Abstract
Ornithine transcarbamylase (OTC) is an important enzyme in the detoxification of ammonia to urea, and its deficiency is the most common inborn error of ureagenesis in humans. Among 24 cases of OTC deficiency previously examined, three unrelated individuals all showed loss of a Taq I site in the OTC gene corresponding to codon 109, suggesting that this Taq I site may be prone to mutation. Two of these patients demonstrated the same C----T transition (in antisense strand) converting Arg109 to Gln. Although these studies implied a strong association between the missense mutation and OTC-deficient phenotype, a causal relationship could not be firmly established. We have investigated this relationship by reconstructing the mutation in vitro. A full-length human OTC cDNA was cloned into an SV40-based expression vector and has been reproducibly expressed at high levels in the cell line Cos1. By site-directed mutagenesis of this wild type sequence, we constructed a missense mutation which contains the C----T transition. Electroporation and transient assay in Cos1 indicated that the specific activity of mutant OTC was 100-fold lower than that of wild type. This result confirms that the Taq I alteration leading to the Gln missense is responsible for the OTC deficiency affecting the above patients.
Full text
PDF
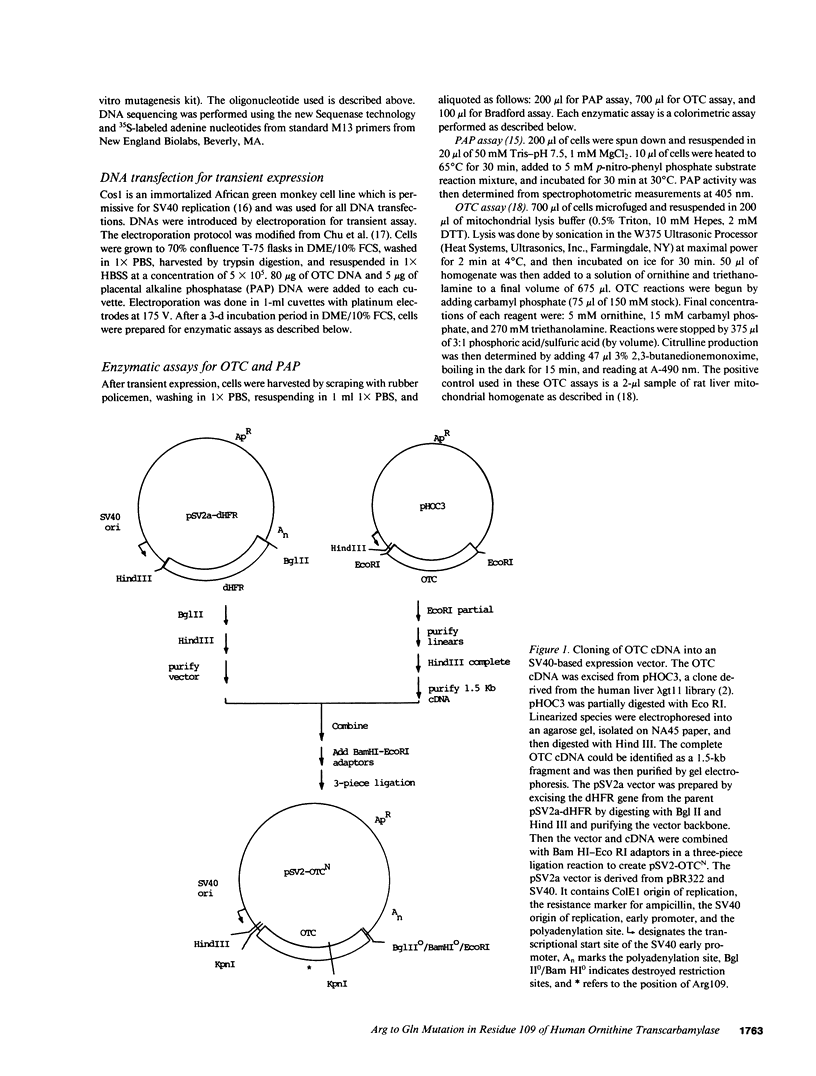



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bencini D. A., Houghton J. E., Hoover T. A., Foltermann K. F., Wild J. R., O'Donovan G. A. The DNA sequence of argI from Escherichia coli K12. Nucleic Acids Res. 1983 Dec 10;11(23):8509–8518. doi: 10.1093/nar/11.23.8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand P., Francois B., Rabier D., Cathelineau L. Ornithine transcarbamylase deficiencies in human males. Kinetic and immunochemical classification. Biochim Biophys Acta. 1982 May 21;704(1):100–106. doi: 10.1016/0167-4838(82)90136-4. [DOI] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas D. H., Skomra C. J., Anderson G. R., Hughes R. G., Jr In situ staining procedure for the detection of ornithine transcarbamylase activity in polyacrylamide gels. Anal Biochem. 1987 Feb 1;160(2):421–428. doi: 10.1016/0003-2697(87)90070-4. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Hata A., Tsuzuki T., Shimada K., Takiguchi M., Mori M., Matsuda I. Structure of the human ornithine transcarbamylase gene. J Biochem. 1988 Feb;103(2):302–308. doi: 10.1093/oxfordjournals.jbchem.a122265. [DOI] [PubMed] [Google Scholar]
