Abstract
This review aims to present an overview of recent clinical trials targeting biomarkers in advanced prostate cancer. We searched ClinicalTrials.gov for early phase clinical trials on treatments of prostate cancer that have been recently completed, are ongoing or are actively recruiting participants. Drug targets and their mechanism of actions were assessed and summarized. Trials were categorized according to prostate cancer biomarkers that have potential as therapeutic targets. A total of 19 new therapeutic agents for the treatment of prostate cancer are included in this review. Trials are summarized according to the targeted biomarkers and are categorized into five therapeutic approaches: prostate cancer vaccine, epigenetic therapy, pro-apoptotic agents, prostate cancer antibodies and anti-angiogenesis approach. Some of the therapeutic agents reviewed showed promising results, warranting further investigation in late phase clinical trials. Recent novel prostate cancer biomarkers that made it through clinical trials and their relevance as drug targets are summarized. This review emphasizes the importance of specific prostate cancer biomarkers and their potentials as targets of the disease. Some clinical trials of targeted treatments in prostate cancer show promising results. Better understanding of disease mechanisms should potentially lead to more specific treatments for individual patients.
Keywords: biomarker, prostate cancer, review
Introduction
Prostate cancer and PSA screening
Prostate cancer is among the most commonly diagnosed male disease and remains a leading cause of death in most Western countries, especially in elderly men [1–3]. More than half of all men diagnosed with cancer are over the age of 70 years, with prostate cancer constituting about 50% of cancers in this age group [4].
Rapid increases in incidence rates for prostate cancer in the past two decades have occurred in part due to the widespread use of screening since the 1980s, by serum prostate-specific antigen (PSA), a glycoprotein produced by the prostate gland [5–8]. Whether PSA screening and earlier detection of prostate cancer has an impact on the decline of mortality is still debated [9–12]. Recently, large scale studies from the US [13] and Europe [14] looking at correlation between PSA screening and prostate cancer mortality have been published. In the US, they found no significant decline in mortality rate between patients receiving annual PSA screening and digital rectal examination (DRE) and those receiving usual care which may sometimes include screening [13]. However a study from European countries found a weak association between increased PSA screening and mortality rate [14]. There is, nonetheless, an urgent need for better biomarkers to replace or work in conjunction with PSA screening, due to the high prevalence of false positive and false negative rates seen with PSA alone [8].
Biomarkers leading to new therapeutic approaches
The knowledge of biomarkers in cancer offers new hope for more specific therapies and provides important understanding of the factors influencing the aggressiveness of prostate cancer and potential new treatments [15]. Molecular genetic approaches examining tumour gene expression profiling using microarrays have been introduced in recent years and analysis using a small panel of genes of interest has the potential for use in clinical practice to allow better diagnosis and classification of the disease, provide information on how each individual patient may respond to treatments and lead to reduced toxicity and identification of new therapeutics [16, 17]. We will review a number of biomarkers in prostate cancer which have involved development of targeted therapies in animal and human trials. Figure 1 schematically summarizes the function of these various biomarkers. Table 1 summarizes these targets and ongoing clinical trials which will be discussed in the following section. Although, as mentioned above, PSA measurement has limitations in prostate cancer management, most studies we reviewed utilized PSA responses as an efficacy endpoint. Some studies also used the RECIST system (to evaluate response rates of tumour lesions) [18].
Figure 1.
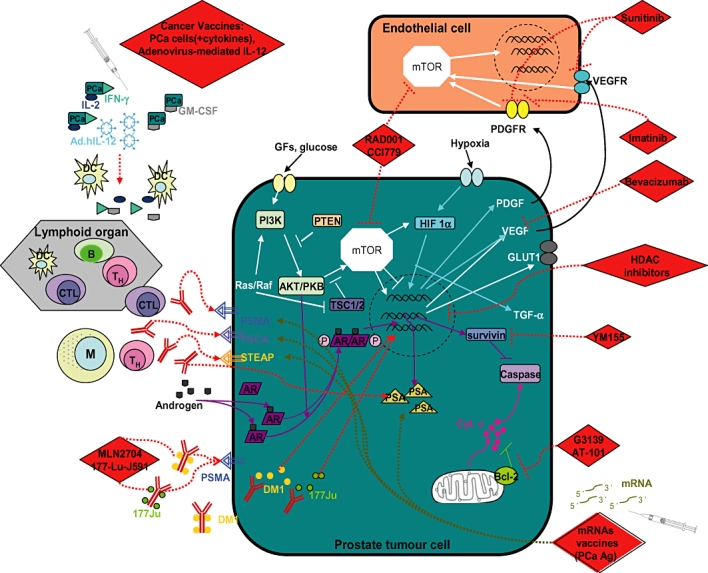
Site of actions of drugs that are currently in clinical trials for treatment of prostate cancer and target biomarkers and their roles in prostate cancer. [Experimental therapeutics: RAD001, CCI779, mRNAs vaccines, MLN2704, 177-Lu-J591, YM155, HDAC inhibitors, G3139, AT-101, bevacizumab, sunitinib, imatinib and cancer vaccines are indicated as RED diamonds], lymphoid cells are indicated as B ( ) for B lymphocyte; CTL (
) for B lymphocyte; CTL ( ) for cytotoxic T lymphocytes); M (
) for cytotoxic T lymphocytes); M ( ) for macrophage; TH (
) for macrophage; TH ( ) for T helper cells and DC (
) for T helper cells and DC ( ) for dendritic cells;
) for dendritic cells;  indicates phosphorylation. (For others see ‘abbreviations and acronyms’ listing for definitions of targets under study
indicates phosphorylation. (For others see ‘abbreviations and acronyms’ listing for definitions of targets under study
Table 1.
Current drugs in clinical trials for treatment of prostate cancer (PCa) and their targets (Source: clinical trial data and drug names are available on http://ClinicalTrial.gov; []= study reference number)
| Drugs | Targets | Mode of action | Stage of development (Trial phase) | Efficacy PSA response | Anti-tumour response (RECIST [18]) | Dose limiting toxicities (DLT) | Treatment modality | Intent of treatment | Ref. | Clinical trials |
|---|---|---|---|---|---|---|---|---|---|---|
| GVAX® vaccine | GM-CSF | • Prolonged immunity against PCa cells via DC differentiation and proliferation • Specific to PCa cells | I, II | Phase I/II • Overall median PSA DT increased compared with pre-treatment [35]• 8/19 (42%) patients had stable PSA during treatment period[35]• 16/21(76%) patients had decreased PSA post-treatments [34]• 42/80 (52%) patients that had decrease PSA concentrations had longer survivals [33] | Phase I/II • T-cell and B-cell stimulations [34, 35]• 28/80 patients had SD[33] | DLT not reached in any trials | Single/combination (ADT) | Palliation | [33–35] | [154, 155] |
| RNActive®-derived PCa vaccine (CV9103) | PSA, PSMA, PSCA and STEAP | • Direct anti-tumour activity via delivery of mRNA encoding prostate-specific Ag | I, II | N/A | N/A | N/A | Single | Palliation | [37] | [38, 39] |
| IL-2-IFN-γ-secreting tumour vaccine | IL-2 and IFN- γ | • Specific anti-tumour responses • Enhancing Ag-presenting cells and T-cells responses to tumour Ags | I, II | Phase I [41]• 2/6 patients had <50% PSA decline • 2/6 patients had PSA stabilization Phase I/II [40]• 22/30 patients had increased PSA DT compared with pre-treatment • 12/30 had plateau PSA for a period of 12 weeks | Phase I [41]• Immunogenic Ag PSGR-1, STEAP, PSA, PRAME (5/6 patients) • Immunogenic Ag PSCA, PSMA and EpCAM (3/6 patients) Phase I/II [40]• Immunogenic Ags including survivin, PSCA, PSMA, PSA, STEAP, PAP and Ep-2H Both trials had T-cell response against PCa Ag (but was not correlated with PSA response) | DLT is not reached in any trials | Single | Palliation | [40, 41] | [36] |
| Adenovirus-mediated IL-12 gene delivery and PSMA-pulsed PBMC with recombinant IL-12 | IL-12 gene | • Increase tumour cells susceptibility to cytotoxic T cells | I | N/A | N/A | N/A | Single | Palliation | [42] | [51, 52] |
| Vorinostat (SAHA), panobinostat, belinostat (PXD101), valproic acid and romidepsin (FK228) | HDACs | • Epigenetic therapy • Prevention of gene modification promoting cancer survival | I, II | Vorinostat Phase I Single agent [66, 68]• Trials of combined solid and haematological malignancies • PSA concentrations were not determined Panobinostat Phase I Combination [71]All patients receiving panobinostat alone had PSA progression | Vorinostat Phase I Single agent [66, 68]• PCa patients did not have anti-tumour response Panobinostat Phase I Combination [71]• 2/7 patients receiving panobinostat + docetaxel had PR | Vorinostat Phase I Single agent [66, 68]• Grade 4 leukopenia • Grade ¾ thrombocytopenia • Grade 4 neutropenia Panobinostat Phase I Combination [71]• Grade 3 neutropenia • Grade 3 dyspnea | Single/Combination (chemothera-py, ADT, isotretinoin and bevacizumab) | Palliation | [66–71, 156] | [53–60, 157, 158] |
| Cont'd | • 5/8 patients receiving panobinostat+ docetaxel had ≥50% PSA decline Phase Ib Combination [69]• 10/18 patients had decline PSA (7 patients <50% decline) Belinostat Phase I Single agent [67]• PSA concentrations not measured | (Including 1 patient who had ≥50% PSA decline • 4/7 patients had receiving panobinostat + docetaxel had SD Phase Ib Combination [69]• 2/13 patients had PR • 6/13 patients had SD Belinostat Phase I Single agent [67]• 18/46 patients had SD • 12/24 (MTD) patients had SD | Phase Ib Combination [69]• Grade 4 bradycardia • Grade 4 neutropenia Belinostat Phase I Single agent [67]• Grade 3 fatigue • Grade 3 atrial fibrillation | |||||||
| Romidepsin Phase II Single agent [70]• 2/35 had ≥50% PSA decline PSA response rate of 5.7% | Romidepsin Phase II Single agent [70]2/35 patients had PR | • Grade 3 diarrhoea • Grade 2 nausea/vomiting Romidepsin Phase II Single agent [70]DLT was not reached due to early trial closure | ||||||||
| Oblimersen sodium (G3139) and R-(-)-gossypol acetic acid (AT-101) | Bcl-2 | • Targeting anti-apoptotic Bcl-2 protein • Increase susceptibility of cancer cells to cytotoxic drugs and radiotherapy | I, II | Oblimersen Combination • Contradicting results on PSA responses [79, 80]• 14/27 patients had PSA response (6 patients had ≥80% PSA decline)[79]• PSA decline of ≥30% was not reached [80] | Oblimersen Combination • Contradicting results[79, 80]• 4/12 had PR [79]• Docetaxel-oblimersen and docetaxel alone showed similar clinical responses [80] | Oblimersen • Grade ¾ neutropenia • Grade ¾ leukopenia • Grade ¾ fatigue | Combination (Chemotherapy and ADT) | Palliation | [79, 80, 84, 85] | [78, 83, 159, 160] |
| AT101 Single agent [84]• 2/23 (8%) patients had ≥50% decline in PSA concentrations Combination [85]• 14/20 (70%) patients had ≥50% decline in PSA concentrations | AT101 Single agent [84]• 2/19 patients SD Combination [85]• 50% PR levels | AT101 • Grade 3 small intestinal obstruction • Grade 3 gastrointestinal toxicities | ||||||||
