Abstract
AIMS
Frailty, a syndrome of decreased physiological reserve that is prevalent in old age, impacts on clinical pharmacology. The aims of the study were to (1) determine whether frailty affects the pharmacokinetics of gentamicin and (2) assess the accuracy of different estimates of body size and renal clearance as estimates of gentamicin pharmacokinetics in older inpatients.
METHODS
This was an observational study of gentamicin pharmacokinetics in a cohort of Australian hospital inpatients aged ≥65 years, who were administered prophylactic intravenous gentamicin.
RESULTS
Of the 31 participants, 14 were frail and 17 non frail on the Reported Edmonton Frail Scale. The mean volume of distribution of gentamicin was 14.8 ± 1.4 l in frail participants and 15.3 ± 2.2 l in non frail (NS). Volume of distribution correlated best with lean bodyweight. Gentamicin clearance was significantly lower in frail participants (46.6 ± 10.7 ml min−1) than in non frail (58.2 ± 12.4 ml min−1, P= 0.01). The Cockcroft Gault estimate of creatinine clearance calculated using ideal bodyweight gave the best estimate of gentamicin clearance (mean error – 0.15 ml min−1, 95% CI −2.67, 2.39). The Cockcroft Gault creatinine clearance calculated using actual bodyweight and the estimated glomerular filtration rate from the modified diet in renal disease equation overestimated gentamicin clearance, with mean errors of −10.15 ml min−1 (95%CI −13.60, −6.71) and −18.86 ml min−1 (95% CI −22.45, −15.27), respectively. The Cockcroft Gault creatinine clearance calculated using lean bodyweight underestimated gentamicin clearance (mean error 6.54 ml min−1, 95% CI 4.18, 8.90).
CONCLUSIONS
Frail older people have significantly lower gentamicin clearance than non frail. The best estimate of gentamicin clearance is obtained from the Cockcroft Gault creatinine clearance calculated using ideal bodyweight.
Keywords: age, frailty, gentamicin, pharmacokinetics
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Gentamicin pharmacokinetics show wide inter-individual variability across all age groups and impaired gentamicin clearance is associated with impaired creatinine clearance in older people.
Changes in body composition and renal function with old age and frailty are likely to affect the pharmacokinetics of gentamicin.
There is current debate on whether the Modification of Diet in Renal Disease (MDRD) equation estimate of glomerular filtration rate (GFR) should replace the Cockcroft Gault equation estimate of creatinine clearance for calculation of doses of renally excreted drugs.
WHAT THIS STUDY ADDS
The volume of distribution of gentamicin is not significantly lower in frail than in non frail older people.
The correlation between volume of distribution of gentamicin and actual bodyweight is poor in frail and moderate in non frail older people.
Gentamicin clearance is significantly lower in frail than in non frail older people.
The Cockcroft Gault calculation of creatinine clearance, calculated using ideal bodyweight, gave the best estimate of gentamicin clearance in this population of frail and non frail older people.
The MDRD estimate of GFR and Cockcroft Gault estimate of creatinine clearance, calculated using actual bodyweight, overestimate gentamicin clearance in frail and non frail older people.
Introduction
Gentamicin is a broad spectrum aminoglycoside bacteriocidal antibiotic that is used frequently in older patients to prevent and treat infections caused by gram negative bacteria [1, 2]. The efficacy of gentamicin requires an adequate peak concentration, and the toxicity relates to the area under the concentration–time curve. Optimal dosing of a narrow therapeutic index drug such as gentamicin is critical in old age and frailty to ensure benefit with minimal adverse effects. Frailty is characterized by high susceptibility to disease, impending decline in physical function and reduced functional reserve [3, 4]. Recently, several validated measures of frailty have been developed that can facilitate research into the clinical pharmacology of frailty [4–6].
Gentamicin is a hydrophilic drug that is distributed to body water and excreted unchanged by the kidneys, predominantly by glomerular filtration. Factors that may affect the pharmacokinetics of gentamicin in old age include reduced lean bodyweight and declining renal function, as well as drug interactions from the increasing prevalence of polypharmacy. These factors are likely to be more prevalent in frail older people [7]. Previous studies of gentamicin pharmacokinetics demonstrated wide inter-individual variability in volume of distribution across all age groups, and impaired gentamicin clearance which was associated with impaired creatinine clearance in older people [2].
