Abstract
AIMS
To examine the predictive performance of limited sampling methods for estimation of tacrolimus exposure in adult kidney transplant recipients.
METHODS
Twenty full tacrolimus area under the concentration–time curve from 0 to 12 h post-dose (AUC0–12) profiles (AUCf) were collected from 20 subjects. Predicted tacrolimus AUC0–12 (AUCp) was calculated using the following: (i) 42 multiple regression-derived limited sampling strategies (LSSs); (ii) five population pharmacokinetic (PK) models in the Bayesian forecasting program TCIWorks; and (iii) a Web-based consultancy service. Correlations (r2) between C0 and AUCf and between AUCp and AUCf were examined. Median percentage prediction error (MPPE) and median absolute percentage prediction error (MAPE) were calculated.
RESULTS
Correlation between C0 and AUCf was 0.53. Using the 42 LSS equations, correlation between AUCp and AUCf ranged from 0.54 to 0.99. The MPPE and MAPE were <15% for 29 of 42 equations (62%), including five of eight equations based on sampling taken ≤2 h post-dose. Using the PK models in TCIWorks, AUCp derived from only C0 values showed poor correlation with AUCf (r2 = 0.27–0.54) and unacceptable imprecision (MAPE 17.5–31.6%). In most cases, correlation, bias and imprecision estimates progressively improved with inclusion of a greater number of concentration time points. When concentration measurements at 0, 1, 2 and 4 h post-dose were applied, correlation between AUCp and AUCf ranged from 0.75 to 0.93, and MPPE and MAPE were <15% for all models examined. Using the Web-based consultancy service, correlation between AUCp and AUCf was 0.74, and MPPE and MAPE were 6.6 and 9.6%, respectively.
CONCLUSIONS
Limited sampling methods better predict tacrolimus exposure compared with C0 measurement. Several LSSs based on sampling taken 2 h or less post-dose predicted exposure with acceptable bias and imprecision. Generally, Bayesian forecasting methods required inclusion of a concentration measurement from <2 h post-dose to adequately predict exposure.
Keywords: area under the concentration–time curve, Bayesian forecasting, kidney transplantation, limited sampling strategy, multiple regression, tacrolimus
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Tacrolimus pre-dose (C0) concentrations are currently used to guide tacrolimus dosing.
However, conflicting data exist regarding the relationship of C0 with tacrolimus area under the concentration–time curve from 0 to 12 h post-dose (AUC0–12) and clinical outcomes.
Previous literature suggests that limited sampling methods, such as multiple linear regression-derived limited sampling strategies or maximum a posteriori (MAP) Bayesian analyses, may provide more reliable estimations of tacrolimus exposure.
WHAT THIS STUDY ADDS
For the first time, the predictive performances of all published limited sampling methods for tacrolimus are compared in an independent cohort of adult kidney transplant recipients.
Limited sampling methods better predict tacrolimus exposure compared with measurement of C0.
However, the predictive power of the methods is highly variable, highlighting the importance of validating any method prior to applying it to an alternative population.
Introduction
Tacrolimus is an immunosuppressive drug that is one of the cornerstones in the prevention of rejection following solid organ transplantation. In 2006, 82.4% of kidney transplant recipients reported to the US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients were prescribed this agent at hospital discharge [1].
Tacrolimus has a narrow therapeutic index [2], making dosing difficult. Adequate exposure is imperative for the prevention of rejection, while overexposure risks serious toxicities that reduce tolerability and impact long-term allograft and patient survival [3, 4]. Tacrolimus also displays considerable between- and within-subject pharmacokinetic (PK) variability, with a poor correlation between dose and blood concentration [2]. Multiple factors have been identified as contributors to PK variability (Table 1) [3, 5–37]. As a consequence, therapeutic drug monitoring is mandatory [38].
Table 1.
Covariates contributing to tacrolimus pharmacokinetic variability
| Covariates | Reference |
|---|---|
| Transplanted organ | [15, 19, 37] |
| Patient age | [26, 27] |
| Patient race | [28–30] |
| Hepatitis C status | [21, 22] |
| Diabetes status | [36] |
| Time from transplantation | [24, 25] |
| Diurnal rhythm | [24, 31] |
| Food administration | [32, 33] |
| Corticosteroid dosage | [12, 13, 24] |
| Co-medication use | [3, 24] |
| Diarrhoea | [34, 35] |
| Albumin concentration | [24] |
| Haematocrit | [24] |
| Liver dysfunction | [15–18, 20] |
| Cytochrome P450 isoenzyme and P-glycoprotein genotype and phenotype | [6–11, 14] |
While full dose interval area under the concentration–time curve (AUC0–12) is generally considered the best marker of drug exposure [38], the requirement for collection of multiple samples over a 12 h period makes this approach impractical for routine clinical use. Subsequently, largely for reasons of practicality and convenience, most transplant centres use pre-dose (C0) concentrations to guide tacrolimus dosing. However, evidence regarding the relationship of C0 with AUC0–12 is conflicting (correlation, r2 = 0.04–0.91) [39–50], and there are minimal prospective data relating C0 values to clinical outcomes [51]. A feasible alternative is the use of limited sampling methods, such as multiple linear regression or maximum a posteriori (MAP) Bayesian analyses [52]. These may offer a better means of estimating tacrolimus exposure, yielding greater accuracy than C0 measurements, while being less cumbersome than full AUC0–12 measurements [52, 53]. The multiple linear regression method uses an equation derived from multiple linear regression analysis to estimate tacrolimus AUC0–12 from a limited number of concentrations measured at predefined times after dosing [52]. Such equations are relatively easy for the clinician to use and do not require specialist software. However, there is heavy reliance on exact sampling times, such that deviation of timing of sample collection may compromise equation predictive power.
Alternatively, Bayesian analysis uses information from a population PK model for tacrolimus. The model provides population PK parameter estimates (such as mean drug clearance and volume of distribution) and expected associated variability, and allows the opportunity to consider the influence of patient variables (covariates) on tacrolimus exposure. Tacrolimus AUC0–12 is determined from individualized PK parameter estimates by combining concentration measurements and data from that individual (such as patient weight or genotype) with available population data. The more individual data provided, the less the reliance on population data [52, 54]. Major advantages of this method include more flexible timing of blood sampling and improved prediction in patients with unusual pharmacokinetics. Disadvantages include reliance on the existence of an appropriate PK model, and a more complex calculation requiring specialist software and user training [38, 52].
It is likely that both multiple regression-derived limited sampling strategies (LSSs) and Bayesian analysis can only be applied with any accuracy to a population similar to the one in which they were developed, as defined by graft type, time post-transplant and analytical technique used for tacrolimus measurement. To ensure reliable predictions, it is essential that limited sampling methods are validated properly, ideally using a separate group of patients from the one in which the LSS equation or population PK model was derived [52].
This manuscript provides a review of all currently published limited sampling methods for tacrolimus in adult kidney transplant recipients. The predictive performances of each of these methods have been evaluated using an independent cohort of adult kidney transplant recipients. These results have then been used to identify the best method for our patient group, and to examine the general applicability (or otherwise) of these methods.
Methods
Patients
Adults who had undergone kidney transplant surgery at the Princess Alexandra Hospital (Brisbane, Queensland, Australia) were considered for inclusion in this study. Eligibility criteria included an immunosuppressive regimen of twice daily tacrolimus (Prograf®; Janssen-Cilag, Dublin, Ireland), twice daily mycophenolate mofetil (MMF; Cellcept®; Roche Pharma, Milan, Italy) and once daily prednisolone (Panafcortelone®; Aspen Pharmacare, St Leonards, New South Wales, Australia). A total of 20 tacrolimus PK profiles were collected from 20 kidney transplant recipients over the period April to June, 2009. The Princess Alexandra Hospital and University of Queensland Ethics Committees approved the study, and all participants provided written informed consent.
Blood sampling and analytical method
Thirteen whole blood samples were collected over a 12 h dosing interval (pre-dose, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 3, 4, 6, 9 and 12 h post-dose) from each subject. Samples were collected into Vacutainer® tubes containing ethylenediaminetetraacetic acid and stored at −20°C until analysis. There was no restriction on food intake prior to or during blood sampling. Tacrolimus concentrations were determined using a validated high-performance liquid chromatography–tandem mass spectrometry method [55, 56]. This assay is specific for the parent drug tacrolimus, and is linear over the range of 0.5–50 ng ml−1 (r2 > 0.99). The within-day and between-day imprecision is <8%.
Limited sampling methods
A literature search was performed using MEDLINE (1982 to current) and PubMed (1995 to current). Search terms included tacrolimus, therapeutic drug monitoring, limited sampling strategies, multiple linear regression, Bayesian forecasting, population pharmacokinetics, area under the concentration–time curve and kidney transplantation. Relevant primary research papers presenting limited sampling methods for tacrolimus derived in adult kidney transplant recipients were identified and evaluated. Articles were included if they were written in English.
Forty-two multiple linear regression-derived LSS equations were identified [42, 44, 46, 50, 57, 58]. These equations were entered into an Excel spreadsheet. Six population PK models of tacrolimus were identified [54, 59–63]. Covariate information included in the population models was collected from patient medical records. The models were entered into the Bayesian forecasting program TCIWorks, version 1 (The TCIWorks Development Team, Brisbane, Queensland, Australia) [64]. Each study subject was added as a new patient in the system with tacrolimus dosing history entered in chronological order. Additionally, a Web-based consulting service that uses Bayesian analysis to estimate tacrolimus AUC0–12 from concentration measurements made at 20 (±10), 60 (±15) and 180 (±30) min post-dose was identified [ImmunoSuppressants Bayesian dose Adjustment (ISBA)][65].
Pharmacogenetic analysis
Cytochrome P450 3A5 (CYP3A5) and multidrug resistant protein-1 (MDR-1) genotype were included as significant covariates in two of the population PK models [61, 62]. The CYP3A5 and MDR-1 genotyping was performed on blood samples from each study patient. Genomic DNA was extracted from whole blood samples using a QIAamp deoxyribonucleic acid mini kit (Qiagen, Hilden, Germany) and was stored at 4°C until analysis. Real-time PCR was performed with a 7900 Real Time PCR System (Applied Biosystems, Melbourne, Victoria, Australia). The PCR conditions were 10 min at 95°C, then 50 cycles of 15 s at 92°C and 1 min 30 s at 69°C. CYP3A5 6986A>G (rs776746) allelic discrimination was undertaken with a Custom TaqMan® Single Nucleotide Polymorphism (SNP) Genotyping Assay (Applied Biosystems) and VIC and FAM reporters. MDR-1 1236 (rs1128503) and MDR-1 3435 (rs1045642) allelic discrimination was undertaken with TaqMan® Drug Metabolism Genotyping Assays (Applied Biosystems), using assays C_7586662-10 and C_7586657-20 for MDR-1 1236 and MDR-1 3435, respectively. MDR-1 2677 (rs2032582) allelic discrimination was undertaken with a custom TaqMan® SNP Genotyping Assay (Applied Biosystems) and VIC and FAM reporters.