| YM155 | Survivin | • Pro-apoptotic agents • Block apoptosis inhibitor protein, survivin | I, II | • 2/9 (22%) PCa patients had decline PSA concentrations | • Only PSA concentrations were determined for PCa patients | • Increase creatinine concentrations • Grade ¾ neutropenia • Grade 3 thrombocytopenia | Single/Combination (Chemotherapy) | Palliation | [89] | [90, 91] |
| MLN2704 and 177Lu-J591 | PSMA | • PCa Ab • Ab-directed cytotoxic drug and radioisotopes | I, II | MLN2704 Single agent [94]• 2/23 (8%) with PSA decline of ≥50% | MLN2704 Single agent [94]• 40% SD | MLN2704 • Uncomplicated febrile neutropenia | Single/Combination (177Lu-J591 with chemotherapy and ADT) | Palliation | [93, 94, 100, 101] | [95–99, 161, 162] |
| Cont'd | 177Lu-J591 Single agent • 2/29 had 70–80% decline in PSA concentrations[101]• 6/29 had PSA stabilization [101]• 4/35 had ≥50% PSA decline [100]• 16/35 had PSA stabilization [100] | 177Lu-J591 Single agent 2/12 patients objective responses[101] | 177Lu-J591 • Grade 4 thrombocytopenia • Grade 4 neutropenia | |||||||
| Bevacizumab (Avastin) | VEGF | • Anti-angiogenesis | I, II | Combination • 11/20 (55%) patients has major PSA response | Combination • 2/8 patients had SD • 3/8 patients had PR • 3/8 patients had PD | • Grade ¾ neutropenia • Grade ¾ thrombocytopenia | Single/Combination (Chemotherapy, ADT, mTOR inhibitor) | Palliation and prevention | [146] | [111, 112, 142, 147, 148, 163–165] |
| Imatinib mesylate (Gleevec®) | PDGFR | • Anti-angiogenesis | I, II | Single agent • 1/19 patients had ≥50% decline and 2/19 had a decline of <50% [118]• Median PSA DT (16/19 patients) were not different pre- and post-treatments [118] | Single agent • 11/20 patients withdrew from study (most due to imatinib-related toxicity) [118]• 6/20 patients had PD [118] | Single agent • Trial was stopped due to toxicity [118]• Grade ¾ neutropenia • Grade 4 dyspnoea | Combination (Chemotherapy and ADT) | Palliation | [118, 120, 166, 167] | [121–123] |
| • 9/16 patients had PSA stabilization [120]• 5/16 patients had PSA progression [120]Combination • PSA changes in imatinib-docetaxel group were similar to docetaxel-placebo group [165] | Combination No apparent benefit when combined with docetaxel [165] | • Grade 3 dyspepsia • Grade 3 rash • Grade 3 diarrhoea • Grade 4 haematuria Combination • 5/89 patients had sigmoid diverticular perforations [165]• 3 deaths related to therapy; pneumonitis, peritonitis and diverticular perforation [165] | ||||||||
| Sunitinib malate (SU011248) | VEGFR and PDGFR | • Anti-angiogenesis | I, II | • Single agent 8/34 patients had ≥30% PSA decline (2 patients had ≥50% decline) • 15/34 (44%) patients had PSA stabilization | Single agent • 1/34 patients had PR • 18/34 patients had SD • 10/34 patients had PD | • Grade ¾ neutropenia • Grade ¾ leukopenia • Grade ¾ hypertension • Grade ¾ diarrhoea and fatigue | Single/Combination (Chemotherapy, ADT, RT) | Palliation and prevention | [124] | [127–129, 149, 168] |
| Everolimus (RAD001) and temsirolimus (CCI-779) | mTOR | • Anti-angiogenesis • Interrupt cancer survival signalling | I, II | Single agent • 1/13 (7.5%) patients had PSA decline of 50% | Single agent • 4/13 patients had SD • 1/13 patients had PR | N/A | Single/Combination (Chemotherapy and bevazicumab) | Palliation and prevention | [141] | [111, 142, 144, 152, 153] |
RECIST, Response Evaluation Criteria in Solid Tumours criteria [18]; CR, (complete response) = disappearance of all target lesions; PR, (partial response) = 30% decrease in the sum of the longest diameter of target lesions; PD, (progressive disease) = 20% increase in the sum of the longest diameter of target lesions; SD, (stable disease) = small changes that do not meet above criteria.
Current clinical trials in prostate cancer biomarker targeting
Prostate cancer vaccines
Cancer vaccines stimulate systemic anti-tumour immune responses which may provide reduced toxicities compared with traditional chemotherapy. Patients who develop an androgen-independent prostate cancer after radiation therapy or hormone ablation therapy and those who have metastatic disease at the time of diagnosis are much less likely to be cured. Therefore it is crucial to identify markers early in the disease process that might be able to distinguish between indolent and aggressive cancers [19–22]. The presence of markers that are prostate-specific and prostate cancer-specific [23] such as PSA, prostate specific membrane antigen (PSMA) (PSCA), and early prostate cancer antigen (EPCA) makes them potential candidates for cancer vaccines.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) vaccine or GVAX® is a promising approach for prostate cancer [24]. This vaccine involves genetically modified irradiated prostate cancer cells expressing the cytokine, GM-CSF which is a known mediator of immune system activation [25]. In mice, irradiated tumour cells expressing GM-CSF need CD4+ and CD8+ T-cells for immune activation and result in a long lasting and specific immune response [26]. In 2000, successful phase I/II trials using dendritic cells (DC) with encoding fusion proteins of prostatic acid phosphatase (PAP) and GM-CSF in hormone-refractory prostate cancer patients demonstrated anti-PAP immune responses in all patients. Almost half of participants involved had a decline in PSA concentrations, with no major adverse effects [27]. The mechanism of action of this immunotherapy involves DC (adaptive) immune responses to tumour cell antigens, thus promoting differentiation of bone marrow-derived progenitors into DCs at the local injection site [28]. GM-CSF can reverse tumour tolerance and produce anti-tumour responses by initiating bone marrow-derived progenitor differentiation into DCs and DC proliferation [24, 28, 29].1
Hege et al. [28] have summarized clinical trials that combined immunotherapy with other treatments to enhance the effectiveness of anti-tumour activity and this includes the use of CD40 and Toll-like receptors (TLRs) for DC activation, anti-CTLA4 and anti-CD25 antibodies. These approaches attempt to inhibit down-modulation of T-cell responses, vascular endothelial growth factor (VEGF) blockade to prevent inhibitory effects of the VEGF receptor, and interferon (IFN)-α for promotion of immunomodulatory responses. Furthermore, chemotherapy, for example with docetaxel, a cytotoxic anti-microtubule agent and anti-androgen therapy, has been combined with cancer vaccines in clinical trials to see if these more traditional treatments can enhance anti-tumour responses [30, 31].
An early phase I trial of eight prostate cancer patients involved treatment with autologous cancer vaccine prepared from the patients' own prostate tumours taken during prostatectomy, which were then irradiated and engineered to express GM-CSF [32]. This trial evaluated patients' T- and B-cell immune responses at the injection sites, hypersensitivity against the un-transduced autologous tumour cells and antibodies against tumour antigens in the serum. There were increased immune responses at the vaccination site including CD4±, CD8± T-cells, T helper cells, macrophages and DCs. Three out of eight patients had antibodies specific to prostate cancer antigens. Clinical trial phase I/II studies using allogeneic human GM-CSF gene transduced irradiated prostate tumour cell lines administered via intradermal injection have demonstrated early evidence of safety and clinical activity, showing dose–response immune responses and good tolerance in patients with metastatic hormone-refractory prostate cancer [33–35].
RNActive®-Derived Prostate Cancer Vaccine, CV9103. is another promising immunotherapy vaccine for prostate cancer which derives from mRNA encoding prostate-specific antigens which have been modified to maintain stability (PSA, PSMA, PSCA and six-transmembrane epithelial antigen of the prostate [STEAP]) [36]. The mechanism of action involves stimulation of cytotoxic T lymphocytes by gene therapy vehicle mRNAs to respond against PSA-, PSMA-, PSCA- and STEAP-expressing prostate tumour cells [37]. The use of naked mRNA vectors has been recently developed and engineered to overcome the issue of instability of mRNA leading to rapid digestion within the body. This mRNA technology is now being tested in prostate cancer and non-small cell lung cancer. Current open clinical trials phase I/IIa and II are now recruiting participants with evidence of hormone refractory prostate cancer showing increasing concentrations of PSA despite hormone deprivation therapy, using CV9103 delivery via multiple intradermal injections [38, 39].
Interleukin-2 (IL-2)-interferon-gamma (IFN-γ)-secreting allogenic tumour vaccine derived from allogeneic prostate cancer cell lines with recombinant human IL-2 and IFN-γ have been involved in early clinical trials via multiple intradermal injections [36, 40, 41] and have shown prostate cancer antigen-specific T-cell responses (reactivity against peptides PSMA, PAP, survivin, PCTA and PSA), but assessment of effectiveness of this combination of the two cytokines requires further investigation.
Pre-clinical data using adult bone marrow cells as a delivery vehicle for Il-12 genes showed satisfactory anti-metastatic effects (and prolonged survival in a mouse model with metastatic prostate cancer, with elevated levels of CD4+ and CD8+ T-cells [42, 43]. Adenovirus pre-infected with Il-12 into the transgenic adenocarcinoma of the mouse prostate (TRAMP) model of prostate cancer also results in tumour-specific immune responses and anti-tumour activity [44, 45]. An orthotopic mouse model of prostate cancer treated with Il-12 recombinant adenoviral vector transduced macrophages survived longer than control treated mice [46]. Although, specific anti-tumour and anti-metastatic activity was seen, phase I trials involving other cancers such as advanced digestive tumours, ovarian cancer, renal cell cancer and metastatic melanoma, whilst encouraging in terms of drug safety, did not show anti-tumour activity [47–50]. A current phase I trial of Il-12 for patients with recurrent non-metastatic prostate cancer after radiation therapy is now ongoing [51]. A phase II trial involving recombinant Il-12 transduced into PSMA-pulsed autologous peripheral blood mononuclear cells (PBMC) in metastatic prostate cancer is also ongoing [52].
