Abstract
Responses of mesenchymal stem cells (MSCs) cultured with zinc-added (2 and 5%) bioactive glass granules were evaluated in terms of cell growth and osteogenic differentiation. MSCs were cultured with different quantities (3, 10 and 30) of glass granules for up to 21 days in the osteogenic medium. Cell growth was stimulated by a small quantity of glasses, particularly those that contained zinc. Osteogenic differentiation, as assessed by alkaline phosphatase activity (ALP) activity, was significantly enhanced by the glasses, particularly with large quantities of glass and for prolonged culturing. Expression of bone-sialo protein (BSP) was significantly up-regulated around the bioactive glass granules. Moreover, the zinc addition significantly altered the ALP and BSP depending on the culture time and glass quantity. Cellular mineralization was improved in all glass samples, and particularly in the 2% zinc-glass. Taken together, the zinc addition to bioactive glass induced the MSCs growth and their osteogenic differentiation, at least to the level of zinc-free glass, and with even higher level observed depending on the quantity and culture time. These findings indicate that the zinc addition to bioactive glass may be useful in development of biomaterials for the stimulation of adult stem cell in bone tissue engineering.
1. Introduction
The reconstruction of defective and degenerative bones has been a significant challenge in oral and maxillofacial surgery [1, 2]. Autologous tissues derived from intra- or extra-oral sites are considered to be a gold-standard in terms of regenerative potential. However, the limited access and amount of bones as well as the pain and psychological fear that patients tend to suffer due to the additional surgical operation required have been raised as concerns [3]. Bone grafts have been introduced as an alternative to the use of autologous bones, and they have been found to be useful for filling bony defects and expanding the quantity and quality of bones available for implantation [4–6]. When compared to allogenic or xenogenic materials, which may cause immune and/or disease problems, synthetic bone grafts are considered relatively safe for use in large quantities [3, 4].
Over the past few decades, several types of synthetic bone grafts have been proved by clinical trials and commercialized, and ceramics or glasses classified as bioactive materials comprise the major category [5–8]. Bioactive glasses obtained from melt-quenching or sol-gel process are known to form direct bonds with hard human tissues through a specific attachment mechanism, such as the formation of a bone mineral like carbonated hydroxyapatite layer that forms on the surface of the glasses under physiological conditions [5]. One of the benefits of bioactive glasses is the ability to easily incorporate elements tuned to the required tissue responses [9]. For example, the addition of magnesium to melt glass 45S5 has been found to improve bioactivity [10], while the addition of silver to bioactive glass was reported to elicit antimicrobial activity [11] and the addition of strontium to sol-gel bioactive glass was shown to enhance bone cell responses [12].
In this study, zinc was added to the sol-gel bioactive glass based on the previous reports demonstrating zinc roles in bone formation [13–15]. As one of the trace elements found in natural bone, zinc has been studied in bone biology, particularly in osteoporotic patients [13, 14]. Furthermore, several recent studies have utilized zinc as an additive to medical materials, including zinc-substituted hydroxyapatite powders, and coatings, tricalcium phosphate powders, and bioactive glass granules [15–20]. Small concentrations of zinc within the bioactive glasses have been shown to affect bone-associated cell responses such as proliferation and matrix synthesis in vitro [18–20].
Stem cell-based tissue engineering is gaining considerable opportunities for the successful reconstruction of bone tissue. Mesenchymal stem cells (MSCs) derived from adult bone marrow are one of the most popular cell sources available for the regenerative therapy, including osteogenesis. Compared to the stem cells of embryonic origin, relatively large quantities of MSCs can be obtained without significant ethical concerns, and these cells can be applied clinically using autologous cells [21, 22]. In this study, as a first step towards bone tissue engineering using zinc-added bioactive glass, the effects of the addition of small concentrations (2 and 5% by mole) of zinc to the sol-gel bioactive glass on the growth and osteogenic potential of MSCs were investigated.
2. Materials and Methods
2.1. Preparation of Zinc-Added Bioactive Glasses
The sol-gel-derived bioactive glass was produced using precursors of tetraethyl orthosilicate and calcium nitrate tetrahydrate (from Sigma-Aldrich) in an ethanol-water solvent, with hydrochloric acid as a catalyst. To incorporate the zinc, calcium nitrate tetrahydrate was replaced with zinc nitrate hexahydrate (from Sigma-Aldrich) at 2 and 5 mol% to produce three different compositions of the bioactive glasses: 70SiO2-30CaO (0% Zn), 70SiO2-28CaO-2ZnO (2% Zn), and 70SiO2-25CaO-5ZnO (5% Zn). The prepared sol was then left to age for 24 h and transformed into a gel, which was subsequently dried in an oven (60°C) and then thermal-treated by heating to 650°C at a ramping rate of 2°C/min, where it was maintained for 3 h, after which the sol was furnace-cooled in the air. The calcined granules containing diameters ranging from 500 to 1000 micrometers were then gathered using metal sieves. For the cell test samples, the glass granules were sterilized and different quantities (3, 10, and 30) of the granules of each composition (0% Zn, 2% Zn, and 5% Zn) were placed in the individual wells of 24 culture well plates.
