Abstract
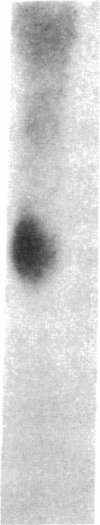
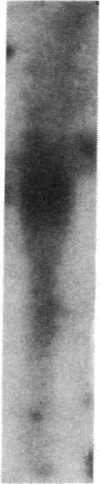
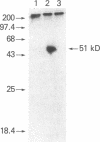
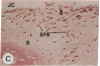
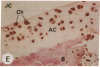
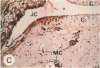
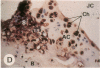
Transin is a neutral metalloproteinase initially isolated from malignantly transformed rat fibroblasts and subsequently shown to be homologous to human stromelysin. We performed Northern blot analysis on synovial tissue specimens from Lewis rats with proliferative and invasive streptococcal cell wall (SCW) arthritis. Transin mRNA was present in abundance, as was the mRNA of the c-myc oncogene, which is associated with cellular proliferation. Immunohistochemical staining of synovia from rats with chronic SCW arthritis showed high-level transin expression in the cells of the lining layer and underlying stroma, as well as in chondrocytes and osteoclasts in subchondral bone. Intense nuclear staining for the Myc oncoprotein was also detected with a cross-reactive antibody to v-Myc. Transin stained similarly in the early, rapid-onset, thymus-independent, acute phase of SCW arthritis. In the T cell-dependent adjuvant arthritis, transin expression was noted by day 4, 6 d before the influx of mononuclear cells and the onset of clinical disease. Athymic rats did not express transin. We concluded that transin is a marker of proliferative, invasive arthritis in rats and appears early in the course of disease development, but requires a competent immune system to sustain its expression in these model arthropathies.
Full text
PDF


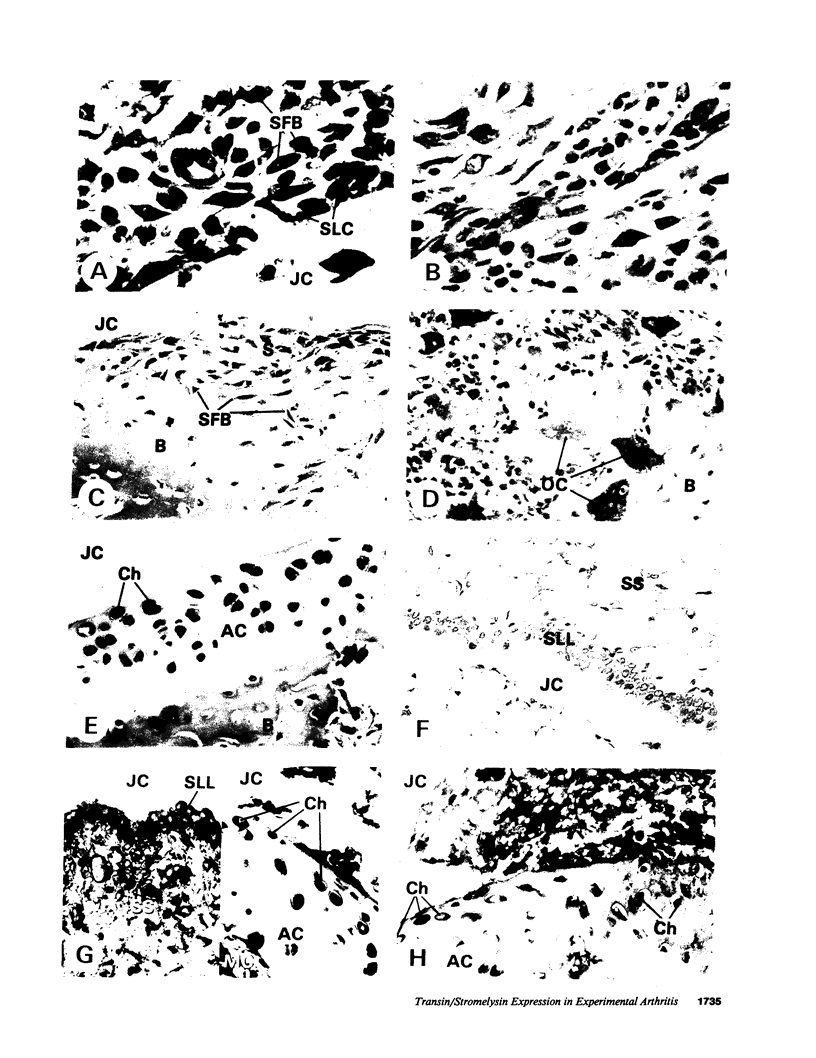





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Bishop J. M., Smith D. H., Chen E. Y., Colby W. W., Levinson A. D. Nucleotide sequence to the v-myc oncogene of avian retrovirus MC29. Proc Natl Acad Sci U S A. 1983 Jan;80(1):100–104. doi: 10.1073/pnas.80.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. B., Malone D. G., Wahl S. M., Calandra G. B., Wilder R. L. Role of the thymus in streptococcal cell wall-induced arthritis and hepatic granuloma formation. Comparative studies of pathology and cell wall distribution in athymic and euthymic rats. J Clin Invest. 1985 Sep;76(3):1042–1056. doi: 10.1172/JCI112057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastassiades T. P., Ley J., Wood A., Irwin D. The growth kinetics of synovial fibroblastic cells from inflammatory and noninflammatory arthropathies. Arthritis Rheum. 1978 May;21(4):461–466. doi: 10.1002/art.1780210410. [DOI] [PubMed] [Google Scholar]
- Berry H., Willoughby D. A., Giroud J. P. Evidence for an endogenous antigen in the adjuvant arthritic rat. J Pathol. 1973 Dec;111(4):229–238. doi: 10.1002/path.1711110403. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., Coffey J. W., Sullivan A. C. Inflammation and collagenase production in rats with adjuvant arthritis reduced with 13-cis-retinoic acid. Science. 1983 Aug 19;221(4612):756–758. doi: 10.1126/science.6308759. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., Harris E. D., Jr Survival of rheumatoid synovium implanted into nude mice. Am J Pathol. 1981 Jun;103(3):411–418. [PMC free article] [PubMed] [Google Scholar]
- Brinckerhoff C. E., Sheldon L. A., Benoit M. C., Burgess D. R., Wilder R. L. Effect of retinoids on rheumatoid arthritis, a proliferative and invasive non-malignant disease. Ciba Found Symp. 1985;113:191–211. doi: 10.1002/9780470720943.ch12. [DOI] [PubMed] [Google Scholar]
