Abstract
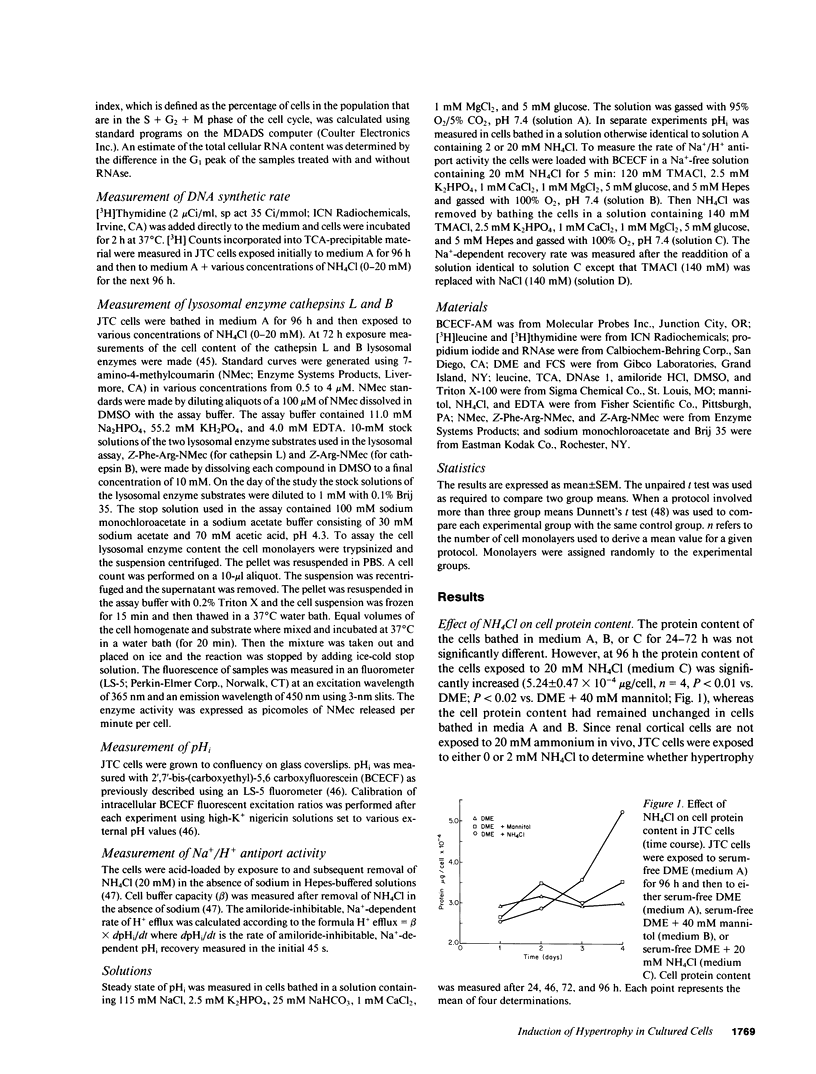
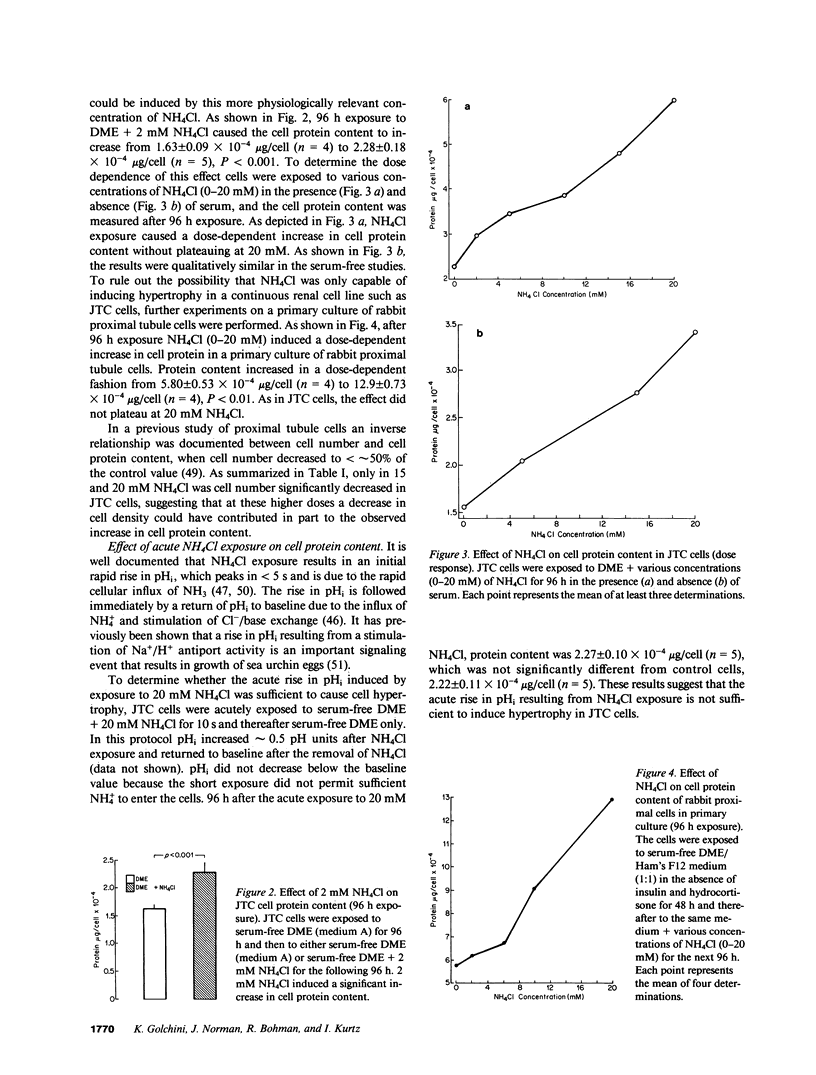
Ammonia production increases in several models of renal hypertrophy in vivo. The present study was designed to determine whether ammonia can directly modulate the growth of renal cells in the absence of a change in extracellular acidity. In serum-free media NH4Cl (0-20 mM) caused JTC cells and a primary culture of rabbit proximal tubule cells to hypertrophy (increase in cell protein content) in a dose-dependent fashion without a change in DNA synthesis. Studies in JTC cells revealed that the cell protein content increased as a result of both an increase in protein synthesis and a decrease in protein degradation. Total cell RNA content and ribosome number increased after NH4Cl exposure and the cell content of the lysosomal enzymes cathepsin B and L decreased. Inhibition of the Na+/H+ antiporter with amiloride did not prevent the hypertrophic response induced by NH4Cl. The results indicate that ammonia is an important modulator of renal cell growth and that hypertrophy can occur in the absence of functioning Na+/H+ antiport activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J. Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem J. 1980 Jun 1;187(3):909–912. doi: 10.1042/bj1870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerkert J., Martin D., Trigg D., Simon E. Effect of reduced renal mass on ammonium handling and net acid formation by the superficial and juxtamedullary nephron of the rat. Evidence for impaired reentrapment rather than decreased production of ammonium in the acidosis of uremia. J Clin Invest. 1983 Jun;71(6):1661–1675. doi: 10.1172/JCI110921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan M. J., Stow J. L., Newman A. P., Madri J., Anderson H. C., Farquhar M. G., Palade G. E., Jamieson J. D. Dependence on pH of polarized sorting of secreted proteins. Nature. 1987 Oct 15;329(6140):632–635. doi: 10.1038/329632a0. [DOI] [PubMed] [Google Scholar]
- Ching S., Rogoff T. M., Gabuzda G. J. Renal ammoniagenesis and tissue glutamine, glutamine synthetase, and glutaminase I levels in potassium-deficient rats. J Lab Clin Med. 1973 Aug;82(2):208–214. [PubMed] [Google Scholar]
- Chung S. D., Alavi N., Livingston D., Hiller S., Taub M. Characterization of primary rabbit kidney cultures that express proximal tubule functions in a hormonally defined medium. J Cell Biol. 1982 Oct;95(1):118–126. doi: 10.1083/jcb.95.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe F. L., Korty P. R. Protein synthesis during compensatory renal hypertrophy. Am J Physiol. 1967 Dec;213(6):1585–1589. doi: 10.1152/ajplegacy.1967.213.6.1585. [DOI] [PubMed] [Google Scholar]