2.2. Characterizations of Glass Granules
The surface morphology of the glass granules was observed by scanning electron microscopy (SEM, Hitachi). For observation of the bone bioactivity, the zinc-added bioactive glass granules were soaked in simulated body fluid (ionic concentration: 142.0 mM Na+, 5 mM K+, 1.5 mM Mg2+, 2.5 mM Ca2+, 147.8 mM Cl−, 4.2 mM HCO3−, 1.0 mM HPO42−, and 0.5 mM SO42−) for 7 days, after which the surface was examined by SEM. The dissolution of ions (Si, Ca and Zn) from the glasses was detected using Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES). For the dissolution test, glass granules were incubated in an acellular culture medium (serum free) for up to 7 days, during which time the medium samples were analyzed.
2.3. Isolation and Culture of MSCs
Mesenchymal stem cells (MSCs) were isolated from the bone marrow of tibia and femora of adult rats (4–8 weeks, Korea) using a method described in our previous study [23]. The bone marrow mixture was then centrifuged, after which the supernatant was gathered and maintained on a culture flask containing growth medium (α-minimal essential medium; MEM, 2 mM glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin) supplemented with 10% fetal bovine serum (FBS). After cell adhesion for 1 day, the cells were washed with phosphate buffered saline solution (PBS) and cultured for up to 7 days until they reached near confluence.
2.4. Cell Growth by MTS
Glass granules (500–1000 micrometers in size) with three different compositions (0, 2, and 5% Zn) were contained within the individual wells of 24-well plates at three different quantities (3, 10, and 30). Thus, a total of ten sample groups were used, including a glass-free blank control. An aliquot of 100 μl of the cell suspension prepared at a density of 5 × 104 cells/ml was then seeded into each well. The medium for the cell growth was supplemented with 50 μg/ml sodium ascorbate, 10−2 M sodium β-glycerol phosphate, and 10−8 M dexamethasone to induce osteoblastic differentiation. The samples were then incubated for 6 h, after which 0.1 ml of culture medium without cells was added to each well. Next, the samples were cultured for up to 21 days, with the medium being refreshed every 2-3 days. The cell growth level was measured at days 3 and 7 based on the mitochondrial NADH/NADPH-dependant dehydrogenase activity, which was measured using a cell proliferation assay kit (CellTiter 96 Aqueous One Solution, Promega). Briefly, 100 μl of MTS regent was added to each sample and incubated for 3 h. The absorbance was then determined at 490 nm using an Elisa Plate Reader. Three replicate samples were tested for each condition (n = 3).
The morphology of cells at each culture time was determined by optical microscopy (Motic). The cell growth on the glass granules was examined by SEM after fixation of the samples with glutaraldehyde, dehydration in a graded series of ethanol, and treatment with hexamethyldisilazane solution. SEM was conducted at an accelerating voltage of 15 kV after gold coating the surface of the samples.
2.5. Alkaline Phosphatase Activity
The effect of the glass granules on the in vitro osteogenic differentiation of the MSCs was assessed based on the alkaline phosphatase activity. Cells were seeded onto each sample at 5 × 104 cells/ml and then cultured for 7 and 14 days. After washing the samples with PBS the cells were gathered and added with cell lysis buffer (10% Triton X-100, 1M Tris-HCL, 0.5 M NaCL, and 0.5 M EDTA) containing protease inhibitor. Total protein content was measured using a commercial DC protein assay kit (BioRad, Hercules, USA). Bovine serum albumin (from Sigma) was used as the reference curve for the protein assay. The quantity of each sample used for an enzymatic reaction was determined when normalized to the total protein content. The enzymatic activity of the ALP produced was determined using an ALP assay kit (Procedure No. ALP-10, Sigma). The p-nitrophenol produced in the presence of ALP was determined based on its absorbance at 405 nm. Three replicate samples were used for the assay (n = 3).
2.6. Immunofluorescence Staining of Bone Sialoprotein
To observe the osteogenic marker, bone sialoprotein (BSP), immunofluorescence staining was performed. After culturing for 14 days, the cellular samples were fixed with formaldehyde and then dehydrated with ethanol. Next, the samples were washed with PBS and incubated with the primary antibody at a concentration of 1 : 500 (polyclonal rabbit antibone sialoprotein: BSP) at 4°C, after which they were washed with PBS. Subsequently, the Alexfluor 555 secondary antibody (molecular probe) was added to the samples at 1 : 200 and incubated at ~25°C in dark condition. After washing with PBS, the samples were mounted and visualized by fluorescence microscopy.
2.7. Cellular Mineralization by Alizarin Red S
For the mineralization study, the cells were cultured under the influence of the eluted products from glass granules that were present within the Transwell insert. After culturing the cells, the samples were rinsed in PBS, fixed with 10% formaldehyde, and then stained with 1% Alizarin red S (ARS) solution (pH 4.1) for 30 min. After washing fully with distilled water, the stained areas were visualized under an optical microscope. The mineralization was quantified by detecting the amount of calcium present on the cellular constructs. After culturing, the cell layer (including extracellular matrix) collected from each specimen was homogenized in diluted HCl solution by vigorous shaking. The cell lysate was centrifuged and the supernatant was used to determine the calcium content. The calcium concentration in each sample was quantified with a regent solution using orthocresolphthalein complexone (OCPC) as a metallochromic indicator. Standard calcium solutions were prepared after suitable dilutions of 1 mg/ml CaCl2 solution. An aliquot of the regent was added to each sample to induce Ca-OCPC complexometric reaction, and the absorbance was read at 590 nm using a microplate reader. Five replicate samples were used at each condition (n = 5).
