Abstract
This study examined the risk for alcoholism, diabetes, and depression (triADD) in American Indian/Alaska Native (AI/AN) populations in the U.S. Using the Behavioral Risk Factor Surveillance System, a series of descriptive statistics and regression models were used to examine the interrelationships among these disorders in AI/AN populations. Despite a small sample size, results indicate that AI/ANs are at elevated risk for the individual and combined presence of triADD (OR=12.5) when compared to the White population. These findings indicate the need for further investigation and prevention focused on effective, culturally appropriate interventions with these populations.
American Indian/Alaska Native (AI/AN) populations are afflicted disproportionately with a number of chronic illnesses (Indian Health Service [IHS], 2001). Specifically, the rates of Type 2 Diabetes, alcohol abuse, and suicide have consistently been higher in these populations for many years (IHS). To date the majority of research has focused on these three disorders alone or in dyads; little attention has been given to the co-occurrence of all three simultaneously in AI/AN populations. The Behavioral Risk Factor Surveillance System (BRFSS) provides annual data on estimates of risk factors and health-related behavior in state populations across the nation. This article reports an analysis of the prevalence and correlates of diabetes and associated risk factors for depression and alcohol abuse in AI/AN adults sampled in the fifty states as part of the 2003 BRFSS survey.
Background
Alcohol abuse, Type 2 diabetes, and depression share a number of common attributes in AI/AN populations. They are expressed at disproportionately high rates, they are increasing in prevalence, and they are considered chronic illnesses, necessitating significant levels of self-management. Data on alcohol use and abuse in AI/AN communities are often misleading. These populations have the lowest overall rates of individuals who consume alcohol but concomitantly have the highest rates of “heavy drinkers” (defined as binge drinking 5 times a month or more) among those aged 26 and older (Substance Abuse and Mental Health Services Administration, 2002). The age-adjusted death rates due to alcohol dependence in AI/ANs have been reported as more than 7 times higher than that of the U.S. All-Races rate. In AI/AN adults aged 25-44, alcohol is associated with the two leading causes of mortality: accidents and chronic liver disease/cirrhosis. (IHS, 2001)
Prevalence rates of Type 2 diabetes in AI/AN populations vary among tribal groups, but consistently rank higher than the rate for non-Hispanic Whites in any given year. It has been estimated that the overall prevalence across tribes is 4 to 8 times higher in AI/ANs than in the general population (Lee et al., 1995). The American Diabetes Association (ADA) reports that, on average, AI/ANs are 2.2 times more likely to have diabetes than the non-Hispanic White population (ADA, n.d.a). Supporting this finding, the Centers for Disease Control reported 15.3% prevalence in AI/ANs compared to 7.3% in the U.S. All-Races population in 2002 (Acton, Burrows, Geiss, & Thompson, 2003). In the southwestern U.S. the Pima Indians have the highest documented prevalence rate in the world, with 50% of the population over the age of 35 diagnosed with diabetes (Knowler, Pettitt, Saad, & Bennett, 1990).
Depression among AI/AN populations has been understudied; however, initial research suggests that rates of some mental illness and depressive symptoms are higher in these populations (Manson, 2001). This scarcity of research has made characterizing the illness from an epidemiological perspective challenging, at best, and much more difficult than characterizing more heavily studied diseases such as diabetes and alcohol abuse. Nonetheless, research has found a high co-occurrence of depressive disorder with suicidal ideation. For example, Roberts and Yeager (2004) found that 72% of an adult psychiatric sample with depressive disorder presented with current suicidal ideation. Due to the startlingly high rates of suicide in these populations, this manifestation of mental distress and other depressive symptoms is often used as a proxy for depression and mental illness. Manson reported 32% of AI elders utilizing an urban IHS facility experienced depressive symptoms, a rate considerably higher than those seen in older White populations. Another study described 38% of AI adults interviewed as having problems with depression (Wilson, Civic, & Glass, 1995). In separate mental health needs assessments of urban and semi-urban Indian populations conducted in Colorado (King, 1999), Montana (Barron, Oge, & Markovich, 1999), and Arizona (Chester, Mahalish, & Davis, 1999; Evaneshko, 1999), high rates of depressive symptoms were described in all of the study populations.
Depression and Diabetes
To date there have been few, if any, studies that have explored the relationship between diabetes and depression in AI/AN communities, other than to document that these two issues are of concern in these populations. In the general population, individuals with diabetes are twice as likely to have a diagnosis of depression as individuals who do not have diabetes (Anderson, 2001). In a meta-analysis that examined 27 studies of the association between diabetes and depression, it was found that an increase in depressive symptoms was associated with an increase in the severity and/or number of diabetes complications (de Groot, Anderson, Freedland, Clouse, & Lustman, 2001). However, the temporal relationship between depression and diabetes is unclear (Iosifescu et al., 2003). Previous studies have found that depression may occur in response to the psychosocial pressure that ensues after a diabetes diagnosis (Egede & Zheng, 2003) or as a result of biochemical changes caused by diabetes or its treatment (Lustman, Anderson, Freedland, de Groot, & Carney, 2000). Others argue that depression not only tends to precede a Type 2 diabetes diagnosis, but it may actually increase the risk for developing Type 2 diabetes, thus suggesting that there is an inverse temporal relationship between the two conditions (Talbot & Nouwen, 2000; Eaton, Armenian, Gallo, Pratt, & Ford, 1996). A correlation has been found between glycemic control and depression (Anderson; Lustman et al.), although the temporal relation is unclear as well. Does better glycemic control reduce depression or vice versa? The association between diabetes and depression is complicated by other factors that are linked to both of these conditions, such as obesity and cardiovascular disease (Nichols & Brown, 2003). A recent study conducted among another minority group (Mexican Americans) suggests that diabetes and depression have a much larger combined effect than they do individually (Black, Markides, & Ray, 2003). More research is necessary to elucidate the comorbidity of these disorders with AI/AN populations.