An equation for estimating lean bodyweight was recently validated in frail and non frail community dwelling older men [8], and hospitalized older women [9], which may provide a good estimate of volume of distribution of hydrophilic drugs like gentamicin. There is currently debate on whether the Modification of Diet in Renal Disease (MDRD) equation estimate of glomerular filtration rate [10] should replace the Cockcroft Gault equation estimate of creatinine clearance [11] for calculation of doses of renally excreted drugs [10, 12], with evidence that MDRD tends to systematically overestimate renal function in geriatric inpatients [13–16]. Stratification of older patients by frailty may allow better prediction of gentamicin pharmacokinetics and identification of clinical measures to estimate these parameters.
The primary aim of this study was to evaluate the pharmacokinetics (volume of distribution and clearance) of gentamicin in frail and non frail older hospital patients in Sydney, Australia. A secondary aim was to assess the accuracy of different estimates of body size and renal clearance as estimates of gentamicin volume of distribution and clearance in this population.
Methods
A prospective, observational study of gentamicin pharmacokinetics was performed in frail and non frail urology inpatients aged ≥65 years receiving a single dose of prophylactic gentamicin. The survey was conducted from February 2008 to September 2009 across three teaching hospitals in Sydney, Australia: The Royal North Shore Hospital (RNSH), Hornsby Hospital and Ryde Hospital. Patients unable to speak English and those with severe cognitive or hearing impairments were excluded. The study was approved by the Human Research Ethics Committee of the Northern Sydney and Central Coast Health Service, Sydney, Australia. Signed informed consent was obtained from participants, or if they were not competent, from the person responsible.
Patients were screened for frailty at the baseline interview using the Reported Edmonton Frailty Scale [6]. The Reported Edmonton Frail Scale is based on performance in 10 different domains including cognitive impairment, balance and mobility, mood, functional independence, medication use, social support, nutrition, health attitudes, continence, burden of medical illness and quality of life. Reports are obtained from the patient, carer and/or medical notes. All patients who received a score of 8 or above were classified as frail and those with scores less than 8 were non frail. Baseline assessments included demographics, use of medications and self reported physician diagnosis of renal disease risk factors. Risk factors for renal disease were derived from the National Kidney Foundation disease outcomes initiative clinical guidelines [17] and from a validated survey to detect occult kidney disease [18]. The Charlson Co-Morbidity Scale was used to screen for co-morbidities [19]. The patient's hospital and medical records were reviewed after the interview for additional or unreported data.
Ideal body weight (IBW) was calculated for each patient based on their gender, height and estimated body frame using the Devine formula [20].
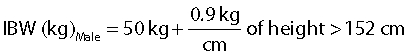 |
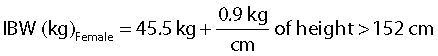 |
Lean bodyweight (LBW) was estimated using the gender specific semi-mechanistic LBW equations [21]:
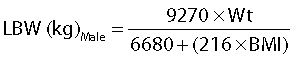 |
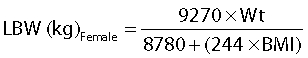 |
where Wt is weight (kg) and BMI is body mass index (kg m−2).
During the urology surgery, the dose of gentamicin administered, time and duration of infusion were recorded for all participants. A venous blood sample was taken 30–60 min after the end of the infusion for measurement of serum creatinine and gentamicin concentration. Additional samples were collected after 2 h and where possible, 4–6 h, for gentamicin concentrations. Blood samples were delivered to hospital pathologists within the Pacific Laboratory Medicine Services for analysis. For each patient, concentrations of serum creatinine were measured on the Roche Modular Autoanalyzer. Gentamicin concenatrations were measured using a Siemens Dimension RxL analyzer with Siemens reagents and calibrators. The calibrators are traceable to United States Pharmacopeia standards. At mean concentrations of 2.5 mg l−1, 5.3 mg l−1 and 7.1 mg l−1 the coefficients of variation were 5.6%, 2.8% and 2.3%, respectively. The lower limit of quantitation was 0.5 mg l−1.