- Bromley M., Woolley D. E. Chondroclasts and osteoclasts at subchondral sites of erosion in the rheumatoid joint. Arthritis Rheum. 1984 Sep;27(9):968–975. doi: 10.1002/art.1780270902. [DOI] [PubMed] [Google Scholar]
- Bromley M., Woolley D. E. Histopathology of the rheumatoid lesion. Identification of cell types at sites of cartilage erosion. Arthritis Rheum. 1984 Aug;27(8):857–863. doi: 10.1002/art.1780270804. [DOI] [PubMed] [Google Scholar]
- Case J. P., Lorberboum-Galski H., Lafyatis R., FitzGerald D., Wilder R. L., Pastan I. Chimeric cytotoxin IL2-PE40 delays and mitigates adjuvant-induced arthritis in rats. Proc Natl Acad Sci U S A. 1989 Jan;86(1):287–291. doi: 10.1073/pnas.86.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Hein A., Dynesius-Trentham R., Trentham D. E. Morphologic demonstration of two stages in the development of type II collagen-induced arthritis. Lab Invest. 1982 Mar;46(3):321–343. [PubMed] [Google Scholar]
- Chin J. R., Murphy G., Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985 Oct 5;260(22):12367–12376. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker J. L., Malone D. G., Haraoui B., Wahl S. M., Schrieber L., Klippel J. H., Steinberg A. D., Wilder R. L. NIH conference. Rheumatoid arthritis: evolving concepts of pathogenesis and treatment. Ann Intern Med. 1984 Dec;101(6):810–824. doi: 10.7326/0003-4819-101-6-810. [DOI] [PubMed] [Google Scholar]
- Fassbender H. G. Histomorphological basis of articular cartilage destruction in rheumatoid arthritis. Coll Relat Res. 1983 Mar;3(2):141–155. doi: 10.1016/s0174-173x(83)80040-5. [DOI] [PubMed] [Google Scholar]
- Fassbender H. G., Simmling-Annefeld M., Stofft E. Transformation der Synovialzellen bei rheumatoider Arthritis. Verh Dtsch Ges Pathol. 1980;64:193–212. [PubMed] [Google Scholar]
- Fini M. E., Karmilowicz M. J., Ruby P. L., Beeman A. M., Borges K. A., Brinckerhoff C. E. Cloning of a complementary DNA for rabbit proactivator. A metalloproteinase that activates synovial cell collagenase, shares homology with stromelysin and transin, and is coordinately regulated with collagenase. Arthritis Rheum. 1987 Nov;30(11):1254–1264. doi: 10.1002/art.1780301108. [DOI] [PubMed] [Google Scholar]
- Fini M. E., Plucinska I. M., Mayer A. S., Gross R. H., Brinckerhoff C. E. A gene for rabbit synovial cell collagenase: member of a family of metalloproteinases that degrade the connective tissue matrix. Biochemistry. 1987 Sep 22;26(19):6156–6165. doi: 10.1021/bi00393a032. [DOI] [PubMed] [Google Scholar]
- Frisch S. M., Clark E. J., Werb Z. Coordinate regulation of stromelysin and collagenase genes determined with cDNA probes. Proc Natl Acad Sci U S A. 1987 May;84(9):2600–2604. doi: 10.1073/pnas.84.9.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch S. M., Ruley H. E. Transcription from the stromelysin promoter is induced by interleukin-1 and repressed by dexamethasone. J Biol Chem. 1987 Dec 5;262(34):16300–16304. [PubMed] [Google Scholar]