Epigenetic therapy
Early phase clinical trials for histone deacetylase (HDAC) inhibitors in a wide range of solid tumours including prostate cancer have involved suberoylanilide hydroxamic acid (SAHA) or vorinostat [53, 54], LBH589 (panobinostat) [55, 56], PXD101 (belinostat) [57], valproic acid [58, 59] and FK228 (romidepsin) [60]. The mechanism of action is via direct inhibition of acetylation or methylation of histones affecting cancer-related gene expression and may also be via an action through non-histone proteins such as p53, heat shock protein 90 (Hsp90) and the androgen receptor, which leads to activation of transcription of certain genes [61–65]. These early phase trials showed satisfactory tolerance, with results examining serum PSA and acetylated histones (measurement of accumulated acetylated histones) across various solid tumour patients and provided good rationale to proceed to further clinical trials. Some of these trials recruited a wide range of patients with solid tumours, and anti-tumour activities were measured via clinical examinations that were analysed as partial response, complete response or stable disease [66–68]. PSA concentrations were measured for those trials that involved castrate-resistant prostate cancer patients and the reduction in PSA concentrations of more that 50% from baseline level was considered to have major anti-tumour activity [69–71]. Measurements of acetylated histones were carried out for all trials via either Western blotting, immunohistochemistry or enzyme immunoassay (ELISA). Other effects of the drugs i.e. pro-apoptitic activities were measured via concurrent monitoring of apoptotic markers in the blood.
Clinical trials of PXD101 and SAHA showed good tolerance, with a maximum tolerated dose determined to be 1000 mg m−2 day−1 and 400 mg day−1, respectively, and both trials showed dose-dependent histone hyperacetylation [66–68]. These trials in patients with advanced solid tumours (and haematological malignancies for SAHA trials) showed some anti-tumour activity (i.e. tumour regression, stable disease and tumour related pain) but this was not evident in patients with advanced prostate cancer. Trials of panobinostat showed impressive anti-tumour effects with more than 50% of patients receiving panobinostat in combination with docetaxel or docetaxel/prednisone having ≥50% decline in PSA concentrations, in contrast to those receiving panobinostat alone, where almost all patients had progressive disease despite dose-related increase histone acetylation [69, 71]. While these trials obtained valuable information on drug safety and dose-limiting toxicities, correlation between levels of acetylated histones and disease progression could not be established. This may suggest the existence of a subpopulation of prostate cancer cells that do not respond to particular HDAC inhibition and therefore implicates narrowing of a selective group of responsive patients within chemo-naive patients.
The exact mechanism by which HDAC inhibitors induces tumour differentiation, cell cycle arrest and enhancing tumour cell sensitivity to chemotherapy is yet to be elucidated. Therefore each HDAC inhibitor may have different effects on prostate cancer [72]. Some of the trials mentioned above resulted in withdrawal of patients from the studies due to ongoing toxicities i.e. gastrointestinal toxic effects including nausea, diarrhoea and vomiting. These early trials suggest that the use of HDAC inhibitors in combination with other therapies may minimize drug-related toxicities. Careful selection of patients to enter the trials and drugs to be used for advanced prostate cancer in the clinical trials may therefore give more information regarding risk-benefit assessment of these compounds.
Pro-apoptotic agents Bcl-2 modulators
Ursolic acid, a pentacyclic triterpenoid compound [73, 74] and the CXC chemokine receptor-4 (CXCR4) antagonist, CTCE-9908 [75], down-regulate Bcl-2 resulting in induction of apoptosis. However, recent findings from Nariculam et al. [76] demonstrate that Bcl-2 and p53, although overexpressed in localized prostate cancer, were not associated with clinical outcomes. Nevertheless, Bcl-2 is a potential target and has been assessed in clinical trials. For example, oblimersen sodium (G3139, Bcl-2 antisense oligonucleotide) therapy targets the Bcl-2 initiation codon region of Bcl-2 mRNA and down-regulates mRNA expression [77, 78]. A trial of G3139 in combination with docetaxel suggested an encouraging correlation between steady-state concentrations and PSA decline, with no serious toxicities reported [79]. However, another trial treating patients with oblimersen-docetaxel combination showed increased toxicities (40% had major toxic events compared with 22% in the docetaxel group) and did not incur better outcomes than docetaxel treatment alone [80]. A more recent study using microarray to evaluate the effects of G3139 and the effects of Bcl-2 silencing via small interfering RNA (siRNA) on gene profiling of PC-3 cell lines has found that the apoptotic effect of G3139 was a result of an off-target response, via up-regulation of growth-inhibitory proteins, rather than of Bcl-2 silencing alone (both treatments showed similar Bcl-2 knock down) [77]. This is important as treatment with Bcl-2 siRNA alone in metastatic prostate cancer cell lines did not induce apoptosis as seen with cells treated with G3139 [77]. Furthermore, although there were some anti-tumour responses as a result of targeting Bcl-2, several issues still need to be addressed. Marked variation in patients' responses to different concentrations of oblimersen was a major factor in defining effective doses for maximal response. Thus assessment of specific mechanisms by which oblimersen induces apoptosis will need to be evaluated to fully understand and predict patients' responses and clinical adverse effects.
Another drug that is in clinical trials targeting Bcl-2 is the small molecule inhibitor AT-101 [81–83]. In contrast to G3139, AT-101 targets the Bcl-2 homologous (BH) region 3 of Bcl-2 and when given alone, there was satisfactory tolerance in most castrate-resistance prostate cancer patients, with some gastrointestinal toxicities leading to reduction in dosage [84]. Preliminary results of phase I/II trials using a combination of AT-101, docetaxel and prednisone showed 70% (14/20) of prostate cancer patients with a ≥50% PSA decline and 54% (6/11 patients with measurable disease) having partial responses by RECIST criteria (Response Evaluation Criteria in Solid Tumors) [18], thus warranting further assessment of this agent [85]. AT-101 may be a better candidate to target Bcl-2 as combination with traditional docetaxel did not result in increased toxicities and these trials were given a more usual dose regimen of 75 mg m−2 docetaxel unlike G3139 trials where docetaxel had to be reduced (75 to 60 and then to 45 mg m−2) due to ongoing toxicities [80, 85].
Survivin modulators
Survivin is a member of the inhibitors of apoptosis (IAPs) gene family and its expression is seen during fetal development and not in normal, terminally differentiated adult human tissues [86]. However, survivin over-expression is seen in various adenocarcinomas including prostate [86]. Moreover, increased expression is associated with disease progression after radical prostatectomy [87]. Pre-clinical data have demonstrated that YM155, a small molecule survivin suppressant, promoted apoptosis in vitro and in an in vivo xenograft mouse model, using hormone-refractory prostate cancer cell lines and that this apoptotic effect was not significantly related to other IAPs or Bcl-2 related proteins [88]. YM155 has now been in early clinical trials and one single-agent trial in various advanced cancers showed some anti-tumour activity (two out of nine prostate cancer patients had a decline in PSA concentrations) [89]. Other recently completed early phase trials according to ClinicalTrials.gov (results unavailable) are either YM155 single agent in advanced cancers or YM155 in combination with docetaxel in hormone refractory prostate cancer patients [90, 91].
Prostate cancer antibodies
Immunoconjugates consisting of a humanized monoclonal Ab which is directed against prostate-specific membrane antigen (PSMA) have been investigated in prostate cancer [92]. One of the current drugs in clinical trials is MLN2704. MLN2704 is an immunoconjugate consisting of humanized monoclonal Ab directed against PSMA (named MLN591 Ab) which was linked to a maytansinoid (DM1). DM1, a potent microtubule-depolymerizing drug, is an analogue of maytansine, a naturally occurring ansa macrolide [93]. The monoclonal antibody moiety of MLN2704 binds to tumour cells expressing PSMA and is then internalized into the tumour cell, where the DM1 maytansinoid moiety binds to tubulin and inhibits tubulin polymerization and microtubule assembly, resulting in a disruption of microtubule activity, cell division and cell death. Pre-clinical data showed MLN2704 efficiency in anti-tumour activity in a mouse xenograft model, in a dose- and schedule-dependent manner [93]. Early phase I/II trials using MLN2704 showed acceptable safety (no antibody responses to either MLN2704, MLN591 or DM1) with minor grade toxicities such as fatigue and headache with only 1/23 patients reaching dose-limiting toxicity of uncomplicated febrile neutropenia, but neuropathy was observed in 35% of patients [94]. The efficacy of MLN2704 was measured by PSA concentrations and tumour regression. Two patients sustained ≥50% decline in PSA concentrations compared with baseline and six other patients treated at doses ≥156 mg m−2 sustained stable PSA concentrations for up to 86 days. Of 10 assessable patients, four had stable disease up to a dose of 343 mg m−2 and one patient receiving 264 mg m−2 had a partial response. This trial provided useful information regarding the dosage and immunogenic responses to the drug. Further trials are ongoing using MLN2704 alone in progressive metastatic prostate cancer patients [95–97].
Radiolabelled monoclonal Ab HuJ591-GS (177Lu-J591) derived from J591, an immunoglobulin G (IgG) monoclonal Ab targeting the extracellular domain of PSMA (tagged with radionuclide lutetium-177) is currently under phase I/II clinical trials [98, 99]. However some results from earlier phase I trials determined dose-limiting toxicity (including grade 4 neutropenia, severe thrombocytopenia, and other severe non-haematologic toxicities), In another trial, some patients had more than 70–80% decline in PSA concentrations which lasted up to 3–8 months and there was a strong correlation between PSA concentrations and measurable disease responses [100, 101]. Both trials did not show anti-immunogenic responses to the drugs.
These trials warrant the potential further use of PSMA as a biomarker for targeted treatments in prostate cancer, suggesting efficacy together with safety and lack of immunogenic responses to the PSMA antibodies, foreshadowing the need for further clinical assessment.
Anti-angiogenesis approaches
Vascular endothelial growth factor (VEGF) is currently being targeted as a treatment for cancer together with traditional cancer treatments. Expression levels of VEGF, an angiogenic stimulator, are elevated in prostate cancer and PIN and found to be down-regulated by androgen deprivation, resulting in changes in patterns of vascularization [102]. In addition, Ferrer et al. [103] found that there was no or diminished VEGF and IL-8 expression in BPH and normal prostate tissue, whilst expression was also reduced in higher grades of prostate cancer. Overexpression of angiogenic factors in malignant tissues, particularly VEGF is proposed to be mediated largely by hypoxia-inducible factor-1 alpha (HIF-1 α), a transcription factor [104]. As a response to oxygen deprivation (hypoxia), HIF-1 heterodimer complex is formed and consists of HIF-1α and HIF-1β transcription factors which in turn regulate activation of angiogenesis to help restore oxygen homeostasis [105]. HIF-1α is found to be overexpressed in prostate cancer specimens compared with BPH, but this overexpression is not associated with progression of the disease [106, 107]. HIF-1α expression is not seen in normal prostate tissues [107, 108]. Targeting of hypoxia-induced angiogenesis with HDAC inhibitors that inhibit HIF-1α activity and hence reduce expression of VEGF (and basic fibroblast growth factor [bFGF]) are currently being investigated in prostate cancer [105]. Other drugs targeting VEGF in phase I and II clinical trials are bevacizumab, a humanized anti-VEGF antibody, which has been approved for treatments of other cancers such as metastatic breast cancer, non-small-cell lung cancer, glioblastoma, renal cell carcinoma and colorectal cancers [58, 109, 110]. Bevacizumab in combination with docetaxel is well tolerated and approximately 50% of patients had decreased concentrations of PSA in a phase II clinical trial for metastatic hormone refractory prostate cancer [111, 112].