Estimation of gentamicin pharmacokinetics and renal function
Gentamicin volume of distribution and clearance were determined using the Target Concentration Intervention (http://www.tciworks.info/) software, which is a population pharmacokinetics software package that uses an algorithm for dose individualization for patients [22]. The precision and validity of TCIWorks at estimating volume of distribution and clearance have been evaluated against the gold standard population pharmacokinetic modelling program NONMEM. In this evaluation, TCIworks provided similar accuracy and precision on all test examples to NONMEM (Personal communication, Carl Kirkpatrick TCIWorks development team).
Creatinine clearance was estimated from the Cockroft Gault equation. For each participant, creatinine clearance was calculated using the participant's actual bodyweight as well as their ideal bodyweight and lean bodyweight. As the lean bodyweight equations are gender specific, the 0.85 correction for females was only applied to the Cockcroft Gault equation when actual and ideal bodyweights were used [23]. Each participant's eGFR, determined by the MDRD equation, was also derived from results of biochemistry reports and normalized to the participant's body surface area using the Dubois-Dubois formula [24].
Data analysis
In this study four different methods were used to estimate renal function: (i) the Cockcroft Gault equation for creatinine clearance using actual bodyweight, (ii) the Cockcroft Gault equation for creatinine clearance using ideal bodyweight, (iii) the Cockcroft Gault equation for creatinine clearance using lean bodyweight and (iv) the MDRD equation for estimated glomerular filtration rate. Each equation generated a different estimate of renal function, which was then compared with gentamicin clearance. The predictive performance of the equations to estimate gentamicin clearance was determined graphically using Bland-Altman plots [25] generated with Statistical Package for the Social Sciences (SPSS) Graduate Pack version 17.0 (Chicago, IL, USA) and quantitatively using precision and bias statistics [26] using Microsoft Office Excel 2007.
Bland-Altman plots demonstrated the agreement between each equation and gentamicin clearance. The limits of agreement were defined as the mean difference between gentamicin clearance and each equation ± two standard deviations (SD) of the difference [25]. Precision and bias statistics were also calculated to determine the predictive performances of the equations: Cockcroft Gault (using actual, ideal and lean body weight) and MDRD in relation to gentamicin clearance [26]. The predictive performance of the equations was assessed in terms of the mean error (ME), a measure of bias, which should include zero in a nonbiased model, and root mean square error (RMSE), a measure of precision, with 95% confidence intervals (CI) [26].
The Statistical Package for the Social Sciences (SPSS) Graduate Pack version 17.0 (Chicago, IL, USA) was used for data entry and generation of descriptive statistics. Cross-tabulations were constructed to summarize nominal variables across different groups and Chi squared tests assessed differences in their distribution. Frail and non frail participant characteristics and pharmacokinetics were compared using Student's t-test in which differences were considered statistically significant when P < 0.05. The association of gentamicin volume of distribution with each measure of bodyweight was measured using Pearson's correlation.
Sample size was estimated using SAS Power and Sample Size 3.1 (SAS Institute Inc., Cary, NC, USA) at 16 participants in each group, based on a difference in gentamicin clearance (effect size) of 10 ml min−1 between frail and non frail participants, and a statistical power of 80% at the 0.05 alpha level, as this was likely to be a clinically significant difference for dosing guideline brackets. Variability was estimated from previous studies [2].
Results
Of 127 patients who were screened, 57 met eligibility criteria and 31 participated in the study. Most of those who were ineligible were under the age of 65 years. The participants had a mean age (±SD) of 77.0 (±7.1) years and were predominantly male (80.6%). The frail participants (n= 14) were significantly older and shorter than the non frail participants (n= 17) and used more medications. The majority of participants underwent cystoscopies, with one non frail patient undergoing an open prostatectomy. Estimated creatinine clearance calculated using ideal bodyweight and lean bodyweight were both significantly lower in the frail than in the non frail participants. Table 1 shows the baseline characteristics of participants stratified by frailty.
Table 1.
