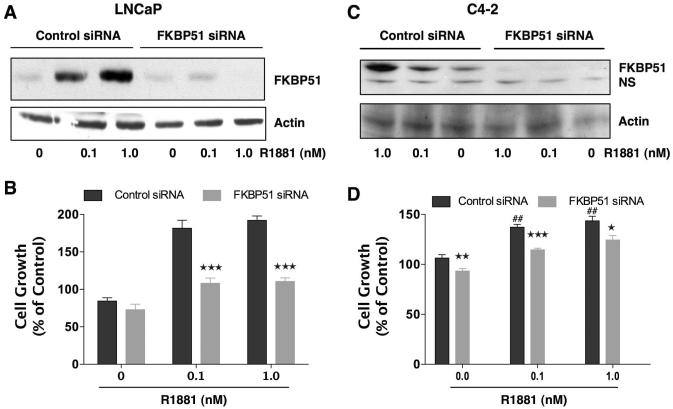
To confirm the significance of FKBP51 to androgen-dependent and -independent growth, levels of FKBP51 in LNCaP and C4-2 cells were reduced using siRNA (Fig. 7). Western blot analysis showed near-complete depletion of the FKBP51 in both cell lines. As expected, FKBP51 expression was upregulated by R1881 in control-siRNA transfected cells in both cell lines, and this upregulation was significantly reduced by FKBP51-siRNA. In LNCaP cells, FKBP51 knock-down caused a greater than 50% reduction in androgen-induced cell proliferation compared to control siRNA cells, while having no effect on hormone-free growth. The magnitude of this reduction is similar to the inhibitory effect FKBP51 knock-down had on AR transcriptional activity in these cells (Fig. 4). In contrast to LNCaP cells, FKBP51 knock-down in C4-2 cells had a smaller, but significant, reduction of proliferation (~15%) under basal and hormone-induced conditions, indicating that FKBP51 is critical for AD, but not AI PCa cell growth.
Figure 7.
Down-regulation of FKBP51 predominantly inhibits androgen-dependent growth in PCa cells. AD LNCaP and AI C4-2 cells were transiently transfected with FKBP51-siRNA or control-siRNA. Six to 8 h after transfection, medium was changed. Twenty-four hours after transfection, cells were plated (1500 cells / well) in 96-well (cell proliferation assay) and 6-well plates (Western blotting). Seventy-two hours after transfection, LNCaP (a) and C4-2 (c) cells were treated with R1881 (0.1 and 1.0 nM) or vehiclel (0) for 24 h. Whole cell extracts were prepared, and expression of Cyp40 and actin (control) was measured by Western blotting. LNCaP (b) and C4-2 (d) cells were treated with R1881 (0.1 and 1.0 nM) or vehicle (0) alone for 3 days. Ninety-six hours after transfection, cell proliferation was determined by means of an MTT assay. Results are means ± SEM of two independent experiments, each performed in quadruplicate. LNCAP: pair-wise comparison of control siRNA vs FKBP51 siRNA at each condition, ***P<0.001. C4-2: pair-wise comparison of control siRNA vs FKBP51 siRNA at each condition, ***P<0.001, **P<0.01,*P<0.05; comparison of control siRNA in presence and absence of R1881, ##P<0.01.
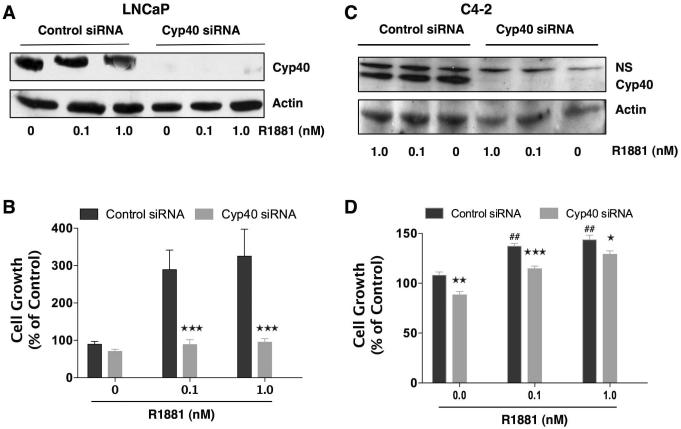
Similar studies in LNCaP and C4-2 cells were performed to determine the contribution of Cyp40 to androgen-dependent and -independent growth (Fig. 8). In response to siRNA knock-down of Cyp40, LNCaP cells showed a dramatic decrease in androgen-induced proliferation. Indeed, loss of Cyp40 made LNCaP cells essentially unresponsive to hormone, while leaving basal growth unaltered. These results are in good agreement with the very strong inhibitory effect that Cyp40 knock-down had on hormone-induced AR transcriptional activity in LNCaP cells (Fig. 5). The effect of Cyp40 loss on C4-2 cells, however, was much less dramatic, showing a decrease of ~20% in growth under all conditions. These results show that Cyp40, like FKBP51, is a positive regulator of AR activity, serving to preferentially promote androgen-induced PCa cell growth. The results also indicate that Cyp40 plays a greater role than FKBP51 in the function of AR in LNCaP cells, consistent with our prior observation that CsA is more effective than FK506 as an inhibitor of androgen-induced cell proliferation (Periyasamy et al., 2007).