Platelet derived growth factor receptor (PDGFR) and platelet derived growth factor (PDGF)
PDGFR and PDGF are frequently expressed in primary and metastatic prostate cancer [113, 114] and PDGF A and its receptor PDGFR alpha are expressed in PIN, a likely precursor of prostate cancer [115]. This suggests that inhibition of PDGFR may be of therapeutic benefit to advanced prostate cancer patients. Imatinib mesylate is a potent inhibitor of PDGFR and has been approved by the FDA for the treatment of chronic myelogenous leukaemia and metastatic gastrointestinal stromal cancers [116]. Treatment with imatinib in an experimental prostate cancer mouse model was better than paclitaxel alone in reducing bone metastases but the anti-tumour effect was strongest with the combination of both [117]. However, several phase II trials evaluating imatinib mesylate alone did not show decline in PSA concentrations and, with one study, severe side effects (grade 3 and 4 haematological toxicities, neutropenia and lymphopenia, or recurrent grade 1 and 2 non-haematological toxicities) had lead to early closure [118–120], although there are several trials that are underway [121–123]. Another drug that inhibits both PDGFR and VEGFR is sunitinib malate, a multiple receptor tyrosine kinase inhibitor [124], which is currently an approved treatment for stomach cancer and gastrointestinal stromal cancer [125]. A phase II trial with only sunitinib given to two groups of patients (docetaxel-resistant prostate cancer patients and patients with no prior treatment) showed no significant decline in PSA levels in the majority of patients but serum angiogenesis biomarkers i.e. VEGFR and PDGFaa, a member of PDGF family, confirmed effects of sunitinib malate upon angiogenesis factors [124]. Even though this trial did not reach the expected PSA responses (confirmed ≥50% decline in PSA concentrations from baseline), an interesting point from this trial was that sunitinib affected PSA changes in both group of patients similarly (only one patient per group had a major PSA response and there were similar numbers of patients with stable PSA for up to 12 weeks post-treatment). Another trial targeting metastatic castration-resistant prostate cancer patients with prior docetaxel treatment demonstrated declines in PSA in 30% of the patients, but half of the participants discontinued the drug due to severe grade 3–4 toxicities of fatigue, anorexia, nausea and leukopenia and there were two deaths deemed to be related to the study [126]. Actively recruiting phase II clinical trials are ongoing for sunitinib malate with either combination with taxotere (docetaxel/prednisone) [127], hormone ablation therapy [128] or radiation therapy [129].
mTOR (mammalian target of rapamycin)
mTOR is a protein kinase that regulates cell growth, cell proliferation, cell motility, cell survival and protein synthesis and mTOR pathway proteins are overexpressed in primary prostate cancer compared with high-grade PIN and BPH tissues [130], expression being correlated with aggressiveness of the disease [131]. The loss of phosphatase and tensin homolog (PTEN) tumour suppressor gene indicating progression towards the malignant form leads to activation of the phosphatidylinositol 3-kinase (PI3K)/Akt (protein kinase B family)/mTOR signalling pathway [132]. PTEN is correlated with increased in mTOR signalling pathway markers [131] and oncogenic Akt activation in prostate cancer [133]. In addition, PTEN expression is often lost where Bcl-2 is often overexpressed as the disease progresses [134, 135]. One study demonstrated that resistance to apoptosis via an mTOR pathway inhibitor can be a response mediated by HIF-1α regulation and Bcl-2 up-regulation, suggesting a combination of a mTOR inhibitor and Bcl-2 inhibitor to prevent mTOR inhibitor resistance may be useful [136]. Pre-clinical data suggest a combination of mTOR inhibitor and chemotherapy may inhibit growth of prostate cancer and prolong survival in a xenograft model [137]. Also, a combination of a mTOR inhibitor and anti-androgen therapy may benefit some patients as there is evidence that androgen receptor stimulation promotes mTOR activation but only at a concentration of up to ∼1 nmol l−1[138] and androgen deprivation has no effect on mTOR activation in PTEN-deficient mice [139]. In vivo experiments using an androgen-independent metastatic cell line in a xenograft mouse model suggested that inhibition of growth via mTOR inhibitor was a result of re-population of PTEN-deficient cancer cells [137]. Pre-clinical data of RAD001 (everolimus) treatment in mice implanted with androgen-independent cell lines with mutated PTEN resulted in reduction of bone metastases and serum PSA concentrations and the result was enhanced with a combination of RAD001, docetaxel and zoledronic acid [140]. A pilot study using rapamycin (sirolimus) in 13 hormone-refractory prostate cancer patients resulted in some patients (1/13) with a 50% PSA decline and stable disease (4/13), with mean survival time after treatments being nearly 24 months [141]. mTOR inhibitors, everolimus and temsirolimus are now in phase II clinical trials in patients with castration-resistant metastatic prostate cancer [111, 142–145].
Discussion
Treatment for hormone-refractory prostate cancer includes docetaxel-based chemotherapy regimens. However there are very limited choices of treatment if there is an inadequate response to this approach. The majority of clinical trials reviewed focused on hormone-refractory prostate cancer patients. However more clinical trials recruiting patients prior to any treatment may also be valuable in studying initial responses of cancer cells to each drug.
The present review focuses on new agents administered in conjunction with traditional systemic therapies. A number of novel drugs, mentioned in this review, offer more specific treatments targeting either prostate cancer cell surface proteins (CV9103, MLN2704 and 177Lu-J591) or introducing irradiated tumour cells (GVAX and IL-2/IFNγ tumour vaccine) which may lead to activation of the host immune system. The obvious benefits of targeted therapeutics would be the potential for minimization of adverse effects and hence better quality of life for patients. Although cancer treatment vaccines may be beneficial for prostate cancer patients, cancer preventive vaccines are yet to be developed.
Anti-angiogenic treatments are one of the most pursued areas in cancer treatments, regulatory approval occurring with bevacizumab (breast cancer, glioblastoma, renal cell carcinoma and colorectal cancer), imatinib mesylate (chronic myelogenous leukaemia and metastatic gastrointestinal stromal cancer) and sunitinib mesylate (stomach cancer and gastrointestinal stromal cancer). In prostate cancer, a trial of bevacizumab as second-line treatment to docetaxel although showing good PSA responses with overall PSA concentrations declining by up to 65% (7/20 patients had major PSA response, which included four patients who were prior non-responders to docetaxel), a contribution by bevacizumab could not be confirmed due to the non-randomized nature of this trial [146]. The results of randomized control trials with patients receiving either docetaxel-based treatment or hormone therapy with and without bevacizumab are not yet fully available or the trials are ongoing [147, 148]. Trials of imatinib mesylate did not yield good responses in prostate cancer and a trial was terminated due to severe toxicities after single-drug treatments [118]. Despite these trial results, there are a number of ongoing clinical trials combining imatinib mesylate and docetaxel-based therapies [121–123]. VEGF may correlate with the development of prostate cancer but HIF-1α, a main regulator of VEGF, does not. Also androgen deprivation may also diminish VEGF expression and thus it would be irrelevant to target VEGF in hormone-refractory prostate cancer patients. Similarly, PDGFR and PDGF are expressed in PIN, primary and metastatic prostate cancer but do not correlate with the prognosis of the disease. In the case of biomarkers which may associate with the development of disease (VEGF, VEGFR), targeting these biomarkers prior to subjecting prostate cancer to any treatment (which several early phase trials are currently undertaking) or prior to evident metastatic disease may be more pertinent as a preventive approach [128, 148–151].
Targeting mTOR does appear to be a more promising approach as there is consistent in vitro evidence of expression of mTOR pathway proteins leading to activation of mTOR signalling, which correlates with the aggressiveness of prostate cancer. Pre-clinical data and a pilot study were also encouraging where treatments of single agent mTOR inhibitors initiated anti-tumour responses. Several trials of temrolimus and everolimus are now ongoing and are being tested in a neoadjuvant setting prior to prostatectomy or hormone therapy [143, 152, 153]. These drugs are also used in combination with bevacizumab in patients with advanced cancer, which may allow targeting of different sub-populations of cancer cells in castrate-resistance patients with metastases.
New generations of drugs targeting prostate cancer biomarkers are novel approaches aiming at different populations of prostate cancer cells that may be resistant or become resistant to traditional therapies. The majority of clinical trials concentrated on finding new agents for treatment of advanced disease in which treatments are currently limited to taxane-based chemotherapy. However, some agents can be applied as preventive treatments and are now being tested in a neoadjuvant setting. From this review, we can appreciate how finding potential biomarkers and understanding their role in cancer developmental processes can lead to promising new treatment targets for prostate cancer.
Conclusions
In prostate cancer, the main treatments are radical prostatectomy, radiotherapy and systemic therapies (hormone-deprivation therapy and chemotherapy). Prostate cancer is known to be a chronic disease and better detection allowing long-term management will improve quality of life of patients. With increasing awareness of the existence of different phenotypes of prostate cancer cell populations, personalized therapy is going to be the future of cancer treatment. By taking advantage of our increasing knowledge of prostate cancer initiation, progression and biomarkers associated with progression of disease, optimal regimens of novel therapeutic agents are now being tested in early clinical trials and will hopefully pave the way toward more targeted and individualized therapy for prostate cancer.
Glossary
Abbreviations and acronyms
- 177Lu
radionuclide lutetium-177
- Ab
antibody
- ADT
androgen deprivation therapy
- Ag(s)
antigen(s)
- Akt/PKB
protein kinase B
- AR
androgen receptor
- Bcl-2
apoptosis regulator protein
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- DLT
dose-limiting toxicity
- DM1
maytansinoid
- Ep-2H
Ep-CAM-derived peptides Ep-2H
- EpCAM
epithelial cell adhesion molecule
- GF(s)
growth factor(s)
- GLUT1
Glucose Transporter 1
- GM-CSF
granulocyte-macrophage colonystimulating factor
- HDACs
histone deacetylases
- HIF-1α
hypoxia-inducible factor-1 alpha
- IFN-γ
interferon gamma
- IL-2
interleukin 2
- IL-12
interleukin 12
- MTD
maximum tolerated dose
- mTOR
mammalian target of rapamycin
- N/A
data not available
- PBMC
peripheral blood mononuclear cell
- PCa
prostate cancer
- PDGF
platelet derived growth factor
- PDGFR
platelet derived growth factor receptor
- PI3K
phosphoinositide 3 kinase
- PRAME
preferentially expressed antigen in melanoma
- PSA
prostate specific antigen
- PSA DT
prostate specific antigen doubling time
- PSCA
prostate stem cell antigen
- PSGR-1
prostate specific G-protein coupled receptor-1
- PSMA
prostate specific membrane antigen
- PTEN
phosphatase and tensin homolog protein
- Pts
patients
- Raf
proto-oncogene serine/threonine-protein kinase
- Ras
a protein superfamily of small GTPases.
- RT
radiation therapy
- SAHA
suberoylanilide hydroxamic acid
- STEAP
six-transmembrane epithelial antigen of the prostate
- TGF-α
transforming growth factor alpha
- TH
T helper cell
- TSC ½
Tuberous sclerosis protein 1 and 2
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Provenge (sipuleucel-T), autologous CD54+ cells activated with PAP-GM-CSF, has recently been approved by the US FDA for the treatment of asymptomatic or minimally symptomatic metastatic hormone refractory prostate cancer.
Competing Interests
There are no competing interests to declare.