2.8. Statistics
Data were presented as mean ± one standard deviation for three or five different sets of samples in each group. Statistical analysis was performed by the student t-test and significant level was considered at P < .05.
3. Results
Figure 1 shows the typical morphology of the glass granules (2% Zn) that were prepared by the sol-gel synthesis and subsequent heat treatment (Figure 1(a)). Granules with sizes of approximately 500 to 1000 μm were chosen for further biological tests. When the glass granules were immersed in a simulated body fluid and incubated for 3 days at 37°C, calcium phosphate crystallites were found to precipitate on the surface of the glass granules, and after 7 days of incubation, the surface of the granules was fully covered by a thick mineral layer (representative image of 2% Zn-added glass shown in Figures 1(b) and 1(c)). Similar in vitro mineralization behavior in SBF was observed in the other compositions (data not shown here).
Figure 1.
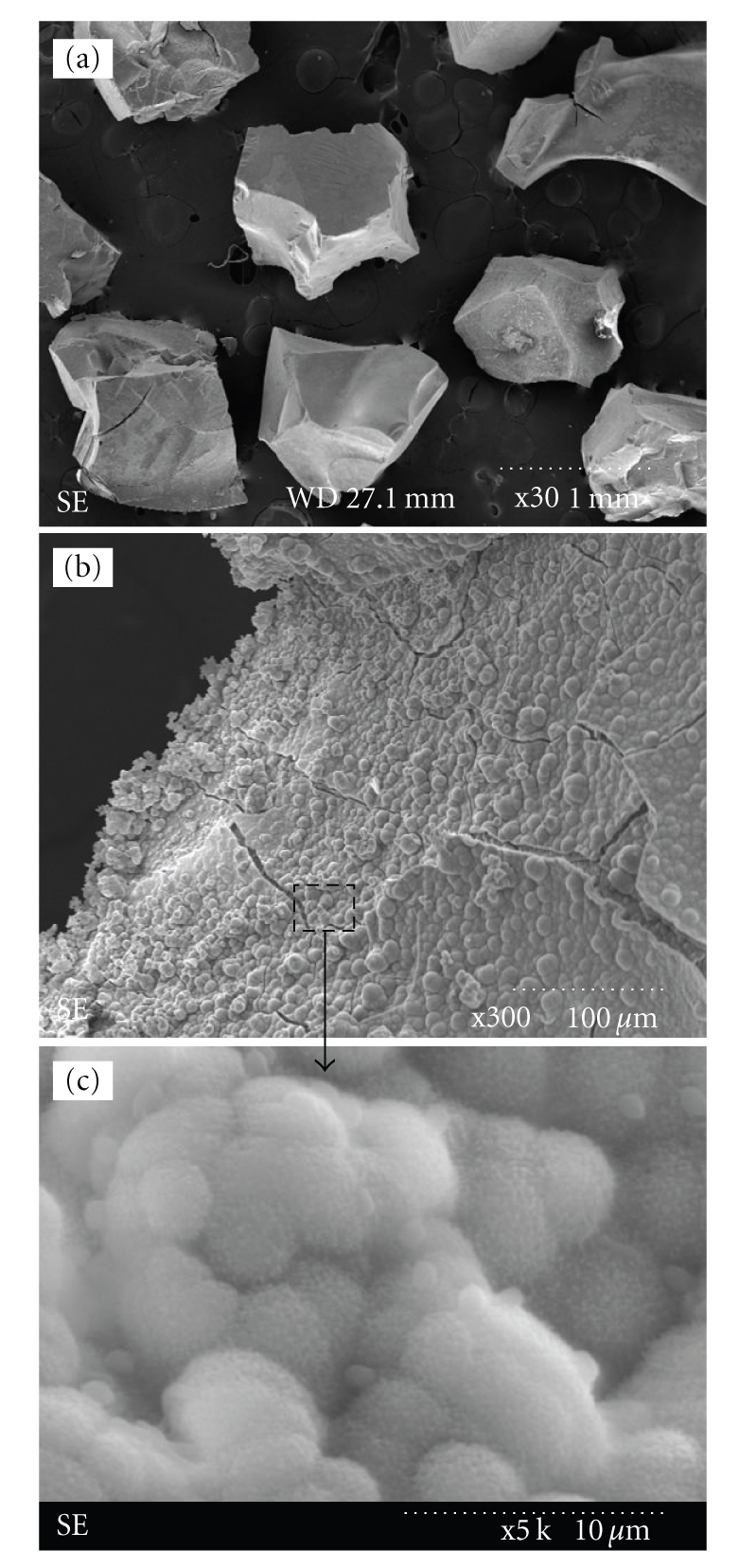
(a) Typical morphology of sol-gel bioactive glass granules (2% Zn). Granules with sizes of 500 to 1000 μm were selected for use in the cellular tests. ((b) and (c)) Formation of apatite crystallites on the glass granules (2% Zn) after immersion in SBF for 7 days to demonstrate the bone bioactivity of the granules. (c) Enlarged image of (b) (bar: 1 mm). Similar morphology was noticed in other glass compositions.
For the biological tests, the glass granules were evaluated at three different concentrations (low (3), medium (10), and high (30)) contained within each well of a 24-well culture dish. The ionic elusion (Si, Ca, and Zn) from glass samples cultured in an acellular medium was measured using ICP-AES for up to 7 days (Table 1). The results revealed that the addition of zinc to the glass led to a slight decrease in the dissolution of silicon and calcium. In addition, a trace amount of zinc was released from the zinc-added glass granules. As the release time increased, the ionic release rate was decreased greatly. The reduced release rate of the cations with time may be due to the depletion of cations with time and the resultant slow-down in further diffusion release process.