Baseline characteristics of frail and non frail participants
| Parameter | Frail (n= 14) | Non frail (n= 17) | P value |
|---|---|---|---|
| Age (years) | 80.4 ± 6.4 | 74.2 ± 6.5 | <0.05 |
| Gender (male/female) | 12/2 | 14/3 | NS |
| Charlson Co-morbidity Score | 3.1 ± 1.9 | 2.4 ± 1.9 | NS |
| Number of medications | 4.6 ± 2.0 | 2.1 ± 1.8 | 0.001 |
| Number of renal disease risk factors | 1.35 ± 1.06 | 1.57 ± 0.94 | NS |
| Height (cm) | 165.1 ± 8.6 | 171.2 ± 7.4 | <0.05 |
| Actual bodyweight (kg) | 77.1 ± 11.7 | 76.8 ± 10.8 | NS |
| Ideal bodyweight (kg) | 62.0 ± 6.5 | 66.4 ± 7.9 | NS |
| Lean bodyweight (kg) | 54.6 ± 8.8 | 55.8 ± 8.4 | NS |
| SCr (mg dl−1) | 1.1 ± 0.1 | 1.0 ± 0.3 | NS |
| CGABW (ml min−1) | 56.7 ± 16.0 | 68.4 ± 15.9 | NS |
| CGIBW (ml min−1) | 45.7 ± 10.9 | 59.4 ± 13.8 | <0.01 |
| CGLBW (ml min−1) | 40.8 ± 10.7 | 51.0 ± 11.5 | <0.05 |
| eGFR (ml min−1) | 65.8 ± 14.4 | 76.8 ± 17.9 | NS |
All values except gender are quoted in mean ± standard deviation (SD); CGABW, Cockcroft Gault estimate of creatinine clearance calculated using actual bodyweight; CGIBW, Cockcroft Gault estimate of creatinine clearance calculated using ideal bodyweight; CGLBW, Cockcroft Gault estimate of creatinine clearance calculated using lean bodyweight; eGFR, estimated glomerular filtration rate calculated using the Modified Diet in Renal Disease equation normalized for body surface area (ml min−1); NS, not significant; SCr, serum creatinine.
The pharmacokinetic parameters of gentamicin in the frail and non frail participants are summarized in Table 2. The volume of gentamicin distribution in frail patients was not significantly lower than in the non frail participants. Gentamicin clearance was significantly lower in the frail than the non frail (P= 0.01). The volume of distribution correlated best with lean bodyweight, and least well with actual bodyweight, particularly in the frail participants (Table 3).
Table 2.
Pharmacokinetic parameters of gentamicin in frail and non frail participants
| Parameter | Frail | Non frail | P value |
|---|---|---|---|
| Vd (l) | 14.8 ± 1.4 | 15.2 ± 2.2 | 0.56 (NS) |
| CL (ml min−1) | 46.6 ± 10.7 | 58.2 ± 12.4 | 0.01 |
All values are quoted as mean ± SD; NS, not significant; Vd, volume of distribution; CL, clearance.
Table 3.
Correlation of volume of distribution (Vd) with different measures of bodyweight in frail and non frail participants
| Measure of weight that Vd (l) is correlated with | r2 for Frail | r2 for Non frail |
|---|---|---|
| Actual bodyweight (kg) | 0.39 | 0.50 |
| Ideal bodyweight (kg) | 0.66 | 0.63 |
| Lean bodyweight (kg) | 0.72 | 0.71 |
Figure 1 represents the Bland–Altman analysis of agreement plots for each estimate of renal function with gentamicin clearance, for all participants stratified by frailty status. Gentamicin clearance was overestimated by both the equation calculated using actual bodyweight (Figure 1A) and ideal bodyweight (Figure 1B), as well as by the MDRD equation (Figure 1D) with mean differences (limits of agreement) of −10.15 ml min−1 (−28.95, 8.65 ml min−1), −0.15 ml min−1 (−13.99, 13.69 ml min−1) and −18.86 ml min−1 (−38.44, 0.72 ml min−1), respectively. The Cockcroft Gault equation calculated using lean bodyweight (Figure 1C) underestimated gentamicin clearance with mean differences (limits of agreement) of 6.54 ml min−1 (−6.32, 19.40 ml min−1).
Figure 1.