Figure 8.
Down-regulation of Cyp40 predominantly inhibits androgen-dependent growth in PCa cells. AD LNCaP and AI C4-2 cells were transiently transfected with Cyp40-siRNA or control-siRNA. Six to 8 h after transfection, medium was changed. Twenty-four hours after transfection, cells were plated (1500 cells / well) in 96-well (cell proliferation assay) and 6-well plates (Western blotting). Seventy-two hours after transfection, LNCaP (a) and C4-2 (c) cells were treated with R1881 (0.1 and 1.0 nM) or vehiclel (0) for 24 h. Whole cell extracts were prepared, and expression of Cyp40 and actin (control) was measured by Western blotting. LNCaP (b) and C4-2 (d) cells were treated with R1881 (0.1 and 1.0 nM) or vehicle (0) alone for 3 days. Ninety-six hours after transfection, cell proliferation was determined by means of an MTT assay. Results are means ± SEM of two independent experiments, each performed in quadruplicate. LNCAP: pair-wise comparison of control siRNA vs Cyp40 siRNA at each condition, ***P<0.001. C4-2: pair-wise comparison of control siRNA vs Cyp40 siRNA at each condition, ***P<0.001, **P< 0.01,*P<0.05; comparison of control siRNA in presence and absence of R1881, ##P<0.01.
Discussion
In prior work, we demonstrated the ability of immunophilin ligands, CsA and FK506, to inhibit growth of PCa cell lines by attenuating AR hormone-binding and nuclear localization functions (Periyasamy et al. 2007). As Cyp40, FKBP51 and FKBP52 have been found in AR complexes (Febbo et al., 2005; Periyasamy et al., 2007; Veldscholte et al., 1992a), we speculated a direct targeting of these receptor-associated TPR proteins in the observed actions of FK506 and CsA. In this report, we extend these observations by measuring expression of Cyp40, FKBP51 and FKBP52 in PCa tissue specimens and by altering their expression levels in PCa cells. We demonstrate high level expression of Cyp40 and FKBP51, but not FKBP52, in PCa tissues and in a variety of PCa cell lines, with expression being highest in AI cell lines. We also demonstrate that Cyp40 and FKBP51 are positive regulators of AR transcription that serve as the molecular targets of CsA and FK506, respectively. Lastly, although both TPR proteins contributed to a smaller magnitude of androgen-independent growth, their principal roles appear to be control of androgen-dependent PCa proliferation. Taken as a whole, the results suggest that selective and effective inhibition of androgen-induced growth is possible through CsA and FK506 drug action.
Recent results in FKBP52-deficient male mice showed aberrant penile development and prostate dysgenesis that correlated with reduced AR activity at endogenous and heterologous genes (Yong et al. 2007). It was, therefore, surprising that levels of FKBP52 were neutral in normal and PCa tissue samples (data not shown). Instead, we observed high levels of FKBP51 and Cyp40 in PCa tissues, suggesting that these proteins, rather than FKBP52, are prime contributors to the PCa phenotype. Interestingly, FKBP51-KO mice showed no defects of penile or prostate gland development (Yong et al. 2007), while similar negative results have been observed by our laboratories in Cyp40-KO male mice (unpublished observations). Based on these findings, we conclude that FKBP51 and Cyp40 must preferentially regulate AR activity in the mature prostate gland, whereas FKBP52 controls AR activity during prostate organogenesis.
Although it is clear that the PCa growth suppressive effects of FK506 and CsA are mediated by the AR, the exact AR pathway affected remains unresolved. We have eliminated involvement by FK506 and CsA in apoptotic pathways, as no DNA fragmentation was observed in LNCaP cells treated with these drugs (Periyasamy et al., 2007). The classical immunosuppressive pathways involving inhibition of calcineurin by Cyp18 or FKBP12 (Harding et al., 1989; Schreiber and Crabtree, 1992) have also been eliminated, since inhibition of calcineurin was found in PCa cell lines whose growth was unaffected by CsA or FK506 (Periyasamy et al., 2007). Lastly, no effect of CsA or FK506 on AR-mediated mammalian target of rapamycin (mTOR) activation was found (unpublished observations), suggesting that the suppressive actions of CsA and FK506 on androgen-induced transcription and proliferation did not extend to all aspects of AR signaling. In this respect, TPR protein ligands represent an attractive alternative to androgen ablation in the treatment of PCa, due to reduced potential for global hypoandrogenic side effects, such as impotence and reduced libido.
