Abstract
Background
We showed that subjects with cerebral palsy had greater transverse and frontal plane hip and knee motion, increased duration of muscle activity, increased cocontraction, and decreased efficiency during recumbent cycling than subjects with typical development. However, it is also important to understand the forces exerted on the pedals. The purpose of this report was to compare pedal forces during cycling between adolescents with and without cerebral palsy.
Methods
Ten subjects (3 male, 7 female) with spastic diplegic or quadriplegic cerebral palsy (15.6 years, SD 1.8) and 10 subjects (3 male, 7 female) with typical development (14.9 years, SD 1.4) cycled on a stationary recumbent cycle at 30 and 60 revolutions per minute if able. Three-dimensional piezoelectric force transducers measured pedal forces. Data were analyzed using two-way ANOVAs.
Findings
Subjects with cerebral palsy spent a smaller percentage (P < .001, r2 = .09, power = 1.0) of the revolution applying positive force (pushing into the pedal during the extension phase) and a greater percentage (P < .001, r2 = .09, power = 1.0) of the revolution applying negative force (pulling away from the pedal during the flexion phase). There was no effect of cadence and no interaction effect.
Interpretation
These findings compliment our earlier findings of altered joint kinematics and muscle activity indicating that subjects with cerebral palsy and typical development have different cycling strategies. Methods to increase the duration of the positive force may allow subjects with CP to cycle more successfully and cycle vigorously enough to reach a heart rate necessary for improving fitness.
Keywords: Cerebral palsy, Force, Cycling, Pediatric, Biomechanics
1. Introduction
Cycling is a potential intervention for fitness and strength training in individuals with cerebral palsy (CP). Our recent study comparing cycling biomechanics between adolescents with and without CP showed that subjects with CP had greater transverse and frontal plane hip and knee motion, increased duration of muscle activity, increased cocontraction, and decreased efficiency during recumbent cycling than subjects with typical development (TD) (Johnston et al., 2007). Increased muscle cocontraction and a decreased smoothness of the cycling pattern has also been reported in children with CP (Kaplan, 1995).
However it is also important to understand the forces being exerted on the pedals as this may provide additional insight into the cycling task as well as potential differences in forces between adolescents with and without CP. Understanding these forces can provide an indication of the pedaling effectiveness. With an ineffective cycling pattern, forces may not be directed in the appropriate movement direction (Franco et al., 1999). In addition during a cycling revolution, movement of the contralateral leg during the flexion phase is partially assisted by the forces generated into extension by the ipsilateral leg (Ericson and Nisell, 1988). Alterations in pedal forces for adolescents with CP, if present, may assist in the understanding of functional effects of altered muscle activity patterns during cycling. The purpose of this brief report was to compare the percent of the revolution in which the pedal forces were positive or negative during recumbent cycling between adolescents with CP and adolescents with TD.
2. Methods
Ten subjects (3 male, 7 female) with a diagnosis of spastic diplegic or quadriplegic CP (15.6 years, SD 1.8) and 10 subjects (3 male, 7 female) with TD (14.9 years, SD 1.4) participated. Inclusion criteria were ages 13–19 years, the ability to maintain a sitting position, and at least 15° of plantarflexion range of motion (required for another portion of the study not reported here). Additional criteria for subjects with CP were a diagnosis of spastic CP with a Gross Motor Function Classification System level of III or IV (Palisano et al., 1997). The exclusion criteria were lower extremity surgery or traumatic fracture within the past six months, joint pain, spinal fusion to the pelvis, joint instability or dislocation, lower extremity stress fractures in the past year, cardiac disease, uncontrolled seizure disorder, and pulmonary disease or asthma treated with oral steroids. Addition exclusion criteria for subjects with CP were severe spasticity (modified Ashworth scale ≥4), severely limited range of motion preventing safe positioning on the cycle, and a diagnosis of athetoid or ataxic CP. Subjects 18 years of age and older and a parent of the subjects less than 18 years old signed an informed consent form approved by the governing Institutional Review Board (IRB). Subjects less than 18 years of age signed an IRB approved assent form.