Diabetes and Alcohol Abuse
Information on the comorbidity of alcohol abuse and diabetes in AI/ANs is also scarce. Saremi, Hansen, and Tulloch-Reid (2004) examined the relationship between Type 2 diabetes, alcohol consumption, and hypertension in an AI population only to determine that there was no association between alcohol consumption and prevalence or incidence of diabetes, although they did find a relationship between alcohol consumption and hypertension. However, other population-based studies have consistently found that high alcohol intake increases one's risk of developing Type 2 diabetes (Carlsson et al., 2000; Holbrook, Barrett-Connor, & Wingard, 1990; Howard, Arnsten, & Gourevitch, 2004; Kao, Puddey, Boland, Watson, & Brancati, 2001), while moderate alcohol intake has not been shown to increase risk (Kao et al.), and in fact may have some protective value (Anderson, 2001; Kao et al., Howard et al.). Differences in risk were noted in men and women (Saremi et al.), with much of the prior research focusing on males (Carlsson et al., Conigrave et al., 2001; Perreira & Sloan, 2002; Wei, Gibbons, Mitchell, Kampert, & Blair, 2000). In their meta-analysis of the effect of alcohol consumption on diabetes, Howard et al. found that individuals who consume 3 or more alcoholic drinks each day have a 43% greater risk of diabetes. Little information has been published on the effects that alcohol has on the behaviors of individuals living with diabetes, and while studies have been conducted that suggest that moderate alcohol consumption is protective against other illnesses associated with diabetes, it is likely that heavy alcohol consumption increases the risk of developing comorbid illnesses (Howard et al.). For individuals living with diabetes, high alcohol consumption can be detrimental because it inhibits glucose metabolism (Holbrook et al.; Franz et al., 2002), and obesity may be a mediating factor by which alcohol abuse influences the development of diabetes (Howard et al., Carlsson et al.).
Depression and Alcohol Abuse
Depressive syndrome has been found to be significantly more common among alcoholics than among non-alcoholics (Merikangas & Gelernter, 1990; Nurnberger, Foroud, Flury, Meyer, & Wiegand, 2002). Eighty percent of alcoholics report depressive symptoms, while 30-40% of individuals with diagnosed alcohol dependence are likely to experience a major depressive episode in their lifetimes (Hitzemann, 2000; Kessler et al., 1996; Regier et al., 1990). While little has been published in recent years regarding the comorbidity of alcohol abuse and depression among AI/ANs, it was observed as early as 1983 that excessive alcohol consumption increases the risk of psychiatric disorders in AI populations (Westermeyer & Peake, 1983). Approximately 95% of binge drinkers in some AI communities are alcoholics, thus increasing risk of psychiatric disorders (Robin, Long, Rasmussen, Albaugh, & Goldman, 1998). Causal determinants have not been established as to whether misuse of alcohol can cause depression or, conversely, depression causes alcohol abuse. While some argue that alcohol can be used as a form of self-medication for individuals with depression (Nurnberger et al.), it has alternately been argued that depression or depressive symptomology may develop as a result of alcohol abuse (Raimo & Schuckit, 1998). Regardless of temporal association, alcoholics with comorbid depression are infinitely more complicated to treat than individuals who have either depression or alcohol abuse alone (Merikangas & Gelernter).
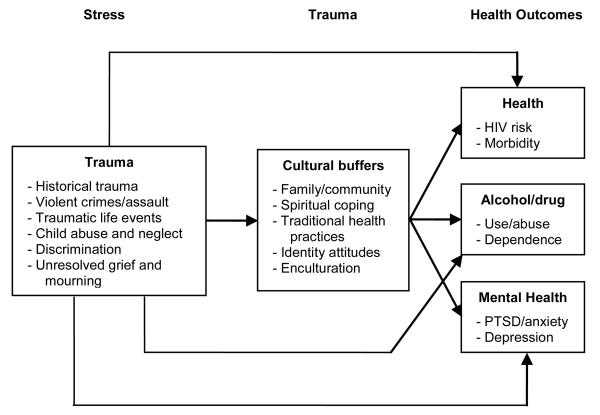
Causal factors related to the multimorbidity of depression, diabetes, and alcoholism in AI/AN populations exist within a unique cultural and sociohistorical context. Walters, Simoni, and Evans-Campbell (2002) propose an Indigenist stress-coping model which identifies not only the environmental stressors that contribute to adverse health outcomes for AI/AN populations, but also the coping mechanisms that can prevent these outcomes (see Figure). In their model, stress manifests itself in the form of trauma (e.g., colonization, violent crimes, and traumatic life events). This stress can lead to adverse health and/or mental health outcomes for AI populations. However, Walters et al. suggest that cultural moderators, such as the family/community, spirituality, traditional health practices, and enculturation, can buffer the impact of traumatic stressors. Proposed as a decolonizing conceptual framework for the etiology of adverse health conditions, their stress-coping model highlights how the sociohistorical context of AI/AN populations contributes to their health status.
Figure. Indigenist Stress-coping Model.
The majority of articles that focused on the dyads of depression and diabetes, diabetes and alcohol abuse, and depression and alcohol abuse discuss risk rather than prevalence. Studies are scarce on the impact that these comorbid conditions have on the health of individuals and communities. Although depression, diabetes, and alcohol abuse have been well documented among AI/ANs, there is a dearth of information on the interplay of all three illnesses. Anecdotal evidence shared among health care practitioners working in Indian country (e.g., one of the authors has many years' experience with tribal 638 health care organizations, and with development of IHS diabetes education standards) – coupled with the existing health research on the comorbidity of these disorders – suggests that AI/ANs are at elevated risk for the simultaneous multimorbidity of these syndromes. However, at this time, no empirical investigations have been published on the inter-relationship of these illnesses as an epidemiological phenomenon in these populations. The occurrence of alcohol abuse, diabetes, and depression in this study is referred to as a multimorbid condition called “triADD.” Using the Behavioral Risk Factor Surveillance System (BRFSS), this pilot study examines the individual and combined relationships between alcohol abuse, diabetes, and depression in AI/AN and other ethnic populations. This analysis has implications for prevention and treatment of these disorders with AI/AN populations.
Method
In 1984 the Centers for Disease Control and Prevention (CDC) launched the BRFSS, a random-digit dialed telephone survey aimed at measuring health risks in the non-institutionalized American population over eighteen years of age. The BRFSS questionnaire is designed by a working group of state coordinators and CDC staff. Standard questions are changed periodically to reflect current research related to known risks of morbidity and mortality. Currently, the questionnaire has three sections: 1) the core component, which is always used in administering the BRFSS and not modifiable in format, 2) optional modules, and 3) state-added questions. The core component is a standard set of questions asked by all states. It includes queries about current behaviors that affect health (e.g., tobacco use, women's health) and questions on demographic characteristics. Optional CDC modules are sets of questions on specific topics (e.g., smokeless tobacco) that states elect to use on their questionnaires. Although the modules are optional, CDC standards require that, if they are used, they must be used without modification.