Predictive methods
Full tacrolimus AUC0–12 (AUCf) was estimated from all measured concentration–time points using noncompartmental analysis (trapezoidal rule) and compartmental analysis (two-compartment model with lag time) in WinNonlin® (Pharsight, version 5.2, Pharsight Corporation, North Carolina, USA). The predicted tacrolimus AUC0–12 (AUCp) was calculated in the following ways.
Applying relevant concentration measurements within each of the multiple regression LSS equations.
Applying concentration measurements taken at 0, 1, 2, 4 and 6 h post-dose along with relevant patient covariate values in the Bayesian forecasting program TCIWorks using each of the population PK models.
Sending concentration measurements taken at 0.25, 1 and 3 h post-dose and requested covariate information to the Web-based consultancy service, ISBA.
The AUCp calculated using each of the limited sampling strategies was compared with the AUCf estimated using noncompartmental analysis. The AUCp calculated using Bayesian forecasting was compared with AUCf estimated using compartmental analysis.
Pharmacokinetic and statistical analysis
Descriptive statistics used were mean ± standard deviation (SD) or median with interquartile range (IQR) for continuous variables, and percentages for categorical variables. For univariate comparisons, χ2, Fisher's exact test, Student's unpaired t-test and Wilcoxon rank sum test were used, where appropriate.
A Pearson correlation coefficient test was applied to assess the correlation between the following variables: (i) C0 and AUCf; and (ii) AUCp and AUCf. The AUCp was compared with the AUCf in terms of bias and imprecision according to the guidelines proposed by Sheiner & Beal [66]. Specifically, the four measures used for assessment were as follows.
Bias:
Median prediction error (MPE) = median (AUCp − AUCf).
-
Median percentage prediction error (MPPE) = median [100% × (AUCp − AUCf)/AUCf].
Imprecision:
Root median squared prediction error
 .
.Median absolute percentage prediction error (MAPE) = median [100% × I(AUCp − AUCf)I/AUCf]
Values of MPPE and MAPE of <15% were considered acceptable, as is the norm in clinical studies [52]. The percentage of AUCp estimates within 15% of AUCf was also calculated as a measure of overall predictive ability [67].
Analyses were carried out using the software packages Stata/SE 10.1 (College Station, TX, USA) and Excel 2007 (Microsoft Corporation). Graphs were completed using GraphPad Prism, version 5.0 (GraphPad Software).
Results
Limited sampling methods for tacrolimus
Tables 2 and 3 summarize the 42 multiple regression-derived LSSs [42, 44, 46, 50, 57, 58] and the six population PK models [54, 59–63] developed for estimation of tacrolimus exposure in adult kidney transplant recipients. One of the population PK models [54] had no between-subject variability and residual random error estimates and thus could not be evaluated further using TCIworks. Information on the population model used by the Web-based consultancy service was not available [65].
Table 2.
Multiple linear regression methods for estimation of tacrolimus exposure in adult kidney transplant recipients
| Eqn no. | Patients (country) | Time post-transplant | Dietary control | Prospective data collection | Assay | Times investigated (h) | Limited sampling equation for estimation of AUC0–12 | Validation method | r2 | MPE(µg h l−1) | MPPE(%) | RMSE(µg h l−1) | MAPE(%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 14* (Spain) | NA | No | No | MEIA | 0, 0.5, 1, 2, 4, 6, 8, 12 | 8.90 + 4.0C0 + 1.77C1 + 5.47C4 | Separate group (n = 13*) | 0.97 | 0.48 | 0.16 | 6.3 | NA | [50] |
| 2 | 29 (India) | 3–6 months | Yes | Yes | MEIA | 0.5, 1, 1.5, 2, 2.5, 4, 6, 8, 12 | 14.73 + 4.38C0 + 2.09C1.5 + 4.06C4 | Jackknife | 0.99 | NA | 0.35 | NA | 4.7 | [46] |
| 3 | 19.16 + 6.75C0 + 3.33C1.5 | 0.95 | NA | 0.53 | NA | 6.4 | ||||||||
| 4 | 23.90 + 2.74C0 + 7.88C4 | 0.93 | NA | −0.01 | NA | 6.5 | ||||||||
| 5 | 18 (Hong Kong) | 1–8 years | Yes | Yes | MEIA | 0, 1, 2, 4, 6, 8, 12 | 1.0 + 0.5C0 + C1 + 1.5C2 + 2C4 + 2C6 + 2.9C8 + 2C12 | None | 1.00 | NA | −0.5 | NA | 0.5 | [44] |
| 6 | 5.4 + 1.1C0 + C1 + 1.4C2 + 2.3C4 + 2C6 + 3.3C8 | 0.99 | NA | −0.2 | NA | 0.8 | ||||||||
| 7 | 8.3 + 1.2C0 + 0.9C1 + 1.6C2 + 2.7C4 + 3.7C6 | 0.99 | NA | 0.5 | NA | 1.7 | ||||||||
| 8 | 10 + 1.4C0 + 0.8C1 + 1.6C2 + 5.5C4 | 0.98 | NA | −0.08 | NA | 2.5 | ||||||||
| 9 | 13.3 + 1.2C0 + 2.4C2 + 5.6C4 | 0.93 | NA | −0.8 | NA | 3.5 | ||||||||
| 10 | 16.2 + 2.4C2 + 5.9C4 | 0.93 | NA | −0.2 | NA | 3.6 | ||||||||
| 11 | 24.5 + 3.8C0 + 0.9C1 + 3.3C2 | 0.82 | NA | 0.5 | NA | 6.8 | ||||||||
| 12 | 44 + 0.8C1 + 3.5C2 | 0.77 | NA | 0.6 | NA | 7.6 | ||||||||
| 13 | 26.2 + 3.6C0 + 4.2C2 | 0.76 | NA | 0.7 | NA | 7.7 | ||||||||
| 14 | 53.22 + 5.26C0 + 1.95C1 | 0.57 | NA | 1.3 | NA | 9.5 | ||||||||
| 15 | 100 (Belgium) | Day 7, 42, 90, 180, 360 | Yes | Yes | MEIA | 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12 | 5.36 + 3.06C0 + 0.98C0.5 + 1.61C2 + 1.13C3 + 3.96C4 | None | 0.96 | NA | −0.57 | NA | 4.7 | [58] |
| 16 | 50† (Japan) | Day 28 | Yes | Yes | MEIA | 0, 1, 2, 3, 4, 6, 9, 12 | 10.11C9 + 41.41 | Separate group (n = 50†) | 0.77 | −7.4 | NA | 28.3 | NA | [57] |
| 17 | 6.46C4 + 59.49 | 0.62 | 0 | NA | 28.3 | NA | ||||||||
| 18 | 12.46C0 + 46.00 | 0.61 | −0.2 | NA | 27.8 | NA | ||||||||
| 19 | 3.73C3 + 7.44C9 + 9.96 | 0.91 | −3.8 | NA | 18.3 | NA | ||||||||
| 20 | 5.19C0 + 7.50C9 + 21.73 | 0.82 | −5.1 | NA | 25.2 | NA | ||||||||
| 21 | 7.63C0 + 4.11C4 + 21.66 | 0.77 | 0.6 | NA | 21.2 | NA | ||||||||
| 22 | 2.25C2 + 5.06C4 + 47.12 | 0.68 | 1.0 | NA | 26.0 | NA | ||||||||
| 23 | 3.45C3 + 2.75C6 + 4.75C9 + 4.75 | 0.95 | −3.3 | NA | 14.7 | NA | ||||||||
| 24 | 2.25C2 + 1.92C4 + 7.27C9 + 6.61 | 0.93 | −3.7 | NA | 17.8 | NA | ||||||||
| 25 | 2.15C0 + 3.34C3 + 6.64C9 + 5.06 | 0.91 | −3.3 | NA | 17.7 | NA | ||||||||
| 26 | 7.04C0 + 1.71C2 + 3.23C4 + 15.19 | 0.80 | 1.3 | NA | 19.9 | NA | ||||||||
| 27 | 1.13C2 + 3.03C3 + 3.65C4 + 37.09 | 0.73 | 1.5 | NA | 23.9 | NA | ||||||||
| 28 | 15 (Thailand) | <3 months | Yes | Yes | MEIA | 0, 1, 2, 4, 6, 8, 12 | −5.385 + 3.337C0 + 0.96C1 + 1.402C2 + 6.01C4 | None | 0.98 | NA | NA | NA | 3.2 | [42] |
| 29 | −5.496 + 7.189C0 + 2.357C1 + 2.131C2 | 0.93 | NA | NA | NA | 5.9 | ||||||||
| 30 | 3.85 + 3.688C0 + 1.355C1 + 6.649C4 | 0.97 | NA | NA | NA | 4.5 | ||||||||
| 31 | −6.103 + 2.383C0 + 1.911C2 + 7.582C4 | 0.97 | NA | NA | NA | 3.9 | ||||||||
| 32 | 1.304 + 0.465C1 + 1.636C2 + 8.256C4 | 0.96 | NA | NA | NA | 4.6 | ||||||||
| 33 | 9.345 + 8.408C0 + 3.23C1 | 0.91 | NA | NA | NA | 7.4 | ||||||||
| 34 | −8.453 + 7.389C0 + 4.902C2 | 0.85 | NA | NA | NA | 9.6 | ||||||||
| 35 | 8.231 + 2.316C0 + 9.636C4 | 0.95 | NA | NA | NA | 4.0 | ||||||||
| 36 | 19.648 + 2.456C1 + 4.049C2 | 0.81 | NA | NA | NA | 11.0 | ||||||||
| 37 | 13.114 + 0.873C1 + 9.291C4 | 0.95 | NA | NA | NA | 5.2 | ||||||||
| 38 | −0.192 + 1.888C2 + 8.783C4 | 0.96 | NA | NA | NA | 4.8 | ||||||||
| 39 | 52.509 + 13.126C0 | 0.61 | NA | NA | NA | 14.7 | ||||||||
| 40 | 62.472 + 4.451C1 | 0.71 | NA | NA | NA | 13.4 | ||||||||
| 41 | 17.295 + 6.995C2 | 0.72 | NA | NA | NA | 13.2 | ||||||||
| 42 | 13.808 + 10.779C4 | 0.94 | NA | NA | NA | 4.8 |
Cx, tacrolimus concentration at given time; MAPE, mean absolute percentage prediction error; MEIA, microparticle enzyme immunoassay; MPE, mean prediction error; MPPE, mean percentage prediction error; NA, not available; and RMSE, root mean squared prediction error.
Number of PK profiles (22 subjects in total in study).
Number of PK profiles (50 subjects in total in study).
Table 3.