Evidence suggests that AR-mediated signaling plays a key role in ablation-resistant PCa (Craft and Sawyers, 1998; Gregory et al., 2001; Grossmann et al., 2001), as re-expression of androgen-regulated genes is seen in recurrent PCa. Thus, factors other than androgens must activate the AR and contribute to PCa progression. Several laboratories have identified FKBP51 as a promising candidate for this function (Amler et al., 2000; Mousses et al., 2001; Tomlins et al., 2007; Velasco et al., 2004). FKBP51 is strongly repressed during hormone-ablation therapy. Conversely, FKBP51 can be 2-fold higher in recurrent compared to primary tumors, and high-level expression of FKBP51 within sites of localized and/or metastatic PCa has been seen. Through our work here, high level expression of Cyp40 in PCa tissues and cells is now known. These results provided strong circumstantial evidence for a role for FKBP51 and Cyp40 in the pathogenesis and progression of PCa. As a direct test of this concept, we measured proliferation of PCa cells in the absence and presence of FKBP51 and Cyp40. The strongest role for each protein was seen for androgen-dependent growth in LNCaP cells, although AI growth of C4-2 cells was affected to a much lesser degree. Taken together, the data indicate that FKBP51 and Cyp40 are predominantly involved in androgen-dependent PCa cell proliferation. Although the etiology of FKBP51 and Cyp40 over-expression in PCa cells is not known, a possible candidate for FKBP51 over-expression is the AR itself. Androgens regulate expression of the FKBP51 gene (fkbp5) via a direct interaction between the AR and distal enhancers located in the fifth and seventh introns of fkbp5 (Magee et al., 2006; Makkonen et al., 2009). Thus, mutations to the AR that upregulate this function are likely to be important contributors to the androgen-independent phenotype.
An important question that arises from our study are the mechanisms by which FKBP51 and Cyp40 regulate AR transcription and proliferation in PCa cells. A mechanism likely to be shared by both proteins is alteration to AR conformation via their common PPIase function, which involves isomerization of the peptide backbone at select proline residues (Lavery and McEwan, 2005; McEwan, 2004; Reid et al., 2002; Simental et al., 1991). Interestingly, the N-terminal activation domain (AF-1) of AR has a high proline content and is required for both AD and AI activity (Dehm and Tindall 2006), whereas the C-terminal AF-2 domain is critical only for ligand-dependent activity (Dehm et al., 2007; Dehm and Tindall, 2006). As such, the AF-1 domain of the AR is the most likely target for FKBP51 or Cyp40 actions. However, both the AF-1 and AF-2 domains of the AR contribute to a variety of AR functions, such as ligand binding, nuclear localization, and recruitment of coactivators. Alterations to any one of these properties could account for our observed effects on AR transcription and cell proliferation. Indeed, we have already shown that CsA and FK506 caused a decrease in AR hormone-binding capacity and nuclear localization in LNCaP cells (Periyasamy et al. 2007). It should be noted, however, that the most recent data from studies of GR show that mutations which abrogate the PPIase function of FKBP52 do not block FKBP52 potentiation of GR (Riggs et al., 2007). Similarly, the PPIase activity of FKBP51 is not important for its inhibitory effect on GR. Yet, it is clear that the PPIase domain (rather than catalytic activity) is critical to FKBP52 actions on GR and PR (Riggs et al., 2003; Tranguch et al., 2005; Wochnik et al., 2005). A similar relationship may exist for FKBP51 and Cyp40 control of the AR in PCa cells. However, we cannot exclude the possibility that PPIase catalytic activity may still be required for the AR, as the mechanism of TPR control of nuclear receptors need not be universal. Indeed, FKBP51 actions on the AR are distinctly different from GR and PR, where FKBP51 is a negative modulator (Denny et al., 2000; Hubler et al., 2003; Reynolds et al., 1999). Our results (Periyasamy et al. 2007 and this work) and others (Febbo et al., 2005) all point to positive regulation of the AR by FKBP51. Therefore, it is likely that FKBP51 exerts receptor-specific actions that could also vary across tissues or cell types. Further experiments are clearly needed to resolve these differences and define the possible mechanisms by which FKBP51 and Cyp40 augment AR transcription and cell proliferation in PCa cells.