Sujitra Detchokul was a recipient of a Medical & Scientific Kidney & Urology related Research Biomedical Research Scholarship from Kidney Health Australia and a University of Melbourne Fee Remission Scholarship from The University of Melbourne. This work was supported by the Austin Hospital Medical Research Foundation and the Sir Edward Dunlop Medical Research Foundation.
REFERENCES
- 1.Schulz WA, Burchardt M, Cronauer MV. Molecular biology of prostate cancer. Mol Hum Reprod. 2003;9:437–48. doi: 10.1093/molehr/gag064. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalgo ML, Isaacs WB. Molecular pathways to prostate cancer. J Urol. 2003;170:2444–52. doi: 10.1097/01.ju.0000085381.20139.b6. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 4.Hayat MJ, Howlader N, Reichman E, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the surveillance, epidemiology, and end results (SEER) program. Oncologist. 2007;12:20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 5.Parkes C, Wald NJ, Murphy P, George L, Watt HC, Kirby R, Knekt P, Helzlsouer KJ, Tuomilehto J. Prospective observational study to assess value of prostate specific antigen as screening test for prostate cancer. BMJ. 1995;311:1340–3. doi: 10.1136/bmj.311.7016.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes C, Murphy A, Martin C, Sheils O, O'Leary J. Molecular pathology of prostate cancer. J Clin Pathol. 2005;58:673–84. doi: 10.1136/jcp.2002.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah JB, Reese AC, McKiernan JM, Benson MC. PSA updated: still relevant in the new millennium? Eur Urol. 2005;47:427–32. doi: 10.1016/j.eururo.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez J, Thomson IM. Prostate-specific antigen: a review of the validation of the most commonly used cancer biomarker. Cancer. 2004;101:894–904. doi: 10.1002/cncr.20480. [DOI] [PubMed] [Google Scholar]
- 9.Coldman AJ, Phillips N, Pickles TA. Trends in prostate cancer incidence and mortality: an analysis of mortality change by screening intensity. CMAJ. 2003;168:31–5. [PMC free article] [PubMed] [Google Scholar]
- 10.Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the U.S. preventive services task force. Ann Intern Med. 2002;137:917–29. doi: 10.7326/0003-4819-137-11-200212030-00014. [DOI] [PubMed] [Google Scholar]
- 11.Hankey BF, Feuer EJ, Clegg LX, Hayes RB, Legler JM, Prorok PC, Ries LA, Merrill RM, Keplan RS. Cancer surveillance series: interpreting trends in prostate cancer- Part I: evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999;91:1017–24. doi: 10.1093/jnci/91.12.1017. [DOI] [PubMed] [Google Scholar]
- 12.Vis AN. Does PSA screening reduce prostate cancer mortality? CMAJ. 2002;166:600–1. [PMC free article] [PubMed] [Google Scholar]
- 13.Andriole GL, Crawford D, Grubb RL, III, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, Clapp JD, Rathmell JM, Riley TL, Hayes RB, Kramer BS, Izmirlian G, Miller AB, Pinsky PF, Prorok PC, Gohagan JK, Berg CD. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Maattanen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 15.Gann PH, Chatterton RT, Lee C. Peptide growth factors as biomarkers of prostate cancer risk. Epidemiol Rev. 2001;23:67–71. doi: 10.1093/oxfordjournals.epirev.a000797. [DOI] [PubMed] [Google Scholar]
- 16.Golias C, Charalabopoulos A, Stagikas D, Giannakopoulos X, Pescho D, Batistatou A, Sofikitis N, Charalabopoulos K. Molecular profiling and genomic microarrays in prostate cancer. Exp Oncol. 2007;29:82–4. [PubMed] [Google Scholar]
- 17.Gimba ERP, Barcinski MA. Molecular aspects of prostate cancer: implications for future directions. Int Braz J Urol. 2003;29:401–11. doi: 10.1590/s1677-55382003000500003. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz LH, Bogaerts J, Ford R, Shankar L, Therasse P, Gwyther S, Eisenhauer EA. Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer. 2009;45:261–7. doi: 10.1016/j.ejca.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 19.Reiter RE, Gu Z, Watabe T, Thomas G, Szigeti K, Davis E, Wahl M, Nisitani S, Yamashiro J, Le Beau MM, Loda M, Witte ON. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci USA. 1998;95:1735–40. doi: 10.1073/pnas.95.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–34. doi: 10.1101/gad.819500. [DOI] [PubMed] [Google Scholar]
- 21.Nelson EC, Cambio AJ, Yang JC, Ok J-H, Lara JPN, Evans CP. Clinical implications of neuroendocrine differentiation in prostate cancer. Prostate Cancer Prostatic Dis. 2007;10:6–14. doi: 10.1038/sj.pcan.4500922. [DOI] [PubMed] [Google Scholar]
- 22.Berruti A, Mosca A, Tucci M, Terrone C, Torta M, Tarabuzzi R, Russo L, Cracco C, Scarpa RM, Angeli A, Dogliptti L. Independent prognostic role of circulating chromogranin A in prostate cancer patients with hormone-refractory disease. Endocr Relat Cancer. 2005;12:109–17. doi: 10.1677/erc.1.00876. [DOI] [PubMed] [Google Scholar]
- 23.Arlen PM, Gulley JL. Current perspectives in prostate cancer vaccines. Anticancer Agents Med Chem. 2009;9:1052–7. doi: 10.2174/187152009789735062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simons JW, Sacks N. Granulocyte-macrophage colony-stimulating factor-transduced allogeneic cancer cellular immunotherapy: the GVAX (TM) vaccine for prostate cancer. Urol Oncol. 2006;24:419–24. doi: 10.1016/j.urolonc.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 25.Greten TF, Jaffee EM. Cancer vaccines. J Clin Oncol. 1999;17:1047–60. doi: 10.1200/JCO.1999.17.3.1047. [DOI] [PubMed] [Google Scholar]
- 26.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor-cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting antitumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, Valone FH. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 28.Hege KM, Jooss K, Pardoll D. GM-CSF gene-modifed cancer cell immunotherapies: of mice and men. Int Rev Immunol. 2006;25:321–52. doi: 10.1080/08830180600992498. [DOI] [PubMed] [Google Scholar]
- 29.Morrissey PJ, Bressler L, Park LS, Alpert A, Gillis S. Granulocyte-macrophage colony-stimulating factor augments the primary antibody-response by enhancing the function of antigen-presenting cells. J Immunol. 1987;139:1113–19. [PubMed] [Google Scholar]
- 30.Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, Beetham P, Tsang KY, Grosenbach DW, Feldman J, Steinberg SM, Jones E, Chen C, Marte J, Schlom J, Dahut W. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–9. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arlen PM, Gulley JL, Todd N, Lieberman R, Steinberg SM, Morin S, Bastian A, Marte J, Tsang KY, Beetham P, Grosenbach DW, Schlom J, Dahut W. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;174:539–46. doi: 10.1097/01.ju.0000165159.33772.5b. [DOI] [PubMed] [Google Scholar]
- 32.Simons JW, Mikhak B, Chang JF, DeMarzo AM, Carducci MA, Lim M, Weber CE, Baccala AA, Goemann MA, Clift SM, Ando DG, Levitsky HI, Cohen LK, Sanda MG, Mulligan RC, Partin AW, Carter HB, Piantadosi S, Marshall FF, Nelson WG. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 1999;59:5160–8. [PubMed] [Google Scholar]
- 33.Higano CS, Corman JM, Smith DC, Centeno AS, Steidle CP, Gittleman M, Simons JW, Sacks N, Aimi J, Small EJ. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer. 2008;113:975–84. doi: 10.1002/cncr.23669. [DOI] [PubMed] [Google Scholar]
- 34.Simons JW, Carducci MA, Mikhak B, Lim M, Biedrzycki B, Borellini F, Clift SM, Hege KM, Ando DG, Piantadosi S, Mulligan R, Nelson WG. Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naive prostate cancer. Clin Cancer Res. 2006;12:3394–401. doi: 10.1158/1078-0432.CCR-06-0145. [DOI] [PubMed] [Google Scholar]
- 35.Urba WJ, Nemunaitis J, Marshall F, Smith DC, Hege KM, Ma J, Nguyen M, Small EJ. Treatment of biochemical recurrence of prostate cancer with granulocyte-macrophage colony-stimulating factor secreting, allogeneic, cellular immunotherapy. J Urol. 2008;180:2011–17. doi: 10.1016/j.juro.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 36.ClinicalTrials.gov. Biological therapy in treating patients with prostate cancer. 1999. ClinicalTrials.gov Available at http://clinicaltrials.gov/ct2/show/NCT00002637 (last accessed 20 August 2010)
- 37.Weide B, Garbe C, Rammensee HG, Pascolo S. Plasmid DNA- and messenger RNA-based anti-cancer vaccination. Immunol Lett. 2008;115:33–42. doi: 10.1016/j.imlet.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 38.ClinicalTrials.gov. RNActive®-derived therapeutic vaccine. 2009. ClinicalTrials.gov Available at http://clinicaltrials.gov/show/NCT00906243 (last accessed 20 August 2010)
- 39.ClinicalTrials.gov. Safety and efficacy trial of a RNActive®-derived prostate cancer vaccine in hormone refractory disease. 2009. ClinicalTrials.gov Available at http://clinicaltrials.gov/ct2/show/NCT00831467 (last accessed 20 August 2010)
- 40.Brill TH, Kubler HR, Pohla H, Buchner A, Fend F, Schuster T, van Randenborgh H, Paul R, Kummer T, Plank C, Eisele B, Breul J, Hartung R, Schendel DJ, Gansbacher B. Therapeutic vaccination with an Interleukin-2-Interferon-gamma-secreting allogeneic tumor vaccine in patients with progressive castration-resistant prostate cancer: a phase I/II trial. Hum Gene Ther. 2009;20:1641–51. doi: 10.1089/hum.2009.101. [DOI] [PubMed] [Google Scholar]
- 41.Brill TH, Kubler HR, von Randenborgh H, Fend F, Pohla H, Breul J, Hartung R, Paul R, Schendel DJ, Gansbacher B. Allogeneic retrovirally transduced, IL-2- and IFN-gamma-secreting cancer cell vaccine in patients with hormone refractory prostate cancer – a phase I clinical trial. J Gene Med. 2007;9:547–60. doi: 10.1002/jgm.1051. [DOI] [PubMed] [Google Scholar]
- 42.Nasu Y, Bangma CH, Hull GW, Lee H-M, Hu J, Wang J, McCurdy MA, Shimura S, Yang G, Timme TL, Thompson TC. Adenovirus-mediated interleukin-12 gene therapy for prostate cancer: suppression of orthotopic tumor growth and pre-established lung metastases in an orthotopic model. Gene Ther. 1999;6:338–49. doi: 10.1038/sj.gt.3300834. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Yang G, Timme TL, Fujita T, Naruishi K, Frolov A, Brenner MK, Kadmon D, Thompson TC. IL-12 gene-modified bone marrow cell therapy suppresses the development of experimental metastatic prostate cancer. Cancer Gene Ther. 2007;14:819–27. doi: 10.1038/sj.cgt.7701069. [DOI] [PubMed] [Google Scholar]
- 44.Varghese S, Rabkin SD, Liu R, Nielsen PG, Ipe T, Martuza RL. Enhanced therapeutic efficacy of IL-12, but not GM-CSF, expressing oncolytic herpes simplex virus for transgenic mouse derived prostate cancers. Cancer Gene Ther. 2006;13:253–65. doi: 10.1038/sj.cgt.7700900. [DOI] [PubMed] [Google Scholar]