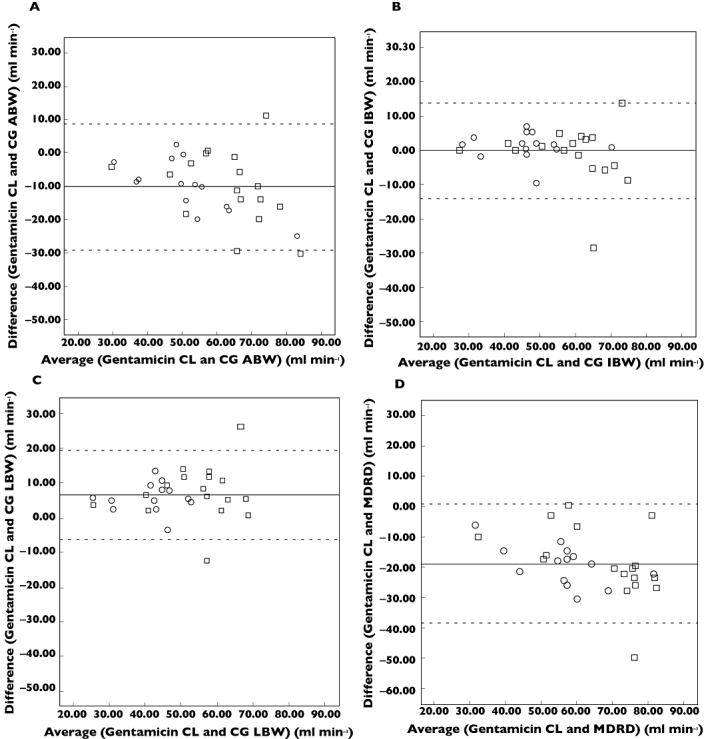
Bland–Altman plots with the mean difference (solid line) and limits of agreement (dashed line) comparing different estimates of renal function with optimized gentamicin clearance. Creatinine clearance determined by the Cockcroft Gault equation using actual bodyweight (1A), ideal bodyweight (1B), lean bodyweight (1C) and estimated glomerular filtration rate determined by the Modified Diet in Renal Disease equation (1D) are plotted against gentamicin clearance. Participants were stratified by frailty, ○ frail, □ non frail. CG ABW, Cockcroft Gault estimate of creatinine clearance calculated using actual bodyweight (ml min−1); CG IBW, Cockcroft Gault estimate of creatinine clearance calculated using ideal bodyweight (ml min−1); CG LBW, Cockcroft Gault estimate of creatinine Clearance calculated using lean bodyweight (ml min−1); Gentamicin CL, Gentamicin clearance (ml min−1); MDRD, Modified Diet in Renal Disease equation estimate of glomerular filtration rate normalized for body surface area (ml min−1)
Table 4 represents the predictive performance of the four estimates of renal function compared with gentamicin clearance, stratified by frailty. The magnitude of bias was consistently smaller in both the frail and non frail patients for creatinine clearance using ideal bodyweight compared with the other three estimates. This indicates that the use of ideal bodyweight in the Cockcroft Gault creatinine clearance calculation gave relatively better estimates of actual renal function with respect to gentamicin clearance. The creatinine clearance estimate using ideal bodyweight gave a smaller RMSE value in both frail and non frail groups, indicating better precision in estimating actual gentamicin clearance. Relative to the other methods, the MDRD estimate gave the largest magnitude of bias and had the poorest precision in both frail and non frail participants.
Table 4.