Table 1.
Ionic concentrations (in ppm) eluted from the sol-gel glass granules during incubation in acellular culture medium for up to 7 days. Ten granules with sizes of 500 to 1000 μm were immersed in the medium at 37°C, and the ions eluted were measured by ICP-AES.
| 3 days | 7 days | |||||
|---|---|---|---|---|---|---|
| 0% Zn | 2% Zn | 5% Zn | 0% Zn | 2% Zn | 5% Zn | |
| Si | 36.3 | 29.4 | 28.1 | 58.1 | 39.3 | 33.1 |
| Ca | 48.8 | 39.2 | 19.1 | 62 | 42.6 | 29.9 |
| Zn | — | 0.8 | 2.4 | — | 1.1 | 3.0 |
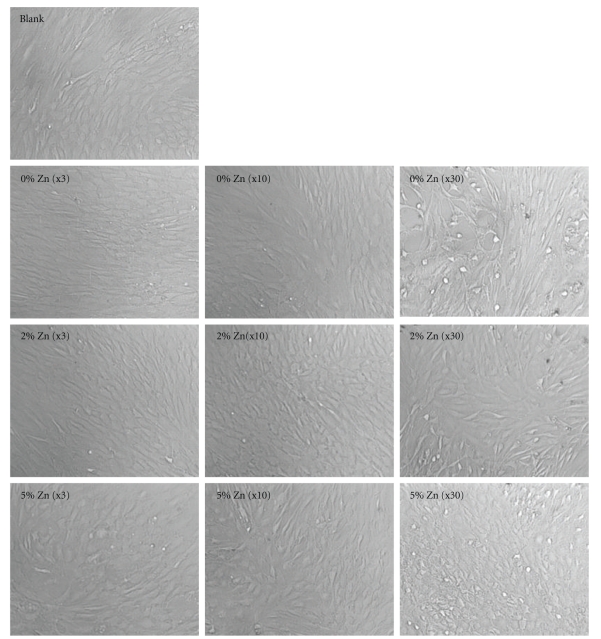
Mesenchymal stem cells (MSCs) extracted from rat bone marrow were cultured in the presence of the bioactive glass granules. Three different quantities (3, 10, and 30) of glass granules were placed in each well of a 24-well culture dish, and cellular responses such as growth and osteoblastic differentiation were investigated. Figure 2 shows the optical morphology of cells grown in culture wells for 7 days, including wells that contained the glass granules (0, 2, and 5% Zn) at different concentrations (3, 10, and 30 granules), as well as a glass-free culture well as a control (blank). When low and medium concentrations (3 and 10) of the glass granules were present, the cells reached near confluence and had an elongated and well-spreading morphology similar to that of the control. However, when the high concentration (30) of the granules was present, the cell morphology became less elongated and more granular-like. Depending on the glass composition, no noticeable difference was observed.
Figure 2.
Optical micrographs of the cells at day 7, showing the cell growth morphology on culture plates without glass granules (blank) and those containing different amounts of granules amended with 0%, 2%, and 5% Zn. The quantity of granules is noted in the bracket. Cell morphologies were similar when cultured in the presence of small quantity of glass granules (x3), while they differed in response to the presence of large quantity of granules (x30), particularly when the granules contained no Zn.
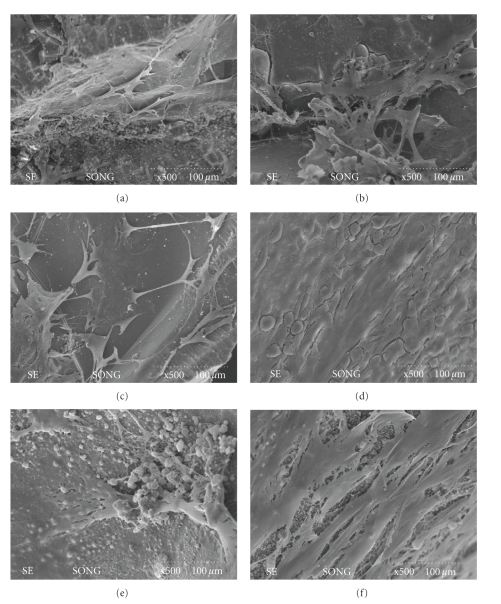
The cell morphology on the contained glass granules was observed with SEM (Figure 3). After 14 and 21 days of culture, the cells were found to actively grow on the surface of glass granules. Furthermore, the cells had flattened bodies and many cytoskeletal extensions that were in intimate contact with the glass substrate. At day 21, the cells appeared to be aggregated (Figure 3(e)), and mineral-like products were noticeable around/below the cellular construct (Figures 3(e) and 3(f)).
Figure 3.
SEM micrographs of mesenchymal stem cells grown for prolonged periods in the presence of bioactive glass granules: 14 days on (a) 0% Zn, (b) 2% Zn, and (c) 5% Zn, and 21 days on (d) 0% Zn, (e) 2% Zn, and (f) 5% Zn. Cells grew actively upon the glass granules at all compositions. Cellular aggregates (e) and mineral formation were noticed at day 21 (f).