- 45.Nikitina EY, Desai SA, Zhao XQ, Song WT, Luo AZ, Gangula RD, Slawin KM, Spencer DM. Versatile prostate cancer treatment with inducible caspase and interleukin-12. Cancer Res. 2005;65:4309–19. doi: 10.1158/0008-5472.CAN-04-3119. [DOI] [PubMed] [Google Scholar]
- 46.Satoh T, Saika T, Ebara S, Kusaka N, Timme TL, Yang G, Wang JX, Mouraviev V, Cao GW, Fattah EMA, Thompson TC. Macrophages transduced with an adenoviral vector expressing interleukin 12 suppress tumor growth and metastasis in a preclinical metastatic prostate cancer model. Cancer Res. 2003;63:7853–60. [PubMed] [Google Scholar]
- 47.Sangro B, Mazzolini G, Ruiz J, Herraiz M, Quiroga J, Herrero I, Benito A, Larrache J, Pueyo J, Subtil JC, Olague C, Sola J, Sadaba B, Lacasa C, Melero I, Qian C, Prieto J. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J Clin Oncol. 2004;22:1389–97. doi: 10.1200/JCO.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 48.Gollob JA, Mier JW, Veenstra K, McDermott DF, Clancy D, Clancy M, Atkins MB. Phase I trial of twice-weekly intravenous interleukin 12 in patients with metastatic renal cell cancer or malignant melanoma: ability to maintain IFN-gamma induction is associated with clinical response. Clin Cancer Res. 2000;6:1678–92. [PubMed] [Google Scholar]
- 49.Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, Munster PN, Sullivan DM, Ugen KE, Messina JL, Heller R. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26:5896–903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anwer K, Barnes MN, Fewell J, Lewis DH, Alvarez RD. Phase-I clinical trial of IL-12 plasmid/lipopolymer complexes for the treatment of recurrent ovarian cancer. Gene Ther. 2010;17:360–9. doi: 10.1038/gt.2009.159. [DOI] [PubMed] [Google Scholar]
- 51.ClinicalTrials.gov. Gene therapy for prostate cancer that returns after radiation therapy. 2005. ClinicalTrials.gov Available at http://clinicaltrials.gov/ct2/show/NCT00110526 (last accessed 20 August 2010)
- 52.ClinicalTrials.gov. Vaccine therapy plus interleukin-12 in treating patients with metastatic prostate cancer that has not responded to hormone therapy. 2001. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00015977 (last accessed 20 August 2010)
- 53.ClinicalTrials.gov. Vorinostat in treating patients with progressive metastatic prostate cancer. 2006. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/study/NCT00330161 (last accessed 20 August 2010)
- 54.ClinicalTrials.gov. Androgen deprivation therapy and vorinostat followed by radical prostatectomy in treating patients with localized prostate cancer. 2007. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00589472 (last accessed 20 August 2010)
- 55.ClinicalTrials.gov. A dose finding study with i.v. panobinostat (LBH589), docetaxel, and prednisone in patients with hormone refractory prostate cancer. 2008. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00663832 (last accessed 20 August 2010)
- 56.ClinicalTrials.gov. Efficacy and safety study of panobinostat in patients with metastatic hormone refractory prostate cancer. 2008. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00667862 (last accessed 20 August 2010)
- 57.ClinicalTrials.gov. PXD101 and isotretinoin in treating patients with solid tumors that are metastatic or that cannot be removed by surgery. 2006. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00334789 (last accessed 20 August 2010)
- 58.ClinicalTrials.gov. Valproic acid and bevacizumab in patients with advanced cancer. 2007. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00530907 (last accessed 20 August 2010)
- 59.ClinicalTrials.gov. Valproic acid in treating patients with progressive, non-metastatic prostate cancer. 2008. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00670046 (last accessed 20 August 2010)
- 60.ClinicalTrials.gov. A research study for patients with prostate cancer. 2005. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00106418 (last accessed 20 August 2010)
- 61.Kudahetti S, Fisher G, Ambroisine L, Foster C, Reuter V, Eastham J, Moller H, Kattan MW, Cooper CS, Scardino P, Cuzick J, Berney D. p53 immunochemistry is an independent prognostic marker for outcome in conservatively treated prostate cancer. BJU Int. 2009;104:20–4. doi: 10.1111/j.1464-410X.2009.08407.x. [DOI] [PubMed] [Google Scholar]
- 62.Concato J, Jain D, Uchio E, Risch H, Li WW, Wells CK. Molecular markers and death from prostate cancer. Ann Intern Med. 2009;150:595–603. doi: 10.7326/0003-4819-150-9-200905050-00005. [DOI] [PubMed] [Google Scholar]
- 63.Apakama I, Robinson MC, Walter NM, Charlton RG, Royds JA, Fuller CE, Neal DE, Hamdy FC. Bcl-2 Overexpression combined with p53 protein accumulation correlates with hormone-refractory prostate cancer. Br J Cancer. 1996;74:1258–62. doi: 10.1038/bjc.1996.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bubendorf L, Sauter G, Moch H, Jordan P, Blochlinger A, Gasser TC, Mihatsch MJ. Prognostic significance of Bcl-2 in clinically localized prostate cancer. Am J Pathol. 1996;148:1557–65. [PMC free article] [PubMed] [Google Scholar]
- 65.Navone NM, Troncoso P, Pisters LL, Goodrow TL, Palmer JL, Nichols WW, Voneschenbach AC, Conti CJ. P53 protein accumulation and gene mutation in the progression of human prostate carcinoma. J Natl Cancer Inst. 1993;85:1657–69. doi: 10.1093/jnci/85.20.1657. [DOI] [PubMed] [Google Scholar]
- 66.Kelly WK, O'Connor OA, Krug LM, Chiao JH, Heaney M, Curley T, MacGregore-Cortelli B, Tong W, Secrist JP, Schwartz L, Richardson S, Chu E, Olgac S, Marks PA, Scher H, Richon VM. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23:3923–31. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steele NL, Plumb JA, Vidal L, Tjornelund J, Knoblauch P, Rasmussen A, Ooi CE, Buhl-Jensen P, Brown R, Evans TRJ, DeBono JS. A phase 1 pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumors. Clin Cancer Res. 2008;14:804–10. doi: 10.1158/1078-0432.CCR-07-1786. [DOI] [PubMed] [Google Scholar]
- 68.Kelly WK, Richon VM, O'Connor O, Curley T, MacGregor-Curtelli B, Tong W, Klang M, Schwartz L, Richardson S, Rosa E, Drobnjak M, Cordon-Cordo C, Chiao JH, Rifkind R, Marks PA, Scher H. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res. 2003;9:3578–88. [PubMed] [Google Scholar]
- 69.Vaishampayan U, Rathkopf D, Chi KN, Hotte S, Vogelzang N, Alumkal J, Agrawal M, Picus J, Fandi A, Scher HI. Phase Ib dose-finding trial of intravenous (i.v.) panobinostat (PAN) with docetaxel (DOC) and prednisone (PRED) in patients (pts) with castration resistant prostate cancer (CRPC) EJC Suppl. 2009;7:413–14. [Google Scholar]
- 70.Molife LR, Attard G, Fong PC, Karavasilis V, Reid AHM, Patterson S, Riggs CE, Higano C, Stadler WM, McCulloch W, Dearnaley D, Parker C, de Bono JS. Phase II, two-stage, single-arm trial of the histone deacetylase inhibitor (HDACi) romidepsin in metastatic castration-resistant prostate cancer (CRPC) Ann Oncol. 2010;21:109–13. doi: 10.1093/annonc/mdp270. [DOI] [PubMed] [Google Scholar]
- 71.Rathkopf DE, Wong BY, Ross RW, George DJ, Picus J, Atadja P, Yang W, Culver KW, Woo MM, Scher HI. A phase I study of oral panobinostat (Lbh589) alone and in combination with docetaxel (Doc) and prednisone in castration-resistant prostate cancer (Crpc) Ann Oncol. 2008;19:199–200. [Google Scholar]
- 72.Pan LN, Lu J, Huang BQ. HDAC inhibitors: a potential new category of anti-tumor agents. Cell Mol Immunol. 2007;4:337–43. [PubMed] [Google Scholar]
- 73.Kassi E, Papoutsi Z, Pratsinis H, Aligiannis N, Manoussakis M, Moutsatsou P. Ursolic acid, a naturally occurring triterpenoid, demonstrates anticancer activity on human prostate cancer cells. J Cancer Res Clin Oncol. 2007;133:493–500. doi: 10.1007/s00432-007-0193-1. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y, Kong C, Wang H, Wang L, Xu C, Sun Y. Phosphorylation of Bcl-2 and activation of caspase-3 via the c-Jun N-terminal kinase pathway in ursolic acid-induced DU145 cells apoptosis. Biochimie. 2009;91:1173–9. doi: 10.1016/j.biochi.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 75.Porvasnik S, Sakamoto N, Kusmartsev S, Eruslanov E, Kim W, Cao W, Urbanek C, Wong D, Goodison S, Rosser CJ. Effects of CXCR4 antagonist CTCE-9908 on prostate tumor growth. Prostate. 2009;69:1460–9. doi: 10.1002/pros.21008. [DOI] [PubMed] [Google Scholar]
- 76.Nariculam J, Freeman A, Bott S, Munson P, Cable N, Brookman-Amissah N, Williamson M, Kirby RS, Masters J, Feneley M. Utility of tissue microarrays for profiling prognostic biomarkers in clinically localized prostate cancer: the expression of Bcl-2, E-cadherin, Ki-67 and p53 as predictors of biochemical failure after radical prostatectomy with nested control for clinical and pathological risk factors. Asian J Androl. 2009;11:109–18. doi: 10.1038/aja.2008.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anderson EM, Miller P, Ilsley D, Marshall W, Khvorova A, Stein CA, Benimetskaya L. Gene profiling study of G3139- and Bcl-2-targeting siRNAs identifies a unique G3139 molecular signature. Cancer Gene Ther. 2006;13:406–14. doi: 10.1038/sj.cgt.7700901. [DOI] [PubMed] [Google Scholar]
- 78.ClinicalTrials.gov. Docetaxel with or without oblimersen in treating patients with hormone-refractory adenocarcinoma (cancer) of the prostate. 2004. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00085228 (last accessed 20 August 2010)
- 79.Tolcher AW, Chi K, Kuhn J, Gleave M, Patnaik A, Takimoto C, Schwartz G, Thompson I, Berg K, D'Aloisio S, Murray N, Frankel SR, Izbicka E, Rowinsky E. A phase II, pharmacokinetic, and biological correlative study of oblimersen sodium and docetaxel in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2005;11:3854–61. doi: 10.1158/1078-0432.CCR-04-2145. [DOI] [PubMed] [Google Scholar]
- 80.Sternberg CN, Dumez H, Van Poppel H, Skoneczna I, Sella A, Daugaard G, Gil T, Graham J, Carpentier P, Calabro F, Collette L, Lacombe D. Docetaxel plus oblimersen sodium (Bcl-2 antisense oligonucleotide): an EORTC multicenter, randomized phase II study in patients with castration-resistant prostate cancer. Ann Oncol. 2009;20:1264–9. doi: 10.1093/annonc/mdn784. [DOI] [PubMed] [Google Scholar]
- 81.Patel MP, Masood A, Patel PS, Chanan-Khan AA. Targeting the Bcl-2. Curr Opin Oncol. 2009;21:516–23. doi: 10.1097/CCO.0b013e328331a7a4. [DOI] [PubMed] [Google Scholar]
- 82.ClinicalTrials.gov. A study of single-agent AT-101 in men with hormone refractory prostate cancer. 2006. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00286806 (last accessed 20 August 2010)
- 83.ClinicalTrials.gov. Safety and efficacy study of AT-101 in combination with docetaxel and prednisone in men with hormone refractory prostate cancer. 2006. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00286793 (last accessed 20 August 2010)