Population pharmacokinetic models for estimation of tacrolimus exposure in adult kidney transplant recipients
| Model | Patients (country) | Time post-transplant | Dietary control | Prospective data collection | Assay | Times investigated (h) | Structural model estimate (%CV) | Statistical model estimate (%CV) | Validation method | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 (Australia) | 2–1475 days | No | No | LC-MS/MS | 12 | CL/F = θ1 + θ2/POD + θ3/AST l h−1V/F = θ4 l ka = 4.48 h−1 (fixed) θ1 = 23.6 (12%) θ2 = 31.9 (50%) θ3 = 76.7 (48%) θ4 = 1070 (27%) | IIV CL/F = 42% (17%) IIV V/F = 111% (47%) RREa = 3.7 ng ml−1 (23%) | None | [59] |
| 2 | 83 (France) | <2 months | No | No | MEIA | 12 | CL = θ1 × [1 + PODθ2/(PODθ2 + θ3θ2)] × PRED l h−1PRED = 1 + θ4 (If prednisolone dose <25 mg or 1 if not) V = θ5 l F = θ6% ka = 4.5 h−1 (fixed) θ1 = 1.81 (12%) θ2 = 3.81 (14%) θ3 = 2.54 (37%) θ4 = 0.575 (6%) θ5 = 98.4 (13%) θ6 = 13.7 (11%) | IIV CL = 31% (55%) IIV V = 79% (48%) IIV F = 32% (56%) RREa = 0.96 ng ml−1 (58%) RREp = 18.6% (53%) | Bootstrap | [60] |
| 3 | 31 (Netherlands) | 2–52 weeks | No | Yes | MEIA | 0, 1, 2, 3, 4, 6, 12 | CL (CYP3A5*3/*3) = θ1 l h−1CL (CYP3A5*1/*1 or *1/*3) = θ2 l h−1Vc = θ3 l Vp = Vc L Q = θ4 l h−1F = 23% (fixed) F = θ5 (If prednisone dose <10 mg) ka = θ6 h−1θ1 = 3.7 (8%) θ2 = 5.5 (10%) θ3 = 42 (10%) θ4 = 10 (10%) θ5 = 19.5 (30%) θ6 = 1.6 (14%) | IIV CL = 19% (32%) IIV Vc = 28% (31%) IOV F = 22% (13%) RREp = 23% (6%) | Bootstrap | [61] |
| 4 | 19* (Belium) | Pre-transplant | Yes | Yes | MEIA | 0, 1, 2, 4, 8, 12 | CL/F = θ1 + CYP3A5 + MDR-1 l h−1CYP3A5 = 34 (19%) (If *1/*1 or *1/*3 or 0 if not) MDR-1 = 10 (21%) (If 1236CC, 2677GG or 3435CC or 0 if not) Vc/F = θ2 l Vp/F = θ3 l Q/F = θ4 l h−1ka = θ5 h−1θ1 = 22 (11%) θ2 = 142 (15%) θ3 = 192 (17%) θ4 = 43 (14%) θ5 = 2.18† (15%) | IIV CL/F = 6% (108%) IIV Vc/F = 33% (45%) IIV Vp/F = 31% (43%) IOV CL/F = 40% (23%) RREa = 0.02 ng ml−1 (12%) RREp = 29% (7%) | Bootstrap, case deletion diagnostics, cross-validation, simulation | [62] |
| 5 | 32 (France) | Weeks 1 and 2 and months 1, 3 and 6 | No | Yes | LC-MS/MS | 0.33, 0.66, 1, 1.5, 2, 3, 4, 6, 9 | CL/F = θ1/HAEM l h−1Vc/F = θ2 l Vp/F = 500 l (fixed) Q/F = θ3 l h−1ka = θ4 h−1θ1 = 863 (7%) θ2 = 147 (16%) θ3 = 60 (20%) θ4 = 6.5‡ (6%) | IIV CL/F = 30% IIV Vc/F = 26% IIV Q/F = 63% IIV ka = 15%‡IOV CL/F = 71% IOV Q/F = 27% IOV ka = 24% RREa = 1.5 ng ml−1RREp = 10% | Bootstrap, cross-validation | [63] |
| 6 | 17 (Netherlands) | 2–52 weeks | No | Yes | MEIA | 0, 1, 2, 3, 4, 6, 8, 12 | ke = θ1 h−1Vc = θ2 l kg−1K12 = θ3 h−1K21 = θ4 h−1F = 23% (fixed) ka = θ5 h−1tlag = θ6 h θ1 = 0.517 (19%) θ2 = 0.180 (35%) θ3 = 2.850 (78%) θ4 = 0.384 (107%) θ5 = 0.580 (90%) θ6 = 0.956 (17%) | Separate group (n = 15) | [54] |
AST, aspartate transaminase; CV, coefficient of variation; CL, clearance, CL/F, apparent clearance; CYP3A5, cytochrome P450 3A5; F, bioavailability; ka, absorption rate constant; HAEM, haematocrit; IIV, interindividual variability; IOV, interoccasional variability; ke, elimination rate constant; K12, distribution rate constant (central to peripheral compartment); K21, distribution rate constant (peripheral to central compartment); LC-MS/MS, liquid chromatography–tandem mass spectrometry; MEIA, microparticle enzyme immunoassay; MDR-1, multiple drug resistant protein 1; POD, postoperative day; Q, intracompartmental clearance; Q/F, apparent intracompartmental clearance; RREa, additive residual random error; RREp, proportional residual random error; tlag, lag time; V, volume of distribution; Vc, volume of distribution of central compartment; Vp, volume of distribution of peripheral compartment; V/F, apparent volume of distribution;
renal transplant candidates;
following the morning dose; and
transfer rate constant.
Baseline characteristics
Study participants were divided into early (3–5 days post-transplant surgery) and late cohorts (<3 months post-transplant surgery). Table 4 shows the baseline demographic and clinical characteristics of study participants according to study group. The serum creatinine concentration was significantly higher and serum albumin concentration and haematocrit significantly lower in the early compared with the late group.
Table 4.
Summary of baseline characteristics of kidney transplant recipients
| Characteristic | All subjects | Early post-transplant group* | Late post-transplant group† | P value |
|---|---|---|---|---|
| Number of patients | 20 | 10 | 10 | 1 |
| Tacrolimus dose (mg day−1) | 7.5 [4, 12.5] | 12.5 [11, 15] | 4.0 [2.0, 5.5] | 0.002 |
| AUCf (µg h l−1) | 24.1 [18.9, 36.0] | 130.6 [87.7, 158.9] | 76.2 [66.5, 94.8] | 0.01 |
| Dose-adjusted AUCf (mg h l mg−1) | 30.2 [20.2, 41.2] | 20.2 [10.7, 26.5] | 41.2 [33.9, 56.6] | 0.002 |
| Age (years) | 49 ± 11 | 45 ± 12 | 53 ± 9 | 0.1 |
| Male (n (%)) | 12 (60) | 6 (60) | 6 (60) | 1 |
| Body weight (kg) | 80 [62, 98] | 91 [74, 104] | 74 [56, 91] | 0.08 |
| Race | ||||
| Caucasian (n (%)) | 19 (90) | 10 (100) | 8 (80) | 0.14 |
| Asian (n (%)) | 2 (10) | 0 (0) | 2 (20) | – |
| Diabetes (n (%)) | 2 (10) | 0 (0) | 2 (20) | 0.14 |
| Aetiology of kidney failure | ||||
| Glomerulonephritis (n (%)) | 6 (30) | 2 (20) | 4 (40) | 0.4 |
| Polycystic kidney disease (n (%)) | 4 (20) | 3 (30) | 1 (10) | – |
| Vesicoureteric reflux (n (%)) | 4 (20) | 3 (30) | 1 (10) | – |
| Diabetes (n (%)) | 1 (5) | 0 (0) | 1 (10) | – |
| Other (n (%)) | 5 (25) | 2 (20) | 3 (30) | – |
| Transplant number | ||||
| 1 (n (%)) | 17 (85) | 9 (90) | 8 (80) | 0.5 |
| 2 (n (%)) | 3 (15) | 1 (10) | 2 (20) | – |
| Transplant type | ||||
| Living donor (n (%)) | 7 (35) | 4 (40) | 3 (30) | 0.6 |
| Deceased donor (n (%)) | 13 (65) | 6 (60) | 7 (70) | – |
| Duration since transplant (days) | 44.5 [4, 569] | 4 [4,4] | 569 [193, 1941] | 0.001 |
| Serum creatinine (µmol l−1) | 140 [103, 220] | 196 [146, 280] | 103 [101, 134] | 0.006 |
| Serum albumin (g l−1) | 32 [28, 38] | 29 [26, 31] | 38 [36, 40] | 0.0008 |
| Haematocrit | 0.33 [0.26, 0.36] | 0.27 [0.25, 0.29] | 0.36 [0.35, 0.39] | 0.002 |
| Serum bilirubin (µmol l−1) | 13 [10, 14] | 12 [9, 14] | 13 [10, 15] | 0.4 |
Values expressed are medians [interquartile range], except mean ± SD for age and median (range) for MMF dose. AUCf, full MPA AUC0–12 calculated using noncompartmental analysis (trapezoidal rule).
Days 3–5 post-transplantation.
<3 months post-transplantation.
Similar values for AUCf were estimated based on noncompartmental and compartmental methods in WinNonlin (mean percentage difference 2.2% and no percentage difference greater than 8.7%). Median (IQR) AUCf (calculated using noncompartmental analysis) was 130.6 (87.7–158.9) µg h l−1vs. 76.2 (66.5–94.8) µg h l−1 (P = 0.01) in the early and late groups, respectively. However, when adjusted for dose, this difference was reversed, with median (IQR) dose-adjusted AUCf being significantly lower in the early post-transplant group compared with the late group [20.2 (10.7–26.5) vs. 41.2 (33.9–56.6) µg h l mg−1 tacrolimus; P = 0.002] (Figure 1).
Figure 1.
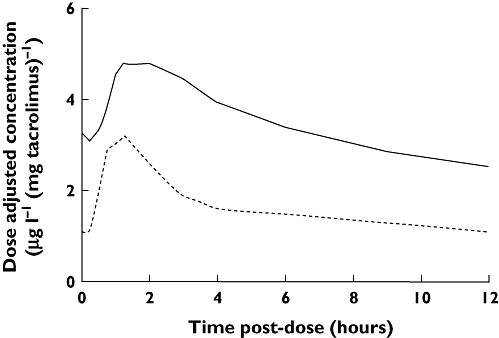
Dose-adjusted tacrolimus concentration vs. time post-dose for the early (3–5 days post-transplant) and late groups (<3 months post-transplant). Solid line is the late post-transplant group and dotted line is early post-transplant group
Predictive performance of the different limited sampling methods
Multiple linear regression equations
The correlation (r2) between C0 and AUCf (calculated using noncompartmental analysis) for the entire study cohort was 0.53 (Figure 2). The correlation between C0 and AUCf was poorer in the late compared with the early post-transplant group (r2 = 0.21 vs. r2 = 0.64, respectively). The correlation (r2) between C12 and AUCf was higher than the correlation between C0 and AUCf (r2 = 0.83 for the study cohort as a whole, r2 = 0.87 in the early group and r2 = 0.63 in the late group). Of all time points, C6 showed the highest correlation with AUCf (r2 = 0.91 for the study cohort as a whole, r2 = 0.90 in the early group and r2 = 0.79 in the late group).
Figure 2.