Variables included in this initial study were derived from the core component questions from a single survey year, 2003. Variables measuring risk factors for triADD include: (1) If the respondent was ever told he or she had diabetes, not including during pregnancy and irrespective of Type 1 or Type 2 (respondents who answered yes are coded 1, respondents who answered no or did not know are coded 0); (2) the risk for heavy drinking, defined in the BRFSS as having, on average, having greater than 2 drinks per day for men and 1 drink per day for women during the past month (men and women at risk are coded 1, others are coded 0); and (3) if the number of days in the previous month in which the respondent felt his or her mental health was poor exceeded 5 (respondents with 5 or more of these days are coded 1, others are coded 0.) Five poor mental health days in the month were used in this analysis as the threshold for “poor” mental health or as a marker for being at risk for depression. This number is fewer than the common number of days (days = 14) often used by clinicians and clinical researchers as a marker for clinical depression (American Psychiatric Association, 2000), and greater than the mean of 3.0 (95% CI = 2.9-3.1) reported by BRFSS respondents between 1995 and 2000 (Kobau, Safran, Zack, Moriarty, & Chapman, 2004). Although these are self-reported measures and do not reflect clinical diagnoses, they are useful measures for identifying respondents with known risk factors. We believe a lower threshold of 5 poor days rather than 14 is justified because our broader aim is to examine individuals who may be at risk for multiple conditions. Thus, while 5 poor mental health days may not be a critical threshold on its own, 5 of these days paired with heavy drinking and diabetes is likely to be a significant burden.
Several variables were used to categorize respondents into subgroups. Race and ethnicity were categorized into White (70.37%), Black (9.59%), Asian (2.68%), Native Hawaiian and Pacific Islander (0.43%), AI/AN (1.06%), Hispanic (13.55%), and Multiracial/Other (2.33%). Following U.S. Census categories, the BRFSS data group Native Hawaiian and Pacific Islanders together. Pacific Islanders include other peoples of Polynesian ethnicity, such as peoples from Samoa and Guam. Additional subgroup indicators were age, marital status, education, and household income. Each of these indicators is measured categorically with dummy variables. For household income, a category for “missing or refused” is created because 13% of BRFSS respondents did not answer this question (this is not unusual, as income is often one of the most sensitive questions asked in surveys). In the descriptive statistics, no adjustments were made to standardize to the 2000 U.S. age distribution. Age groups, however, are included as controls in the multivariate analyses.
Because the BRFSS data involve a complex multistage survey design, the SURVEYMEANS, SURVEYFREQ, and SURVEYLOGISTIC procedures in SAS 9.1 were used to conduct weighted analyses that account for multiple strata and primary sampling units. Procedures that use only sample weights but ignore survey information about strata and sampling units will have correct point estimates, but the standard errors will be incorrect. The survey procedures we used will properly estimate the confidence intervals for means and logistic regression parameter estimates. The 2003 BRFSS interviewed 264,684 respondents. Some respondents (11,695 or 4.4%) were dropped from the analysis due to missing data, primarily from the questions on self-reported mental health (4,622 or 1.7%), drinking (2,473 or .9%), and age (2,054 or .8%). Prevalence and odds ratios for the effects of the predictors on having diabetes, heavy drinking, and poor mental health days were conducted using data from 252,989 respondents. For reference, descriptive statistics are presented in Table 1 and Table 2. Table 1 shows the means for the demographic factors used in the analysis, and Table 2 presents crosstabulations of selected demographic factors by race/ethnicity.
Table 1.
Descriptive Demographic Statistics
| % (95% CI) | |
|---|---|
| Race/Ethnicity (N = 262,381) | |
| White | 70.1 (69.8, 70.5) |
| Black | 9.7 (9.5, 9.9) |
| Asian | 2.7 (2.5, 2.8) |
| Native Hawaiian/Pacifi c Islander | 0.4 (0.4, 0.5) |
| American Indian | 1.1 (1.0, 1.1) |
| Hispanic | 13.7 (13.3, 14) |
| Multiracial/Other | 2.4 (2.3, 2.5) |
| Age (N = 262,630) | |
| 18 – 24 | 13.1 (12.8, 13.4) |
| 25 – 34 | 18 (17.7, 18.3) |
| 35 – 44 | 20.5 (20.2 – 20.8) |
| 45 – 54 | 18.4 (18.1, 18.7) |
| 55 – 65 | 13.1 (12.9, 13.4) |
| 65 – 74 | 8.8 (8.6, 9) |
| 75+ | 81. (7.9, 8.3) |
| Household Income (N = 264,684) | |
| $0 – $14,999 | 10.1 (9.8, 10.3) |
| $15,000 – $24,999 | 15.6 (15.3, 15.9) |
| $25,000 – $34,999 | 12.1 (11.9, 12.3) |
| $35,000 – $49,999 | 14.9 (14.6, 15.1) |
| $50,000 and more | 34.3 (34, 34.7) |
| Missing or Refused | 13.0 (12.8, 13.3) |
| Education (N = 264,039) | |
| Less than High School | 12.3 (12, 12.6) |
| High School | 30.4 (30.1, 30.8) |
| Some College | 26.8 (26.4, 27.1) |
| College Degree | 30.5 (30.2, 30.8) |
| Marital Status (N = 263,836) | |
| Married | 58.8 (58.4, 59.2) |
| Divorced or Separated | 11.6 (11.4, 11.8) |
| Widowed | 6.9 (6.8, 7.1) |
| Single | 18.7 (18.4, 19.1) |
| Cohabiting | 3.9 (3.8, 4.1) |
Table 2.