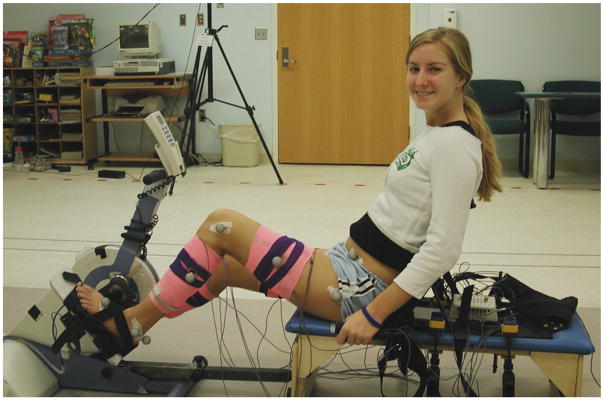
The recumbent stationary cycle (Restorative-Therapies, Inc., Baltimore, MD, USA) had adjustable-length crank arms, adjustable pedals, an adjustable seat back and an attached therapy bench (Kaye Products, Hillsborough, NC, USA) as the seat (Fig. 1). The cycle was adjusted to each subject’s anthropometric measurements (Johnston et al., 2007). After receiving 1–2 short training sessions (10–20 min), subjects were tested while cycling at cadences of 30 rpm and 60 rpm if able. Further details on the cycle and testing are described in a previous report (Johnston et al., 2007).
Fig. 1.
A subject with typical development on the cycle.
The cycle pedals were instrumented with tri-axial piezoelectric force transducers (PCB Piezotronics, Depew, NY, USA) capable of maximum force measurements of 5.87 kN in the Z direction and 2.94 kN in the X and Y directions. The transducers had a resolution of 0.56 mV per Newton of force in the Z and 2.25 mV per Newton of force in the X and Y directions and a decay constant of 1000 s. Each instrumented pedal consisted of the transducer sandwiched in series between two plates, one of which served as the pedal surface (Fig. 2). A four-pin output connection from each sensor connected to a bundled cable that split into three BNC connectors, one for each axis of force measurement. The cables were routed to signal conditioners, which also provided power to the sensors. A total of six channels of signal conditioning were needed to measure all forces.
Fig. 2.
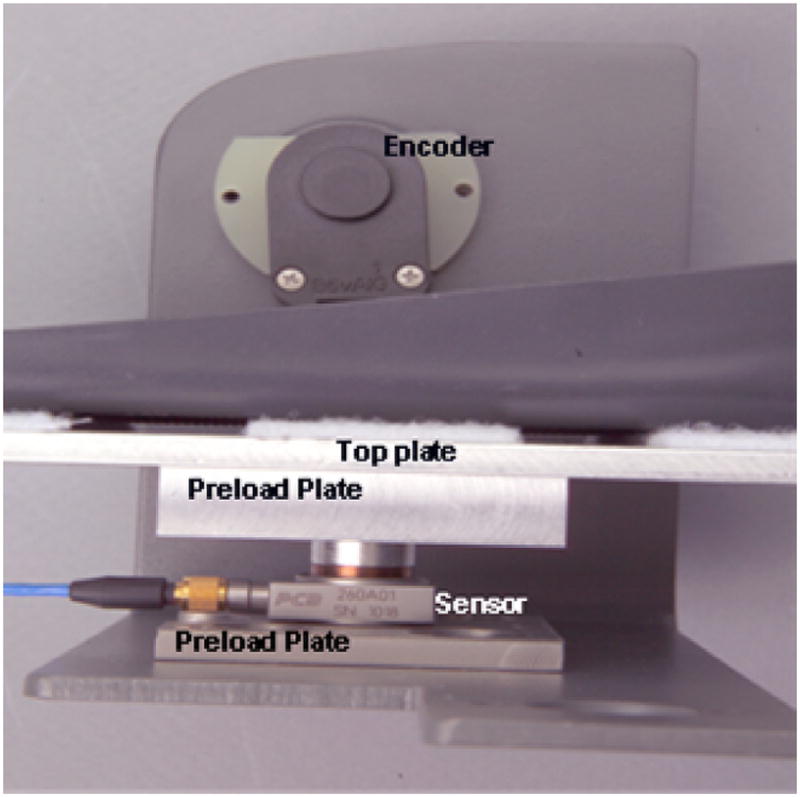
Components of the pedal system.
Pedal force data were collected in a motion analysis laboratory simultaneously with previously reported kinematic and electromyographic data (Johnston et al., 2007). Force data were collected at 1200 Hz and down-sampled to 360 points, each representing 1° of the cycling revolution. A rotary encoder (US Digital Corporation, Vancouver, WA, USA) mounted on the crank indicated the degree of the revolution. Three trials of 10–15 s duration were captured at each cadence once the targeted cadence was reached. Six of the 10 subjects with CP were able to attain and maintain 60 rpm for these short duration trials. Four subjects with CP who could not cycle at 60 rpm could cycle at 30 rpm for these short duration trials and were tested only at this lower cadence. Resistance provided was based upon each subject’s weight and the following formula adapted from Dore et al. (2000) Load (N m) = 0.49 N/kg × body weight (kg) × crank arm length (m).