- Henthorn P., Zervos P., Raducha M., Harris H., Kadesch T. Expression of a human placental alkaline phosphatase gene in transfected cells: use as a reporter for studies of gene expression. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6342–6346. doi: 10.1073/pnas.85.17.6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover T. A., Roof W. D., Foltermann K. F., O'Donovan G. A., Bencini D. A., Wild J. R. Nucleotide sequence of the structural gene (pyrB) that encodes the catalytic polypeptide of aspartate transcarbamoylase of Escherichia coli. Proc Natl Acad Sci U S A. 1983 May;80(9):2462–2466. doi: 10.1073/pnas.80.9.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A. L., Fenton W. A., Williams K. R., Kalousek F., Kraus J. P., Doolittle R. F., Konigsberg W., Rosenberg L. E. Structure and expression of a complementary DNA for the nuclear coded precursor of human mitochondrial ornithine transcarbamylase. Science. 1984 Jun 8;224(4653):1068–1074. doi: 10.1126/science.6372096. [DOI] [PubMed] [Google Scholar]
- Huygen R., Crabeel M., Glansdorff N. Nucleotide sequence of the ARG3 gene of the yeast Saccharomyces cerevisiae encoding ornithine carbamoyltransferase. Comparison with other carbamoyltransferases. Eur J Biochem. 1987 Jul 15;166(2):371–377. doi: 10.1111/j.1432-1033.1987.tb13525.x. [DOI] [PubMed] [Google Scholar]
- Kadesch T., Berg P. Effects of the position of the simian virus 40 enhancer on expression of multiple transcription units in a single plasmid. Mol Cell Biol. 1986 Jul;6(7):2593–2601. doi: 10.1128/mcb.6.7.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus J. P., Hodges P. E., Williamson C. L., Horwich A. L., Kalousek F., Williams K. R., Rosenberg L. E. A cDNA clone for the precursor of rat mitochondrial ornithine transcarbamylase: comparison of rat and human leader sequences and conservation of catalytic sites. Nucleic Acids Res. 1985 Feb 11;13(3):943–952. doi: 10.1093/nar/13.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddalena A., Spence J. E., O'Brien W. E., Nussbaum R. L. Characterization of point mutations in the same arginine codon in three unrelated patients with ornithine transcarbamylase deficiency. J Clin Invest. 1988 Oct;82(4):1353–1358. doi: 10.1172/JCI113738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Evidence for an exceptionally reactive arginyl residue at the binding site for carbamyl phosphate in bovine ornithine transcarbamylase. J Biol Chem. 1980 Aug 10;255(15):7301–7305. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylases. Ordering of S-cyano peptides and location of characteristically reactive cysteinyl residues within the sequence. J Biol Chem. 1980 Aug 10;255(15):7287–7290. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylases. Ordering of S-cyano peptides and location of characteristically reactive cysteinyl residues within the sequence. J Biol Chem. 1980 Aug 10;255(15):7287–7290. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. The essential sulfhydryl group of ornithine transcarbamylases. pH dependence of the spectra of its 2-mercuri-4-nitrophenol derivative. J Biol Chem. 1980 Aug 10;255(15):7296–7300. [PubMed] [Google Scholar]