Crosstabulations of Racial/Ethnic Groups and Demographic Background Factors
| Education | |||||
|---|---|---|---|---|---|
| Less than High School |
High School |
Some College |
College Degree |
Total | |
| White | 7.8 | 30.4 | 28.0 | 33.8 | 100% |
| Black | 15.7 | 36.3 | 27.5 | 20.6 | 100% |
| Asian | 3.0 | 16.1 | 19.3 | 61.6 | 100% |
| Native Hawaiian/ Pacific Islander |
9.2 | 30.0 | 30.5 | 30.2 | 100% |
| American Indian | 16.0 | 34.8 | 30.7 | 18.5 | 100% |
| Hispanic | 33.6 | 29.5 | 20.8 | 16.1 | 100% |
| Multiracial/Other | 13.0 | 29.6 | 29.5 | 27.9 | 100% |
| Crosstab N = 261,812 |
|||||
| Marital Status | ||||||
|---|---|---|---|---|---|---|
| Married | Divorced/ Separated |
Widowed | Single | Cohabiting | Total | |
| White | 62.4 | 11.0 | 7.5 | 15.8 | 3.3 | 100% |
| Black | 39.4 | 17.5 | 7.5 | 32.0 | 3.5 | 100% |
| Asian | 63.1 | 4.4 | 1.9 | 28.6 | 2.0 | 100% |
| Native Hawaiian/ Pacific Islander |
55.0 | 10.2 | 1.8 | 30.8 | 2.2 | 100% |
| American Indian | 51.3 | 16.6 | 7.2 | 17.7 | 7.2 | 100% |
| Hispanic | 55.5 | 11.3 | 4.3 | 21.3 | 7.7 | 100% |
| Multiracial/Other | 49.9 | 14.7 | 5.8 | 24.7 | 5.0 | 100% |
| Crosstab N = 261,694 |
||||||
| Household Income | |||||||
|---|---|---|---|---|---|---|---|
| $0- $14,999 |
$15,000- $24,999 |
$25,000- $34,999 |
$35,000- $49,999 |
$50,000 and more |
Missing or Refused |
Total | |
| White | 7.0 | 13.2 | 11.6 | 16.0 | 39.8 | 12.5 | 100% |
| Black | 16.2 | 21.9 | 14.7 | 13.6 | 20.6 | 13.0 | 100% |
| Asian | 8.7 | 10.1 | 10.3 | 11.8 | 44.6 | 14.4 | 100% |
| Native Hawaiian/ Pacific Islander |
11.0 | 14.9 | 10.5 | 17.7 | 36.0 | 9.9 | 100% |
| American Indian | 13.5 | 22.1 | 13.3 | 16.4 | 22.5 | 12.1 | 100% |
| Hispanic | 21.1 | 24.4 | 13.3 | 11.0 | 16.4 | 13.8 | 100% |
| Multiracial/Other | 12.7 | 15.7 | 13.0 | 13.9 | 30.5 | 14.3 | 100% |
| Crosstab N = 262,381 |
|||||||
Results
Results indicate that risk factors vary across racial and ethnic groups (Table 3). Blacks (11.5%) and AI/ANs (12.5%) were the groups with the highest estimated prevalence of diabetes. The groups at highest risk for heavy drinking were Native Hawaiians/Pacific Islanders (8.0%) and AI/ANs (6.7%), both considered “indigenous” populations. The groups with the highest number of self-reported poor mental health days in the previous month were Multiracial/Other (4.6 days) and AIs (5.3 days). The differences between the top two groups in each of these risk categories are not statistically significant; e.g., diabetes levels for Blacks are not statistically different from levels for AI/ANs. Nevertheless, most notable about these descriptive results is that AI/ANs are the subgroup most at risk for the components of triADD. Although Native Hawaiians/Pacific Islanders had higher prevalence of risk for heavy drinking, they reported a lower prevalence of both diabetes and poor mental health days than did the AI/AN respondents. The higher responses reported by AI/ANs, however, may be associated with other demographic factors such as educational, marital, or income status. Thus, we conducted multivariate logistic regression analyses that control for these additional factors.
Table 3.
Prevalence of Ever Having Been Diagnosed with Diabetes, Risk for Heavy Drinkinga, and Mean Number of Poor Mental Health Days
| Diabetes % (95% CI) |
Heavy Drinking % (95% CI) |
Poor Mental Health Days Mean (95% CI) |
|
|---|---|---|---|
| Race/Ethnicity (N = 262,381) | |||
| White | 6.9 (6.8, 7.1) | 6.2 (6.0, 6.4) | 3.3 (3.2, 3.3) |
| Black | 11.5 (10.8, 12.3) | 3.6 (3.1, 4.1) | 4.0 (3.8, 4.2) |
| Asian | 5.4 (3.7, 7.1) | 1.7 (1.0, 2.3) | 2.5 (2.2, 2.9) |
| Native Hawaiian/Pacifi c Islander | 5.2 (2.1, 8.4) | 8.0 (3.8, 12.1) | 3.2 (2.2, 4.2) |
| American Indian | 12.5 (9.5, 15.6) | 6.7 (4.7, 8.7) | 5.3 (4.5, 6.0) |
| Hispanic | 7.5 (6.8, 8.2) | 4.6 (4.0, 5.3) | 3.6 (3.4, 3.9) |
| Multiracial/Other | 8.3 (7.0, 9.6) | 5.7 (4.6, 6.7) | 4.6 (4.2, 5.0) |
| Age (N = 262,630) | |||
| 18 – 24 | 0.9 (0.6, 1.2) | 10.5 (9.7, 11.3) | 4.2 (4.0, 4.4) |
| 25 – 34 | 1.9 (1.6, 2.2) | 5.7 (5.3, 6.2) | 3.7 (3.6, 3.9) |
| 35 – 44 | 4.2 (3.9, 4.5) | 5.4 (5.1, 5.7) | 3.6 (3.5, 3.7) |
| 45 – 54 | 8.0 (7.6, 8.5) | 5.4 (5.0, 5.8) | 3.7 (3.6, 3.8) |
| 55 – 64 | 14.6 (13.9, 15.3) | 4.7 (4.3, 5.1) | 3.1 (3.0, 3.2) |
| 65 – 74 | 17.4 (16.6, 18.2) | 3.6 (3.3, 4.0) | 2.2 (2.1, 2.4) |
| 75+ | 15.8 (15.0, 16.6) | 2.4 (1.9, 2.8) | 2.1 (1.9, 2.2) |
| Education (N = 264,039) | |||
| Less than High School | 12.6 (11.9, 13.3) | 4.7 (4.1, 5.2) | 4.9 (4.6, 5.1) |
| High School | 8.4 (8.0, 8.7) | 6.2 (5.9, 6.5) | 3.7 (3.6, 3.8) |
| Some College | 6.9 (6.6, 7.2) | 6.3 (5.9, 6.6) | 3.6 (3.5, 3.7) |
| College Degree | 5.2 (4.9, 5.5) | 4.8 (4.6, 5.1) | 2.4 (2.3, 2.5) |
| Marital Status (N = 263,836) | |||
| Married | 7.7 (7.4, 8.0) | 4.2 (4.0, 4.4) | 2.8 (2.7, 2.8) |
| Divorced or Separated | 9.5 (8.9, 10.1) | 6.7 (6.2, 7.1) | 5.3 (5.1, 5.5) |
| Widowed | 16.3 (15.5, 17.2) | 3.0 (2.5, 3.5) | 3.2 (3.0, 3.5) |
| Single | 3.5 (3.2, 3.9) | 9.4 (8.8, 9.9) | 4.1 (4.0, 4.3) |
| Cohabiting | 2.7 (2.0, 3.4) | 10.1 (8.9, 11.4) | 4.5 (4.2, 4.8) |
| Household Income | |||
| $0 – $14,999 | 13.7 (12.9, 14.5) | 4.9 (4.3, 5.5) | 6.1 (5.9, 6.4) |
| $15,000 – $24,999 | 10.1 (9.5, 10.6) | 5.8 (5.3, 6.3) | 4.3 (4.1, 4.4) |
| $25,000 – $34,999 | 7.9 (7.4, 8.5) | 5.6 (5.2, 6.1) | 3.4 (3.3, 3.6) |
| $35,000 – $49,999 | 6.5 (6.0, 6.9) | 6.4 (5.9, 6.9) | 3.1 (3.0, 3.3) |
| $50,000 and more | 4.6 (4.3, 4.8) | 5.9 (5.6, 6.1) | 2.4 (2.4, 2.5) |
| Missing or Refused | 8.4 (7.8, 8.9) | 4.4 (3.9, 4.8) | 3.2 (3.1, 3.4) |
Risk for heavy drinking is defi ned by CDC staff as more than 2 drinks per day for men, and more than 1 drink per day for women.