In addition to the above mentioned direct mechanism on the AR, FKBP51 and Cyp40 proteins might indirectly activate AR transcription and cell proliferation through other signaling pathways. For example, FKBP51 has been shown to activate STAT5 (Giraudier et al., 2002; Komura et al., 2003) and NF-kB signaling pathways (Avellino et al., 2005; Jiang et al., 2008; Romano et al., 2004), over-activity of which correlates with several non-PCa malignancies. Because cross-talk between STAT5 and AR (Ahonen et al., 2003; Li et al., 2007) as well as NF-kB and AR signaling (Chen and Sawyers, 2002; Cinar et al., 2004; Lee et al., 2005) in PCa cells has been demonstrated, careful consideration of these and other non-direct pathways will be incorporated into future mechanistic studies.
In conclusion, our findings are the first evidence that CsA and FK506 negatively modulate androgen-dependent activity and PCa proliferation by directly targeting Cyp40 and FKBP51, respectively. Cyp40 and FKBP51 can now be considered positive regulators of androgen-induced AR-mediated cell growth and transcription, providing new and potentially important targets for the drug treatment of PCa.
Materials and Methods
Cell culture
The human PCa cell lines, LNCaP, C4-2, C-81, CWR22R, DuCaP and VCaP, were routinely cultured and maintained in RPMI 1640 medium containing 5–10% FBS and 0.05% vol/vol penicillin/streptomycin (pen/strep). The human PCa LAPC-4 cells were routinely cultured and maintained in Iscove's Modified Dulbecco's Medium (IMDM) containing 10% FBS and 0.05% vol/vol pen/strep. The normal human prostate epithelial cells (hPrECs) were cultured and maintained in the media recommended by the manufacturer (Cambrex Corp., San Diego, CA).
Prostate tissues
Normal and PCa tissues were purchased from the CHTN as frozen samples. The CHTN represents a group of hospitals that obtain tissues from either surgeries or autopsies and make these tissues available for research. The information provided with the tissues indicates a diagnosis of the stage of cancer, which may include a Gleason score and type of therapy used (radiation and/or chemotherapy).
Real-time reverse transcription-PCR
Total RNA from normal and PCa tissues, and control and treated LNCaP cells were extracted using the Trizol reagent according to the manufacturer's protocol (Invitrogen). Reverse transcription (2 μg total RNA) was performed by the iScript cDNA Synthesis Kit (Bio-Rad) following the manufacturer's instructions. Real-time reverse transcription-PCR (RT-PCR) was done using SYBR Green PCR master mix (Applied Biosystems) on a ABI Prism 7700 sequence detection system (Applied Biosystems). The gene-specific forward and reverse primer sequences were selected using the TaqMan probe and primer design function of the Primer Express version 1.5 software (Applied Biosystems). The primers used are listed in Supplementary Table 1. The fold-change in expression levels (using ribosomal 18S as control) was determined by a comparative C T method using the formula 2-ΔΔCT; where CT is the threshold cycle of amplification.
SDS-PAGE and Western blot
Whole cell extracts (WCEs) of prostate cells and tissues were prepared as previously described (Periyasamy et al., 2007). Equal amounts of WCEs were resolved by denaturing SDS-PAGE using either 7 or 10% polyacrylamide gels, followed by transfer to Immobilon polyvinylidene difluoride membranes and immunoblotting, as previously described (Banerjee et al., 2008). The human anti-AR antibody (SC815, Santa Cruz Biotechnology) was used to probe for the AR, whereas various antibodies were used to probe for FKBP51 (SC11514), actin (SC8432), Cyp40 (PA3–022; Affinity BioReagents). After being probed, blots were incubated with appropriate HRP-conjugated counter antibodies. Proteins were detected using luminol (A8511; Sigma), coumaric acid (C9008; Sigma), and hydrogen peroxide (H1009; Sigma) via enhanced chemiluminescence.
Transient transfection and luciferase reporter assays
LNCaP and C4-2 cells were plated either at a density of 5 × 105 in six-well plates or 1 × 106 in 6-cm culture dishes and incubated with RPMI 1640 medium containing 5% dextran-coated charcoal-treated (DCC) serum. At 95% confluence, the cells were cotransfected with a prostate-specific antigen luciferase (PSA-Luc) construct and a ß-gal plasmid using Lipofectamine 2000 reagent (Invitrogen Corp., Carlsbad, CA) according to the manufacturer's protocol, and incubated for 6 h at 37°C in a serum-free RPMI 1640 medium. For TPR protein overexpression and knockdown studies, PCa cells were co-transfected with Flag-tagged FKBP51 (2.0 μg) or FKBP51-siRNA or Cyp40-siRNA or control siRNA (100 nM; Dharmacon RNA Technologies) along with and without PSA-Luc construct and a ß-gal plasmid as described above. Twenty-four to forty-eight hours after transfection, the cells were washed and refed with RPMI 1640 medium containing 5% DCC serum, and then treated with CsA (5.0 μM) or FK506 (10.0 μM) for 3 h, followed by R1881 (1 nM) for an additional 20 h as previously described (Periyasamy et al., 2007). Cell extracts were prepared with reporter lysis buffer (Promega Corp., Madison, WI), and luciferase activity was measured using a luminometer and expressed as a percentage of control after normalization to ß-gal activity.