Precision and bias calculated according to the methods of Sheiner & Beal comparing different estimates of renal function with gentamicin clearance in frail, non frail and all participants
| Renal function estimate | ME (95% CI) | %ME (95% CI) | RMSE (95% CI) | r2 |
|---|---|---|---|---|
| CGABW | ||||
| Frail | −10.12 (−14.62, −5.63) | −21.25 (−29.36, −13.14) | 12.60 (−90.91, 116.11) | 0.82 |
| Non frail | −10.18 (−15.73, −4.63) | −18.40 (−27.81, −8.98) | 14.60 (−131.24, 160.42) | 0.54 |
| Combined | −10.15 (−13.60, −6.71) | −19.69 (−25.67, −13.70) | 13.73 (−74.30, 101.77) | 0.69 |
| CGIBW | ||||
| Frail | 1.12 (−1.17, 3.42) | 2.26 (−2.84, 7.36) | 4.00 (−10.57, 18.55 | 0.87 |
| Non frail | −1.20 (−5.63, 3.24) | −2.52 (−10.46, 5.42) | 8.45 (−91.29, 108.20) | 0.62 |
| Combined | −0.15 (−2.67, 2.39) | −0.36 (−5.10, 4.38) | 6.81 (−46.52, 60.14) | 0.76 |
| CGLBW | ||||
| Frail | 5.75 (3.38, 8.11) | 12.54 (7.49, 17.60) | 6.97 (−22.10, 36.04) | 0.86 |
| Non frail | 7.19 (3.11, 11.27) | 11.80 (5.42, 18.18) | 10.54 (−71.83, 92.90) | 0.61 |
| Combined | 6.54 (4.18, 8.90) | 12.14 (8.20, 16.08) | 9.10 (−36.98, 55.18) | 0.76 |
| eGFR (MDRD) | ||||
| Frail | −19.24 (−23.06, −15.42) | −41.98 (−50.63, −33.33) | 20.27 (−126.04, 166.57) | 0.82 |
| Non frail | −18.55 (−24.71, −12.38) | −32.87 (−44.14, −21.60) | 21.89 (−273.75, 317.54) | 0.56 |
| Combined | −18.86 (−22.45, −15.27) | −36.98 (−44.09, −29.88) | 21.17 (−145.05, 187.40) | 0.68 |
ME, Mean Error (ml min−1), a measure of bias; %ME, Mean Error (bias) expressed as a percentage of optimized gentamicin clearance; RMSE, Root Mean Square Error (ml min−1), a measure of precision; CGIBW, Cockcroft Gault estimate of creatinine clearance calculated using ideal bodyweight; CGABW; Cockcroft Gault estimate of creatinine clearance calculated using actual bodyweight; CGLBW; Cockcroft Gault estimate of creatinine clearance calculated using lean bodyweight; eGFR(MDRD), estimated glomerular filtration rate calculated using the Modified Diet in Renal Disease equation normalized for body surface area (ml min−1).
Discussion
In this study of older inpatients receiving prophylactic gentamicin, we observed no significant difference in volume of distribution and a significant reduction in clearance of gentamicin in the frail compared with the non frail participants. Volume of distribution correlated best with lean body weight in all participants, and particularly poorly with actual bodyweight in the frail participants. Gentamicin clearance was best predicted by the Cockcroft Gault equation for creatinine clearance calculated using ideal bodyweight. Both the Cockcroft Gault equation for creatinine clearance calculated using actual bodyweight and the MDRD equation normalized for body surface area overestimated gentamicin clearance. The Cockcroft Gault equation for creatinine clearance calculated using lean bodyweight underestimated gentamicin clearance.
We found that frail older participants had higher actual body weight and lower lean body weight than the non frail (Table 1). This is consistent with the increased prevalence and severity of sarcopenia in frailty [4]. The volume of distribution of gentamicin in our population was lower than that reported in other older populations when normalized for actual bodyweight (0.20 ± 0.02 l kg−1), although comparable when normalized for lean bodyweight (0.28 ± 0.03 l kg−1) [2]. This could be because our population was older, with a higher prevalence of sarcopenia than those studied previously, and because unlike the participants in other studies, ours were not septic, and sepsis increases the volume of distribution of gentamicin [27]. Our findings (Table 3) suggest that the loading dose calculation, which depends on volume of distribution [28], would be more accurate in older patients if lean bodyweight was used rather than actual bodyweight, particularly in the frail. Dosing based on actual bodyweight may result in potentially toxic doses, particularly in frail older patients.
The gentamicin clearance that we observed in both frail and non frail participants was lower than that reported in most previous studies of older patients [2]. This is consistent with the high prevalence of renal disease risk factors and renal impairment in our urologic surgery population.
Renal function, calculated with the Cockcroft Gault estimate of creatinine clearance using ideal or lean bodyweight, and with the MDRD equation, was significantly poorer in the frail participants than the non frail. There is a complex relationship between frailty, ageing and chronic disease [3]. A previous study demonstrated an association between frailty and chronic renal insufficiency indicated by elevated serum creatinine [29]. However, another pharmaco-epidemiologic study found that impaired physical function, which is closely linked to frailty, was associated with elevated Cystatin C, but not with impairment of creatinine-based measures of renal function [30]. Cystatin C is increased with inflammation [31], which is also part of the frailty syndrome. An increased prevalence of diseases or medicines that are associated with renal impairment may partly explain the association between frailty and impaired renal function.