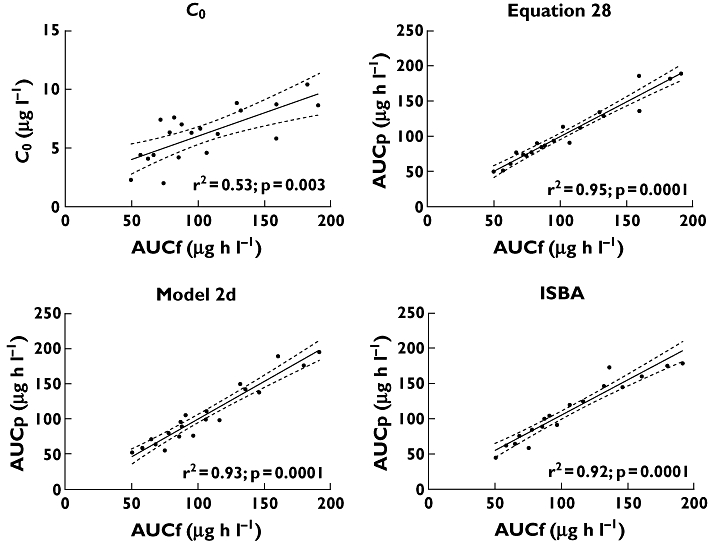
Correlation between AUCf and C0, equation 28, model 2d and ISBA estimates. Continuous lines represent the linear regression lines. Dotted lines represent the 95% confidence intervals for each linear regression line
Based on the 42 multiple regression equations, the r2 between AUCp and AUCf ranged from 0.54 to 0.99. Table 5 summarizes the predictive performance of each of the LSSs. All equations showed a better correlation with AUCf than did C0. The MPPE varied from 0.1 to 33.5%, and the MAPE varied from 2.0 to 33.5%. Both MPPE and MAPE were <15% for 29 of the 42 equations (62%). The two equations that incorporated only C0 measurements displayed the lowest correlations and the greatest bias and imprecision of all of the equations (for equation 18, r2 = 0.54, MPPE 23.3% and MAPE 25.7%; and for equation 39, r2 = 0.54, MPPE 33.5% and MAPE 33.5%).
Table 5.
Predictive performance of multiple linear regression methods for prediction of tacrolimus exposure in adult kidney transplant recipients
| Eqn no. | Limited sampling equation for estimation of AUC0–12 | Times used(h) | r2 | MPE(µg h l−1) | MPPE(%) | RMSE(µg h l−1) | MAPE(%) | % AUCp within 15% AUCf | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1* | 8.90 + 4.0C0 + 1.77C1 + 5.47C4 | 0, 1, 4 | 0.95 | 7.6 | 9.3 | 8.1 | 7.3 | 85 | [50] |
| 2* | 14.73 + 4.38C0 + 2.09C1.5 + 4.06C4 | 0, 1.5, 4 | 0.92 | 6.9 | 7.7 | 10.4 | 12.2 | 60 | [46] |
| 3* | 19.16 + 6.75C0 + 3.33C1.5 | 0.86 | 2.4 | 2.5 | 12.2 | 13.2 | 60 | ||
| 4 | 23.90 + 2.74C0 + 7.88C4 | 0.80 | 17.9 | 20.6 | 20.9 | 23.2 | 30 | ||
| 5* | 1.0 + 0.5C0 + C1 + 1.5C2 + 2C4 + 2C6 + 2.9C8 + 2C12 | 0, 1, 2, 4, 6, 8, 12 | 0.99 | 1.0 | 1.6 | 2.0 | 2.0 | 95 | [44] |
| 6* | 5.4 + 1.1C0 + C1 + 1.4C2 + 2.3C4 + 2C6 + 3.3C8 | 0.99 | 1.0 | 0.9 | 2.4 | 2.1 | 90 | ||
| 7* | 8.3 + 1.2C0 + 0.9C1 + 1.6C2 + 2.7C4 + 3.7C6 | 0.99 | −0.7 | 1.1 | 3.2 | 3.2 | 100 | ||
| 8* | 10 + 1.4C0 + 0.8C1 + 1.6C2 + 5.5C4 | 0.95 | −0.7 | −0.7 | 5.7 | 6.2 | 85 | ||
| 9* | 13.3 + 1.2C0 + 2.4C2 + 5.6C4 | 0.90 | 1.3 | 1.6 | 7.5 | 9.1 | 80 | ||
| 10* | 16.2 + 2.4C2 + 5.9C4 | 0.88 | −0.8 | −1.0 | 8.7 | 9.6 | 80 | ||
| 11* | 24.5 + 3.8C0 + 0.9C1 + 3.3C2 | 0.89 | 1.8 | 1.9 | 11.8 | 12.8 | 60 | ||
| 12* | 44 + 0.8C1 + 3.5C2 | 0.86 | −1.1 | −1.4 | 13.8 | 14.5 | 50 | ||
| 13* | 26.2 + 3.6C0 + 4.2C2 | 0.89 | 1.1 | 0.9 | 9.4 | 8.4 | 65 | ||
| 14 | 53.22 + 5.26C0 + 1.95C1 | 0.80 | 15.0 | 13.0 | 19.4 | 20.0 | 45 | ||
| 15* | 5.36 + 3.06C0 + 0.98C0.5 + 1.61C2 + 1.13C3 + 3.96C4 | 0, 0.5, 2, 3, 4 | 0.97 | 2.0 | 3.2 | 7.1 | 5.0 | 85 | [58] |
| 16 | 10.11C9 + 41.41 | 0, 2, 3, 4, 9 | 0.90 | 5.0 | 5.6 | 18.6 | 20.7 | 40 | [57] |
| 17 | 6.46C4 + 59.49 | 0.77 | 24.1 | 25.0 | 24.8 | 25.0 | 30 | ||
| 18 | 12.46C0 + 46.00 | 0.54 | 25.7 | 23.3 | 28.1 | 25.7 | 30 | ||
| 19* | 3.73C3 + 7.44C9 + 9.96 | 0.91 | −1.2 | −1.1 | 7.7 | 9.3 | 75 | ||
| 20* | 5.19C0 + 7.50C9 + 21.73 | 0.82 | −3.2 | −2.7 | 11.7 | 13.9 | 60 | ||
| 21 | 7.63C0 + 4.11C4 + 21.66 | 0.77 | 8.6 | 10.2 | 15.4 | 18.1 | 35 | ||
| 22 | 2.25C2 + 5.06C4 + 47.12 | 0.89 | 22.2 | 23.7 | 22.2 | 23.7 | 30 | ||
| 23* | 3.45C3 + 2.75C6 + 4.75C9 + 4.75 | 0.94 | −5.0 | −5.1 | 6.8 | 7.4 | 75 | ||
| 24* | 2.25C2 + 1.92C4 + 7.27C9 + 6.61 | 0.94 | −2.3 | 0.1 | 3.7 | 3.9 | 90 | ||
| 25* | 2.15C0 + 3.34C3 + 6.64C9 + 5.06 | 0.91 | −0.6 | −1.0 | 6.2 | 7.5 | 80 | ||
| 26* | 7.04C0 + 1.71C2 + 3.23C4 + 15.19 | 0.86 | 13.2 | 10.9 | 15.3 | 15.0 | 50 | ||
| 27 | 1.13C2 + 3.03C3 + 3.65C4 + 37.09 | 0.88 | 16.4 | 19.5 | 19.2 | 19.5 | 45 | ||
| 28† | −5.385 + 3.337C0 + 0.96C1 + 1.402C2 + 6.01C4 | 0, 1, 2, 4 | 0.95 | −1.1 | −1.1 | 2.8 | 2.8 | 90 | [42] |
| 29* | −5.496 + 7.189C0 + 2.357C1 + 2.131C2 | 0.87 | −3.5 | −3.4 | 13.6 | 11.6 | 65 | ||
| 30* | 3.85 + 3.688C0 + 1.355C1 + 6.649C4 | 0.94 | 5.7 | 7.2 | 6.7 | 8.1 | 75 | ||
| 31* | −6.103 + 2.383C0 + 1.911C2 + 7.582C4 | 0.88 | 0.3 | 0.3 | 8.3 | 8.8 | 65 | ||
| 32* | 1.304 + 0.465C1 + 1.636C2 + 8.256C4 | 0.96 | 1.9 | 2.2 | 6.9 | 7.7 | 70 | ||
| 33 | 9.345 + 8.408C0 + 3.23C1 | 0.80 | 6.5 | 6.1 | 14.5 | 17.9 | 40 | ||
| 34* | −8.453 + 7.389C0 + 4.902C2 | 0.85 | 0.6 | 0.6 | 8.7 | 7.8 | 75 | ||
| 35 | 8.231 + 2.316C0 + 9.636C4 | 0.80 | 11.6 | 14.9 | 15.1 | 17.5 | 40 | ||
| 36* | 19.648 + 2.456C1 + 4.049C2 | 0.83 | 4.2 | 3.8 | 10.5 | 12.8 | 65 | ||
| 37* | 13.114 + 0.873C1 + 9.291C4 | 0.88 | 8.6 | 9.2 | 10.3 | 13.1 | 50 | ||
| 38* | −0.192 + 1.888C2 + 8.783C4 | 0.86 | 0.9 | 1.5 | 10.9 | 9.3 | 65 | ||
| 39 | 52.509 + 13.126C0 | 0.54 | 35.6 | 33.5 | 35.2 | 33.5 | 25 | ||
| 40 | 62.472 + 4.451C1 | 0.66 | 27.2 | 22.3 | 29.7 | 24.0 | 25 | ||
| 41* | 17.295 + 6.995C2 | 0.83 | 4.1 | 4.5 | 11.1 | 11.3 | 60 | ||
| 42 | 13.808 + 10.779C4 | 0.77 | 13.1 | 12.2 | 17.3 | 18.9 | 45 |
Cx, tacrolimus concentration at given time; MAPE, median absolute percentage prediction error; MPE, median prediction error; MPPE, median percentage prediction error; RMSE, root median squared prediction error.
Acceptable bias and imprecision.
Best performance of all equations with regard to practicality and performance.
Equation 28 was superior to all other equations with regard to practicality and performance (sampling confined to the first 4 h post-dose, r2 = 0.95, MPPE −1.1% and MAPE 2.8%). Figure 2 displays the correlation between AUCp and AUCf for each study participant based on this equation. Bias and imprecision are depicted in Figures 3 and 4, respectively, using Bland–Altman plots. As shown in Figure 3, there was no consistent pattern to the direction of bias, with AUCp both over- and underestimating AUCf. However, there was a suggestion of increasing bias at increasing values of AUCf. Figure 4 shows that for equation 28, 18 of 20 AUCp values (90%) fell within 15% of AUCf.
Figure 3.
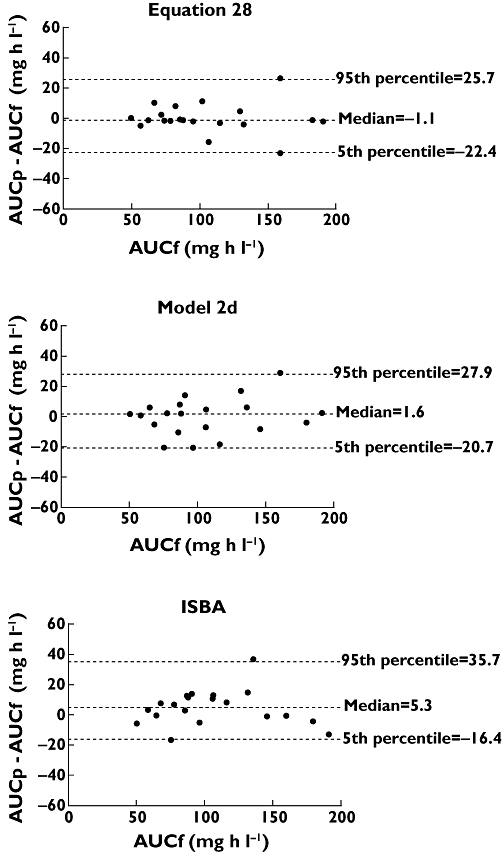
Bland–Altman plots comparing the difference between AUCf and AUCp and the average of AUCf and AUCp, when AUCp was estimated from equation 28 or model 2d and using ISBA. Dotted lines represent median differences and the 5th and 95th percentiles
Figure 4.