- Galloway W. A., Murphy G., Sandy J. D., Gavrilovic J., Cawston T. E., Reynolds J. J. Purification and characterization of a rabbit bone metalloproteinase that degrades proteoglycan and other connective-tissue components. Biochem J. 1983 Mar 1;209(3):741–752. doi: 10.1042/bj2090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- HE C. S., Wilhelm S. M., Pentland A. P., Marmer B. L., Grant G. A., Eisen A. Z., Goldberg G. I. Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2632–2636. doi: 10.1073/pnas.86.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A. Hypothesis: in vitro evidence for the invasive and tumor-like properties of the rheumatoid pannus. J Rheumatol. 1983 Dec;10(6):845–851. [PubMed] [Google Scholar]
- Haraoui B., Wilder R. L., Allen J. B., Sporn M. B., Helfgott R. K., Brinckerhoff C. E. Dose-dependent suppression by the synthetic retinoid, 4-hydroxyphenyl retinamide, of streptococcal cell wall-induced arthritis in rats. Int J Immunopharmacol. 1985;7(6):903–916. doi: 10.1016/0192-0561(85)90054-2. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr Recent insights into the pathogenesis of the proliferative lesion in rheumatoid arthritis. Arthritis Rheum. 1976 Jan-Feb;19(1):68–72. doi: 10.1002/art.1780190111. [DOI] [PubMed] [Google Scholar]
- Henderson B., Glynn L. E., Bitensky L., Chayen J. Evidence for cell division in synoviocytes in acutely inflamed rabbit joints. Ann Rheum Dis. 1981 Apr;40(2):177–181. doi: 10.1136/ard.40.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B., Glynn L. E., Chayen J. Cell division in the synovial lining in experimental allergic arthritis: proliferation of cells during the development of chronic arthritis. Ann Rheum Dis. 1982 Jun;41(3):275–281. doi: 10.1136/ard.41.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B., Glynn L. E., Chayen J. Experimental allergic arthritis in the rabbit: alterations in the cellularity and the rate of cellular proliferation in the synovial linings of the challenged joints of rabbits immunized with antigen in Freund's incomplete adjuvant. Br J Exp Pathol. 1982 Feb;63(1):5–12. [PMC free article] [PubMed] [Google Scholar]
- Henderson B., Pettipher E. R. The synovial lining cell: biology and pathobiology. Semin Arthritis Rheum. 1985 Aug;15(1):1–32. doi: 10.1016/0049-0172(85)90007-1. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Naparstek Y., Ben-Nun A., Cohen I. R. Lines of T lymphocytes induce or vaccinate against autoimmune arthritis. Science. 1983 Jan 7;219(4580):56–58. doi: 10.1126/science.6336851. [DOI] [PubMed] [Google Scholar]
- Jones D. J., Ghosh A. K., Moore M., Schofield P. F. A critical appraisal of the immunohistochemical detection of the c-myc oncogene product in colorectal cancer. Br J Cancer. 1987 Dec;56(6):779–783. doi: 10.1038/bjc.1987.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KULKA J. P., BOCKING D., ROPES M. W., BAUER W. Early joint lesions of rheumatoid arthritis; report of eight cases, with knee biopsies of lesions of less than one year's duration. AMA Arch Pathol. 1955 Feb;59(2):129–150. [PubMed] [Google Scholar]
- Kayashima K., Koga T., Onoue K. Role of T lymphocytes in adjuvant arthritis. I. Evidence for the regulatory function of thymus-derived cells in the induction of the disease. J Immunol. 1976 Nov;117(5 PT2):1878–1882. [PubMed] [Google Scholar]
- Klein G., Klein E. Evolution of tumours and the impact of molecular oncology. Nature. 1985 May 16;315(6016):190–195. doi: 10.1038/315190a0. [DOI] [PubMed] [Google Scholar]
- Kohashi O., Aihara K., Ozawa A., Kotani S., Azuma I. New model of a synthetic adjuvant, N-acetylmuramyl-L-alanyl-D-isoglutamine- induced arthritis: clinical and histologic studies in athymic nude and euthymic rats. Lab Invest. 1982 Jul;47(1):27–36. [PubMed] [Google Scholar]
