Abstract
We have proposed that 4β-hydroxycholesterol (4β-OHC) may be used as an endogenous marker of CYP3A activity. The cholesterol metabolite 4β-OHC is formed by CYP3A4. Treatment of patients with strong inducers of CYP3A enzymes, e.g. anti-epileptic drugs, resulted in 10-fold increased concentrations of plasma 4β-OHC, while treatment with CYP3A inhibitors such as ritonavir or itraconazole resulted in decreased plasma concentrations. There was a relationship between the 4β-OHC concentration and the number of active CYP3A5*1 alleles showing that 4β-OHC was not only formed by CYP3A4, but also by CYP3A5. The concentration of 4β-OHC was higher in women than in men, confirming previous studies indicating a gender difference in CYP3A4/5-activity. The rate of elimination of 4β-OHC is slow (half-life 17 days) which results in stable plasma concentrations within individuals, but limits its use to study rapid changes in CYP3A activity. In short-term studies exogenous markers such as midazolam or quinine may be superior, but in long-term studies 4β-OHC is a sensitive marker of CYP3A activity, especially to assess induction but also inhibition. Under conditions where the cholesterol concentration is changing, the ratio of 4β-OHC : cholesterol may be used as an alternative to 4β-OHC itself. The use of an endogenous CYP3A marker has obvious advantages and may be of value both during drug development and for monitoring CYP3A activity in patients.
Keywords: 4β-hydroxycholesterol, anti-epileptic drugs, biomarker, CYP3A4, CYP3A5, ethnicity, gender, induction, inhibition, rifampicin
Introduction
Cholesterol is an important structural element of cell membranes but is also an essential substrate for biosynthesis of bile acids and steroid hormones. The cholesterol molecule is easily oxidized and may be transformed into numerous oxidation products known as oxysterols. Such oxysterols may be formed by different mechanisms such as cholesterol autoxidation [1], secondary to lipid peroxidation [2] or as a consequence of enzymatic cholesterol metabolism [3]. The quantitatively dominating oxysterols in the human circulation are 27-hydroxycholesterol, 24-hydroxycholesterol, 7α-hydroxycholesterol and 4β-hydroxycholesterol (4β-OHC) [4, 5]. An early meeting abstract from 1985 reported that human serum contained significant amounts of 4β-OHC [6]. An assay for 4β-OHC based on isotope-dilution gas chromatography-mass spectrometry with a deuterium labelled internal standard was developed and the presence of the oxysterol in human plasma was confirmed [5]. An 18O2 inhalation technique was used to identify oxysterols formed in vivo in rats. It was suggested that 4β-OHC was formed in vivo and was not an artefact formed during sampling or sample preparation [7]. Furthermore, in vitro oxidation of low density lipoprotein with either copper ions or soybean lipoxygenase resulted in massive production of 7-ketocholesterol but only very little 4β-OHC suggesting that cholesterol autoxidation is not a major pathway for 4β-OHC formation in vivo[8].
The average plasma concentration of 4β-OHC in 125 healthy Swedish volunteers was 30 ng ml−1 with a range of 10 to 60 ng ml−1. A plasma sample from a subject treated with the anti-epileptic drug carbamazepine for more than 10 years had a 4β-OHC concentration exceeding 600 ng ml−1[9]. As carbamazepine induces cytochrome P450 (CYP) enzymes [10], it seemed likely that 4β-OHC could be formed by a CYP enzyme. Samples from patients treated with different anti-epileptic drugs were analyzed and it was shown that treatment with carbamazepine, phenytoin or phenobarbital, drugs known as strong inducers of CYP3A4, resulted in highly elevated concentrations of 4β-OHC in the circulation, as shown in Table 1. In contrast, patients treated with valproate, which does not induce CYP3A4, had concentrations comparable with untreated healthy volunteers. In vitro experiments with recombinant CYP enzymes showed that CYP3A4 was able to convert cholesterol into 4β-OHC while no conversion was observed when CYP1A2, CYP2B6 or CYP2C9 were used. Treatment of gall-stone patients with the weak CYP3A-inducer ursodeoxycholic acid resulted in modestly increased (50%) plasma concentrations of 4β-OHC [9]. In parallel with 4β-OHC, the epimer 4α-OHC (Figure 1) was determined in all plasma samples. The plasma concentration of 4α-OHC was lower than that of 4β-OHC and did not change when patients were treated with CYP3A4-inducers, suggesting that 4α-OHC is not formed by CYP3A4 [9]. The metabolism of 4β-OHC was studied in healthy volunteers and different cell systems and it was shown that the elimination from the circulation was much slower than for other oxysterols, possibly due to slow 7α-hydroxylation [11]. Taken together, this suggested to us that 4β-OHC may be used as a biomarker for CYP3A activity.