- 84.Liu G, Kelly WK, Wilding G, Leopold L, Brill K, Somer B. An open-label, multicenter, phase I/II study of single-agent AT-101 in men with castrate-resistant prostate cancer. Clin Cancer Res. 2009;15:3172–6. doi: 10.1158/1078-0432.CCR-08-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.MacVicar G, Greco FA, Reeves J, Curti B, Poiesz B, Somer B, Brill K, Leopold L. An open-label, multicenter, phase 1/2 study of AT-101 in combination with docetaxel and prednisone in men with hormone refractory prostate cancer. In: 20th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics, Geneva, Switzerland. EJC Suppl. 2008:66. [Google Scholar]
- 86.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–21. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 87.Shariat SF, Lotan Y, Saboorian H, Khoddami SM, Roehrborn CG, Slawin KM, Ashfaq R. Survivin expression is associated with features of biologically aggressive prostate carcinoma. Cancer. 2004;100:751–7. doi: 10.1002/cncr.20039. [DOI] [PubMed] [Google Scholar]
- 88.Nakahara T, Takeuchi M, Kinoyama I, Minematsu T, Shirasuna K, Matsuhisa A, Kita A, Tominaga F, Yamanaka K, Kudoh M, Sasamata M. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 2007;67:8014–21. doi: 10.1158/0008-5472.CAN-07-1343. [DOI] [PubMed] [Google Scholar]
- 89.Tolcher AW, Mita A, Lewis LD, Garrett CR, Till E, Daud AI, Patnaik A, Papadopoulos K, Takimoto C, Bartels P, Keating A, Antonia S. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J Clin Oncol. 2008;26:5198–203. doi: 10.1200/JCO.2008.17.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.ClinicalTrials.gov. An open-label study of YM155 + docetaxel in subjects with advanced hormone refractory prostate. 2007. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00514267 (last accessed 20 August 2010)
- 91.ClinicalTrials.gov. A clinical pharmacological study of YM155 after intravenous infusion in patients with advanced cancer. 2009. ClinicalTrials.gov; Available at http://clinicaltrials.gov/show/NCT01023386 (last accessed 20 August 2010)
- 92.Tagawa ST, Beltran H, Vallabhajosula S, Goldsmith SJ, Osborne J, Matulich D, Petrillo K, Parmar S, Nanus DM, Bander NH. Anti-prostate-specific membrane antigen-based radioimmunotherapy for prostate cancer. Cancer. 2010;116:1075–83. doi: 10.1002/cncr.24795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Henry MD, Wen SH, Silva MD, Chandra S, Milton M, Worland PJ. A prostate-specific membrane antigen-targeted monoclonal antibody-chemotherapeutic conjugate designed for the treatment of prostate cancer. Cancer Res. 2004;64:7995–8001. doi: 10.1158/0008-5472.CAN-04-1722. [DOI] [PubMed] [Google Scholar]
- 94.Galsky MD, Eisenberger M, Moore-Cooper S, Kelly WK, Slovin SF, Delacruz A, Lee Y, Webb IJ, Scher HI. Phase I trial of the prostate-specific membrane antigen directed immunoconjugate MLN2704 in patients with progressive metastatic castration-resistant prostate cancer. J Clin Oncol. 2008;26:2147–54. doi: 10.1200/JCO.2007.15.0532. [DOI] [PubMed] [Google Scholar]
- 95.ClinicalTrials.gov. A trial of MLN2704 in subjects with metastatic androgen independent prostate cancer. 2003. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00052000 (last accessed 20 August 2010)
- 96.ClinicalTrials.gov. MLN2704 in treating patients with progressive metastatic prostate cancer. 2003. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00058409 (last accessed 20 August 2010)
- 97.ClinicalTrials.gov. MLN2704 in subjects with metastatic androgen-independent prostate cancer. 2003. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00070837 (last accessed 20 August 2010)
- 98.ClinicalTrials.gov. Radioimmunotherapy in prostate cancer using 177Lu-J591 antibody. 2007. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00538668 (last accessed 20 August 2010)
- 99.ClinicalTrials.gov. Treatment with radiolabeled monoclonal antibody HuJ591-GS (177Lu-J591) in patients with metastatic prostate cancer. 2005. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00195039 (last accessed 20 August 2010)
- 100.Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of (177)lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005;23:4591–601. doi: 10.1200/JCO.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 101.Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ, Bander NH. Phase I trial of yttrium-90-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. J Clin Oncol. 2004;22:2522–31. doi: 10.1200/JCO.2004.09.154. [DOI] [PubMed] [Google Scholar]
- 102.Mazzucchelli R, Montironi R, Santinelli A, Lucarini G, Pugnaloni A, Biagini G. Vascular endothelial growth factor expression and capillary architecture in high-grade PIN and prostate cancer in untreated and androgen-ablated patients. Prostate. 2000;45:72–9. doi: 10.1002/1097-0045(20000915)45:1<72::aid-pros9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 103.Ferrer FA, Miller LJ, Andrawis RI, Kurtzman SH, Albertsen PC, Laudone VP, Kreutzer DL. Angiogenesis and prostate cancer: in vivo and in vitro expression of angiogenesis factors by prostate cancer cells. Urology. 1998;51:161–7. doi: 10.1016/s0090-4295(97)00491-3. [DOI] [PubMed] [Google Scholar]
- 104.Ellis L, Hammers H, Pili R. Targeting tumor angiogenesis with histone deacetylase inhibitors. Cancer Lett. 2009;280:145–53. doi: 10.1016/j.canlet.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim MS, Kwon HJ, Lee YM, Baek JH, Jang JE, Lee SW, Moon EJ, Kim HS, Lee SK, Chung HY, Kim CW, Kim KW. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001;7:437–43. doi: 10.1038/86507. [DOI] [PubMed] [Google Scholar]
- 106.Du ZX, Fujiyama C, Chen YX, Masaki Z. Expression of hypoxia-inducible factor 1 alpha in human normal, benign, and malignant prostate tissue. Chin Med J. 2003;116:1936–9. [PubMed] [Google Scholar]
- 107.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1 alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5. [PubMed] [Google Scholar]
- 108.Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. The expression and distribution of the hypoxia-inducible factors HIF-1 alpha and HIF-2 alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411–21. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kluetz PG, Figg WD, Dahut WL. Angiogenesis inhibitors in the treatment of prostate cancer. Expert Opin Pharmacother. 2010;11:233–47. doi: 10.1517/14656560903451716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.FDA. FDA label information for bevacizumab. 2009. FDA; Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125085s0168lbl.pdf (last accessed 20 August 2010)
- 111.ClinicalTrials.gov. A study to test the safety and effectiveness of docetaxel with RAD001 and bevacizumab in men with advanced prostate cancer. 2007. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00574769 (last accessed 20 August 2010)
- 112.ClinicalTrials.gov. Docetaxel, bevacizumab, thalidomide, and prednisone in treating patients with metastatic androgen-independent prostate cancer. 2004. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00091364 (last accessed 20 August 2010)
- 113.Ko YJ, Small EJ, Kabbinavar F, Chachoua A, Taneja S, Reese D, DePaoli A, Hannah A, Balk SP, Bubley GJ. A multi-institutional Phase II study of SU101, a platelet-derived growth factor receptor inhibitor, for patients with hormone-refractory prostate cancer. Clin Cancer Res. 2001;7:800–5. [PubMed] [Google Scholar]
- 114.Chott A, Sun Z, Morganstern D, Pan J, Li T, Susani M, Mosberger I, Upton MP, Bubley GJ, Balk SP. Tyrosine kinases expressed in vivo by human prostate cancer bone marrow metastases and loss of the type 1 insulin-like growth factor receptor. Am J Pathol. 1999;155:1271–9. doi: 10.1016/S0002-9440(10)65229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fudge K, Bostwick DG, Stearns ME. Platelet-derived growth factor A and B chains and the alpha and beta receptors in prostatic intraepithelial neoplasia. Prostate. 1996;29:282–6. doi: 10.1002/(SICI)1097-0045(199611)29:5<282::AID-PROS2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 116.FDA. FDA label information for Gleevec. 2003. FDA; Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021588s026s028lbl.pdf (last accessed 20 August 2010)
- 117.Kim SJ, Uehara H, Yazici S, Langley RR, He JQ, Tsan R, Fan D, Killion JJ, Fidler IJ. Simultaneous blockade of platelet-derived growth factor-receptor and epidermal growth factor-receptor signaling and systemic administration of paclitaxel as therapy for human prostate cancer metastasis in bone of nude mice. Cancer Res. 2004;64:4201–8. doi: 10.1158/0008-5472.CAN-03-3763. [DOI] [PubMed] [Google Scholar]
- 118.Lin AM, Rini BI, Weinberg V, Fong K, Ryan CJ, Rosenberg JE, Fong L, Small EJ. A phase II trial of imatinib mesylate in patients with biochemical relapse of prostate cancer after definitive local therapy. BJU Int. 2006;98:763–9. doi: 10.1111/j.1464-410X.2006.06396.x. [DOI] [PubMed] [Google Scholar]
- 119.Bajaj GK, Zhang Z, Garrett-Mayer E, Drew R, Sinibaldi V, Pili R, Denmeade SR, Carducci MA, Eisenberger MA, DeWeese TL. Phase II study of imatinib mesylate in patients with prostate cancer with evidence of biochemical relapse after definitive radical retropubic prostatectomy or radiotherapy. Urology. 2007;69:526–31. doi: 10.1016/j.urology.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 120.Rao K, Goodin S, Levitt MJ, Dave N, Shih WJ, Lin Y, Capanna T, Doyle-Lindrud S, Juvidian P, DiPaola RS. A phase II trial of imatinib mesylate in patients with prostate specific antigen progression after local therapy for prostate cancer. Prostate. 2005;62:115–22. doi: 10.1002/pros.20130. [DOI] [PubMed] [Google Scholar]
- 121.ClinicalTrials.gov. Docetaxel and imatinib mesylate (Gleevec) in hormone refractory prostate cancer. 2009. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00861471 (last accessed 20 August 2010)
- 122.ClinicalTrials.gov. Docetaxel with or without imatinib mesylate in treating patients with androgen-independent prostate cancer and bone metastases. 2004. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00080678 (last accessed 20 August 2010)
- 123.ClinicalTrials.gov. A study of imatinib and docetaxel in prostate cancer. 2005. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00251225 (last accessed 20 August 2010)
- 124.Michaelson MD, Regan MM, Oh WK, Kaufman DS, Olivier K, Michaelson SZ, Spicer B, Gurski C, Kantoff PW, Smith MR. Phase II study of sunitinib in men with advanced prostate cancer. Ann Oncol. 2009;20:913–20. doi: 10.1093/annonc/mdp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.FDA. FDA label information for sunitinib. 2007. FDA; Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021938s009lbl.pdf (last accessed 20 August 2010)