- Lafyatis R., Remmers E. F., Roberts A. B., Yocum D. E., Sporn M. B., Wilder R. L. Anchorage-independent growth of synoviocytes from arthritic and normal joints. Stimulation by exogenous platelet-derived growth factor and inhibition by transforming growth factor-beta and retinoids. J Clin Invest. 1989 Apr;83(4):1267–1276. doi: 10.1172/JCI114011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewi G., Dorling J., Glynn L. E. The origin of antibody-producing cells in experimental synovitis. Int Arch Allergy Appl Immunol. 1971;41(1):132–137. doi: 10.1159/000230502. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M., Bowden G. T., Krieg P., Fürstenberger G., Briand J. P., Leroy P., Breathnach R. The mRNA coding for the secreted protease transin is expressed more abundantly in malignant than in benign tumors. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9413–9417. doi: 10.1073/pnas.83.24.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Glaichenhaus N., Gesnel M. C., Breathnach R. Epidermal growth factor and oncogenes induce transcription of the same cellular mRNA in rat fibroblasts. EMBO J. 1985 Jun;4(6):1435–1440. doi: 10.1002/j.1460-2075.1985.tb03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Leroy P., Ruhlmann C., Gesnel M. C., Breathnach R. Isolation of the oncogene and epidermal growth factor-induced transin gene: complex control in rat fibroblasts. Mol Cell Biol. 1986 May;6(5):1679–1686. doi: 10.1128/mcb.6.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Stephens P. E., Smith B. J., Docherty A. J. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987 Nov 15;248(1):265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Hembry R. M., Reynolds J. J. Characterization of a specific antiserum to rabbit stromelysin and demonstration of the synthesis of collagenase and stromelysin by stimulated rabbit articular chondrocytes. Coll Relat Res. 1986 Oct;6(4):351–363. doi: 10.1016/s0174-173x(86)80005-x. [DOI] [PubMed] [Google Scholar]
- NEWBOULD B. B. LYMPHATIC DRAINAGE AND ADJUVANT-INDUCED ARTHRITIS IN RATS. Br J Exp Pathol. 1964 Aug;45:375–383. [PMC free article] [PubMed] [Google Scholar]
- NEWBOULD B. B. ROLE OF LYMPH NODES IN ADJUVANT-INDUCED ARTHRITIS IN RATS. Ann Rheum Dis. 1964 Sep;23:392–396. doi: 10.1136/ard.23.5.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Nagase H., Harris E. D., Jr A metalloproteinase from human rheumatoid synovial fibroblasts that digests connective tissue matrix components. Purification and characterization. J Biol Chem. 1986 Oct 25;261(30):14245–14255. [PubMed] [Google Scholar]
- Ostrowski L. E., Finch J., Krieg P., Matrisian L., Patskan G., O'Connell J. F., Phillips J., Slaga T. J., Breathnach R., Bowden G. T. Expression pattern of a gene for a secreted metalloproteinase during late stages of tumor progression. Mol Carcinog. 1988;1(1):13–19. doi: 10.1002/mc.2940010106. [DOI] [PubMed] [Google Scholar]
- Saus J., Quinones S., Otani Y., Nagase H., Harris E. D., Jr, Kurkinen M. The complete primary structure of human matrix metalloproteinase-3. Identity with stromelysin. J Biol Chem. 1988 May 15;263(14):6742–6745. [PubMed] [Google Scholar]
- Stanton L. W., Watt R., Marcu K. B. Translocation, breakage and truncated transcripts of c-myc oncogene in murine plasmacytomas. Nature. 1983 Jun 2;303(5916):401–406. doi: 10.1038/303401a0. [DOI] [PubMed] [Google Scholar]