Table 1.
Plasma concentrations of cholesterol, 4α-hydroxycholesterol and 4β-hydroxy cholesterol in patients with epilepsy treated with different antiepileptic drugs*
| Treatment | Cholesterol (mmoL l−1†) | 4α-hydroxycholesterol (ng ml−1†) | 4β-hydroxycholesterol (ng ml−1†) |
|---|---|---|---|
| Valproic acid | 4.5 ± 0.8 | 5.6 ± 1.1 | 28 ± 15 |
| Carbamazepine | 5.8 ± 1.5 | 7.0 ± 2.8 | 240 ± 142 |
| Phenytoin | 5.1 ± 1.0 | 5.9 ± 1.6 | 214 ± 154 |
| Phenobarbital | 4.9 ± 1.1 | 6.1 ± 2.7 | 239 ± 226 |
Data from [9].
Mean ± standard deviation (n= 5–15).
Figure 1.
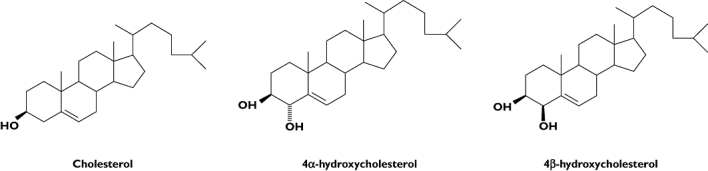
Chemical structures of cholesterol and 4α- and 4β-hydroxycholesterol
CYP3A4/5 phenotypes and genotypes
The major cytochrome P450 3A enzyme in humans is CYP3A4 [12]. This is the most important drug metabolizing enzyme and has more than 50% of registered drugs as substrates [12]. Also, many endogenous substances, e.g. steroids, are metabolized by CYP3A4. 6β-hydroxylation of cortisol has been used to detect strong induction of CYP3A4 in vivo, but a major problem is the diurnal secretion of cortisol [13]. Many drugs, e.g. alprazolam, erythromycin, midazolam, and our group has promoted quinine [14, 15], have been used as exogenous markers of CYP3A.
Some mutations have been reported in the CYP3A4 gene (http://www.imm.ki.se/CYPalleles), but they are rare. The sister enzyme CYP3A5 with 85% amino acid homology, has very similar substrate specificity to CYP3A4 and is highly polymorphic. CYP3A5 is expressed in most Africans, but in few Caucasians. When we determined the most common CYP3A5*3, *6 and *7 mutated alleles, the wildtype functional *1 was present in 14% of Swedes, 33% of Koreans and 74% of Tanzanians [16].
Influence of ethnicity, CYP3A5 haplotype and gender on basal plasma 4β-OHC
We have shown that the mean plasma concentration of 4β-OHC was significantly different (P < 0.00001) in 161 Swedes (26.8 ng ml−1), 149 Koreans (29.3) and 138 Tanzanians (21.9) [16]. That there are interethnic differences in CYP3A activity was confirmed by administering quinine to the same subjects in these populations [16]. Figure 2 shows the 4β-OHC concentrations in subjects with 0, 1 and 2 CYP3A5*1 alleles in the three populations. In subjects without the CYP3A5*1 allele, i.e. only CYP3A4 expressed, Tanzanian subjects had lower concentrations of 4β-OHC than both Swedes and Koreans (P < 0.000001). In Figure 2 it can also be seen that within each population the concentration of 4β-OHC increased with the number of active CYP3A5*1 alleles. These results presented in Figure 2 show that 1) there are interethnic differences in both the CYP3A4 activity and the frequency of active CYP3A5 alleles and 2) both CYP3A4 and CYP3A5 catalyze the formation of 4β-OHC. In that same study [16] we showed that women have higher concentrations of 4β-OHC than men among Koreans (P < 0.00001), Tanzanians (P < 0.001) and Swedes (not significant). This confirms a study with tirilazad [17] and our own with quinine [15] showing a higher CYP3A activity in women compared with men.
Figure 2.