- Cohn D. E., Hruska K. A., Klahr S., Hammerman M. R. Increased Na+-H+ exchange in brush border vesicles from dogs with renal failure. Am J Physiol. 1982 Sep;243(3):F293–F299. doi: 10.1152/ajprenal.1982.243.3.F293. [DOI] [PubMed] [Google Scholar]
- Crissman H. A., Mullaney P. F., Steinkamp J. A. Methods and applications of flow systems for analysis and sorting of mammalian cells. Methods Cell Biol. 1975;9(0):179–246. doi: 10.1016/s0091-679x(08)60076-x. [DOI] [PubMed] [Google Scholar]
- Dean R. T. Protein degradation in cell cultures: general considerations on mechanisms and regulation. Fed Proc. 1980 Jan;39(1):15–19. [PubMed] [Google Scholar]
- Dubé F., Epel D. The relation between intracellular pH and rate of protein synthesis in sea urchin eggs and the existence of a pH-independent event triggered by ammonia. Exp Cell Res. 1986 Jan;162(1):191–204. doi: 10.1016/0014-4827(86)90438-6. [DOI] [PubMed] [Google Scholar]
- Dubé F., Schmidt T., Johnson C. H., Epel D. The hierarchy of requirements for an elevated intracellular pH during early development of sea urchin embryos. Cell. 1985 Mar;40(3):657–666. doi: 10.1016/0092-8674(85)90214-4. [DOI] [PubMed] [Google Scholar]
- Eagle H. The effect of environmental pH on the growth of normal and malignant cells. J Cell Physiol. 1973 Aug;82(1):1–8. doi: 10.1002/jcp.1040820102. [DOI] [PubMed] [Google Scholar]
- Fine L. G., Badie-Dezfooly B., Lowe A. G., Hamzeh A., Wells J., Salehmoghaddam S. Stimulation of Na+/H+ antiport is an early event in hypertrophy of renal proximal tubular cells. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1736–1740. doi: 10.1073/pnas.82.6.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine L. The biology of renal hypertrophy. Kidney Int. 1986 Mar;29(3):619–634. doi: 10.1038/ki.1986.45. [DOI] [PubMed] [Google Scholar]
- Fraley D. S., Adler S., Rankin B., Curthoys N., Zett B. Relationship of phosphate-dependent glutaminase activity to ammonia excretion in potassium deficiency and acidosis. Miner Electrolyte Metab. 1985;11(3):140–149. [PubMed] [Google Scholar]
- GOSS R. J. RENAL AND ADRENAL RELATIONSHIPS IN COMPENSATORY HYPERPLASIA. Proc Soc Exp Biol Med. 1965 Feb;118:342–346. doi: 10.3181/00379727-118-29837. [DOI] [PubMed] [Google Scholar]