Pedal force data were processed using custom software. The forces in the Z direction (forces perpendicular to the pedal surface) were calculated and the percentage of the revolution in which positive (pushing into the pedal during the extension phase) and negative (pulling away from the pedal during the flexion phase) forces were applied was calculated. Five revolutions closest to the target cadence were selected for analysis. Data were analyzed via two-way repeated measures ANOVAs (group × cadence) using SPSS 11.0 (SPSS Inc., Chicago, IL, USA).
3. Results
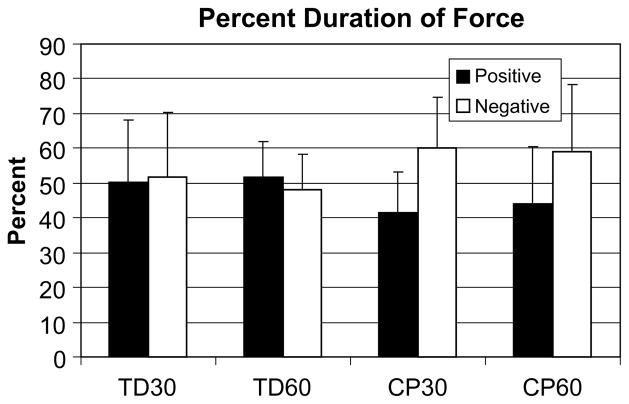
The pedaling cadences for the analyzed trials were 31.2 rpm (SD 1.6) and 59.9 rpm (SD 0.8) for subjects with TD and 29.7 rpm (SD 5.7) and 59.9 rpm (SD 1.5) for subjects with CP at the 30 and 60 rpm targets, respectively. Pedal force data (Fig. 3) showed that subjects with CP spent a smaller percentage (30 rpm: CP 41.4% (SD 11.7), TD 50.4% (SD 17.7); 60 rpm: CP 43.9% (SD 58.9.7), TD 51.9% (SD 10.2)) of the revolution applying positive force (pushing into the pedal) and a greater percentage of the revolution applying negative force (pulling away from the pedal) than did subjects with TD regardless of the cycling cadence (P < .001). There was no main effect of cadence (P > .05) and no interaction effect of group and cadence (P > .05). Fig. 4 shows example force tracings for one subject in each group.
Fig. 3.
Percent duration of force for both groups and cadences. Subjects with CP spent a smaller percentage of the revolution applying positive force (pushing into the pedal) and a greater percentage of the revolution applying negative force (pulling away from the pedal) than did subjects with TD regardless of the cycling cadence (P < .001). There was no main effect of cadence (P > .05) and no interaction effect of group and cadence (P > .05). TD30 = subjects with typical development cycling at 30 rpm, TD60 – subjects with typical development cycling at 60 rpm, CP30 –subjects with cerebral palsy cycling at 30 rpm, CP60 – subjects with cerebral palsy cycling at 60 rpm.
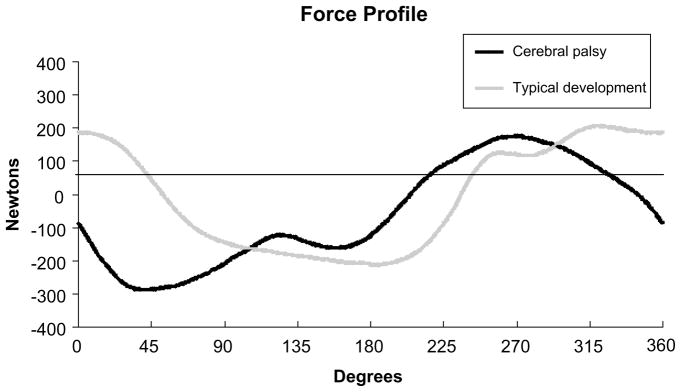
Fig. 4.
Force tracings for one subject with cerebral palsy and one subject with typical development cycling at 60 rpm showing the differences in the phasing of force patterns in relation to the position of the crank. These two subjects were of comparable body weight. Positive force indicates pushing into the pedal and negative force indicates pulling away from the pedal, and 0° represents when the crank arm of the cycle is horizontal and farthest away from the subject. As can be seen starting at approximately 270°, the forces for the subject with cerebral palsy began reversing direction from positive to negative sooner than for the subject with typical development. For these subjects, positive forces were present for 139.5° and 176.7° for the subject with cerebral palsy and the subject with typical development, respectively.