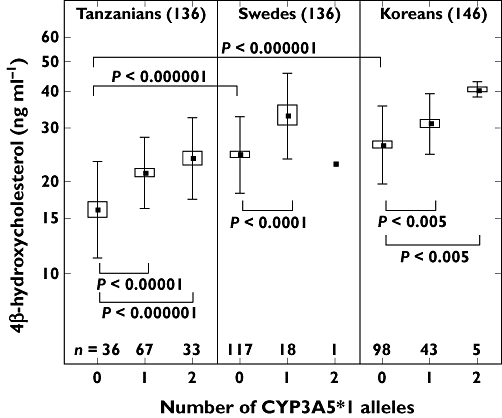
Plasma concentrations of 4β-hydroxycholesterol in Tanzanian, Swedish and Korean populations with different numbers of CYP3A5*1 alleles. Originally published by Diczfalusy et al. [16]. Mean ( ); Mean ± SE (□); Mean ± SD (
); Mean ± SE (□); Mean ± SD ( )
)
Recently a study in Ethiopian healthy subjects [18] showed a higher mean 4β-OHC plasma concentration of 35.4 ng ml−1 than in the above three populations (P= 0.0001). The concentration of 4β-OHC was in these Ethiopians related to the CYP3A5 genotype (P= 0.003) and women had higher concentrations than men (P= 0.0001) confirming our previous study in the three populations [16]. These studies in the three major racial populations i.e. Africans (Ethiopia and Tanzania), Asians (Korea), and Caucasians (Sweden) show differences in the mean concentration of 4β-OHC reflecting CYP3A activity. Consistently they also show that both CYP3A4 and CYP3A5 catalyze the formation of this cholesterol metabolite.
Basal concentration of 4β-OHC and exogenous markers of CYP3A, e.g. quinine and midazolam
In our studies on 4β-OHC in populations we have also given quinine as a marker of CYP3A4/5 [15, 16]. A correlation between the quinine : 3-hydroxyquinine ratio and concentration of 4β-OHC was found especially in subjects with no active CYP3A5*1 alleles (i.e. only CYP3A4 expressed) in Koreans (r=−0.49; n= 99; P= 0.0000003), Swedes (r=−0.24; n= 113; P= 0.009) and Tanzanians (r=−0.50; n= 36; P= 0.002) [16]. This indicates that these two markers of CYP3A measure at least some common factor of CYP3A activity regulation. Tomalik-Scharte et al. [19] assessed both 4β-OHC and the 4β-OHC : cholesterol ratio as markers of CYP3A in comparison with midazolam clearance after i.v and oral administration reflecting hepatic and overall activity. In untreated healthy subjects they found weak, but significant relationships between 4β-OHC and midazolam measurements (rs= 0.24–0.35; P < 0.05). The authors [19] concluded that only a small part of the variation in the midazolam and cholesterol based measures shared the same source.
Induction and inhibition of CYP3A4/5 and change of plasma concentrations of 4β-OHC
As discussed above long-term treatment with the anti-epileptic drugs carbamazepine, phenytoin and phenobarbital induces CYP3A and increases plasma concentrations of 4β-OHC by about 10-fold [9]. The time course of the increase of 4β-OHC in plasma was studied in eight paediatric patients with newly diagnosed epilepsy and subsequently treated with carbamazepine [20]. The mean basal 4β-OHC concentration (42.7 ng ml−1) increased during 7–9 weeks of treatment (296 ng ml−1; P= 0.00006) and then leveled off at 15–23 weeks of treatment (321 ng ml−1) and was not significantly different from that at 7–9 weeks [20]. The increase in plasma 4β-OHC was pronounced (5–10 fold), but the increase took several weeks to reach a plateau. The CYP3A4/5 induction was essentially complete within 1–2 weeks, as judged by the plasma concentrations of carbamazepine and its 10,11-epoxide metabolite [20]. This fairly rapid completion of induction is in accord with our earlier observation [10]. We believe that the increase in the concentration of 4β-OHC reflects the induction, but the time-course of the induction is faster than the rate of increase of 4β-OHC.
We studied the inducing effect of rifampicin at doses of 20, 100 and 500 mg day−1 given for 2 weeks to eight healthy subjects in each dose group [21]. The Karolinska cocktail [22] with marker drugs for different cytochrome P450 enzymes was given before and after rifampicin to evaluate the induction potential of the different CYPs. As markers for CYP3A4/5 we used quinine and 4β-OHC. The subjects included had no functional CYP3A5*1 allele and therefore we studied only CYP3A4. A significant induction of CYP3A4 by all three doses of rifampicin could be demonstrated by both quinine and 4β-OHC (Figure 3). As seen in Figure 3 the clinically used daily dose of 500 mg rifampicin caused a four-fold increase in 4β-OHC. With the subtherapeutic dose of 20 mg a small but significant (P < 0.01) increase could be demonstrated by intra-individual comparison before and during rifampicin treatment (Figures 3, [21]). The effect of rifampicin treatment on the two markers, quinine and 4β-OHC, was highly correlated [21].