Proliferation assays
LNCaP and C4-2 cells (3 × 103 cells per well) were plated in 96-well plates in RPMI 1640 medium containing 5% DCC serum, and the growth rate was determined as a function of time. To determine the effect of androgen-stimulated cell growth, LNCaP and C4-2 cells grown in RPMI 1640 medium containing 2% DCC serum were treated with increasing concentrations of R1881 (0–100 nM) with media and ligand changes on d 2, 4, and 6. At the end of d 7, cell proliferation was determined by a calorimetric assay using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-dipheyltetrazoline bromide) as previously described (Periyasamy et al., 2007). In experiments assessing the effects of TPR protein knockdown on cell growth, PCa cells were transfected with FKBP51-siRNA or Cyp40-siRNA or control siRNA as described. Twenty-four hours after transfection, cells were plated (1500 cells / well) in 96-well plates and treated with R1881 (0.1 and 1.0 nM) or vehicle (0) alone for 3 days. Ninety-six hours after transfection, cell proliferation was determined by means of an MTT assay and protein expression by Western blot as described.
Statistical Analysis
The Student's unpaired t test was used throughout this study. The threshold for significance was set at P < 0.05.
Supplementary Material
Acknowledgements
We thank Drs. Theo Rein (Max Planck Institute of Psychiatry) and Marianne Sadar (British Columbia Cancer Agency) for the generous gift of Flag-tagged FKBP51 and PSA-luciferase reporter plasmid, respectively. This study was supported in part by National Institutes of Health grants DK73402 to W.S. and DK70127 to E.R.S.
References
- Ahonen TJ, Xie J, LeBaron MJ, Zhu J, Nurmi M, Alanen K, et al. Inhibition of transcription factor Stat5 induces cell death of human prostate cancer cells. J Biol Chem. 2003;278:27287–92. doi: 10.1074/jbc.M304307200. [DOI] [PubMed] [Google Scholar]
- Amler LC, Agus DB, LeDuc C, Sapinoso ML, Fox WD, Kern S, et al. Dysregulated expression of androgen-responsive and nonresponsive genes in the androgen-independent prostate cancer xenograft model CWR22-R1. Cancer Res. 2000;60:6134–41. [PubMed] [Google Scholar]
- Avellino R, Romano S, Parasole R, Bisogni R, Lamberti A, Poggi V, et al. Rapamycin stimulates apoptosis of childhood acute lymphoblastic leukemia cells. Blood. 2005;106:1400–6. doi: 10.1182/blood-2005-03-0929. [DOI] [PubMed] [Google Scholar]
- Balk SP. Androgen receptor as a target in androgen-independent prostate cancer. Urology. 2002;60:132–8. doi: 10.1016/s0090-4295(02)01593-5. discussion 138–9. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Periyasamy S, Wolf IM, Hinds TD, Jr., Yong W, Shou W, et al. Control of glucocorticoid and progesterone receptor subcellular localization by the ligand-binding domain is mediated by distinct interactions with tetratricopeptide repeat proteins. Biochemistry. 2008;47:10471–80. doi: 10.1021/bi8011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batch JA, Williams DM, Davies HR, Brown BD, Evans BA, Hughes IA, et al. Androgen receptor gene mutations identified by SSCP in fourteen subjects with androgen insensitivity syndrome. Hum Mol Genet. 1992;1:497–503. doi: 10.1093/hmg/1.7.497. [DOI] [PubMed] [Google Scholar]
- Chang CS, Kokontis J, Liao ST. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240:324–6. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- Chen CD, Sawyers CL. NF-kappa B activates prostate-specific antigen expression and is upregulated in androgen-independent prostate cancer. Mol Cell Biol. 2002;22:2862–70. doi: 10.1128/MCB.22.8.2862-2870.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- Cheung-Flynn J, Prapapanich V, Cox MB, Riggs DL, Suarez-Quian C, Smith DF. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol Endocrinol. 2005;19:1654–66. doi: 10.1210/me.2005-0071. [DOI] [PubMed] [Google Scholar]
- Cheung-Flynn J, Roberts PJ, Riggs DL, Smith DF. C-terminal sequences outside the tetratricopeptide repeat domain of FKBP51 and FKBP52 cause differential binding to Hsp90. J Biol Chem. 2003;278:17388–94. doi: 10.1074/jbc.M300955200. [DOI] [PubMed] [Google Scholar]