The Cockcroft Gault equation for creatinine clearance calculated using ideal body weight gave the most accurate estimate of gentamicin clearance in frail and non frail older patients. Gentamicin clearance was overestimated by the use of actual bodyweight in the Cockcroft Gault equation and by the MDRD equation. However gentamicin clearance was underestimated by Cockcroft Gault using lean bodyweight (Figure 1, Table 4). These findings are consistent with those of another study in older hospitalized patients [14], which also found that the MDRD equation overestimated gentamicin clearance and the Cockcroft Gault equation using ideal bodyweight provided a better estimate of gentamicin clearance. The findings are also consistent with observations in young obese people: use of actual bodyweight in the Cockcroft Gault equation overestimates gentamicin clearance by more than 50%, whereas use of LBW and IBW give the best predictions of gentamicin clearance [32].
The present study had several strengths. It incorporated an accurate and detailed data collection protocol on gentamicin dosing, the timing of blood sample collection and recording of patient information. The study used validated clinical tools including the Reported Edmonton Frailty Scale [6] and Charlson Co-morbidity Scale [19]. Serum concentrations of gentamicin were measured by a National Association of Testing Authorities accredited laboratory and validated population pharmacokinetic software [22] was used to calculate gentamicin volume of distribution and clearance.
There were also several important limitations to the present study. The sample size was quite small (31 patients), which may have limited the power to detect differences in volume of distribution between frail and non frail participants. The response rate was 65% of eligible patients, which may limit generalizability of the findings. Multiple investigators (n = 3) collected the data, which could have resulted in information bias. The study population consisted of urology patients who were receiving prophylactic gentamicin. This limits the generalizability of our results in other populations of older hospital patients such as critically ill septic patients. These patients generally show an increased volume of gentamicin distribution and decreased gentamicin clearance [27]. Our participants were predominantly males, which further limits the generalizability of the results to the predominantly female older population.
Further investigation is needed to determine whether frailty is associated with differences in gentamicin pharmacokinetics in other populations of older hospitalized patients. Pharmacokinetic–pharmacodynamic studies are also required to establish the therapeutic window of gentamicin in frail older people. Frail patients may have poorer functioning immune systems [33], and thus may require higher concentrations of gentamicin to control sepsis, and may also be increasingly susceptible to gentamicin toxicity.
In conclusion, we found that while there were some differences in pharmacokinetic parameters between frail and non frail older participants, the same prescribing guidelines could be used to optimize the dose of gentamicin for frail and non frail patients. It is important to use lean bodyweight rather than actual bodyweight to calculate the loading dose of gentamicin, especially in the frail. Gentamicin clearance, which influences the loading and maintenance doses, was significantly lower in the frail than the non frail participants. However in all participants, gentamicin clearance correlated best with creatinine clearance determined by the Cockroft and Gault equation using ideal bodyweight. Current Australian [34] and British [1] dosing guidelines for gentamicin are given per kilogram of actual body weight, and only recommend using ideal bodyweight in those who weigh over 20% more than ideal bodyweight [34] or are obese [1], without specifying directions for sarcopenic obese frail patients. Our study has established that use of ideal bodyweight in the Cockcroft Gault equation results in an estimate of renal function that better predicts gentamicin clearance in older, and particularly in frail, hospitalized patients receiving prophylactic gentamicin.
Acknowledgments
We gratefully acknowledge the financial support of the Geoff and Elaine Penney Ageing Research Unit at Royal North Shore Hospital.
We thank the Departments of Biochemistry, Urology and Anaesthetics at Royal North Shore, Hornsby and Ryde hospitals for their co-operation with this project.