Figure 3.
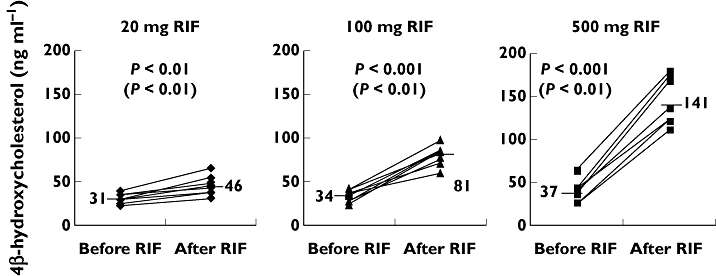
Plasma concentrations of 4β-hydroxycholesterol before and after 2 weeks of treatment with daily doses of 20, 100 and 500 mg rifampicin (RIF). Mean values are displayed and marked with a line. Statistically significant changes calculated using two tailed t-test are shown (Wilcoxon signed rank). Originally published by Kanebratt et al. [21]
It has thus been shown that known inducers of CYP3A4/5 i.e. carbamazepine [9, 20], phenytoin and phenobarbital [9] and rifampicin [21, 23] all increase the concentration of 4β-OHC in plasma. Other inducers of CYP3A4/5 such as ursodeoxycholic acid [9], efavirenz [24] and St Johns Worth (unpublished) also have this effect. Thus, measurement of the degree of increase of 4β-OHC is a useful tool in vivo to detect even minor induction of CYP3A4/5.
It is clear that 4β-OHC measurements can detect induction, but how about inhibition of CYP3A4/5? We have shown that in 22 HIV patients undergoing ritonavir-boosted treatment with atazanavir the concentration of plasma 4β-OHC decreased significantly (P= 0.0003) [24]. Similarly, patients with onychomycosis were treated with 400 mg itraconazole daily for 1 week on two occasions [25]. Although only eight patients were studied this CYP3A4/5 inhibitor caused significant decrease in serum 4β-OHC during both treatment periods. The percentage decrease for each individual on both occasions was comparable [25]. These two studies show that the 4β-OHC measure can be used to demonstrate inhibition of CYP3A4/5 in vivo in patients.
Tomalik-Scharte et al. [19] measured 4β-OHC and cholesterol in samples from four previous cocktail phenotyping studies with or without comedication with potential CYP3A inhibitors. In two of these studies (C and D) no statistically significant effect on cholesterol or midazolam metrics by an ‘investigational drug’ (not characterized) after 2 and 7 days, respectively, was seen. In another study, B, Tomalik-Scharte et al. [19] gave propiverine for 7 days and found a mild but significant increase in the cholesterol measurements (4β-OHC and 4β-OHC : cholesterol), but on the contrary a small decrease of midazolam clearance was shown. Opposite effects on 4β-OHC (induction of CYP3A) and midazolam (inhibition of CYP3A) could not be explained by the authors. In a fourth study E with ritanovir-boosted lopinavir treatment a moderate inhibition of CYP3A was found by 4β-OHC while a strong inhibition was shown by midazolam measurements. These data are similar to our data [24] showing a minor decrease of 11% in 4β-OHC caused by 4 weeks of treatment of HIV with ritonavir-boosted lopinavir.