- Golchini K., Kurtz I. NH3 permeation through the apical membrane of MDCK cells is via a lipid pathway. Am J Physiol. 1988 Jul;255(1 Pt 2):F135–F141. doi: 10.1152/ajprenal.1988.255.1.F135. [DOI] [PubMed] [Google Scholar]
- Goldstein L., Boylan J. M., Schröck H. Adaptation of renal ammonia production in the diabetic ketoacidotic rat. Kidney Int. 1980 Jan;17(1):57–65. doi: 10.1038/ki.1980.7. [DOI] [PubMed] [Google Scholar]
- Good D. W., Burg M. B. Ammonia production by individual segments of the rat nephron. J Clin Invest. 1984 Mar;73(3):602–610. doi: 10.1172/JCI111250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good D. W., Caflisch C. R., DuBose T. D., Jr Transepithelial ammonia concentration gradients in inner medulla of the rat. Am J Physiol. 1987 Mar;252(3 Pt 2):F491–F500. doi: 10.1152/ajprenal.1987.252.3.F491. [DOI] [PubMed] [Google Scholar]
- Good D. W., DuBose T. D., Jr Ammonia transport by early and late proximal convoluted tubule of the rat. J Clin Invest. 1987 Mar;79(3):684–691. doi: 10.1172/JCI112871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling K. K., Berk B. C., Alexander R. W. Evidence that Na+/H+ exchange regulates angiotensin II-stimulated diacylglycerol accumulation in vascular smooth muscle cells. J Biol Chem. 1988 Aug 5;263(22):10620–10624. [PubMed] [Google Scholar]
- Haas M. J., Simpson D. P. The effects of chronic metabolic acidosis on patterns of protein synthesis in rat renal cortex. Comp Biochem Physiol B. 1984;79(2):187–194. doi: 10.1016/0305-0491(84)90012-9. [DOI] [PubMed] [Google Scholar]
- Halliburton I. W., Thomson R. Y. The effect of diet and of unilateral nephrectomy on the composition of the kidney. Cancer Res. 1967 Sep;27(9):1632–1638. [PubMed] [Google Scholar]
- Harris R. C., Brenner B. M., Seifter J. L. Sodium-hydrogen exchange and glucose transport in renal microvillus membrane vesicles from rats with diabetes mellitus. J Clin Invest. 1986 Mar;77(3):724–733. doi: 10.1172/JCI112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. C., Seifter J. L., Brenner B. M. Adaptation of Na+-H+ exchange in renal microvillus membrane vesicles. Role of dietary protein and uninephrectomy. J Clin Invest. 1984 Dec;74(6):1979–1987. doi: 10.1172/JCI111619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgeland K. NH4Cl and protein metabolism in human gingival fibroblasts. Scand J Dent Res. 1981 Oct;89(5):400–406. doi: 10.1111/j.1600-0722.1981.tb01699.x. [DOI] [PubMed] [Google Scholar]