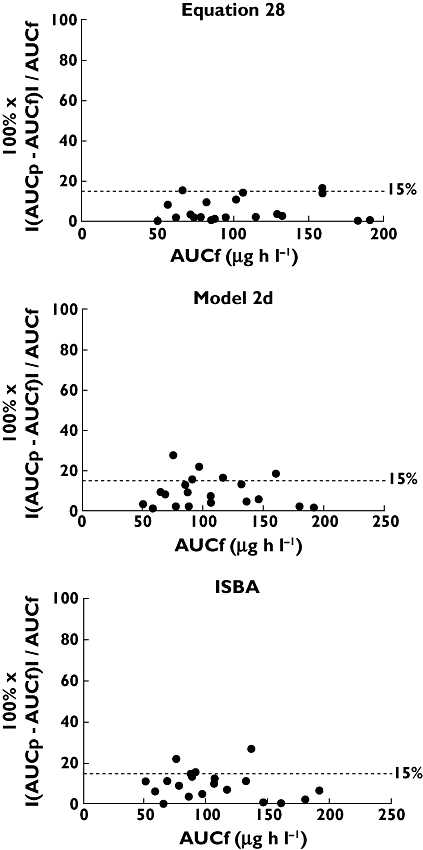
Scatter plots of the absolute percentage prediction error vs. AUCf, when AUCp was estimated from equation 28 or model 2d and using ISBA. Accuracy within 15% of AUCf is represented by the dotted lines
When patients in the early post-transplant group were considered separately, MPPE and MAPE were <15% for 35 (83%) of the 42 equations. When patients in the late group were considered separately, this was the case for 24 of the 42 equations (52%). Equation 28 remained superior regardless of duration post-transplant (early group, r2 = 0.92, MPPE −0.8% and MAPE 2.1%; and late group, r2 = 0.91, MPPE −1.9% and MAPE 3.1%).
The AUCp was also compared with AUCf estimated using compartmental analysis. Generally, bias and imprecision estimates were slightly inferior (data not shown). However, equation 28 remained superior regardless of metric used, and 26 of the 29 equations that had previously been identified as yielding clinically acceptable bias and imprecision estimates continued to do so (all except equations 12, 20 and 37).
MAP Bayesian estimators
Table 6 summarizes the predictive performance of each of population PK models in TCIWorks when varying concentration time points from 0 to 6 h post-dose were applied. It also shows the predictive performance of the Web-based consultancy service when concentration time points at 0.25, 1 and 3 h post-dose were used. Regardless of the model used, Bayesian AUC estimates derived from only C0 values showed poor correlation with AUCf (calculated using compartmental analysis; r2 = 0.27–0.54) and unacceptable imprecision (MAPE 17.5–31.6%). In most cases, correlation, bias and imprecision estimates progressively improved with inclusion of a greater number of concentration time points. Generally, population models required at least one concentration time point greater than 2 h post-dose to predict tacrolimus AUCf with acceptable bias and imprecision.
Table 6.
Predictive performance of population models for prediction of tacrolimus exposure in adult kidney transplant recipients
| Model | Limited sampling equations | Times used (h) | r2 | MPE(µg h l−1) | MPPE(%) | RMSE(µg h l−1) | MAPE(%) | % AUCp within 15% AUCf | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1a | CL/F = 23.6 + 31.9/POD + 76.7/AST l h−1 | 0 | 0.34 | −5.9 | −4.9 | 22.3 | 26.4 | 30 | [59] |
| 1b | V/F = 1070 l | 0, 1 | 0.65 | 2.9 | 5.0 | 27.0 | 22.9 | 30 | |
| 1c | ka = 4.48 h−1 (fixed) | 0, 1, 2 | 0.71 | 5.2 | 5.0 | 14.6 | 19.2 | 45 | |
| 1d | IIV CL/F = 42% | 0, 1, 2, 4 | 0.75 | 1.9 | 1.3 | 12.5 | 12.5 | 55 | |
| 1e* | IIV V/F = 111% | 0, 1, 2, 4, 6 | 0.83 | 3.2 | 2.3 | 9.2 | 8.6 | 70 | |
| 1f* | RREa = 3.7 ng ml−1 | 0, 1, 4 | 0.74 | 0.1 | 0 | 12.9 | 13.0 | 60 | |
| 1g | 0, 4 | 0.68 | −7.4 | −5.2 | 16.2 | 15.9 | 45 | ||
| 2a | CL = 1.81 × [1 + POD2.54 / (POD2.54 + 3.812.54)] × PRED l h−1 | 0 | 0.27 | 8.2 | 9.3 | 20.1 | 17.5 | 40 | [60] |
| 2b | PRED = 1 + 0.575 (If prednisolone dose >25 mg or 1 if not) | 0, 1 | 0.75 | 0.7 | 1.2 | 30.0 | 21.8 | 25 | |
| 2c | V = 98.4 l | 0, 1, 2 | 0.83 | 3.9 | 4.7 | 13.7 | 15.4 | 50 | |
| 2d† | F = 13.7% | 0, 1, 2, 4 | 0.93 | 1.6 | 1.6 | 6.6 | 7.4 | 75 | |
| 2e* | ka = 4.5 h−1 (fixed) | 0, 1, 2, 4, 6 | 0.95 | −2.1 | −1.4 | 4.9 | 4.6 | 90 | |
| 2f* | IIV CL = 31% | 0, 1, 4 | 0.91 | −0.1 | −0.1 | 8.1 | 9.2 | 70 | |
| 2g* | IIV V = 79% | 0, 4 | 0.88 | −3.3 | −4.1 | 9.9 | 9.7 | 75 | |
| IIV F = 32% | |||||||||
| RREa = 0.96 ng ml−1 | |||||||||
| RREp = 18.6% | |||||||||
| 3a | CL (CYP3A5*3/*3) = 3.7 l h−1 | 0 | 0.52 | 30.7 | 25.7 | 43.0 | 31.6 | 20 | [61] |
| 3b | CL (CYP3A5*1/*3) = 5.5 l h−1 | 0, 1 | 0.64 | 11.0 | 14.4 | 21.1 | 20.8 | 40 | |
| 3c | Vc = 42 l | 0, 1, 2 | 0.73 | 8.2 | 10.0 | 18.3 | 16.4 | 50 | |
| 3d | Vp = 42 l | 0, 1, 2, 4 | 0.86 | 6.4 | 6.5 | 14.9 | 12.7 | 55 | |
| 3e* | Q = 10 l h−1 | 0, 1, 2, 4, 6 | 0.89 | 4.7 | 6.7 | 12.1 | 9.7 | 60 | |
| 3f* | F = 23% (fixed) | 0, 1, 4 | 0.82 | 6.7 | 9.2 | 17.7 | 13.6 | 55 | |
| 3g | F = 19.5% (If prednisone dose >10 mg) | 0, 4 | 0.72 | 18.3 | 14.8 | 28.3 | 23.8 | 40 | |
| ka = 1.6 h−1 | |||||||||
| IIV CL = 19% | |||||||||
| IIV Vc = 28% | |||||||||
| RREp = 23% | |||||||||
| 4a | CL/F = 22 + 34 (if CYP3A5*1/*1 or *1/*3) + 10 (if MDR-1 | 0 | 0.54 | 20.9 | 24.2 | 31.9 | 28.1 | 35 | [62] |
| 4b | 1236CC, 2677GG or 3435CC) l h−1 | 0, 1 | 0.69 | −1.2 | −0.5 | 29.1 | 25.1 | 40 | |
| 4c | Vc/F = 142 l | 0, 1, 2 | 0.82 | −2.4 | −2.4 | 14.2 | 14.1 | 50 | |
| 4d* | Vp/F = 192 l | 0, 1, 2, 4 | 0.93 | −8.6 | −7.2 | 9.4 | 9.4 | 65 | |
| 4e* | Q/F = 43 l h−1 | 0, 1, 2, 4, 6 | 0.94 | −4.7 | −5.0 | 5.4 | 6.5 | 65 | |
| 4f* | ka = 2.18 h−1 | 0, 1, 4 | 0.83 | −4.6 | −4.6 | 13.0 | 14.1 | 55 | |
| 4g | IIV CL/F = 46% | 0, 4 | 0.82 | 9.8 | 8.0 | 14.8 | 15.8 | 50 | |
| IIV Vc = 33% | |||||||||
| IIV Vp = 31% | |||||||||
| RREa = 0.02 ng ml−1 | |||||||||
| RREp = 29% | |||||||||
| 5a | CL/F = 863/HAEM | 0 | 0.42 | 18.9 | 17.0 | 29.4 | 22.5 | 30 | [63] |
| 5b | Vc/F = 147 l | 0, 1 | 0.70 | −6.3 | −6.7 | 15.8 | 13.8 | 50 | |
| 5c | Vp/F = 500 l (fixed) | 0, 1, 2 | 0.76 | −2.4 | −2.7 | 13.5 | 13.5 | 50 | |
| 5d* | Q/F = 60 l h−1 | 0, 1, 2, 4 | 0.87 | −1.1 | −1.3 | 9.5 | 9.8 | 80 | |
| 5e* | ka = 6.5 h−1 | 0, 1, 2, 4, 6 | 0.91 | −0.2 | −0.2 | 8.1 | 7.7 | 80 | |
| 5f* | IIV CL/F = 30% | 0, 1, 4 | 0.86 | −0.7 | −0.3 | 10.1 | 8.1 | 65 | |
| 5g | IIV Vc/F = 26% | 0, 4 | 0.69 | 20.1 | 17.3 | 26.1 | 22.9 | 25 | |
| IIV Q/F = 63% | |||||||||
| IIV ka = 15% | |||||||||
| RREa = 1.5 ng ml−1 | |||||||||
| RREp = 10% | |||||||||
| ISBA | Population model not published | 0.25, 1, 3 | 0.92 | 5.3 | 6.6 | 8.0 | 9.6 | 85 | [54] |
| Web-based | Information on covariates (postoperative day, | ||||||||
| service* | diabetes status, assay used) is supplied by the user |
AST, aspartate transaminase; CV, coefficient of variation; CL, clearance, CL/F, apparent clearance; CYP3A5, cytochrome P450 3A5; F, bioavailability; ka, absorption rate constant; HAEM, haematocrit; IIV, interindividual variability; IOV, interoccasional variability; MAPE, median absolute percentage prediction error; MDR-1, multiple drug resistant protein 1; MPE, median prediction error; MPPE, median percentage prediction error; POD, postoperative day; Q, intracompartmental clearance; Q/F, apparent intracompartmental clearance; RMSE, root median squared prediction error; RREa, additive residual random error; RREp, proportional residual random error; V, volume of distribution; Vc, volume of distribution of central compartment; Vp, volume of distribution of peripheral compartment; V/F, apparent volume of distribution; IIV + IOV.