Methodology
Plasma concentrations of 4β-OHC were determined by gas chromatography-mass spectrometry using deuterium labelled 4β-OHC as internal standard as described in [9]. Plasma and serum may be used equally well. As the major part of 4β-OHC is present in esterified form in blood the sample workup includes a hydrolysis step followed by liquid-liquid extraction and solid phase extraction [9]. The sample preparation procedure is very laborious which limits the number of samples that can be analyzed in a day. We have therefore developed a simplified sample preparation procedure that increases the number of samples processed during a day by a factor of 3–4 (unpublished). The modified procedure utilizes 250 µl plasma instead of 1 ml in the original method. Briefly, 1 ml of 0.7 m ethanolic potassium hydroxide was added to 250 µl plasma together with 200 µg EDTA, 50 µg butylated hydroxytoluene (BHT) and 100 ng internal standard. The samples were hydrolyzed at room temperature for 30 min. The hydrolysis was terminated by addition of phosphoric acid to pH 7 and the samples were centrifuged and loaded onto Strata-X solid phase extraction columns. The columns were washed with 10% methanol in water and 4β-OHC was then eluted with 1 ml 85% acetonitrile in water. After evaporation of the solvent the samples were derivatized to the tert-butyl-dimethylsilyl ether and analyzed by gas chromatography-mass spectrometry as described [9]. Ninety samples were analyzed in parallel with the two different sample preparation procedures and linear correlation between the resulting 4β-OHC concentrations gave a line with the equation y= 1.0052x− 2.0953. The r2 value was 0.9878 (y= new procedure, n= 90). The within day variation with the new procedure was 4.5% (n= 16) compared with 3.7% for the original procedure (n= 12) and the between day variations 8.2% (n= 59) and 7.7% (n= 78), respectively. The method was linear up to 600 ng ml−1.
When we analyze 4β-OHC by GC-MS, we simultaneously measure 4α-OHC which has a separate retention time by GC. While the major part of 4β-OHC is formed enzymatically by, e.g. CYP3A4/5, the 4α isomer seems to be formed by nonenzymatic oxidation. The plasma/serum concentration of 4α-OHC is lower (5–10 ng ml−1) than that of 4β-OHC (Table 1) [9]. The two 4-isomers are stable for a few years if samples are stored at −70°C. We have come across samples stored for longer periods of time (and thawed a few times), where the concentrations of 4α-OHC had increased up to 100 ng ml−1 and were closely related to the also increased concentrations of 4β-OHC (r2= 0.98). We believe that this is due to nonenzymatic formation of both 4α- and 4β–OHC by time during uncontrolled storage conditions. This retrospective study was therefore never evaluated. We always use low concentrations of 4α-OHC as an indicator of proper handling of samples and this has been used in all our studies on 4β-OHC as a marker of CYP3A4/5 [16, 18, 20, 21, 24–26]. In samples properly stored we find no significant relationship between concentrations of 4α- and 4β-OHC. In the retrospective studies reported by Tomalik-Scharte et al. [19] evaluating 4β-OHC as a CYP3A metric, concentrations of 4α-OHC were not mentioned. Nonenzymatic formation of 4β-OHC might have contributed to partly unexpected results in that report [19].
Concentrations of 4β-OHC are very similar in plasma and serum. We always use plasma drawn in EDTA containing tubes, as EDTA binds metal ions, which might catalyze oxidation. In serum, which is prepared without EDTA, lipid peroxidation might occur during storage.
Discussion
A major point to discuss is how well 4β-OHC in plasma or serum reflects CYP3A activity. It seems established that the concentration of this cholesterol metabolite is regulated by both the activity of CYP3A4 and CYP3A5 [16, 18]. The more pronounced correlation between 4β-OHC and quinine kinetics [16] compared with midazolam [19] might at least partly be related to the participation of CYP3A5 in the metabolism of quinine, but not of midazolam.
Tomalik-Scharte et al. [19] tried to explain their weak correlation between cholesterol- and midazolam-based metrics by pointing out that these two methods do not measure exactly the same thing. There are many examples of a lack of correlation between different CYP3A markers, e.g. erythromycin breath test and midazolam clearance, two widely used CYP3A metrics [27]. Masica et al. [28] compared the clearance of alprazolam, midazolam and triazolam and concluded that CYP3A phenotyping cannot accurately predict the kinetics of any other CYP3A substrate. One important difference between 4β-OHC and probe drugs is that for probe drugs the CYP3A metrics reflect only elimination and distribution while the 4β-OHC metric depends on its formation as well as its elimination. Tomalik-Scharte et al. have further investigated the reasons for differences in the use of 4β-OHC and midazolam as CYP3A markers. In addition it should be remembered that while midazolam and quinine have relatively short half-lives of a few hours to 1 day, 4β-OHC has a long half-life of 1–3 weeks (Figure 4) [26]. The long half-life of 4β-OHC contributes to the fact that the concentration of 4β-OHC is very stable over time within individuals [26]. This is positive for 4β-OHC, but at the same time negative as this marker cannot be used to follow rapid changes in decreased activity of CYP3A. Therefore, 4β-OHC may be more appropriate to use to assess induction rather than inhibition. Midazolam is a widely used probe drug used to assess CYP3A activity. It seems very important to perform a prospective study to compare 4β-OHC and midazolam as CYP3A metrics.