- Cinar B, Yeung F, Konaka H, Mayo MW, Freeman MR, Zhau HE, et al. Identification of a negative regulatory cis-element in the enhancer core region of the prostate-specific antigen promoter: implications for intersection of androgen receptor and nuclear factor-kappaB signalling in prostate cancer cells. Biochem J. 2004;379:421–31. doi: 10.1042/BJ20031661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft N, Sawyers CL. Mechanistic concepts in androgen-dependence of prostate cancer. Cancer Metastasis Rev. 1998;17:421–7. doi: 10.1023/a:1006141806801. [DOI] [PubMed] [Google Scholar]
- Dehm SM, Regan KM, Schmidt LJ, Tindall DJ. Selective role of an NH2-terminal WxxLF motif for aberrant androgen receptor activation in androgen depletion independent prostate cancer cells. Cancer Res. 2007;67:10067–77. doi: 10.1158/0008-5472.CAN-07-1267. [DOI] [PubMed] [Google Scholar]
- Dehm SM, Tindall DJ. Ligand-independent androgen receptor activity is activation function-2-independent and resistant to antiandrogens in androgen refractory prostate cancer cells. J Biol Chem. 2006;281:27882–93. doi: 10.1074/jbc.M605002200. [DOI] [PubMed] [Google Scholar]
- Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141:4107–13. doi: 10.1210/endo.141.11.7785. [DOI] [PubMed] [Google Scholar]
- Febbo PG, Lowenberg M, Thorner AR, Brown M, Loda M, Golub TR. Androgen mediated regulation and functional implications of fkbp51 expression in prostate cancer. J Urol. 2005;173:1772–7. doi: 10.1097/01.ju.0000155845.44729.ba. [DOI] [PubMed] [Google Scholar]
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- Galat A. Peptidylprolyl cis/trans isomerases (immunophilins): biological diversity--targets--functions. Curr Top Med Chem. 2003;3:1315–47. doi: 10.2174/1568026033451862. [DOI] [PubMed] [Google Scholar]
- Giraudier S, Chagraoui H, Komura E, Barnache S, Blanchet B, LeCouedic JP, et al. Overexpression of FKBP51 in idiopathic myelofibrosis regulates the growth factor independence of megakaryocyte progenitors. Blood. 2002;100:2932–40. doi: 10.1182/blood-2002-02-0485. [DOI] [PubMed] [Google Scholar]
- Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, et al. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61:4315–9. [PubMed] [Google Scholar]
- Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93:1687–97. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- Haag P, Bektic J, Bartsch G, Klocker H, Eder IE. Androgen receptor down regulation by small interference RNA induces cell growth inhibition in androgen sensitive as well as in androgen independent prostate cancer cells. J Steroid Biochem Mol Biol. 2005;96:251–8. doi: 10.1016/j.jsbmb.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Han G, Buchanan G, Ittmann M, Harris JM, Yu X, Demayo FJ, et al. Mutation of the androgen receptor causes oncogenic transformation of the prostate. Proc Natl Acad Sci U S A. 2005;102:1151–6. doi: 10.1073/pnas.0408925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding MW, Galat A, Uehling DE, Schreiber SL. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989;341:758–60. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003;144:2380–7. doi: 10.1210/en.2003-0092. [DOI] [PubMed] [Google Scholar]
- Igawa T, Lin FF, Lee MS, Karan D, Batra SK, Lin MF. Establishment and characterization of androgen-independent human prostate cancer LNCaP cell model. Prostate. 2002;50:222–35. doi: 10.1002/pros.10054. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Jiang W, Cazacu S, Xiang C, Zenklusen JC, Fine HA, Berens M, et al. FK506 binding protein mediates glioma cell growth and sensitivity to rapamycin treatment by regulating NF-kappaB signaling pathway. Neoplasia. 2008;10:235–43. doi: 10.1593/neo.07929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komura E, Chagraoui H, Mansat de Mas V, Blanchet B, de Sepulveda P, Larbret F, et al. Spontaneous STAT5 activation induces growth factor independence in idiopathic myelofibrosis: possible relationship with FKBP51 overexpression. Exp Hematol. 2003;31:622–30. doi: 10.1016/s0301-472x(03)00085-7. [DOI] [PubMed] [Google Scholar]
- Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8–31. 1. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- Lavery DN, McEwan IJ. Structure and function of steroid receptor AF1 transactivation domains: induction of active conformations. Biochem J. 2005;391:449–64. doi: 10.1042/BJ20050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SO, Lou W, Nadiminty N, Lin X, Gao AC. Requirement for NF-(kappa)B in interleukin-4-induced androgen receptor activation in prostate cancer cells. Prostate. 2005;64:160–7. doi: 10.1002/pros.20218. [DOI] [PubMed] [Google Scholar]