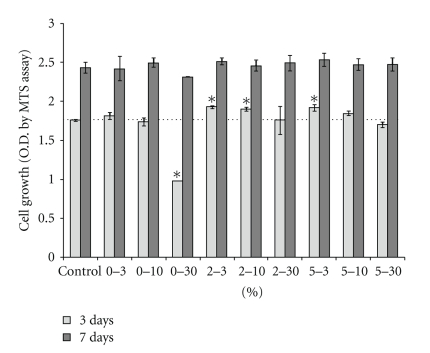
Figure 4 summarizes the cell viability for periods of up to 7 days in the presence of bioactive glass granules with different compositions and concentrations, as measured by an MTS method. When cultured in the presence of low and medium concentrations of granules for 3 and 7 days, the cell growth was equal to or higher than that of the control. However, a slight decrease in cell growth was observed with a high quantity (30) of the glass (2% and 5% Zn-added), and even significant decrease was noticed in the 0% Zn-added glass. In particular, zinc-containing glasses induced significantly higher cell growth at day 3 when they were contained at low (3) and medium (10) quantity. After prolonged culture periods (7 days), the cell viability affected by the glass granules became similar to that of the blank control, regardless of the composition of the glass.
Figure 4.
MSCs viability in response to the presence of glass granules as measured for up to 7 days by an MTS assay. Culture wells containing bioactive glass granules with different compositions (1, 2, and 5% Zn) and different concentrations (3, 10 and 30) differed significantly. (*P < .05 as determined by student t-test, n = 3).
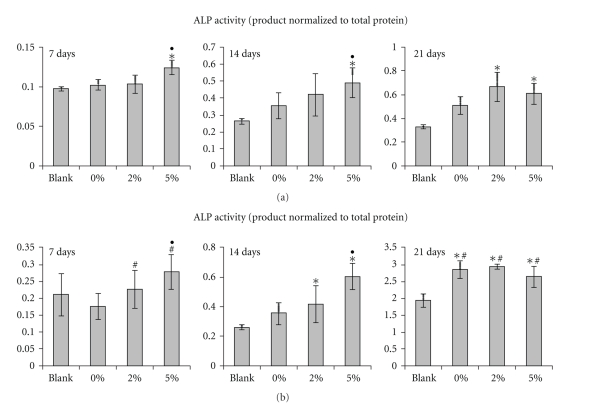
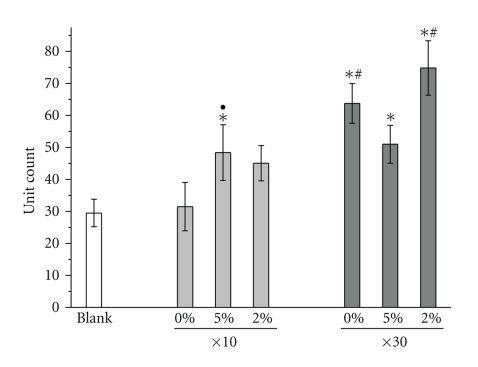
The alkaline phosphatase (ALP) activity of the MSCs cultured in the presence of various glasses was evaluated (Figure 5). Two sets of experiments were conducted using different quantities of glasses (10 and 30 granules). At day 14, the addition of medium quantity (10) of glass granules significantly increased the ALP level, and this improvement increased as the zinc content within the glass increased (Figure 5(a)). A similar trend was observed in samples containing a high quantity of glass granules (30 granules) (Figure 5(b)).
Figure 5.
ALP activity of MSCs grown on wells containing bioactive glasses (0%, 2%, and 5% Zn-glass) or blank control at 7, 14, and 21 days of culture, as determined by an enzymatic reaction method. Culture wells containing different quantities of bioactive glass granules (a) x10 and (b) x30. The incorporation of glass granules significantly altered the ALP level at day 14 and day 21, and the effect of Zn on ALP stimulation was significant. A larger quantity of granules significantly enhanced the ALP level (*P < .05: comparison with respect to blank, #P < .05: comparison between glass quantity 10 versus 30, and ●P < .05: comparison between zinc-free and zinc-added glass). Note the different scales in the y-axis.
The expression of the osteogenic marker bone sialoprotein (BSP) produced by the cells in the presence of bioactive glass granules was observed by immunofluorescence staining. As shown in Figure 6, the red-colored spots representing the expressed BSP were obvious (2% Zn shown as a representative sample), particularly near the bioactive glass granules. The immunofluorescence stain was more obvious in the samples that contained the glass granules than in the control. As shown in Figure 7, the stained BSP spots were quantified. When compared to the blank control, the addition of glass granules significantly enhanced the expression of BSP. Moreover, the stimulation of BSP was greater in response to a higher concentration of glass granules.
Figure 6.