Figure 4.
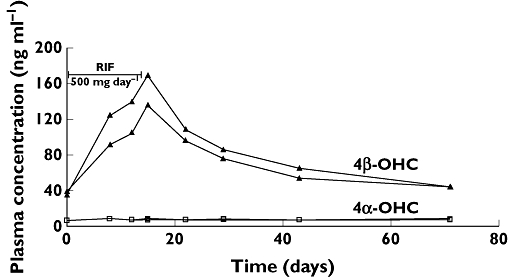
Plasma concentration of both 4α- and 4β-hydroxycholesterol in two healthy subjects given a daily dose of 500 mg of rifampicin (RIF) for 2 weeks. These data were originally published by Diczfalusy et al. [26]
Yang & Rodrigues [29] in a recent and very interesting paper described pharmacokinetic models of how the long half-life of 4β-OHC influences the time course of CYP3A induction and inhibition. Using the half-life of 17 days [26] simulation curves for carbamazepine [20] and rifampicin [21] induction were very similar to the curves of the original publications. Also Yang & Rodrigues found that the concentration vs. time curves of 4β-OHC after various CYP3A inhibitors fitted the simulated ones. We reported [25] a mean decrease of 4β-OHC by 20.8% (P= 0.006) when 400 mg itraconazole was given daily for 7 days and Yang & Rodrigues [29] found 19.4% using their pharmacokinetic model. However, prolonged exposure with experimental drugs is required especially to monitor inhibition of CYP3A activity with 4β-OHC [29]. As pointed out by the authors of this model it could be of great value to predict and use 4β-OHC as an endogenous marker of changes in CYP3A activity. Yang & Rodrigues work in a pharmaceutical company and it is quite clear from their paper that an endogenous marker of CYP3A activity has an obvious use in the development of novel drugs.
In untreated subjects there is a more pronounced variation between subjects in the concentration of 4β-OHC than of cholesterol. Among the 440 subjects presented by Diczfalusy et al. [16] there was a significant, but weak correlation (r= 0.30, P < 0.0001) between 4β-OHC and cholesterol. This indicates that only 9% of the variation in 4β-OHC was due to variation in cholesterol concentration and 91% due to variation in other factors, e.g. CYP3A activity. The concentration of cholesterol did not differ among different populations [16] and was not affected by the treatment with different drugs such as anti-epileptics [9, 20] (Table 1). However other drugs, e.g. itraconazole [25] and antiretrovirals [19, 24] might have an effect on plasma cholesterol. Therefore, it is sometimes more appropriate to use the 4β-OHC : cholesterol ratio instead of only 4β-OHC as a CYP3A measure. The use of the ratio is also promoted by Yang & Rodrigues [29] and we agree with this.
Competing Interests
There are no competing interests to declare.
We thank the coauthors of our individual reports using 4β–OHC to phenotype for CYP3A for pleasant collaboration. These studies were supported by Swedish Research Council, Medicine (3902), Torsten och Ragnar Söderbergs Stiftelser, EDCTP (CG ct05-32030-001), AstraZeneca and through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet (SLL 560177, SLL 581107).
REFERENCES
- 1.Smith LL. Cholesterol Autoxidation. New York: Plenum Press; 1981. [Google Scholar]
- 2.Lund E, Diczfalusy U, Björkhem I. On the mechanism of oxidation of cholesterol at C-7 in a lipoxygenase system. J Biol Chem. 1992;267:12462–67. [PubMed] [Google Scholar]
- 3.Russell DW. Oxysterol biosynthetic enzymes. Biochim Biophys Acta. 2000;1529:126–35. doi: 10.1016/s1388-1981(00)00142-6. [DOI] [PubMed] [Google Scholar]
- 4.Dzeletovic S, Breuer O, Lund E, Diczfalusy U. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal Biochem. 1995;225:73–80. doi: 10.1006/abio.1995.1110. [DOI] [PubMed] [Google Scholar]
- 5.Breuer O. Identification and quantitation of cholest-5-ene-3β,4β-diol in rat liver and human plasma. J Lipid Res. 1995;36:2275–81. [PubMed] [Google Scholar]