Acceptable bias and imprecision.
Best performance of all equations with regard to practicality and performance.
Model 2, using time points at 0, 1, 2 and 4 h post-dose (so-called model 2d) [60], showed slightly superior bias and imprecision estimates compared with all other models (sampling confined to 4 h post-dose, r2 = 0.93, MPPE 1.6% and MAPE 7.4%). Figure 2 displays the correlation between AUCp and AUCf for each study participant based on model 2d. Bias and imprecision are depicted in Figures 3 and 4, respectively. As shown in Figure 3, there was no consistent pattern to the direction of bias, with AUCp both over- and underestimating AUCf. Again, there was a suggestion of increasing bias at increasing values of AUCf. The dotted line in Figure 4 demonstrates that 15 of 20 AUCp values (75%) fell within 15% of AUCf.
Utilizing three concentration time points over the first 3 h post-dose (0.25, 1 and 3 h post-dose), the Web-based consultancy service also showed clinically acceptable predictive power (r2 = 0.92, MPPE 6.6% and MAPE 9.6%; Figures 3 and 4).
Very similar results were obtained when AUCp was compared with AUCf estimated using noncompartmental analysis (data not shown).
Discussion
This study evaluated the performance of published limited sampling methods for tacrolimus using an independent cohort of 20 adult kidney transplant recipients co-treated with mycophenolate mofetil and prednisolone. Poor correlation between C0 and AUCf was demonstrated, particularly in those further from transplantation. Alternatively, the majority of the multiple regression-derived LSSs showed acceptable predictive power, regardless of post-transplant duration. This included several LSSs based on time points 2 h or less post-dose. When population PK models were applied in a Bayesian forecasting program, at least one concentration time point greater than 2 h post-dose appeared necessary to predict tacrolimus AUC0–12 with acceptable levels of bias and imprecision.
This study provides a summary of all currently published limited sampling methods for tacrolimus in adult kidney transplant recipients. The majority of LSSs and population PK models developed to date have been based on small patient numbers, with 42% involving ≤20 and 75% involving ≤50 participants. Most (83%) were derived from tacrolimus concentrations measured by microparticle enzyme immunoassay (MEIA) rather than liquid chromatography–tandem mass spectrometry technology. Ethnicities of participants varied, as did the time post-transplant when sampling occurred. While most (75%) used prospectively collected data, only a minority (25%) were externally validated using a separate group. When reported for the LSS studies, bias and imprecision estimates were generally within clinically acceptable limits. For the PK models, proportional residual random error was as high as 29%.
Measurement of pre-dose (C0) tacrolimus concentrations is currently routine clinical practice. However, consistent with the majority of previous studies, we saw only moderate correlation between C0 and AUCf (r2 = 0.53), with the wide range of the 95% confidence interval suggesting suboptimal imprecision (depicted in Figure 2). Similarly, we found that limited sampling methods that relied solely on C0 values showed poor ability to predict AUCf (Tables 5 and 6). For unclear reasons, but also consistent with previous studies [5, 38], the relationship between C0 and AUCf was particularly weak during the late post-transplant phase (r2 = 0.64 in the early group vs. 0.21 in the late group). The correlation between C12 and AUCf was substantially higher than the correlation between C0 and AUCf (r2 = 0.83 vs. 0.53). Possibly, failure by patients to self-administer their evening dose of medication at the specified time on the night prior to blood sampling may have been responsible, an occurrence that would be even more likely outside of the trial setting.
The majority (69%) of multiple regression-derived LSSs showed acceptable predictive power for AUCf (bias and imprecision <15% for both parameters), regardless of duration post-transplantation. This was particularly the case for equation 28 (AUC0–12 = −5.385 + 3.337C0 + 0.96C1 + 1.402C2 + 6.01C4), which not only demonstrated the highest predictive power in our cohort as whole (r2 = 0.95, MPPE −1.1% and MAPE 2.8%), but also maintained superior predictive power when applied separately to early and late groups. This equation was derived in a study involving 15 Thai kidney transplant recipients, all of whom were <3 months post-transplant [42]. Tacrolimus was administered in the fasting state, and concentrations were measured with MEIA (known to overestimate tacrolimus concentrations by up to 30% due to interference by metabolites [23]). Given the markedly different study conditions and demographic of the derivation population compared with our population, the superior performance of this equation was surprising. However, by showing that applicability cannot always be predicted, it highlights the importance of validating any LSS prior to applying it to an alternative population. Of note, five of eight equations based on time points 2 h or less post-dose showed bias and imprecision estimates of <15%.
Alternatively, in our cohort, on first application of the population PK models developed to date in a Bayesian forecasting program, at least one concentration time point greater than 2 h post-dose appeared necessary to predict tacrolimus AUC0–12 with acceptable bias and imprecision. The predictive power of the models progressively increased with the inclusion of a greater number of concentration time points. This was expected, as it allows for greater reliance on measured data rather than on the predictive power of the underlying model. Model 2 [60], which was developed from the largest number of patients (n = 83; Table 2) and considered postoperative day and prednisolone dosage as covariate parameters, was marginally superior to all other models. However, even when concentration time points greater than 2 h post-dose were employed, its predictive ability was inferior to the performance of the highest performing multiple regression-derived LSS.
These data suggest some limitations with the population models developed to date. All models were derived from small, relatively homogeneous populations, lessening the likelihood of applicability to alternative groups. Additionally, all were associated with reasonably large residual random variability (greater than 20% in most cases). Furthermore, there was inconsistent consideration of the influence of relevant covariates on tacrolimus pharmacokinetics. Staatz et al. [5] included postoperative day and aspartate aminotransferase (AST), while Antignac et al. [60] included postoperative day and prednisolone dose. Neither considered the influence of genotype, despite its well-documented contribution to variable tacrolimus exposure. Alternatively, Press et al. [61], Musuamba et al. [62] and Benkali et al. [63] considered genotype [variably examining the influence of polymorphisms in CYP 3A4 and 3A5, P-glycoprotein (ABCB1/MDR1), and the pregnane X receptor (PXR) genes], but failed to consider the influence of days of therapy. The Web-based consultancy service requested provision of only postoperative day, assay used for tacrolimus measurement and diabetic status, while the study of Scholten et al. [38] considered only the impact of patient weight. Haematocrit and time of drug administration (morning vs. evening) were also found to be significant covariates in some studies [46, 47], but were not considered in others. Additional concerns with the studies of Staatz et al. [5] and Antignac et al. [60] included use of only C0 values to derive population PK parameters and the retrospective nature of data collection.
It is important to note in this study that LSS and Bayesian forecasting methods were tested in a controlled setting, where strict adherence to sampling times was possible. Compared with Bayesian analysis, multiple regression-derived LSSs are dependent on reasonably exact timing of concentration measurements. Accurate timing may be more difficult to achieve in ‘real-world’ practice, thereby potentially affecting the clinical utility of this method. As well as allowing greater flexibility of timing of samples, another advantage of Bayesian predictions is that the population models on which they are based can be continually improved as more patient-specific data become available. As the ability of population models to reflect drug pharmacokinetics improves, the ability of Bayesian estimators to predict AUC0–12 reliably improves simultaneously. Thus, despite the weaknesses apparent in the population models published to date, the abovementioned theoretical advantages of Bayesian analysis mean that, in the future, this methodology may prove to be the most desirable to derive limited sampling methods for use in clinical practice. In this regard, the clinically acceptable AUC estimates returned by the Web-based consultancy service are encouraging. Use of such a service removes the requirement for specialist software and user expertise, making this methodology more accessible to the clinician.
Another interesting finding from our study was higher dose-adjusted tacrolimus AUCf in those <3 months post-transplant compared with those in their first post-transplant week (Figure 1). A similar increase in dose-adjusted exposure over time was seen in the study of Scholten et al. [38]. In our cohort, this may be the consequence of the significantly lower serum albumin and haematocrit concentrations observed in the early post-transplant group (Table 2). Given that tacrolimus binds extensively to albumin and haemoglobin [68], a decrease in albumin and haematocrit should be associated with an increase in tacrolimus free fraction [5]. This in turn should lead to an increase in apparent oral total clearance and a decrease in total tacrolimus whole blood concentrations. Alternatively, given that CYP3A enzymes involved in tacrolimus metabolism are induced by corticosteroids [69], steroid tapering over time may be contributory. Another possible explanation may be poor gut motility, impairing absorption in the early post-transplant group. Regardless of mechanism, the increase in dose-adjusted AUCf over time is of particular interest when viewed in conjunction with our finding of poorer correlation of C0 with AUCf in the later post-transplant period. Together, these findings suggest a risk of misinterpretation of chronic drug exposure if C0 values alone are used for tacrolimus therapeutic drug monitoring.
The primary limitation of our study relates to the relatively small sample size, which exposes our data to potential ascertainment bias. However, our study population was similar in overall demographic to our larger transplant population, and our results are concordant with those of previous studies. A further limitation is that where concentration time points specified by models did not correspond with our sampling time points, linear extrapolation from measured concentrations was required (multiple regression-derived LSS equations 5 and 6). Given the potential error inherent in this process, our bias and imprecision estimates may not truly demonstrate the predictive power of these particular equations. Additionally, although we calculated AUC0–12 using compartmental and noncompartmental analyses, values obtained are still estimates, and thus may not be truly reflective.
Despite these limitations, our study clearly shows that limited sampling methods have superior ability to predict tacrolimus exposure compared with C0 monitoring. Given that collection of multiple samples is likely to incur significant costs and prove inconvenient and time consuming for patients and medical personnel, particularly in the outpatient setting, it is likely that these limited sampling methods may be of particular use on an infrequent basis in the later post-transplant period when the relationship between C0 and AUC0–12 appears to be especially poor, or in patients having a particularly complicated post-transplant course. Future research should be aimed at improving existing population models so as to improve the predictive power of Bayesian methodology. Additionally, it is important to note that this study addresses only those methods available for tacrolimus therapeutic drug monitoring. It provides no data showing clinical relevance of any methodology. Prospective randomized controlled trials are required to establish a target range for AUC0–12 and to confirm that the improved AUC predictions afforded by limited sampling methods translate into improved clinical outcomes.
Acknowledgments
K. Barraclough is currently supported by a National Health and Medical Research Council Medical/Dental Post-graduate Research Scholarship. C. Staatz is currently supported by a Lions Medical Research Fellowship. This research is supported by a National Health and Medical Research Council Project Grant, number 511109, and an Amgen-Transplantation Society of Australia and New Zealand Research Grant. The authors would like to thank the nursing staff of the Department of Nephrology at the Princess Alexandra Hospital for helping with sample collection.
Competing Interests
SC has been on Advisory Boards for Janssen-Cilag. DJ has received speakers' grants and honoraria from Janssen-Cilag. The other authors have no competing interests to declare.
REFERENCES
- 1.U.S. Department of Health and Human Services. U.S. organ procurement and transplantation network and the scientific registry of transplant recipients. Available at http://www.ustransplant.org/annual_reports/current/ (last accessed 18 August 2009.