- Taurog J. D., Argentieri D. C., McReynolds R. A. Adjuvant arthritis. Methods Enzymol. 1988;162:339–355. doi: 10.1016/0076-6879(88)62089-1. [DOI] [PubMed] [Google Scholar]
- Taurog J. D., Sandberg G. P., Mahowald M. L. The cellular basis of adjuvant arthritis. II. Characterization of the cells mediating passive transfer. Cell Immunol. 1983 Aug;80(1):198–204. doi: 10.1016/0008-8749(83)90106-5. [DOI] [PubMed] [Google Scholar]
- Vater C. A., Nagase H., Harris E. D., Jr Purification of an endogenous activator of procollagenase from rabbit synovial fibroblast culture medium. J Biol Chem. 1983 Aug 10;258(15):9374–9382. [PubMed] [Google Scholar]
- Vernon-Roberts B., Liyanage S. P., Currey H. L. Adjuvant arthritis in the rat. Distribution of fluorescent material after footpad injection of rhodamine-labelled tubercle bacilli. Ann Rheum Dis. 1975 Oct;35(5):389–397. doi: 10.1136/ard.35.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]
- Whitham S. E., Murphy G., Angel P., Rahmsdorf H. J., Smith B. J., Lyons A., Harris T. J., Reynolds J. J., Herrlich P., Docherty A. J. Comparison of human stromelysin and collagenase by cloning and sequence analysis. Biochem J. 1986 Dec 15;240(3):913–916. doi: 10.1042/bj2400913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder R. L., Allen J. B., Hansen C. Thymus-dependent and -independent regulation of Ia antigen expression in situ by cells in the synovium of rats with streptococcal cell wall-induced arthritis. Differences in site and intensity of expression in euthymic, athymic, and cyclosporin A-treated LEW and F344 rats. J Clin Invest. 1987 Apr;79(4):1160–1171. doi: 10.1172/JCI112933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder R. L., Calandra G. B., Garvin A. J., Wright K. D., Hansen C. T. Strain and sex variation in the susceptibility to streptococcal cell wall-induced polyarthritis in the rat. Arthritis Rheum. 1982 Sep;25(9):1064–1072. doi: 10.1002/art.1780250906. [DOI] [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Kronberger A., Eisen A. Z., Marmer B. L., Grant G. A., Bauer E. A., Goldberg G. I. Human skin fibroblast stromelysin: structure, glycosylation, substrate specificity, and differential expression in normal and tumorigenic cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6725–6729. doi: 10.1073/pnas.84.19.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley D. E., Brinckerhoff C. E., Mainardi C. L., Vater C. A., Evanson J. M., Harris E. D., Jr Collagenase production by rheumatoid synovial cells: morphological and immunohistochemical studies of the dendritic cell. Ann Rheum Dis. 1979 Jun;38(3):262–270. doi: 10.1136/ard.38.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley D. E., Crossley M. J., Evanson J. M. Collagenase at sites of cartilage erosion in the rheumatoid joint. Arthritis Rheum. 1977 Jul-Aug;20(6):1231–1239. doi: 10.1002/art.1780200612. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Harris E. D., Jr, Mainardi C. L., Brinckerhoff C. E. Collagenase immunolocalization in cultures of rheumatoid synovial cells. Science. 1978 May 19;200(4343):773–775. doi: 10.1126/science.205952. [DOI] [PubMed] [Google Scholar]
- Yocum D. E., Lafyatis R., Remmers E. F., Schumacher H. R., Wilder R. L. Hyperplastic synoviocytes from rats with streptococcal cell wall-induced arthritis exhibit a transformed phenotype that is thymic-dependent and retinoid inhibitable. Am J Pathol. 1988 Jul;132(1):38–48. [PMC free article] [PubMed] [Google Scholar]