- 126.Sonpavde G, Periman PO, Bernold D, Weckstein D, Fleming MT, Galsky MD, Berry WR, Zhan F, Boehm KA, Asmar L, Hutson TE. Sunitinib malate for metastatic castration-resistant prostate cancer following docetaxel-based chemotherapy. Ann Oncol. 2010;21:319–24. doi: 10.1093/annonc/mdp323. [DOI] [PubMed] [Google Scholar]
- 127.ClinicalTrials.gov. Trail of Taxotere plus sunitinib on newly diagnosed, hormone refractory, metastatic prostate cancer. 2009. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00879619 (last accessed 20 August 2010)
- 128.ClinicalTrials.gov. Sunitinib malate with hormonal ablation for patients who will have prostatectomy. 2006. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00329043 (last accessed 20 August 2010)
- 129.ClinicalTrials.gov. Sunitinib malate, hormone ablation and radiation therapy in patients with prostate cancer. 2008. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00631527 (last accessed 20 August 2010)
- 130.Dai B, Kong YY, Ye DW, Ma CG, Zhou XY, Yao XD. Activation of the mammalian target of rapamycin signalling pathway in prostate cancer and its association with patient clinicopathological characteristics. BJU Int. 2009;104:1009–16. doi: 10.1111/j.1464-410X.2009.08538.x. [DOI] [PubMed] [Google Scholar]
- 131.Kremer CL, Klein RR, Mendelson J, Browne W, Samadzedeh LK, Vanpatten K, Highstrom L, Pestano GA, Nagle RB. Expression of mTOR signaling pathway markers in prostate cancer progression. Prostate. 2006;66:1203–12. doi: 10.1002/pros.20410. [DOI] [PubMed] [Google Scholar]
- 132.Schmitz M, Grignard G, Marguel C, Dippel W, Capesius C, Mossong J, Nathan M, Giacchi S, Scheiden R, Kieffer N. Complete loss of PTEN expression as a possible early prognostic marker for prostate cancer metastasis. Int J Cancer. 2007;120:1284–92. doi: 10.1002/ijc.22359. [DOI] [PubMed] [Google Scholar]
- 133.Thomas GV, Horvath S, Smith BL, Crosby K, Lebel LA, Schrage M, Said J, De Kernion J, Reiter RE, Sawyers CL. Antibody-based profiling of the phosphoinositide 3-kinase pathway in clinical prostate cancer. Clin Cancer Res. 2004;10:8351–6. doi: 10.1158/1078-0432.CCR-04-0130. [DOI] [PubMed] [Google Scholar]
- 134.McMenamin ME, Soung P, Perera S, Kaplan I, Loda M, Sellers WR. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high gleason score and advanced stage. Cancer Res. 1999;59:4291–6. [PubMed] [Google Scholar]
- 135.Krajewska M, Krajewski S, Epstein JI, Shabaik A, Sauvageot J, Song K, Kitada S, Reed JC. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol. 1996;148:1567–76. [PMC free article] [PubMed] [Google Scholar]
- 136.Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, Loda M, Lane HA, Sellers WR. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 137.Wu LC, Birle DC, Tannock IF. Effects of the mammalian target of rapamycin inhibitor CCI-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res. 2005;65:2825–31. doi: 10.1158/0008-5472.CAN-04-3137. [DOI] [PubMed] [Google Scholar]
- 138.Xu YY, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66:7783–92. doi: 10.1158/0008-5472.CAN-05-4472. [DOI] [PubMed] [Google Scholar]
- 139.Muders MH, Zhang HY, Wang E, Tindall DJ, Datta K. Vascular endothelial growth factor-c protects prostate cancer cells from oxidative stress by the activation of mammalian target of rapamycin complex-2 and AKT-1. Cancer Res. 2009;69:6042–8. doi: 10.1158/0008-5472.CAN-09-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morgan TM, Pitts TEM, Gross TS, Poliachik SL, Vessella RL, Corey E. RAD001 (Everolimus) inhibits growth of prostate cancer in the bone and the inhibitory effects are increased by combination with docetaxel and zoledronic acid. Prostate. 2008;68:861–71. doi: 10.1002/pros.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Amato RJ, Jac J, Mohammad T, Saxena S. Pilot study of rapamycin in patients with hormone-refractory prostate cancer. Clin Genitourin Cancer. 2008;6:97–102. doi: 10.3816/CGC.2008.n.015. [DOI] [PubMed] [Google Scholar]
- 142.ClinicalTrials.gov. Temsirolimus and bevacizumab in treating patients with hormone-resistant metastatic prostate cancer That did not respond to chemotherapy. 2010. ClinicalTrials.gov; [updated 12 March 2010]; Available at http://clinicaltrials.gov/ct2/show/NCT01083368 (last accessed 20 August 2010)
- 143.ClinicalTrials.gov. Everolimus as first-line therapy in treating patients with prostate cancer. 2009. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00976755 (last accessed 20 August 2010)
- 144.ClinicalTrials.gov. Impact of temsirolimus therapy on circulating tumor cell biology in men with castration resistant metastatic prostate cancer. 2009. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00887640 (last accessed 20 August 2010)
- 145.ClinicalTrials.gov. Single agent temsirolimus (Torisel®) in chemotherapy-naïve castration-resistant prostate cancer patients. 2009. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00919035 (last accessed 20 August 2010)
- 146.Di Lorenzo G, Figg WD, Fossa SD, Mirone V, Autorino R, Longo N, Imbimbo C, Perdona S, Giordano A, Giuliano M, Labianca R, De Placido S. Combination of bevacizumab and docetaxel in docetaxel-pretreated hormone-refractory prostate cancer: a phase 2 study. Eur Urol. 2008;54:1089–96. doi: 10.1016/j.eururo.2008.01.082. [DOI] [PubMed] [Google Scholar]
- 147.ClinicalTrials.gov. Docetaxel and prednisone with or without bevacizumab in treating patients with prostate cancer that did not respond to hormone therapy. 2005. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00110214 (last accessed 20 August 2010)
- 148.ClinicalTrials.gov. Androgen deprivation therapy +/− bevacizumab for PSA recurrence of prostate cancer after definitive local therapy. 2008. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00776594 (last accessed 20 August 2010)
- 149.ClinicalTrials.gov. Study of sunitinib malate in patients with newly diagnosed prostate cancer prior to prostatectomy. 2008. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00672594 (last accessed 20 August 2010)
- 150.ClinicalTrials.gov. Bevacizumab, hormone therapy, and radiation therapy in treating patients with locally advanced prostate cancer. 2006. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00348998 (last accessed 20 August 2010)
- 151.ClinicalTrials.gov. Bevacizumab with hormonal and radiotherapy for high-risk prostate cancer. 2006. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00349557 (last accessed 20 August 2010)
- 152.ClinicalTrials.gov. Phase II study of RAD001 in a neoadjuvant setting in men with intermediate or high risk prostate cancer. 2008. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00657982 (last accessed 20 August 2010)
- 153.ClinicalTrials.gov. An open label exploratory study in newly diagnosed prostate cancer patients. 2005. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00235794 (last accessed 20 August 2010)
- 154.ClinicalTrials.gov. Dose escalation and efficacy trial of GVAX® prostate cancer vaccine. 2005. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/study/NCT00140348 (last accessed 20 August 2010)
- 155.ClinicalTrials.gov. Androgen ablation therapy with or without vaccine therapy in treating patients with prostate cancer. 2008. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00771017 (last accessed 20 August 2010)
- 156.ClinicalTrials.gov. Safety and efficacy studies of panobinostat and bicalutamide in patients with recurrent prostate cancer after castration. 2009. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00878436 (last accessed 20 August 2010)
- 157.ClinicalTrials.gov. Study of oral PXD101 in patients with advanced solid tumors or lymphoma. 2006. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00413075 (last accessed 20 August 2010)
- 158.ClinicalTrials.gov. Gossypol and androgen ablation therapy in treating patients with newly diagnosed metastatic prostate cancer. 2008. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00666666 (last accessed 20 August 2010)
- 159.ClinicalTrials.gov. Gossypol, paclitaxel, and carboplatin in treating patients with solid tumors that are metastatic or cannot be removed by surgery. 2009. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00891072 (last accessed 20 August 2010)
- 160.ClinicalTrials.gov. Docetaxel/prednisone plus fractionated 177Lu- J591 antibody for metastatic, castrate-resistant prostate cancer. 2009. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00916123 (last accessed 20 August 2010)
- 161.ClinicalTrials.gov. 177Lu radiolabeled monoclonal antibody HuJ591 (177Lu-J591) and ketoconazole in patients with prostate cancer. 2009. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00859781 (last accessed 20 August 2010)
- 162.ClinicalTrials.gov. Bevacizumab in treating patients with relapsed prostate cancer that did not respond to hormone therapy. 2007. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00478413 (last accessed 20 August 2010)
- 163.ClinicalTrials.gov. Avastin, docetaxel and androgen deprivation followed by continued avastin and androgen deprivation for men with a rising PSA after local therapy. 2008. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00658697 (last accessed 20 August 2010)
- 164.ClinicalTrials.gov. Treatment of prostate cancer with adjuvant bevacizumab plus erlotinib. 2005. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00203424 (last accessed 20 August 2010)
- 165.Mathew P, Thall PF, Bucana CD, Oh WK, Morris MJ, Jones DM, Johnson MM, Wen S, Pagliaro LC, Tannir NM, Tu SM, Meluch AA, Smith L, Cohen L, Kim SJ, Troncoso P, Fidler IJ, Logothetis C. Platelet-derived growth factor receptor inhibition and chemotherapy for castration-resistant prostate cancer with bone metastases. Clin Cancer Res. 2007;13:5816–24. doi: 10.1158/1078-0432.CCR-07-1269. [DOI] [PubMed] [Google Scholar]
- 166.Mathew P, Thall PF, Jones D, Perez C, Bucana C, Troncoso P, Kim SJ, Fidler IJ, Logothetis C. Platelet-derived growth factor receptor inhibitor imatinib mesylate and docetaxel: a modular phase I trial in androgen-independent prostate cancer. J Clin Oncol. 2004;22:3323–9. doi: 10.1200/JCO.2004.10.116. [DOI] [PubMed] [Google Scholar]
- 167.ClinicalTrials.gov. Clinical study of SU011248 in subjects with high risk prostate cancer who have elected to undergo radical prostatectomy. 2008. ClinicalTrials.gov; Available at http://clinicaltrials.gov/ct2/show/NCT00790595 (last accessed 20 August 2010) [DOI] [PubMed]