- 2.Venkataramanan R, Swaminathan A, Prasad T, Jain A, Zuckerman S, Warty V, McMichael J, Lever J, Burckart G, Starzl T. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet. 1995;29:404–30. doi: 10.2165/00003088-199529060-00003. [DOI] [PubMed] [Google Scholar]
- 3.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43:623–53. doi: 10.2165/00003088-200443100-00001. [DOI] [PubMed] [Google Scholar]
- 4.Kuypers DR. Immunosuppressive drug monitoring – what to use in clinical practice today to improve renal graft outcome. Transpl Int. 2005;18:140–50. doi: 10.1111/j.1432-2277.2004.00041.x. [DOI] [PubMed] [Google Scholar]
- 5.Staatz CE, Willis C, Taylor PJ, Tett SE. Population pharmacokinetics of tacrolimus in adult kidney transplant recipients. Clin Pharmacol Ther. 2002;72:660–9. doi: 10.1067/mcp.2002.129304. [DOI] [PubMed] [Google Scholar]
- 6.Haufroid V, Wallemacq P, VanKerckhove V, Elens L, De Meyer M, Eddour DC, Malaise J, Lison D, Mourad M. CYP3A5 and ABCB1 polymorphisms and tacrolimus pharmacokinetics in renal transplant candidates: guidelines from an experimental study. Am J Transplant. 2006;6:2706–13. doi: 10.1111/j.1600-6143.2006.01518.x. [DOI] [PubMed] [Google Scholar]
- 7.van Schaik RH, van der Heiden IP, van den Anker JN, Lindemans J. CYP3A5 variant allele frequencies in Dutch Caucasians. Clin Chem. 2002;48:1668–71. [PubMed] [Google Scholar]
- 8.Thervet E, Anglicheau D, King B, Schlageter MH, Cassinat B, Beaune P, Legendre C, Daly AK. Impact of cytochrome p450 3A5 genetic polymorphism on tacrolimus doses and concentration-to-dose ratio in renal transplant recipients. Transplantation. 2003;76:1233–5. doi: 10.1097/01.TP.0000090753.99170.89. [DOI] [PubMed] [Google Scholar]
- 9.Macphee IA, Fredericks S, Mohamed M, Moreton M, Carter ND, Johnston A, Goldberg L, Holt DW. Tacrolimus pharmacogenetics: the CYP3A5*1 allele predicts low dose-normalized tacrolimus blood concentrations in whites and South Asians. Transplantation. 2005;79:499–502. doi: 10.1097/01.tp.0000151766.73249.12. [DOI] [PubMed] [Google Scholar]
- 10.Kamdem LK, Streit F, Zanger UM, Brockmoller J, Oellerich M, Armstrong VW, Wojnowski L. Contribution of CYP3A5 to the in vitro hepatic clearance of tacrolimus. Clin Chem. 2005;51:1374–81. doi: 10.1373/clinchem.2005.050047. [DOI] [PubMed] [Google Scholar]
- 11.Shimada T, Terada A, Yokogawa K, Kaneko H, Nomura M, Kaji K, Kaneko S, Kobayashi K, Miyamoto K. Lowered blood concentration of tacrolimus and its recovery with changes in expression of CYP3A and P-glycoprotein after high-dose steroid therapy. Transplantation. 2002;74:1419–24. doi: 10.1097/00007890-200211270-00014. [DOI] [PubMed] [Google Scholar]
- 12.Hesselink DA, Ngyuen H, Wabbijn M, Gregoor PJ, Steyerberg EW, van Riemsdijk IC, Weimar W, van Gelder T. Tacrolimus dose requirement in renal transplant recipients is significantly higher when used in combination with corticosteroids. Br J Clin Pharmacol. 2003;56:327–30. doi: 10.1046/j.0306-5251.2003.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anglicheau D, Flamant M, Schlageter MH, Martinez F, Cassinat B, Beaune P, Legendre C, Thervet E. Pharmacokinetic interaction between corticosteroids and tacrolimus after renal transplantation. Nephrol Dial Transplant. 2003;18:2409–14. doi: 10.1093/ndt/gfg381. [DOI] [PubMed] [Google Scholar]
- 14.Hesselink DA, van Schaik RH, van der Heiden IP, van der Werf M, Gregoor PJ, Lindemans J, Weimar W, van Gelder T. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther. 2003;74:245–54. doi: 10.1016/S0009-9236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 15.Jain AB, Venkataramanan R, Cadoff E, Fung JJ, Todo S, Krajack A, Starzl TE. Effect of hepatic dysfunction and T tube clamping on FK 506 pharmacokinetics and trough concentrations. Transplant Proc. 1990;22:57–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Jain AB, Abu-Elmagd K, Abdallah H, Warty V, Fung J, Todo S, Starzl TE, Venkataramanan R. Pharmacokinetics of FK506 in liver transplant recipients after continuous intravenous infusion. J Clin Pharmacol. 1993;33:606–11. doi: 10.1002/j.1552-4604.1993.tb04712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu-Elmagd K, Fung JJ, Alessiani M, Jain A, Venkataramanan R, Warty VS, Takaya S, Todo S, Shannon WD, Starzl TE. The effect of graft function on FK506 plasma levels, dosages, and renal function, with particular reference to the liver. Transplantation. 1991;52:71–7. doi: 10.1097/00007890-199107000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu-Elmagd KM, Fung JJ, Alessiani M, Jain A, Takaya S, Venkataramanan R, Warty VS, Shannon W, Todo S, Tzakis A, Van Thiel D, Sterzl TE. Strategy of FK 506 therapy in liver transplant patients: effect of graft function. Transplant Proc. 1991;23:2771–4. [PMC free article] [PubMed] [Google Scholar]
- 19.McMaster P, Mirza DF, Ismail T, Vennarecci G, Patapis P, Mayer AD. Therapeutic drug monitoring of tacrolimus in clinical transplantation. Ther Drug Monit. 1995;17:602–5. doi: 10.1097/00007691-199512000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Bekersky I, Dressler D, Alak A, Boswell GW, Mekki QA. Comparative tacrolimus pharmacokinetics: normal versus mildly hepatically impaired subjects. J Clin Pharmacol. 2001;41:628–35. doi: 10.1177/00912700122010519. [DOI] [PubMed] [Google Scholar]
- 21.Manzanares C, Moreno M, Castellanos F, Cubas A, Herrero JC, Morales-Ruiz E, Segura J, Andres A, Morales JM. Influence of hepatitis C virus infection on FK 506 blood levels in renal transplant patients. Transplant Proc. 1998;30:1264–5. doi: 10.1016/s0041-1345(98)00235-8. [DOI] [PubMed] [Google Scholar]
- 22.van den Berg AP, Haagsma EB, Gouw AS, Slooff MJ, Jansen PL. Recurrent HCV infection reduces the requirement for tacrolimus after liver transplantation. Transplant Proc. 2001;33:1467. doi: 10.1016/s0041-1345(00)02553-7. [DOI] [PubMed] [Google Scholar]
- 23.Gruber SA, Hewitt JM, Sorenson AL, Barber DL, Bowers L, Rynders G, Arrazola L, Matas AJ, Rosenberg ME, Canafax DM. Pharmacokinetics of FK506 after intravenous and oral administration in patients awaiting renal transplantation. J Clin Pharmacol. 1994;34:859–64. doi: 10.1002/j.1552-4604.1994.tb02052.x. [DOI] [PubMed] [Google Scholar]
- 24.Undre NA, Schafer A. Factors affecting the pharmacokinetics of tacrolimus in the first year after renal transplantation. European Tacrolimus Multicentre Renal Study Group. Transplant Proc. 1998;30:1261–3. doi: 10.1016/s0041-1345(98)00234-6. [DOI] [PubMed] [Google Scholar]
- 25.Christiaans M, van Duijnhoven E, Beysens T, Undre N, Schafer A, van Hooff J. Effect of breakfast on the oral bioavailability of tacrolimus and changes in pharmacokinetics at different times posttransplant in renal transplant recipients. Transplant Proc. 1998;30:1271–3. doi: 10.1016/s0041-1345(98)00238-3. [DOI] [PubMed] [Google Scholar]
- 26.McDiarmid SV, Colonna JO, 2nd, Shaked A, Vargas J, Ament ME, Busuttil RW. Differences in oral FK506 dose requirements between adult and pediatric liver transplant patients. Transplantation. 1993;55:1328–32. doi: 10.1097/00007890-199306000-00022. [DOI] [PubMed] [Google Scholar]
- 27.Uemoto S, Tanaka K, Honda K, Tokunaga Y, Sano K, Katoh H, Yamamoto E, Takada Y, Ozawa K. Experience with FK506 in living-related liver transplantation. Transplantation. 1993;55:288–92. doi: 10.1097/00007890-199302000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Neylan JF. Racial differences in renal transplantation after immunosuppression with tacrolimus versus cyclosporine. FK506 Kidney Transplant Study Group. Transplantation. 1998;65:515–23. doi: 10.1097/00007890-199802270-00011. [DOI] [PubMed] [Google Scholar]
- 29.Neylan JF. Effect of race and immunosuppression in renal transplantation: three-year survival results from a US multicenter, randomized trial. FK506 Kidney Transplant Study Group. Transplant Proc. 1998;30:1355–8. doi: 10.1016/s0041-1345(98)00274-7. [DOI] [PubMed] [Google Scholar]
- 30.Felipe CR, Garcia C, Moreira S, Olsen N, Silva HT, Pestana OM. Choosing the right dose of new immunossuppressive drugs for new populations: importance of pharmacokinetic studies. Transplant Proc. 2001;33:1095–6. doi: 10.1016/s0041-1345(00)02432-5. [DOI] [PubMed] [Google Scholar]
- 31.Min DI, Chen HY, Fabrega A, Ukah FO, Wu YM, Corwin C, Ashton MK, Martin M. Circadian variation of tacrolimus disposition in liver allograft recipients. Transplantation. 1996;62:1190–2. doi: 10.1097/00007890-199610270-00031. [DOI] [PubMed] [Google Scholar]
- 32.Bekersky I, Dressler D, Mekki QA. Effect of low- and high-fat meals on tacrolimus absorption following 5 mg single oral doses to healthy human subjects. J Clin Pharmacol. 2001;41:176–82. doi: 10.1177/00912700122009999. [DOI] [PubMed] [Google Scholar]
- 33.Bekersky I, Dressler D, Mekki Q. Effect of time of meal consumption on bioavailability of a single oral 5 mg tacrolimus dose. J Clin Pharmacol. 2001;41:289–97. doi: 10.1177/00912700122010104. [DOI] [PubMed] [Google Scholar]
- 34.Eades SK, Boineau FG, Christensen ML. Increased tacrolimus levels in a pediatric renal transplant patient attributed to chronic diarrhea. Pediatr Transplant. 2000;4:63–6. doi: 10.1034/j.1399-3046.2000.00086.x. [DOI] [PubMed] [Google Scholar]