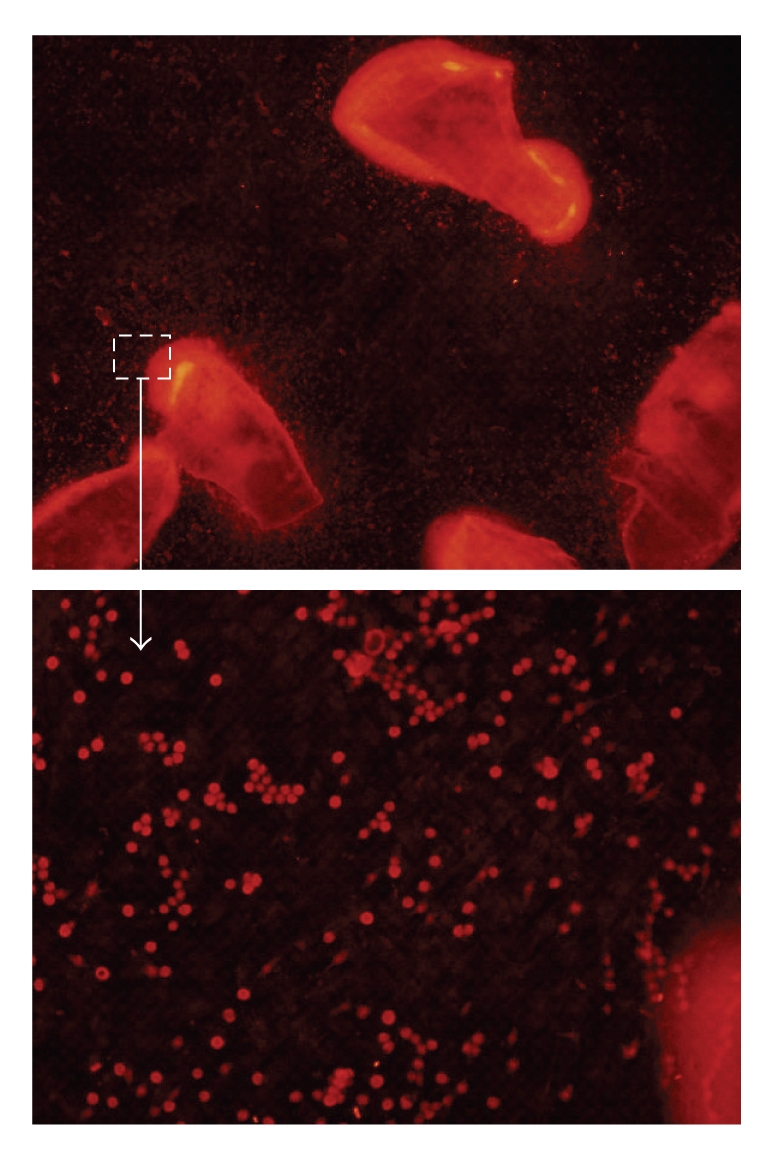
Bone sialoprotein immunofluorescence staining image of MSCs grown in the presence of bioactive glass granules (2% Zn, 30 quantity) for 14 days, showing red-colored dots distributed in a large quantity around the glass granules.
Figure 7.
Measurement of the BPS immunofluorescence stains after 14 days of culture. Five arbitrarily selected areas were counted for each sample. Compared to the blank control, the presence of glass granules (0%, 2%, and 5% Zn) significantly enhanced the production of BSP, and this effect was greater in the presence of larger quantity of granules (10 < 30). Data were analyzed by student t-test for n = 5 (*P < .05: comparison with respect to blank, #P < .05: comparison between glass quantity 10 versus 30, and ●P < .05: comparison between zinc-free and zinc-added glass).
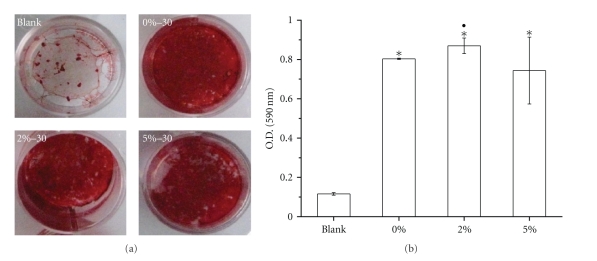
The mineralization behavior of the MSCs was investigated by Alizarin red S (ARS) staining. Specifically, 30 glass granules for each composition (0, 2, and 5% Zn) were placed in a Transwell insert, and the MSCs were then cultured under the influence of the ionic eluants from the glass granules. This was conducted to eliminate the possible interference between Ca within the glass and ARS dye. As shown in Figure 8(a), all the glass-containing groups showed the cellular constructs stained dark-red in response to the influence of the glass eluants, confirming significant level of cellular mineralization. The mineralized level was assessed by detection of calcium quantity of the cellular constructs. As presented in Figure 8(b), the mineralization was significantly higher on all the glass-containing samples than on the blank control, and furthermore, the 2% Zn-added glass sample showed significantly higher level of mineralization than the pure bioactive glass.
Figure 8.
(a) Alizarin red S staining of MSCs grown on the culture dish to determine the effects of the ionic eluants of the Zn-bioactive glass granules. (b) Quantification of the mineralization by the Ca detection. A transwell insert containing the bioactive glass granules (30 granules) with different compositions (0, 2, and 5% Zn) was used for the ARS test (*P < .05: comparison with respect to blank, and ●P < .05: comparison between zinc-free and zinc-added glass).
4. Discussion
The use of bioactive inorganics is considered a promising tool in the reconstruction of oral and maxillofacial defects [1, 2]. Among the various compositions evaluated, bioactive glasses have been widely studied due to their excellent bioactivity and ability to direct-bonding to bone, which is primarily associated with the bone mineral-like phase created on their surface [8, 9]. Moreover, the composition of bioactive glasses is believed to be easily controlled by the introduction of biologically relevant elements, such as silicon, magnesium, strontium, silver, and zinc [10–12]. Zinc is considered a key element present in bone and has been shown to regulate osteoblastic function and bone formation. Osteoporotic patients have often been found to have a lack of zinc; therefore, it is considered to be useful for the treatment of osteoporosis [13, 14]. Additionally, several recent studies have been conducted to utilize zinc within the composition of biomaterials. Those studies have shown that zinc played an effective role in the stimulation of bone cell functions when it was present in calcium phosphate compounds such as hydroxyapatite and tricalcium phosphate [15–18].