- Hertz L., Murthy C. R., Lai J. C., Fitzpatrick S. M., Cooper A. J. Some metabolic effects of ammonia on astrocytes and neurons in primary cultures. Neurochem Pathol. 1987 Feb-Apr;6(1-2):97–129. doi: 10.1007/BF02833602. [DOI] [PubMed] [Google Scholar]
- Hopgood M. F., Clark M. G., Ballard F. J. Inhibition of protein degradation in isolated rat hepatocytes. Biochem J. 1977 May 15;164(2):399–407. doi: 10.1042/bj1640399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulter H. N., Sigala J. F., Sebastian A. K+ deprivation potentiates the renal alkalosis-producing effect of mineralocorticoid. Am J Physiol. 1978 Oct;235(4):F298–F309. doi: 10.1152/ajprenal.1978.235.4.F298. [DOI] [PubMed] [Google Scholar]
- Jaeger P., Karlmark B., Giebisch G. Ammonium transport in rat cortical tubule: relationship to potassium metabolism. Am J Physiol. 1983 Nov;245(5 Pt 1):F593–F600. doi: 10.1152/ajprenal.1983.245.5.F593. [DOI] [PubMed] [Google Scholar]
- Jobin J., Taylor C. M., Caverzasio J., Bonjour J. P. Calcium restriction and parathyroid hormone enhance renal compensatory growth. Am J Physiol. 1984 May;246(5 Pt 2):F685–F690. doi: 10.1152/ajprenal.1984.246.5.F685. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Interaction of NH4+ and Li+ with the renal microvillus membrane Na+-H+ exchanger. Am J Physiol. 1981 Nov;241(5):C220–C226. doi: 10.1152/ajpcell.1981.241.5.C220. [DOI] [PubMed] [Google Scholar]
- Kinsella J., Cujdik T., Sacktor B. Na+-H+ exchange activity in renal brush border membrane vesicles in response to metabolic acidosis: The role of glucocorticoids. Proc Natl Acad Sci U S A. 1984 Jan;81(2):630–634. doi: 10.1073/pnas.81.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knepper M. A., Burg M. B. Increased fluid absorption and cell volume in isolated rabbit proximal straight tubules after in vivo DOCA administration. Am J Physiol. 1981 Nov;241(5):F502–F508. doi: 10.1152/ajprenal.1981.241.5.F502. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes. FASEB J. 1987 Dec;1(6):462–468. doi: 10.1096/fasebj.1.6.3315809. [DOI] [PubMed] [Google Scholar]
- Kurtz I., Golchini K. Na+-independent Cl(-)-HCO-3- exchange in Madin-Darby canine kidney cells. Role in intracellular pH regulation. J Biol Chem. 1987 Apr 5;262(10):4516–4520. [PubMed] [Google Scholar]
- LOTSPEICH W. D. RENAL HYPERTROPHY IN METABOLIC ACIDOSIS AND ITS RELATION TO AMMONIA EXCRETION. Am J Physiol. 1965 Jun;208:1135–1142. doi: 10.1152/ajplegacy.1965.208.6.1135. [DOI] [PubMed] [Google Scholar]
- Long W. M., Chua B. H., Munger B. L., Morgan H. E. Effects of insulin on cardiac lysosomes and protein degradation. Fed Proc. 1984 Apr;43(5):1295–1300. [PubMed] [Google Scholar]