- 35.Matsui A, Arakawa Y, Momoya T, Sasaki N, Kawasaki S, Tanaka K. Apparently increased trough levels of tacrolimus caused by acute infantile diarrhea in two infants with biliary atresia after liver transplantation. Acta Paediatr Jpn. 1996;38:699–701. doi: 10.1111/j.1442-200x.1996.tb03736.x. [DOI] [PubMed] [Google Scholar]
- 36.van Duijnhoven E, Christiaans M, Schafer A, Undre N, van Hooff J. Tacrolimus dosing requirements in diabetic and nondiabetic patients calculated from pretransplantation data. Transplant Proc. 1998;30:1266–7. doi: 10.1016/s0041-1345(98)00236-x. [DOI] [PubMed] [Google Scholar]
- 37.Walker S, Habib S, Rose M, Yacoub M, Banner N. Clinical use and bioavailability of tacrolimus in heart-lung and double lung transplant recipients with cystic fibrosis. Transplant Proc. 1998;30:1519–20. doi: 10.1016/s0041-1345(98)00341-8. [DOI] [PubMed] [Google Scholar]
- 38.Wallemacq P, Armstrong VW, Brunet M, Haufroid V, Holt DW, Johnston A, Kuypers D, Le Meur Y, Marquet P, Oellerich M, Thervet E, Toenshoff B, Undre N, Weber LT, Westley IS, Mourad M. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit. 2009;31:139–52. doi: 10.1097/FTD.0b013e318198d092. [DOI] [PubMed] [Google Scholar]
- 39.Tada H, Satoh S, Iinuma M, Shimoda N, Murakami M, Hayase Y, Kato T, Suzuki T. Chronopharmacokinetics of tacrolimus in kidney transplant recipients: occurrence of acute rejection. J Clin Pharmacol. 2003;43:859–65. doi: 10.1177/0091270003254797. [DOI] [PubMed] [Google Scholar]
- 40.Braun F, Schutz E, Peters B, Talaulicar R, Grupp C, Undre N, Schafer A, Armstrong VW, Oellerich M, Ringe B. Pharmacokinetics of tacrolimus primary immunosuppression in kidney transplant recipients. Transplant Proc. 2001;33:2127–8. doi: 10.1016/s0041-1345(01)01970-4. [DOI] [PubMed] [Google Scholar]
- 41.Kimikawa M, Kamoya K, Toma H, Teraoka S. Effective oral administration of tacrolimus in renal transplant recipients. Clin Transplant. 2001;15:324–9. doi: 10.1034/j.1399-0012.2001.150504.x. [DOI] [PubMed] [Google Scholar]
- 42.Pisitkun T, Eiam-Ong S, Chusil S, Praditpornsilpa K, Pansin P, Tungsanga K. The roles of C4 and AUC0-4 in monitoring of tacrolimus in stable kidney transplant patients. Transplant Proc. 2002;34:3173–5. doi: 10.1016/s0041-1345(02)03684-9. [DOI] [PubMed] [Google Scholar]
- 43.Bottiger Y, Undre NA, Sawe J, Stevenson PJ, Ericzon BG. Effect of bile flow on the absorption of tacrolimus in liver allograft transplantation. Transplant Proc. 2002;34:1544–5. doi: 10.1016/s0041-1345(02)03013-0. [DOI] [PubMed] [Google Scholar]
- 44.Wong KM, Shek CC, Chau KF, Li CS. Abbreviated tacrolimus area-under-the-curve monitoring for renal transplant recipients. Am J Kidney Dis. 2000;35:660–6. doi: 10.1016/s0272-6386(00)70013-8. [DOI] [PubMed] [Google Scholar]
- 45.Stolk LM, Van Duijnhoven EM, Christiaans MH, van Hooff JP. Trough levels of tacrolimus. Ther Drug Monit. 2002;24:573. doi: 10.1097/00007691-200208000-00019. author reply 73–4. [DOI] [PubMed] [Google Scholar]
- 46.Mathew BS, Fleming DH, Jeyaseelan V, Chandy SJ, Annapandian VM, Subbanna PK, John GT. A limited sampling strategy for tacrolimus in renal transplant patients. Br J Clin Pharmacol. 2008;66:467–72. doi: 10.1111/j.1365-2125.2008.03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jusko WJ, Piekoszewski W, Klintmalm GB, Shaefer MS, Hebert MF, Piergies AA, Lee CC, Schechter P, Mekki QA. Pharmacokinetics of tacrolimus in liver transplant patients. Clin Pharmacol Ther. 1995;57:281–90. doi: 10.1016/0009-9236(95)90153-1. [DOI] [PubMed] [Google Scholar]
- 48.Jorgensen K, Povlsen J, Madsen S, Madsen M, Hansen H, Pedersen A, Heinsvig EM, Poulsen J. C2 (2-h) levels are not superior to trough levels as estimates of the area under the curve in tacrolimus-treated renal-transplant patients. Nephrol Dial Transplant. 2002;17:1487–90. doi: 10.1093/ndt/17.8.1487. [DOI] [PubMed] [Google Scholar]
- 49.Cantarovich M, Fridell J, Barkun J, Metrakos P, Besner JG, Deschenes M, Alpert E, Aalamian Z, Tchervenkov JI. Optimal time points for the prediction of the area-under-the-curve in liver transplant patients receiving tacrolimus. Transplant Proc. 1998;30:1460–1. doi: 10.1016/s0041-1345(98)00315-7. [DOI] [PubMed] [Google Scholar]
- 50.Armendariz Y, Pou L, Cantarell C, Lopez R, Perello M, Capdevila L. Evaluation of a limited sampling strategy to estimate area under the curve of tacrolimus in adult renal transplant patients. Ther Drug Monit. 2005;27:431–4. doi: 10.1097/01.ftd.0000158080.61201.65. [DOI] [PubMed] [Google Scholar]
- 51.Laskow DA, Vincenti F, Neylan JF, Mendez R, Matas AJ. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: a report of the United States Multicenter FK506 Kidney Transplant Group. Transplantation. 1996;62:900–5. doi: 10.1097/00007890-199610150-00005. [DOI] [PubMed] [Google Scholar]
- 52.Ting LS, Villeneuve E, Ensom MH. Beyond cyclosporine: a systematic review of limited sampling strategies for other immunosuppressants. Ther Drug Monit. 2006;28:419–30. doi: 10.1097/01.ftd.0000211810.19935.44. [DOI] [PubMed] [Google Scholar]
- 53.Op den Buijsch RA, van de Plas A, Stolk LM, Christiaans MH, van Hooff JP, Undre NA, van Dieijen-Visser MP, Bekers O. Evaluation of limited sampling strategies for tacrolimus. Eur J Clin Pharmacol. 2007;63:1039–44. doi: 10.1007/s00228-007-0354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scholten EM, Cremers SC, Schoemaker RC, Rowshani AT, van Kan EJ, den Hartigh J, Paul LC, de Fijter JW. AUC-guided dosing of tacrolimus prevents progressive systemic overexposure in renal transplant recipients. Kidney Int. 2005;67:2440–7. doi: 10.1111/j.1523-1755.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- 55.Taylor PJ, Brown SR, Cooper DP, Lynch SV. PI P. A high-throughput HPLC-MS/MS method for tacrolimus measurement (abstract) Ther Drug Monit. 2005;27:256. [Google Scholar]
- 56.Keevil BG, McCann SJ, Cooper DP, Morris MR. Evaluation of a rapid micro-scale assay for tacrolimus by liquid chromatography-tandem mass spectrometry. Ann Clin Biochem. 2002;39:487–92. doi: 10.1258/000456302320314502. [DOI] [PubMed] [Google Scholar]
- 57.Miura M, Satoh S, Niioka T, Kagaya H, Saito M, Hayakari M, Habuchi T, Suzuki T. Limited sampling strategy for simultaneous estimation of the area under the concentration-time curve of tacrolimus and mycophenolic acid in adult renal transplant recipients. Ther Drug Monit. 2008;30:52–9. doi: 10.1097/FTD.0b013e31815f5416. [DOI] [PubMed] [Google Scholar]
- 58.Kuypers DR, Claes K, Evenepoel P, Maes B, Coosemans W, Pirenne J, Vanrenterghem Y. Time-related clinical determinants of long-term tacrolimus pharmacokinetics in combination therapy with mycophenolic acid and corticosteroids: a prospective study in one hundred de novo renal transplant recipients. Clin Pharmacokinet. 2004;43:741–62. doi: 10.2165/00003088-200443110-00005. [DOI] [PubMed] [Google Scholar]
- 59.Staatz CE, Tett SE. Comparison of two population pharmacokinetic programs, NONMEM and P-PHARM, for tacrolimus. Eur J Clin Pharmacol. 2002;58:597–605. doi: 10.1007/s00228-002-0517-7. [DOI] [PubMed] [Google Scholar]
- 60.Antignac M, Barrou B, Farinotti R, Lechat P, Urien S. Population pharmacokinetics and bioavailability of tacrolimus in kidney transplant patients. Br J Clin Pharmacol. 2007;64:750–7. doi: 10.1111/j.1365-2125.2007.02895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Press RR, Ploeger BA, den Hartigh J, van der Straaten T, van Pelt J, Danhof M, de Fijter JW, Guchelaar HJ. Explaining variability in tacrolimus pharmacokinetics to optimize early exposure in adult kidney transplant recipients. Ther Drug Monit. 2009;31:187–97. doi: 10.1097/FTD.0b013e31819c3d6d. [DOI] [PubMed] [Google Scholar]
- 62.Musuamba FT, Mourad M, Haufroid V, Delattre IK, Verbeeck RK, Wallemacq P. Time of drug administration, CYP3A5 and ABCB1 genotypes, and analytical method influence tacrolimus pharmacokinetics: a population pharmacokinetic study. Ther Drug Monit. 2009;31:734–42. doi: 10.1097/FTD.0b013e3181bf8623. [DOI] [PubMed] [Google Scholar]
- 63.Benkali K, Premaud A, Picard N, Rerolle JP, Toupance O, Hoizey G, Turcant A, Villemain F, Le Meur Y, Marquet P, Rousseau A. Tacrolimus population pharmacokinetic-pharmacogenetic analysis and Bayesian estimation in renal transplant recipients. Clin Pharmacokinet. 2009;48:805–16. doi: 10.2165/11318080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 64.Limoges University Hospital Laboratory of Pharmacology. Target Concentration Intervention Software (TCIworks) Available at http://www.tciworks.info/ (last accessed 20 May 2010)
- 65.Limoges University Hospital Laboratory of Pharmacology. ImmunoSuppressants Bayesian dose Adjustment (ISBA) Available at https://pharmaco.chu-limoges.fr/abis.htm (last accessed 20 May 2010)
- 66.Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9:503–12. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 67.Coresh J, Stevens LA. Kidney function estimating equations: where do we stand? Curr Opin Nephrol Hypertens. 2006;15:276–84. doi: 10.1097/01.mnh.0000222695.84464.61. [DOI] [PubMed] [Google Scholar]
- 68.Beysens AJ, Wijnen RM, Beuman GH, van der Heyden J, Kootstra G, van As H. FK 506: monitoring in plasma or in whole blood? Transplant Proc. 1991;23:2745–7. [PubMed] [Google Scholar]
- 69.Undre NA. Pharmacokinetics of tacrolimus-based combination therapies. Nephrol Dial Transplant. 2003;18(Suppl. 1):i12–5. doi: 10.1093/ndt/gfg1029. [DOI] [PubMed] [Google Scholar]