- McClead R. E., Jr, Rozen R., Fox J., Rosenberg L., Menke J., Bickers R., Morrow G., 3rd Clinical application of DNA analysis in a family with OTC deficiency. Am J Med Genet. 1986 Nov;25(3):513–518. doi: 10.1002/ajmg.1320250313. [DOI] [PubMed] [Google Scholar]
- Nussbaum R. L., Boggs B. A., Beaudet A. L., Doyle S., Potter J. L., O'Brien W. E. New mutation and prenatal diagnosis in ornithine transcarbamylase deficiency. Am J Hum Genet. 1986 Feb;38(2):149–158. [PMC free article] [PubMed] [Google Scholar]
- Old J. M., Briand P. L., Purvis-Smith S., Howard N. J., Wilcken B., Hammond J., Pearson P., Cathelineau L., Williamson R., Davies K. E. Prenatal exclusion of ornithine transcarbamylase deficiency by direct gene analysis. Lancet. 1985 Jan 12;1(8420):73–75. doi: 10.1016/s0140-6736(85)91966-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. E., Kalousek F., Orsulak M. D. Biogenesis of ornithine transcarbamylase in spfash mutant mice: two cytoplasmic precursors, one mitochondrial enzyme. Science. 1983 Oct 28;222(4622):426–428. doi: 10.1126/science.6623083. [DOI] [PubMed] [Google Scholar]
- Rozen R., Fox J. E., Hack A. M., Fenton W. A., Horwich A. L., Rosenberg L. E. DNA analysis for ornithine transcarbamylase deficiency. J Inherit Metab Dis. 1986;9 (Suppl 1):49–57. doi: 10.1007/BF01800858. [DOI] [PubMed] [Google Scholar]
- Rozen R., Fox J., Fenton W. A., Horwich A. L., Rosenberg L. E. Gene deletion and restriction fragment length polymorphisms at the human ornithine transcarbamylase locus. 1985 Feb 28-Mar 6Nature. 313(6005):815–817. doi: 10.1038/313815a0. [DOI] [PubMed] [Google Scholar]
- Saheki T., Imamura Y., Inoue I., Miura S., Mori M., Ohtake A., Tatibana M., Katsumata N., Ohno T. Molecular basis of ornithine transcarbamylase deficiency lacking enzyme protein. J Inherit Metab Dis. 1984;7(1):2–8. doi: 10.1007/BF01805609. [DOI] [PubMed] [Google Scholar]
- Van Vliet F., Cunin R., Jacobs A., Piette J., Gigot D., Lauwereys M., Piérard A., Glansdorff N. Evolutionary divergence of genes for ornithine and aspartate carbamoyl-transferases--complete sequence and mode of regulation of the Escherichia coli argF gene; comparison of argF with argI and pyrB. Nucleic Acids Res. 1984 Aug 10;12(15):6277–6289. doi: 10.1093/nar/12.15.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veres G., Gibbs R. A., Scherer S. E., Caskey C. T. The molecular basis of the sparse fur mouse mutation. Science. 1987 Jul 24;237(4813):415–417. doi: 10.1126/science.3603027. [DOI] [PubMed] [Google Scholar]