- Lotspeich W. D. Metabolic aspects of acid-base change. Science. 1967 Mar 3;155(3766):1066–1075. doi: 10.1126/science.155.3766.1066. [DOI] [PubMed] [Google Scholar]
- MacClean A. J., Hayslett J. P. Adaptive change in ammonia excretion in renal insufficiency. Kidney Int. 1980 May;17(5):595–606. doi: 10.1038/ki.1980.70. [DOI] [PubMed] [Google Scholar]
- Nagami G. T., Kurokawa K. Regulation of ammonia production by mouse proximal tubules perfused in vitro. Effect of luminal perfusion. J Clin Invest. 1985 Mar;75(3):844–849. doi: 10.1172/JCI111781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath K. A., Hostetter M. K., Hostetter T. H. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest. 1985 Aug;76(2):667–675. doi: 10.1172/JCI112020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neave N., Sobolewski A., Weeks G. The effect of ammonia on stalk cell formation in submerged monolayers of Dictyostelium discoideum. Cell Differ. 1983 Dec;13(4):301–307. doi: 10.1016/0045-6039(83)90040-4. [DOI] [PubMed] [Google Scholar]
- Nord E. P., Hafezi A., Kaunitz J. D., Trizna W., Fine L. G. pH gradient-dependent increased Na+-H+ antiport capacity of the rabbit remnant kidney. Am J Physiol. 1985 Jul;249(1 Pt 2):F90–F98. doi: 10.1152/ajprenal.1985.249.1.F90. [DOI] [PubMed] [Google Scholar]
- Norman J., Badie-Dezfooly B., Nord E. P., Kurtz I., Schlosser J., Chaudhari A., Fine L. G. EGF-induced mitogenesis in proximal tubular cells: potentiation by angiotensin II. Am J Physiol. 1987 Aug;253(2 Pt 2):F299–F309. doi: 10.1152/ajprenal.1987.253.2.F299. [DOI] [PubMed] [Google Scholar]
- Poole B., Ohkuma S., Warburton M. J. The accumulation of weakly basic substances in lysosomes and the inhibition of intracellular protein degradation. Acta Biol Med Ger. 1977;36(11-12):1777–1788. [PubMed] [Google Scholar]
- Rodriguez-Nichols F., Laughrey E., Tannen R. L. Response of renal NH3 production to chronic respiratory acidosis. Am J Physiol. 1984 Dec;247(6 Pt 2):F896–F903. doi: 10.1152/ajprenal.1984.247.6.F896. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sajo I. M., Goldstein M. B., Sonnenberg H., Stinebaugh B. J., Wilson D. R., Halperin M. L. Sites of ammonia addition to tubular fluid in rats with chronic metabolic acidosis. Kidney Int. 1981 Sep;20(3):353–358. doi: 10.1038/ki.1981.146. [DOI] [PubMed] [Google Scholar]