- Li TH, Zhao H, Peng Y, Beliakoff J, Brooks JD, Sun Z. A promoting role of androgen receptor in androgen-sensitive and -insensitive prostate cancer cells. Nucleic Acids Res. 2007;35:2767–76. doi: 10.1093/nar/gkm198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Tang S, Thrasher JB, Griebling TL, Li B. Small-interfering RNA-induced androgen receptor silencing leads to apoptotic cell death in prostate cancer. Mol Cancer Ther. 2005;4:505–15. doi: 10.1158/1535-7163.MCT-04-0313. [DOI] [PubMed] [Google Scholar]
- Magee JA, Chang LW, Stormo GD, Milbrandt J. Direct, androgen receptor-mediated regulation of the FKBP5 gene via a distal enhancer element. Endocrinology. 2006;147:590–8. doi: 10.1210/en.2005-1001. [DOI] [PubMed] [Google Scholar]
- Makkonen H, Kauhanen M, Paakinaho V, Jaaskelainen T, Palvimo JJ. Long-range activation of FKBP51 transcription by the androgen receptor via distal intronic enhancers. Nucleic Acids Res. 2009a doi: 10.1093/nar/gkp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkonen H, Kauhanen M, Paakinaho V, Jaaskelainen T, Palvimo JJ. Long-range activation of FKBP51 transcription by the androgen receptor via distal intronic enhancers. Nucleic Acids Res. 2009b;37:4135–48. doi: 10.1093/nar/gkp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwan IJ. Molecular mechanisms of androgen receptor-mediated gene regulation: structure-function analysis of the AF-1 domain. Endocr Relat Cancer. 2004;11:281–93. doi: 10.1677/erc.0.0110281. [DOI] [PubMed] [Google Scholar]
- Mousses S, Wagner U, Chen Y, Kim JW, Bubendorf L, Bittner M, et al. Failure of hormone therapy in prostate cancer involves systematic restoration of androgen responsive genes and activation of rapamycin sensitive signaling. Oncogene. 2001;20:6718–23. doi: 10.1038/sj.onc.1204889. [DOI] [PubMed] [Google Scholar]
- Nakao R, Haji M, Yanase T, Ogo A, Takayanagi R, Katsube T, et al. A single amino acid substitution (Met786----Val) in the steroid-binding domain of human androgen receptor leads to complete androgen insensitivity syndrome. J Clin Endocrinol Metab. 1992;74:1152–7. doi: 10.1210/jcem.74.5.1569163. [DOI] [PubMed] [Google Scholar]
- Periyasamy S, Warrier M, Tillekeratne MP, Shou W, Sanchez ER. The immunophilin ligands cyclosporin A and FK506 suppress prostate cancer cell growth by androgen receptor-dependent and -independent mechanisms. Endocrinology. 2007;148:4716–26. doi: 10.1210/en.2007-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WB, Galigniana MD, Harrell JM, DeFranco DB. Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement. Cell Signal. 2004;16:857–72. doi: 10.1016/j.cellsig.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Ratajczak T, Ward BK, Minchin RF. Immunophilin chaperones in steroid receptor signalling. Curr Top Med Chem. 2003;3:1348–57. doi: 10.2174/1568026033451934. [DOI] [PubMed] [Google Scholar]
- Reid J, Murray I, Watt K, Betney R, McEwan IJ. The androgen receptor interacts with multiple regions of the large subunit of general transcription factor TFIIF. J Biol Chem. 2002;277:41247–53. doi: 10.1074/jbc.M205220200. [DOI] [PubMed] [Google Scholar]
- Reynolds PD, Ruan Y, Smith DF, Scammell JG. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J Clin Endocrinol Metab. 1999;84:663–9. doi: 10.1210/jcem.84.2.5429. [DOI] [PubMed] [Google Scholar]
- Riggs DL, Cox MB, Tardif HL, Hessling M, Buchner J, Smith DF. Noncatalytic role of the FKBP52 peptidyl-prolyl isomerase domain in the regulation of steroid hormone signaling. Mol Cell Biol. 2007;27:8658–69. doi: 10.1128/MCB.00985-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs DL, Roberts PJ, Chirillo SC, Cheung-Flynn J, Prapapanich V, Ratajczak T, et al. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. Embo J. 2003;22:1158–67. doi: 10.1093/emboj/cdg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano MF, Avellino R, Petrella A, Bisogni R, Romano S, Venuta S. Rapamycin inhibits doxorubicin-induced NF-kappaB/Rel nuclear activity and enhances the apoptosis of melanoma cells. Eur J Cancer. 2004;40:2829–36. doi: 10.1016/j.ejca.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Scherr D, Swindle PW, Scardino PT. National Comprehensive Cancer Network guidelines for the management of prostate cancer. Urology. 2003;61:14–24. doi: 10.1016/s0090-4295(02)02395-6. [DOI] [PubMed] [Google Scholar]
- Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–42. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Simental JA, Sar M, Lane MV, French FS, Wilson EM. Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem. 1991;266:510–8. [PubMed] [Google Scholar]
- Thalmann GN, Sikes RA, Wu TT, Degeorges A, Chang SM, Ozen M, et al. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate. 2000;44:91–103. doi: 10.1002/1097-0045(20000701)44:2<91::aid-pros1>3.0.co;2-l. Jul 1;44(2) [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Mehra R, Rhodes DR, Cao X, Wang L, Dhanasekaran SM, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39:41–51. doi: 10.1038/ng1935. [DOI] [PubMed] [Google Scholar]
- Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A. 2005;102:14326–31. doi: 10.1073/pnas.0505775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–86. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Vanaja DK, Mitchell SH, Toft DO, Young CY. Effect of geldanamycin on androgen receptor function and stability. Cell Stress Chaperones. 2002;7:55–64. doi: 10.1379/1466-1268(2002)007<0055:eogoar>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco AM, Gillis KA, Li Y, Brown EL, Sadler TM, Achilleos M, et al. Identification and validation of novel androgen-regulated genes in prostate cancer. Endocrinology. 2004;145:3913–24. doi: 10.1210/en.2004-0311. [DOI] [PubMed] [Google Scholar]
- Veldscholte J, Berrevoets CA, Brinkmann AO, Grootegoed JA, Mulder E. Antiandrogens and the mutated androgen receptor of LNCaP cells: differential effects on binding affinity, heat-shock protein interaction, and transcription activation. Biochemistry. 1992a;31:2393–9. doi: 10.1021/bi00123a026. [DOI] [PubMed] [Google Scholar]
- Veldscholte J, Berrevoets CA, Zegers ND, van der Kwast TH, Grootegoed JA, Mulder E. Hormone-induced dissociation of the androgen receptor-heat-shock protein complex: use of a new monoclonal antibody to distinguish transformed from nontransformed receptors. Biochemistry. 1992b;31:7422–30. doi: 10.1021/bi00147a029. [DOI] [PubMed] [Google Scholar]
- Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609–16. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- Yong W, Yang Z, Periyasamy S, Chen H, Yucel S, Li W, et al. Essential role for Co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology. J Biol Chem. 2007;282:5026–36. doi: 10.1074/jbc.M609360200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Zhang JS, Young CY. Silymarin inhibits function of the androgen receptor by reducing nuclear localization of the receptor in the human prostate cancer cell line LNCaP. Carcinogenesis. 2001;22:1399–403. doi: 10.1093/carcin/22.9.1399. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Galigniana MD, Harrell JM, DeFranco DB. Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement. Cell Signal. 2004;16:857–72. doi: 10.1016/j.cellsig.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Ratajczak T, Ward BK, Minchin RF. Immunophilin chaperones in steroid receptor signalling. Curr Top Med Chem. 2003;3:1348–57. doi: 10.2174/1568026033451934. [DOI] [PubMed] [Google Scholar]
- Reid J, Murray I, Watt K, Betney R, McEwan IJ. The androgen receptor interacts with multiple regions of the large subunit of general transcription factor TFIIF. J Biol Chem. 2002;277:41247–53. doi: 10.1074/jbc.M205220200. [DOI] [PubMed] [Google Scholar]
- Reynolds PD, Ruan Y, Smith DF, Scammell JG. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J Clin Endocrinol Metab. 1999;84:663–9. doi: 10.1210/jcem.84.2.5429. [DOI] [PubMed] [Google Scholar]
- Riggs DL, Cox MB, Tardif HL, Hessling M, Buchner J, Smith DF. Noncatalytic role of the FKBP52 peptidyl-prolyl isomerase domain in the regulation of steroid hormone signaling. Mol Cell Biol. 2007;27:8658–69. doi: 10.1128/MCB.00985-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs DL, Roberts PJ, Chirillo SC, Cheung-Flynn J, Prapapanich V, Ratajczak T, et al. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. Embo J. 2003;22:1158–67. doi: 10.1093/emboj/cdg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano MF, Avellino R, Petrella A, Bisogni R, Romano S, Venuta S. Rapamycin inhibits doxorubicin-induced NF-kappaB/Rel nuclear activity and enhances the apoptosis of melanoma cells. Eur J Cancer. 2004;40:2829–36. doi: 10.1016/j.ejca.2004.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.