4. Discussion
The finding of differences in the percentage of the revolution in which positive and negative forces were applied was unanticipated due to similar task requirements between the two groups. However, these findings compliment our earlier findings of altered joint kinematics and muscle activity between groups, indicating that the subjects with CP and TD appeared to use different strategies to cycle. In that study, subjects with CP showed increased hip flexion, internal rotation, and abduction, increased knee extension and varus, and increased dorsiflexion, as well as increased duration of muscle activity and increased cocontraction (Johnston et al., 2007). The results of the current study add to these data, and indicate that subjects with CP terminated the extension phase sooner than did subjects with TD despite having increased duration of muscle activity. If the extension phase is terminated early, the contralateral limb that is flexing may have to work harder as it cannot take advantage of the prolonged extensor force (Ericson and Nisell, 1988).
Possible reasons for the early termination of the extension phase are increased hip flexion and dorsiflexion seen during this phase, as knee extension was greater than what was seen with subject with TD. This pattern may be due to decreased strength of the hip extensors and plantarflexors and of the knee extensors as the recumbent position encourages gravity to extend the knee. Decreased movement toward plantarflexion during the extension phase of cycling despite having sufficient passive range of motion may have also contributed. (Johnston et al., 2007). Finally, altered motor control for subjects with CP may be an additional factor as cocontraction is an important contributor in decreased motor control for children with CP (Giuliani, 1991).
Based on the findings, methods to increase the duration of positive force at the appropriate portions of the crank cycle should be investigated to potentially allow subjects with CP to cycle more successfully and work vigorously enough to reach a heart rate necessary for improving overall fitness. One possible technique for this is functional electrical stimulation (FES). FES cycling has been studied in adults with spinal cord injury and typically provides cyclical stimulation to the quadriceps, hamstrings, and gluteal muscles (Trumbower and Faghri, 2005). For individuals with CP, FES may be used to provide cutaneous cues for timing of muscle activation or to provide a stronger muscle contraction than individuals can produce volitionally. The combined results of this and our previous study indicate that extensor muscles, including the gluteus maximus, quadriceps femoris, and gastrocnemius muscles may be the best choices of muscles to stimulate during cycling for adolescents with CP. This combination of muscles may lengthen the extension phase of the cycling revolution, which may especially be important for overground cycling to enhance forward propulsion of the cycle. However, further research is needed to evaluate the effects of FES on cycling in this population.
5. Conclusions
Subjects with CP displayed a shorter extension phase for cycling as indicated by a decrease in the duration of positive force applied through the pedals. Methods such as FES may assist individuals with CP to cycle more successfully and more vigorously.
Acknowledgments
Funding was provided by Shriners Hospitals for Children, Grant #8530 and a Clinical Research Grant from the Pediatric Section of the American Physical Therapy Association. Dr. Lee is also funded by NIH Grant HD043859.
The authors thank Richard T. Lauer, PhD and Carrie A. Stackhouse, MS for their assistance.
References
- Dore E, Bedu M, Franca NM, Diallo O, Duche P, Van Praagh E. Testing peak cycling performance: effects of braking force during growth. Med Sci Sport Exerc. 2000;32:493–498. doi: 10.1097/00005768-200002000-00035. [DOI] [PubMed] [Google Scholar]
- Ericson MO, Nisell R. Efficiency of pedal forces during ergometer cycling. Int J Sports Med. 1988;9:118–122. doi: 10.1055/s-2007-1024991. [DOI] [PubMed] [Google Scholar]
- Franco JC, Perell KL, Gregor RJ, Scremin AM. Knee kinetics during functional electrical stimulation induced cycling in subjects with spinal cord injury: a preliminary study. J Rehabil Res Dev. 1999;36:207–216. [PubMed] [Google Scholar]
- Giuliani CA. Dorsal rhizotomy for children with cerebral palsy: support for concepts of motor control. Phys Ther. 1991;71:248–259. doi: 10.1093/ptj/71.3.248. [DOI] [PubMed] [Google Scholar]
- Johnston TE, Barr AE, Lee SC. Biomechanics of submaximal recumbent cycling in adolescents with and without cerebral palsy. Phys Ther. 2007;78:572–585. doi: 10.2522/ptj.20060261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan SL. Cycling patterns in children with and without cerebral palsy. Dev Med Child Neurol. 1995;37:620–630. doi: 10.1111/j.1469-8749.1995.tb12050.x. [DOI] [PubMed] [Google Scholar]
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Faghri PD. Kinematic analyses of semireclined leg cycling in able-bodied and spinal cord injured individuals. Spinal Cord. 2005;43:543–549. doi: 10.1038/sj.sc.3101756. [DOI] [PubMed] [Google Scholar]