- 6.Brooks CJW, Cole WJ. Some aspects of the analysis of minor oxygenated sterols in serum and in serum lipoprotein fractions. J Am Oil Chem Soc. 1985;62:622. [Google Scholar]
- 7.Breuer O, Björkhem I. Use of an 18O2 inhalation technique and mass isotopomer distribution analysis to study oxygenation of cholesterol in rat. Evidence for in vivo formation of 7-oxo-, 7β-hydroxy-, 24-hydroxy-, and 25-hydroxycholesterol. J Biol Chem. 1995;270:20278–84. doi: 10.1074/jbc.270.35.20278. [DOI] [PubMed] [Google Scholar]
- 8.Breuer O, Dzeletovic S, Lund E, Diczfalusy U. The oxysterols cholest-5-ene-3β,4α-diol, cholest-5-ene-3β,4β-diol and cholestane-3β,5α,6α-triol are formed during in vitro oxidation of low density lipoprotein, and are present in human atherosclerotic plaques. Biochim Biophys Acta. 1996;1302:145–52. doi: 10.1016/0005-2760(96)00052-5. [DOI] [PubMed] [Google Scholar]
- 9.Bodin K, Bretillon L, Aden Y, Bertilsson L, Broomé U, Einarsson C, Diczfalusy U. Antiepileptic drugs increase plasma levels of 4β-hydroxycholesterol in humans. Evidence for involvement of cytochrome P450 3A4. J Biol Chem. 2001;276:38685–89. doi: 10.1074/jbc.M105127200. [DOI] [PubMed] [Google Scholar]
- 10.Bertilsson L, Höjer B, Tybring G, Osterloh J, Rane A. Autoinduction of carbamazepine metabolism in children by a stable isotope technique. Clin Pharmacol Ther. 1980;27:83–8. doi: 10.1038/clpt.1980.13. [DOI] [PubMed] [Google Scholar]
- 11.Bodin K, Andersson U, Rystedt E, Ellis E, Norlin M, Pikuleva I, Eggertsen G, Björkhem I, Diczfalusy U. Metabolism of 4β-hydroxycholesterol in humans. J Biol Chem. 2002;277:31534–40. doi: 10.1074/jbc.M201712200. [DOI] [PubMed] [Google Scholar]
- 12.Eichelbaum M, Burk O. CYP3A genetics in drug metabolism. Nat Med. 2001;7:285–87. doi: 10.1038/85417. [DOI] [PubMed] [Google Scholar]
- 13.Galteau MM, Shamsa F. Urinary 6β-hydroxycortisol: a validated test for evaluating drug induction or drug inhibition mediated through CYP3A in humans and in animals. Eur J Clin Pharmacol. 2003;59:713–33. doi: 10.1007/s00228-003-0690-3. [DOI] [PubMed] [Google Scholar]
- 14.Mirghani RA, Ericsson O, Tybring G, Gustafsson LL, Bertilsson L. Quinine 3-hydroxylation as a biomarker reaction for the activity of CYP3A4 in man. Eur J Clin Pharmacol. 2003;59:23–8. doi: 10.1007/s00228-003-0575-5. [DOI] [PubMed] [Google Scholar]
- 15.Mirghani RA, Sayi J, Aklillu E, Allqvist A, Jande M, Wennerholm A, Ericksen J, Herben VMM, Jones BC, Gustafsson LL, Bertilsson L. CYP3A5 genotype has significant effect on quinine 3-hydroxylation in Tanzanians, who have lower total CYP3A activity than a Swedish population. Pharmacogenet Genomics. 2006;16:637–45. doi: 10.1097/01.fpc.0000230411.89973.1b. [DOI] [PubMed] [Google Scholar]
- 16.Diczfalusy U, Miura J, Roh H-K, Mirghani RA, Sayi J, Larsson H, Bodin KG, Allqvist A, Jande M, Kim J-W, Aklillu E, Gustafsson LL, Bertilsson L. 4β-hydroxycholesterol is a new endogenous CYP3A marker: relationship to CYP3A5 genotype, quinine 3-hydroxylation and sex in Koreans, Swedes and Tanzanians. Pharmacogenet Genomics. 2008;18:201–08. doi: 10.1097/FPC.0b013e3282f50ee9. [DOI] [PubMed] [Google Scholar]
- 17.Hulst LK, Fleishaker JC, Peters GR, Harry JD, Wright DM, Ward P. Effect of age and gender on tirilazad pharmacokinetics in humans. Clin Pharmacol Ther. 1994;55:378–84. doi: 10.1038/clpt.1994.45. [DOI] [PubMed] [Google Scholar]