Table 4 is a model of the odds of having the components of triADD (ever having been diagnosed with diabetes, risk for heavy drinking, and five or more poor mental health days in the previous month) with race/ethnicity, age, education, marital status, and income as predictors. One unexpected finding is that being at risk for heavy drinking is negatively associated with the odds of diabetes (OR = .40; 95% CI = .31, .52). Each self-reported poor mental health day was positively associated with the odds of diabetes (OR = 1.02; 95% CI = 1.02, 1.02). One finding of note in Table 4 is that even after controlling for age, education, marital status, and income, significant race and ethnic differences in the odds of self-reported diabetes diagnoses remain. Compared to Whites, AI/ANs have the highest odds of diabetes (OR = 2.02; 95% CI = 1.50, 2.70), followed by Blacks (OR = 1.90; 95% CI = 1.73, 2.08).
Table 4.
Odds Ratios and 95% Confi dence Intervals from Multivariate Logistic Regression models of Ever Diagnosed with Diabetes, Risk for Heavy Drinking, and Five or more Poor Mental Health Days
| Ever Diagnosed with Diabetes |
Risk for Heavy Drinking |
Five or more Poor Mental Health Days |
|
|---|---|---|---|
| At risk for heavy drinking | 0.40(0.31,0.52) | 1.45(1.34,1.58) | |
| Number poor mental health days | 1.02(1.02,1.02) | 1.02(1.02,1.02) | |
| Ever diagnosed with diabetes | 0.39(0.30,0.50) | 1.43(1.33,1.53) | |
| Race/Ethnicity | |||
| White | (reference) | (reference) | (reference) |
| Black | 1.90(1.73,2.08) | 0.48(0.41,0.55) | 0.87(0.81,0.93) |
| Asian | 1.35(0.94,1.95) | 0.25(0.17,0.37) | 0.86(0.71,1.04) |
| Native Hawaiian/Pacific Islander | 1.26(0.66,2.39) | 1.10(0.61,1.98) | 1.07(0.67,1.70) |
| American Indian | 2.02(1.50,2.70) | 0.96(0.69,1.34) | 1.22(1.02,1.47) |
| Hispanic | 1.28(1.13,1.44) | 0.66(0.56,0.77) | 0.73(0.67,0.80) |
| Multiracial/Other | 1.33(1.11,1.60) | 0.79(0.65,0.97) | 1.16(1.04,1.31) |
| Age | |||
| 18 – 24 | (reference) | (reference) | (reference) |
| 25 – 34 | 2.56(1.71,3.83) | 0.69(0.61,0.78) | 0.96(0.88,1.04) |
| 35 – 44 | 6.16(4.18,9.06) | 0.71(0.62,0.80) | 0.89(0.82,0.96) |
| 45 – 54 | 0.72(0.63,0.82) | 0.83(0.76,0.90) | |
| 55 – 64 | 0.69(0.60,0.80) | 0.60(0.55,0.65) | |
| 65 – 74 | 0.59(0.51,0.70) | 0.34(0.31,0.38) | |
| 75+ | 0.39(0.30,0.49) | 0.28(0.25,0.31) | |
| Education | |||
| Less than High School | 1.47(1.32,1.63) | 1.19(1.02,1.39) | 1.56(1.44,1.68) |
| High School | 1.24(1.14,1.36) | 1.34(1.22,1.47) | 1.28(1.21,1.35) |
| Some College | 1.23(1.13,1.34) | 1.23(1.12,1.34) | 1.29(1.22,1.36) |
| College Degree | (reference) | (reference) | (reference) |
| Marital Status | |||
| Married | (reference) | (reference) | (reference) |
| Divorced or Separated | 0.91(0.83,0.99) | 1.68(1.53,1.85) | 1.64(1.55,1.73) |
| Widowed | 0.91(0.83,0.99) | 1.08(0.91,1.29) | 1.43(1.31,1.56) |
| Single | 1.00(0.89,1.12) | 2.09(1.90,2.31) | 1.23(1.16,1.31) |
| Cohabiting | 0.73(0.55,0.96) | 2.27(1.94,2.65) | 1.35(1.21,1.50) |
| Income | |||
| $0 – $14,999 | 2.28(2.02,2.57) | 0.68(0.58,0.79) | 2.24(2.06,2.43) |
| $15,000 – $24,999 | 1.76(1.59,1.95) | 0.85(0.75,0.95) | 1.62(1.51,1.74) |
| $25,000 – $34,999 | 1.47(1.32,1.63) | 0.85(0.76,0.95) | 1.29(1.20,1.39) |
| $35,000 – $49,999 | 1.32(1.20,1.46) | 0.99(0.89,1.10) | 1.18(1.11,1.26) |
| $50,000 and more | (reference) | (reference) | (reference) |
| Missing or Refused | 1.32(1.18,1.46) | 0.67(0.58,0.76) | 1.16(1.07,1.25) |
| N | 252,989 | 252,989 | 252,989 |
Table 4 also models the odds of being at risk for heavy drinking, with diabetes, poor mental health days, and the other subgroup indicators as predictors. As with the negative association between diabetes and heavy drinking described earlier, the negative relationship between diabetes and heavy drinking persists (OR = .39; 95% CI = .30, .50) when using heavy drinking as the reference rather than diabetes. Each poor mental health day was also positively associated with the odds of being at risk for heavy drinking (OR = 1.02; 95% CI = 1.02, 1.02). The race and ethnic subgroup indicators suggest that, compared to Whites, Blacks, Asians, Hispanics, and Multiracial/Other individuals had significantly lower odds of being at risk for heavy drinking. The odds for Native Hawaiians/Pacific Islanders and AI/ANs were not significantly different than the odds for Whites.
The odds of having 5 or more self-reported poor mental health days, used as a proxy for risk of depression, is also modeled in Table 4. Both diabetes (OR = 1.43; 95% CI = 1.33, 1.53) and heavy drinking (OR = 1.45; 95% CI = 1.34, 1.58) increased the odds of having 5 or more poor mental health days. Compared to Whites, Blacks and Hispanics had significantly lower odds of having 5 or more poor mental health days. Asians, Multiracial/Other, and Native Hawaiians/Pacific Islanders were no different from Whites. American Indians/Alaska Natives, however, had significantly higher odds of 5 or more poor mental health days, compared to Whites (OR = 1.22; 95% CI = 1.02, 1.47).