In the present study, zinc was added to the composition of bioactive glass during the sol-gel synthesis, and the effects of zinc addition (2 and 5%) on the responses of rat bone marrow MSCs were evaluated. MSCs are considered a potential cell source for application in regenerative medicine, including the treatment of bone disorders [21–23]. For stem cell-based bone tissue engineering, the material substrate should guide the initial anchorage of cells and their multiplication and further stimulate the differentiation into an osteogenic lineage. In order to utilize the zinc-bioactive glass as a scaffold for the MSCs, this study was carried out as a first step to gain insight into the behavior of MSCs in response to the glasses that contain zinc.
Different quantities of granules (3, 10, and 30) with variable glass compositions (0, 2, and 5% Zn-added) were used to evaluate the MSCs responses. For all compositions, the initial cell adhesion and growth were shown to be favorable in the presence of small quantities of glass granules (Figures 2 and 4). Although some downregulation of cell viability was noticed initially, particularly in the case of the high concentration of 0% Zn-added glass, the cell growth level became almost equal to that of the blank control after a prolonged culture time (7 days). Conversely, a small quantity of glass granules led to a significant increase in cell viability, suggesting that the existence of glasses and their eluted products should favor the proliferative potential of MSCs. Based on the ionic release data (Table 1), the addition of zinc to the glass led to a slight decrease in the elution of ions such as Si and Ca. Thus, the initial high release of ions from the 0% Zn-added glass might be one of the possible reasons for the decreased cell viability. Although the initial ionic release was high, the release rate decreased with time, with cells eventually reaching their normal proliferation level after a prolonged culture period (over 7 days). The images of the cells grown directly on the glass particles at day 14 (Figure 3) confirmed the viable cellular status, that is, the presence of extensive cytoskeletal processes and the formation of collagenous fibrillar extracellular matrix by the proliferative cells.
The effects of the zinc-added bioactive glass on the osteogenic differentiation of the MSCs were evaluated based on the alkaline phosphatase (ALP) activity of cells. ALP is a marker of osteoblastic cells that is evident at relatively early stage of osteogenic differentiation of progenitor or stem cells [24]. In the present study, ALP was significantly upregulated when grown in the presence of bioactive glass granules, particularly with larger quantity of granules and at later culture times (over 14 days) (Figure 5). Moreover, the increase appeared to be greater in the presence of zinc-added glasses. As another marker for osteogenic differentiation, bone sialo-protein (BSP) was evident in the presence of glass, particularly near the glass granules (Figures 6 and 7). These results indicate that MSCs are triggered to undergo osteogenic development by the eluants of the bioactive glass particles.
The ions released from the bioactive glasses further stimulated cellular mineralization, as confirmed by the ARS staining and calcium quantification of samples (Figure 8). The released calcium ion should participate directly in the mineralization of extracellular matrices (ECMs), which would be secreted by the differentiated cells. Although the MSCs differentiate down to an osteogenic lineage within the total culture medium used herein, the presence of bioactive glasses should alter the secretion of proteins, and thus the ECM components. As noticed, the mineralization behavior under the influence of the glasses was noteworthy with respect to that of glass-free control which was conditioned only with the osteogenic medium. These results indicate that the differentiated cells and the synthesized ECM must be properly conditioned to induce mineralization under the influence of the bioactive glasses eluants.
As to the effects of zinc, the results demonstrated that the MSCs expressed ALP and BSP at levels largely similar to those of zinc-free bioactive glass, and sometimes at even higher levels depending on the zinc concentration and the quantity of glass used. Moreover, the mineralization behavior was slightly stimulated with the addition of 2% Zn. The different levels of ions released from the zinc-added bioactive glasses should affect the differentiation responses of stem cells and further cellular mineralization, and the zinc ions released may participate in the regulation of cellular functions to some extent. Although no significant difference in BSP levels was noticed, the upregulations of ALP and mineralization by the zinc-added glass support the possible role of zinc in the differentiation of MSCs to the osteoblastic pathway.
Taken together this study may provide some useful information regarding the effects of zinc addition to bioactive glasses on the MSCs growth and their stimulation into osteogenic differentiation. Accordingly, we are currently developing zinc-added bioactive glasses as 3D scaffolds to use them in bone tissue engineering with the MSCs.
5. Conclusions
The cell growth and osteogenic differentiation of MSCs with respect to zinc-added bioactive glasses (0, 2, and 5%) in granular form were investigated. The presence of a small quantity of glass enhanced the initial cell growth, whereas a downregulation was noticed in response to a large quantity of zinc-free glass. The osteogenic development, as determined based on the alkaline phosphatase (ALP) activity and bone-sialo protein (BSP) secretion, was significantly enhanced by the bioactive glass granules. Moreover, the zinc-added glass induced cells to differentiate to a level similar to or even greater than that of zinc-free glass. The cellular mineralization was also significantly stimulated by the zinc addition to the bioactive glass. Taken together, zinc-added bioactive glasses may be useful in bone regeneration and tissue engineering with adult stem cells because they preserve the active growth of MSCs and stimulate further osteogenic differentiation.