- 18.Gebeyehu E, Engidawork E, Bijnsdorp A, Aminy A, Diczfalusy U, Aklillu E. Sex and CYP3A5 genotype influence total CYP3A activity: high CYP3A activity and a unique distribution of CYP3A5 variant alleles in Ethiopians. Pharmacogenom J. 2010 doi: 10.1038/tpj.2010.16. doi: 10.1038/tpj.2010.16. [DOI] [PubMed] [Google Scholar]
- 19.Tomalik-Scharte D, Lütjohann D, Doroshyenko O, Frank D, Jetter A, Fuhr U. Plasma 4β-hydroxycholesterol: an endogenous CYP3A metric? Clin Pharmacol Ther. 2009;86:147–53. doi: 10.1038/clpt.2009.72. [DOI] [PubMed] [Google Scholar]
- 20.Wide K, Larsson H, Bertilsson L, Diczfalusy U. Time course of the increase in 4β-hydroxycholesterol concentration during carbamazepine treatment of pediatric patients with epilepsy. Br J Clin Pharmacol. 2008;65:708–15. doi: 10.1111/j.1365-2125.2007.03078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanebratt KP, Diczfalusy U, Bäckström T, Sparve E, Bredberg E, Böttiger Y, Andersson TB, Bertilsson L. Cytochrome P450 induction by rifampicin in healthy subjects: determination using the Karolinska cocktail and the endogenous CYP3A4 marker 4β-hydroxycholesterol. Clin Pharmacol Ther. 2008;84:589–94. doi: 10.1038/clpt.2008.132. [DOI] [PubMed] [Google Scholar]
- 22.Christensen M, Andersson K, Dalén P, Mirghani RA, Muirhead GJ, Nordmark A, Tybring G, Wahlberg A. Yasar Ü, Bertilsson L. The Karolinska cocktail for phenotyping of five human cytochrome P450 enzymes. Clin Pharmacol Ther. 2003;73:517–28. doi: 10.1016/S0009-9236(03)00050-X. [DOI] [PubMed] [Google Scholar]
- 23.Niemi M, Kivistö KT, Diczfalusy U, Bodin K, Bertilsson L, Fromm MF, Eichelbaum M. Effect of SLCO1B1 polymorphism on induction of CYP3A4 by rifampin. Pharmacogenet Genomics. 2006;16:565–68. doi: 10.1097/01.fpc.0000215070.52212.0e. [DOI] [PubMed] [Google Scholar]
- 24.Josephson F, Bertilsson L, Böttiger Y, Flamholc L, Gisslén M, Ormaasen V, Sönnerborg A, Diczfalusy U. CYP3A induction and inhibition by different antiretroviral regimens reflected by changes in plasama 4β-hydroxycholesterol. Eur J Clin Pharmacol. 2008;64:775–81. doi: 10.1007/s00228-008-0492-8. [DOI] [PubMed] [Google Scholar]
- 25.Lütjohann D, Marinova M, Schneider B, Oldenburg J, von Bergmann K, Bieber T, Björkhem I, Diczfalusy U. 4β-hydroxycholesterol as a marker of CYP3A4 inhibition in vivo – effects of itraconazole in man. Int J Clin Pharmacol Ther. 2009;47:709–15. doi: 10.5414/cpp47709. [DOI] [PubMed] [Google Scholar]
- 26.Diczfalusy U, Kanebratt KP, Bredberg E, Andersson TB, Böttiger Y, Bertilsson L. 4β-hydroxycholesterol as an endogenous marker for CYP3A4/5 activity. Stability and half-life of elimination after induction with rifampicin. Br J Clin Pharmacol. 2009;67:38–43. doi: 10.1111/j.1365-2125.2008.03309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinirons MT, O'Shea D, Kim RB, Groopman JD, Thummel KE, Wood AJ, Wilkinson GR. Failure of erythromycin breath test to correlate with midazolam clearance as a probe of cytochrome P4503A. Clin Pharmacol Ther. 1999;66:224–31. doi: 10.1016/S0009-9236(99)70029-9. [DOI] [PubMed] [Google Scholar]
- 28.Masica AL, Mayo G, Wilkinson GR. In vivo comparisons of constitutive cytochrome P450 3A activity assessed by alprazolam, triazolam, and midazolam. Clin Pharmacol Ther. 2004;76:341–9. doi: 10.1016/j.clpt.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Yang Z, Rodrigues AD. Does the long plasma half-life of 4β-hydroxycholesterol impact on its utility as a cytochrome P450 3A (CYP3A) metric? J Clin Pharmacol. 2010 doi: 10.1177/0091270009360041. doi: 10.1177/0091270009360041. [DOI] [PubMed] [Google Scholar]