We next examined which subgroups had the highest odds of triADD, which was defined as having all three risk factors: diabetes, heavy drinking, and five or more reported poor mental health days. The incidence of triADD in the sample was found to be exceptionally small: 91 cases. The Asian and Native Hawaiian/Pacific Islander cases were removed from this analysis due to the low occurrences of triADD in these groups (n=1, n=0, respectively). Thus the following analysis should be interpreted with caution and considered exploratory. The comparisons made in the triADD model included 90 individuals exhibiting all three predictors. Although relatively small in comparison to the entire dataset, statistics were adjusted for the sampling scheme and the confidence intervals were computed accordingly to accurately reflect significant effects. Results indicated that AI/ANs had the highest risk of triADD compared to Whites (OR = 10.95; 95% CI = 2.98, 40.32). The second highest subgroup was Multiracial/Other (OR = 4.85; 95% CI = 1.08, 21.90).
Discussion
This study examined the risk for triADD, or the risk for co-occurring diabetes, alcohol abuse, and depression, in AI/AN populations in the U.S. Using response items from the BRFSS as an approximation of risk for the presence of these three serious health conditions, we examined their separate and combined effects for AI/ANs throughout the U.S. Aside from having the second highest prevalence of heavy drinking after the Native Hawaiian population, the separate and combined presence of each of these conditions is highest in AI/AN populations, even after controlling for marital status, income, education, and age group. Although the overall number of triADD cases was low, those experiencing this multimorbid condition most frequently were AI/ANs. Although this inquiry substantiates the need for further study into the actual prevalence of triADD and possible causal relationships, it is important to recognize the study limitations. BRFSS sampling is limited to those who have landline telephones. This excludes those who do not have telephones or who use only cellular phones, both of which are common scenarios on many reservations in the U.S. The 2003 BRFSS includes post-stratification weights to attempt to adjust for noncoverage of households without telephones, which may reduce (although not totally eliminate) bias. Not having a telephone is likely to be positively correlated with disadvantaged health outcomes. Thus, if the estimates contain noncoverage bias due to homes not having telephones, this bias is likely to cause our estimates of diabetes, heavy drinking, and poor mental health days to be lower than the actual population prevalence. Additionally, data related to race/ethnicity and the variables related to triADD are self-reported. Due to issues of social desirability, the number of alcoholic drinks consumed per day and the number of poor mental health days may be underreported. Like phone noncoverage bias, the bias introduced by social desirability probably exerts a downward pressure on estimates, since it is likely to make people less forthcoming about admitting risky behaviors and sensitive health information. Therefore, the differences we report may very well be conservative estimates of the prevalence of these poor health outcomes.
Prevalence of diabetes and the number of poor days may also be underreported, as the ADA has estimated that nearly one-third of diabetic cases are undiagnosed and therefore unknown to the individuals who have the disease (ADA, n.d.b), and individuals experiencing a high number of poor days may be underrepresented due to severe impairment and inability to participate in the survey.
Additionally, research suggests that survey nonresponse bias may be correlated with health status (Rowland & Forthofer, 1993). Related to this finding, research suggests that the direction of bias in recall of health events tends toward underreporting. Patten (2003) investigated the role of recall bias in lifetime prevalence reports of major depression. He suggested that the most likely direction of the bias is to exert downward pressure on the estimates of lifetime prevalence, since people might not recall prior depressive events. Okura, Urban, Mahoney, Jacobsen, and Rodeheffer (2004) compared questionnaire reports to medical record data and found that questionnaires tended to underestimate the prevalence of diabetes. These findings suggest that recall bias may be downwardly biasing the components of triADD, indicating that the prevalence of triADD could be higher than observed in the BRFSS data.
The results indicating a negative association between heavy drinking and diabetes may be attributed to a number of factors, including health precautions taken by those with diabetes (as alcohol consumption is discouraged), or possibly to the fact that some individuals are unaware of their diabetic status. Further, it is important to note that the number of drinks per day defined by the CDC as “heavy drinking” is not a clinical threshold for alcohol abuse or dependence. Additionally, the variables selected from the BRFSS may not be accurate indicators of the three conditions investigated, as they are not intended to be diagnostic items. However, they do suggest an elevated risk for the conditions investigated in this study, and are consistent with empirical accounts of these disorders in AI/AN populations (many of which are referenced throughout this article).
Implications for Health Promotion
Examining triADD can create a better understanding of health needs within AI/AN populations and may provide insight into more appropriate primary and secondary prevention and treatment measures when these illnesses are presented concurrently. Further, these findings indicate the need for prevention interventions focused on health promotion that are specifically tailored toward these populations. This method might incorporate culturally specific interventions similar to those from other Indigenous populations, such as Native Hawaiians (Ka'opua, 2004; Ka'opua & Mueller, 2004). Specific examples of these types of interventions include those which incorporate spirituality, relational wellness, perceptions of self in relation to significant others, and interpretations and translations of culturally specific beliefs and practices into prevention interventions (Ka'opua). Many of these recommendations are consistent with the enhancement of cultural moderators proposed by Walters et al. (2002), and may serve to buffer the impact of historical trauma and discrimination on AI/AN populations. American Indian/Alaska Native-specific interventions based on cultural moderators are similar to those developed for Hawaiian populations, and may include the promotion of familial relational bonds and community support, and the use of spiritual and traditional healers. In the clinical setting, accurately recognizing triADD in AI/AN populations has significant implications for improved health outcomes, as each component of triADD is a chronic illness and requires significant self-management behaviors. Adapting approaches that have been developed and supported through the grant-funded Special Diabetes Program for Indians (IHS, 2000) and expanding them to include mental illness and substance abuse may prove to be an effective method for identifying individuals affected by these multimorbid conditions and treating them within a culturally specific context. More specifically, this federally-funded grant program allowed tribal communities to identify their diabetes-related priorities and develop interventions refl ecting their specific tribal traditions, customs, and beliefs surrounding illness, health, and wellness. Approaches have included talking circles, use of traditional herbs or medicines, storytelling, incorporation of traditional healers, and other indigenous methods aimed at primary, secondary, and tertiary prevention (IHS).
With the high prevalence of the triADD components in AI/AN populations, the possible impact of this phenomenon as a multimorbid condition may have severe societal consequences stemming from complex care needs and high levels of disability within community members. Accurate diagnosis of multiple conditions can improve the health of populations through appropriate management (van den Akker, Buntinx, Metsemakers, Roos, & Knottnerus, 1998). With appropriate medical care and acquisition of self-management skills, individuals may be more likely to avoid the stress of physical, social, and financial disability that has been associated with multimorbidity (Rice, & LaPlanta, 1988; Verbrugge, Lepkowski, & Imanaka, 1989).