Acknowledgment
This work was supported by Priority Research Centers Program (grant no. 2009-0093829) and WCU (World Class University) program (grant no. R31-10069) through the National Research Foundation (NRF) funded by the Ministry of Education, Science and Technology.
References
- 1.Bodic F, Hamel L, Lerouxel E, Baslé MF, Chappard D. Bone loss and teeth. Joint Bone Spine. 2005;72(3):215–221. doi: 10.1016/j.jbspin.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Sindet-Pedersen S, Enemark H. Mandibular bone grafts for reconstruction of alveolar clefts. Journal of Oral and Maxillofacial Surgery. 1988;46(7):533–537. doi: 10.1016/0278-2391(88)90142-5. [DOI] [PubMed] [Google Scholar]
- 3.Damien CJ, Parsons JR. Bone graft and bone graft substitutes: a review of current technology and applications. Journal of Applied Biomaterials. 1991;2(3):187–208. doi: 10.1002/jab.770020307. [DOI] [PubMed] [Google Scholar]
- 4.Vaccaro AR, Chiba K, Heller JG, et al. Bone grafting alternatives in spinal surgery. Spine Journal. 2002;2(3):206–215. doi: 10.1016/s1529-9430(02)00180-8. [DOI] [PubMed] [Google Scholar]
- 5.Yamamuro T, Hench LL, Wilson J. CRC Handbook of Bioactive Ceramics. Vol. 2. CRC Press; 1990. [Google Scholar]
- 6.Wilson J, Low SB. Bioactive ceramics for periodontal treatment: comparative studies in the Patus monkey. Journal of Applied Biomaterials. 1992;3(2):123–129. doi: 10.1002/jab.770030208. [DOI] [PubMed] [Google Scholar]
- 7.Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002;295(5557):1014–1017. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 8.Kim HW, Kim HE, Knowles JC. Composite nanofiber of bioactive glass nanofiller incorporated poly(lactic acid) for bone regeneration. Advanced Functional Materials. 2010;64(7):802–805. [Google Scholar]
- 9.Hench LL. Bioceramics. Journal of the American Ceramic Society. 1998;81(7):1705–1727. [Google Scholar]
- 10.Hu S, Chang J, Liu M, Ning C. Study on antibacterial effect of 45S5 Bioglass. Journal of Materials Science: Materials in Medicine. 2009;20(1):281–286. doi: 10.1007/s10856-008-3564-5. [DOI] [PubMed] [Google Scholar]
- 11.Bellantone M, Williams HD, Hench LL. Broad-spectrum bactericidal activity of Ag2O-doped bioactive glass. Antimicrobial Agents and Chemotherapy. 2002;46(6):1940–1945. doi: 10.1128/AAC.46.6.1940-1945.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorustovich A, Steimetz T, Cabrini RL, Porto López JM. Osteoconductivity of strontium-doped bioactive glass particles. Bone. 2007;41(6):p. S4. doi: 10.1002/jbm.a.32355. [DOI] [PubMed] [Google Scholar]
- 13.Moonga BS, Dempster DW. Zinc is a potent inhibitor of osteoclastic bone resorption in vitro. Journal of Bone and Mineral Research. 1995;10(3):453–457. doi: 10.1002/jbmr.5650100317. [DOI] [PubMed] [Google Scholar]
- 14.Rico H, Villa LF. Zinc, a new coherent therapy for osteoporosis? Calcified Tissue International. 2000;67(5):422–423. doi: 10.1007/s002230001138. [DOI] [PubMed] [Google Scholar]
- 15.Ito A, Otsuka M, Kawamura H, et al. Zinc-containing tricalcium phosphate and related materials for promoting bone formation. Current Applied Physics. 2005;5(5):402–406. [Google Scholar]
- 16.Hayakawa S, Ando K, Tsuru K, et al. Structural characterization and protein adsorption property of hydroxyapatite particles modified with zinc ions. Journal of the American Ceramic Society. 2007;90(2):565–569. [Google Scholar]
- 17.Miyaji F, Kono Y, Suyama Y. Formation and structure of zinc-substituted calcium hydroxyapatite. Materials Research Bulletin. 2005;40(2):209–220. [Google Scholar]
- 18.Ito A, Kawamura H, Otsuka M, et al. Zinc-releasing calcium phosphate for stimulating bone formation. Materials Science and Engineering. 2002;22(1):21–25. [Google Scholar]
- 19.Du RL, Chang J, Ni SIY, Zhai WY, Wang JY. Characterization and in vitro bioactivity of zinc-containing bioactive glass and glass-ceramics. Journal of Biomaterials Applications. 2006;20(4):341–360. doi: 10.1177/0885328206054535. [DOI] [PubMed] [Google Scholar]
- 20.Ramaswamy Y, Wu C, Zhou H, Zreiqat H. Biological response of human bone cells to zinc-modified Ca-Si-based ceramics. Acta Biomaterialia. 2008;4(5):1487–1497. doi: 10.1016/j.actbio.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19(3):180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 22.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 23.Wilson T, Stark C, Holmbom J, et al. Fate of bone marrow-derived stromal cells after intraperitoneal infusion or implantation into femoral bone defects in the host animal. Journal of Tissue Engineering. 2010;2010 doi: 10.4061/2010/345806. Article ID 345806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Risteli L, Risteli J. Biochemical markers of bone metabolism. Annals of Medicine. 1993;25(4):385–393. doi: 10.3109/07853899309147301. [DOI] [PubMed] [Google Scholar]