- Sakhrani L. M., Badie-Dezfooly B., Trizna W., Mikhail N., Lowe A. G., Taub M., Fine L. G. Transport and metabolism of glucose by renal proximal tubular cells in primary culture. Am J Physiol. 1984 Jun;246(6 Pt 2):F757–F764. doi: 10.1152/ajprenal.1984.246.6.F757. [DOI] [PubMed] [Google Scholar]
- Sartorius O. W., Roemmelt J. C., Pitts R. F., Calhoon D., Miner P. THE RENAL REGULATION OF ACID-BASE BALANCE IN MAN. IV. THE NATURE OF THE RENAL COMPENSATIONS IN AMMONIUM CHLORIDE ACIDOSIS. J Clin Invest. 1949 May;28(3):423–439. doi: 10.1172/JCI102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolwerth A. C., Sandler R. S., Hoffman P. M., Klahr S. Effects of nephron reduction and dietary protein content on renal ammoniagenesis in the rat. Kidney Int. 1975 Jun;7(6):397–404. doi: 10.1038/ki.1975.57. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Shiokawa K., Kawazoe Y., Nomura H., Miura T., Nakakura N., Horiuchi T., Yamana K. Ammonium ion as a possible regulator of the commencement of rRNA synthesis in Xenopus laevis embryogenesis. Dev Biol. 1986 Jun;115(2):380–391. doi: 10.1016/0012-1606(86)90257-5. [DOI] [PubMed] [Google Scholar]
- Simon E., Martin D., Buerkert J. Contribution of individual superficial nephron segments to ammonium handling in chronic metabolic acidosis in the rat. Evidence for ammonia disequilibrium in the renal cortex. J Clin Invest. 1985 Aug;76(2):855–864. doi: 10.1172/JCI112043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolins J. P., Hostetter M. K., Hostetter T. H. Hypokalemic nephropathy in the rat. Role of ammonia in chronic tubular injury. J Clin Invest. 1987 May;79(5):1447–1458. doi: 10.1172/JCI112973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. J., Ives H. E., Alpern R. J., Yee V. J., Warnock D. G., Rector F. C., Jr Increased Vmax for Na+/H+ antiporter activity in proximal tubule brush border vesicles from rabbits with metabolic acidosis. Am J Physiol. 1984 Aug;247(2 Pt 2):F339–F343. doi: 10.1152/ajprenal.1984.247.2.F339. [DOI] [PubMed] [Google Scholar]
- Van Thiel D. H., Gavaler J. S., Little J. M., Lester R. Alcohol: its effect on the kidney. Metabolism. 1977 Aug;26(8):857–866. doi: 10.1016/0026-0495(77)90004-x. [DOI] [PubMed] [Google Scholar]
- Vavatsi-Manos O., Preuss H. G. The effects of high calcium concentrations on renal ammoniagenesis by rat kidney slices. Nephron. 1976;17(6):474–482. doi: 10.1159/000180755. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L., Seifter J. L. Regulation of Na/H exchange in renal microvillus vesicles in chronic hypercapnia. Kidney Int. 1988 Jul;34(1):60–66. doi: 10.1038/ki.1988.145. [DOI] [PubMed] [Google Scholar]