Conclusion
Our analysis suggests that further epidemiological study into the prevalence of triADD in AI/ANs is warranted. Although examining trends is an important step, this pilot study, which focuses on one point in time, represents our first examination of the topic. The goal of the pilot study is to examine this issue at a single cross-section before studying the more complex issue of changes and trends over time. Numerous questions remain as to the etiology of the described phenomenon. Do regional/tribal differences exist in regard to epidemiology? Does one or two of these disorders affect the severity of the other? Does triADD place one at higher risk for other adverse health outcomes compared to individual or dyadic manifestations of these disorders? If so, what is the treatment protocol and how is it influenced by cultural differences? Until these answers can be explored, screening of these conditions in the clinical setting for AI/AN populations with a high prevalence of diabetes, alcohol abuse, and/or depression may improve the health of communities through early detection and appropriate intervention.
Acknowledgments
This study was supported by National Institutes of Health/National Institute on Drug Abuse funding for the Southwest Interdisciplinary Research Center at Arizona State University (R-24 DA 13937-01).
References
- Acton KJ, Burrows NR, Geiss LS, Thompson T. Diabetes prevalence among American Indians and Alaska Natives and the overall population--United States, 1994-2002. MMWR Weekly. 2003 August;52(30):702–704. [PubMed] [Google Scholar]
- American Diabetes Association Total prevalence of Diabetes and Pre-Diabetes. n.d.a. Retrieved February 20, 2007 from http://www.diabetes.org/diabetes-statistics/prevalence.jsp.
- American Diabetes Association All About Diabetes. n.d.b. Retrieved February 20, 2007 from http://www.diabetes.org/about-diabetes.jsp.
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Publishing; Washington, D.C.: 2000. Text Revision. [Google Scholar]
- Anderson RJ. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care. 2001;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Barron L, Oge LL, Markovich J. North American Indian Alliance mental health needs assessment report. American Indian and Alaska Native Mental Health Research: Journal of the National Center. 1999;8(3):13–24. doi: 10.5820/aian.0803.1999.13. [DOI] [PubMed] [Google Scholar]
- Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with Type 2 diabetes. Diabetes Care. 2003;26(10):2822–2828. doi: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- Carlsson S, Hammar N, Efendic S, Persson PG, Östenson CG, Grill V. Alcohol consumption, Type 2 diabetes mellitus, and impaired glucose tolerance in middle-aged Swedish men. Diabetic Medicine. 2000;17:776–781. doi: 10.1046/j.1464-5491.2000.00387.x. [DOI] [PubMed] [Google Scholar]
- Chester B, Mahalish P, Davis D. Mental health needs assessment of off-reservation American Indian people in northern Arizona. American Indian and Alaska Native Mental Health Research: Journal of the National Center. 1999;8(3):25–40. doi: 10.5820/aian.0803.1999.25. [DOI] [PubMed] [Google Scholar]
- Conigrave KM, Hu BF, Camargo CA, Stampfer MJ, Willett WC, Rimm EB. A prospective study of drinking patterns in relation to risk of Type 2 diabetes among men. Diabetes. 2001;50(10):2390–2395. doi: 10.2337/diabetes.50.10.2390. [DOI] [PubMed] [Google Scholar]
- de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: A meta-analysis. Psychosomatic Medicine. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and risk for onset of type II diabetes: A prospective population-based study. Diabetes Care. 1996;19:1097–1020. doi: 10.2337/diacare.19.10.1097. [DOI] [PubMed] [Google Scholar]
- Egede LE, Zheng D. Independent factors associated with major depressive disorder in a national sample of individuals with diabetes. Diabetes Care. 2003;26(1):104–111. doi: 10.2337/diacare.26.1.104. [DOI] [PubMed] [Google Scholar]
- Evaneshko V. Mental health needs assessment of Tucson's urban Native American population. American Indian and Alaska Native Mental Health Research: Journal of the National Center. 1999;8(3):41–60. doi: 10.5820/aian.0803.1999.41. [DOI] [PubMed] [Google Scholar]
- Franz MJ, Bantle JP, Beebe CA, Brunzell JD, Chiasson JL, Garg A, et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2002;25(1):148–198. doi: 10.2337/diacare.25.1.148. [DOI] [PubMed] [Google Scholar]
- Hitzemann R. Animal models of psychiatric disorders and their relevance to alcoholism. Alcohol Research & Health. 2000;24(3):149–158. [PMC free article] [PubMed] [Google Scholar]
- Holbrook TL, Barrett-Connor E, Wingard DL. A prospective population-based study of alcohol use and non-insulin-dependent diabetes mellitus. American Journal of Epidemiology. 1990;132(5):902–909. doi: 10.1093/oxfordjournals.aje.a115733. [DOI] [PubMed] [Google Scholar]
- Howard AA, Arnsten JH, Gourevitch MN. Eff ect of alcohol consumption on diabetes mellitus: A systematic review. Annals of Internal Medicine. 2004;140(3):211–219. doi: 10.7326/0003-4819-140-6-200403160-00011. [DOI] [PubMed] [Google Scholar]
- Indian Health Service . IHS. National Diabetes Program Report to Congress: Special Diabetes Program for Indians. U.S. Department of Health and Human Services; Rockville, MD: 2000. [Google Scholar]
- Indian Health Service . Trends in Indian Health 1998-1999. U.S. Department of Health and Human Services; Rockville, MD: 2001. [Google Scholar]
- Iosifescu DV, Nierenberg AA, Alpert JE, Smith M, Bitran S, Dording C, et al. The impact of medical comorbidity on acute treatment in major depressive disorder. American Journal of Psychiatry. 2003;160(12):2122–2127. doi: 10.1176/appi.ajp.160.12.2122. [DOI] [PubMed] [Google Scholar]
- Kao WHL, Puddey IB, Boland LL, Watson RL, Brancati FL. Alcohol consumption and the risk of Type 2 diabetes mellitus: Atherosclerosis risk in communities study. American Journal of Epidemiology. 2001;154(8):748–57. doi: 10.1093/aje/154.8.748. [DOI] [PubMed] [Google Scholar]
- Ka'opua LSI. Promoting a legacy of Native Hawaiian health through culturally based solutions. Lecture presented in the Southwest Interdisciplinary Research Center Colloquia Series; Tempe, AZ: 2004. February. [Google Scholar]
- Ka'opua LSI, Mueller CW. Treatment adherence among Native Hawaiians living with HIV. Social Work. 2004;49(1):55–63. doi: 10.1093/sw/49.1.55. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Nelson CB, McGonagle KA, Eclund MJ, Frank RG, Leof PJ. The epidemiology of co-occurring addictive and mental disorders. American Journal of Orthopsychiatry. 1996;66:17–31. doi: 10.1037/h0080151. [DOI] [PubMed] [Google Scholar]
- King J. Denver American Indian mental health needs survey. American Indian and Alaska Native Mental Health Research: Journal of the National Center. 1999;8(3):1–12. doi: 10.5820/aian.0803.1999.1. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Pettitt DJ, Saad MF, Bennett PH. Diabetes mellitus in the Pima Indians: Incidence, risk factors and pathogenesis. Diabetes/Metabolism Reviews. 1990;6(1):1–27. doi: 10.1002/dmr.5610060101. [DOI] [PubMed] [Google Scholar]
- Kobau R, Safran MA, Zack MM, Moriarty DG, Chapman D. Sad, blue, or depressed days, health behaviors and health-related quality of life, Behavioral Risk Factor Surveillance System, 1995-2000. Health and Quality of Life Outcomes. 2004;2:40. doi: 10.1186/1477-7525-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ET, Howard BV, Savage PJ, Cowan LD, Fabsitz RR, Oopik AJ, et al. Diabetes and impaired glucose tolerance in three American Indian populations aged 45-74 years. The Strong Heart study. Diabetes Care. 1995;18:599–610. doi: 10.2337/diacare.18.5.599. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM. Depression and poor glycemic control: A meta-analytic review of the literature. Diabetes Care. 2000;23:434–442. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- Manson SP. Behavioral health services for American Indians: Need, use, and barriers to eff ective care. In: Dixon M, Roubideaux Y, editors. Promises to keep: Public health policy for American Indians and Alaska Natives in the 21st century. American Public Health Association; Washington, D.C.: 2001. pp. 167–192. [Google Scholar]
- Merikangas KR, Gelernter CS. Co-morbidity for alcoholism and depression. Psychiatric Clinics of North America. 1990;13(4):613–632. [PubMed] [Google Scholar]
- Nichols GA, Brown JB. Unadjusted and adjusted prevalence of diagnosed depression in Type 2 diabetes. Diabetes Care. 2003;26(3):744–749. doi: 10.2337/diacare.26.3.744. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Foroud T, Flury L, Meyer ET, Wiegand R. Is there a genetic relationship between alcoholism and depression? Alcohol Research & Health. 2002;26(3):233–240. [PMC free article] [PubMed] [Google Scholar]
- Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheff er RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. Journal of Clinical Epidemiology. 2004;57(10):1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Patten SB. Recall bias and major depression lifetime prevalence. Journal of Social Psychiatry and Psychiatric Epidemiology. 2003;38(6):290–296. doi: 10.1007/s00127-003-0649-9. [DOI] [PubMed] [Google Scholar]
- Perreira KM, Sloan FA. Excess alcohol consumption and health outcomes: a 6-year follow-up of men over age 50 from the health and retirement study. Addiction. 2002;97:301–310. doi: 10.1046/j.1360-0443.2002.00067.x. [DOI] [PubMed] [Google Scholar]
- Raimo EB, Schuckit MA. Alcohol dependence and mood disorders. Addictive Behaviors. 1998;23(6):933–946. doi: 10.1016/s0306-4603(98)00068-9. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse: Results from the Epidemiologic Catchment Area (ECA) study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Rice DP, LaPlanta MP. Chronic Illness, disability, and increasing longevity. In: Sullivan S, Ein-Lewin M, editors. The economics and ethics of long-term care and disability. American Enterprise Institute for Public Policy Research; Washington, DC: 1988. pp. 9–55. [Google Scholar]
- Roberts AR, Yeager K. Mental illness, substance dependence, and suicidality: Secondary data analysis. In: Roberts AR, Yeager K, editors. Evidence based practice manual: Research and outcome measures in health and human services. Oxford; New York: 2004. pp. 70–75. [Google Scholar]
- Robin RW, Long JC, Rasmussen JK, Albaugh B, Goldman D. Relationship of binge drinking to alcohol dependence, other psychiatric disorders, and behavioral problems in an American Indian tribe. Alcoholism: Clinical and Experimental Research. 1998;22(2):518–523. [PubMed] [Google Scholar]
- Rowland ML, Forthofer RN. Adjusting for nonresponse bias in a health examination survey. Public Health Reports. 1993;108(3):380–386. [PMC free article] [PubMed] [Google Scholar]
- Saremi A, Hanson RL, Tulloch-Reid M. Alcohol consumption predicts hypertension but not diabetes. Journal of Studies on Alcohol. 2004;65(2):184–190. doi: 10.15288/jsa.2004.65.184. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . American Indians/Alaska Natives and Substance Abuse. U.S. Department of Health and Human Services; Rockville, MD: 2002. [Google Scholar]
- Talbot F, Nouwen A. A review of the relationship between depression and diabetes in adults. Diabetes Care. 2000;23(10):1556–1562. doi: 10.2337/diacare.23.10.1556. [DOI] [PubMed] [Google Scholar]
- van den Akker M, Buntinx F, Metsemakers JF, Roos S, Knottnerus JA. Multimorbidity in general practice: Prevalence, incidence, and determinants of co-occurring chronic and recurrent diseases. Journal of Clinical Epidemiology. 1998;51(5):367–75. doi: 10.1016/s0895-4356(97)00306-5. [DOI] [PubMed] [Google Scholar]
- Verbrugge LM, Lepkowski JM, Imanaka Y. Comorbidity and its impact on disability. The Milbank Quarterly. 1989;67(3-4):450–484. [PubMed] [Google Scholar]
- Walters KL, Simoni JM, Evans-Campbell T. Substance use among American Indians and Alaska Natives: Incorporating culture in an “Indigenist” stress coping paradigm. Public Health Reports. 2002;117(suppl 1):S104–S117. [PMC free article] [PubMed] [Google Scholar]
- Westermeyer J, Peake E. A ten-year follow-up of alcoholic American Indians in Minnesota. American Journal of Psychiatry. 1983;140:189–194. doi: 10.1176/ajp.140.2.189. [DOI] [PubMed] [Google Scholar]
- Wei M, Gibbons LW, Mitchell TL, Kampert JB, Blair SN. Alcohol intake and incidence of Type 2 diabetes in men. Diabetes Care. 2000;23(1):18–22. doi: 10.2337/diacare.23.1.18. [DOI] [PubMed] [Google Scholar]
- Wilson C, Civic D, Glass D. Prevalence and correlates of depressive syndromes among adults visiting an Indian Health Service primary care clinic. American Indian and Alaska Native Mental Health Research: Journal of the National Center. 1995;6(2):1–12. doi: 10.5820/aian.0602.1995.1. [DOI] [PubMed] [Google Scholar]