We acknowledge the assistance of A/Prof Carl Kirkpatrick with TCIWorks software.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Joint Formulary Committee (editor) British National Formulary. 59th edn. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2010. [Google Scholar]
- 2.Triggs E, Charles B. Pharmacokinetics and therapeutic drug monitoring of gentamicin in the elderly. Clin Pharmacokinet. 1999;37:331–41. doi: 10.2165/00003088-199937040-00004. [DOI] [PubMed] [Google Scholar]
- 3.Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, Wolfson C. Frailty: an emerging research and clinical paradigm – issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–7. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–36. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hilmer SN, Perera V, Mitchell S, Murnion BP, Dent J, Bajorek B, Matthews S, Rolfson DB. The assessment of frailty in older people in acute care. Australas J Ageing. 2009;28:182–8. doi: 10.1111/j.1741-6612.2009.00367.x. [DOI] [PubMed] [Google Scholar]
- 7.Hilmer SN, McLachlan AJ, LeCouteur DG. Clinical pharmacology in the geriatric patient. Fundam Clin Pharmacol. 2007;21:217–30. doi: 10.1111/j.1472-8206.2007.00473.x. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell SJ, Kirkpatrick CM, Le Couteur DG, Naganathan V, Sambrook PN, Seibel MJ, Blyth FM, Waite LM, Handelsman DJ, Cumming RG, Hilmer SN. Estimation of lean body weight in older community-dwelling men. Br J Clin Pharmacol. 2010;69:118–27. doi: 10.1111/j.1365-2125.2009.03586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell SJ, Hilmer SN, Kirkpatrick CMJ, Hansen RD, Williamson DA, Singh NA, Finnegan TP, Allen BJ, Diamond TH, Diwan AD, Lloyd BD, Smith EUR, Fiatarone Singh MA. Estimation of lean body weight in older women with hip fracture. J Nutr Health Aging. 2010. In Press. [DOI] [PubMed]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 11.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 12.Mathew TH. Chronic kidney disease and automatic reporting of estimated glomerular filtration rate: a position statement. Med J Aust. 2005;183:138–41. doi: 10.5694/j.1326-5377.2005.tb06958.x. [DOI] [PubMed] [Google Scholar]
- 13.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16:763–73. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- 14.Roberts GW, Ibsen PM, Schioler CT. Modified diet in renal disease method overestimates renal function in selected elderly patients. Age Ageing. 2009;38:698–703. doi: 10.1093/ageing/afp168. [DOI] [PubMed] [Google Scholar]
- 15.Gill J, Malyuk R, Djurdjev O, Levin A. Use of GFR equations to adjust drug doses in an elderly multi-ethnic group – a cautionary tale. Nephrol Dial Transplant. 2007 doi: 10.1093/ndt/gfm289. [DOI] [PubMed] [Google Scholar]
- 16.Pequignot R, Belmin J, Chauvelier S, Gaubert JY, Konrat C, Duron E, Hanon O. Renal function in older hospital patients is more accurately estimated using the Cockcroft-Gault formula than the modification diet in renal disease formula. J Am Geriatr Soc. 2009;57:1638–43. doi: 10.1111/j.1532-5415.2009.02385.x. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National kidney foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–47. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 18.Bang H, Vupputuri S, Shoham DA, Klemmer PJ, Falk RJ, Mazumdar M, Gipson D, Colindres RE, Kshirsagar AV. SCreening for Occult Renal Disease (SCORED): a simple prediction model for chronic kidney disease. Arch Intern Med. 2007;167:374–81. doi: 10.1001/archinte.167.4.374. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Devine BJ. Gentamicin therapy. Drug Intell Clin Pharm. 1974;8:650–55. [Google Scholar]
- 21.Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44:1051–65. doi: 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
- 22.Duffull SB, Kirkpatrick CMJ, Begg EJ. Comparison of two Bayesian approaches to dose-individualization for once-daily aminoglycoside regimens. Br J Clin Pharmacol. 1997;43:125–35. doi: 10.1046/j.1365-2125.1997.05341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H-K, Kirkpatrick CMJ, Green B. Does the use of Lean Body Weight (LBW) in a modified Cockcroft-Gault (C-G) equation provide a better prediction of gentamicin clearance? Proceedings of the 11th Annual Population Approach Group in Australia and New Zealand (PAGANZ) Meeting, 2009 Feb 4-6; Newcastle, Australia.
- 24.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–71. [PubMed] [Google Scholar]
- 25.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 26.Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9:503–12. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 27.Triginer C, Izquierdo I, Fernandez R, Rello J, Torrent J, Benito S, Net A. Gentamicin volume of distribution in critically ill septic patients. Intensive Care Med. 1990;16:303–6. doi: 10.1007/BF01706354. [DOI] [PubMed] [Google Scholar]
- 28.Hansen M, Christrup LL, Jarløv JO, Kampmann JP, Bonde J. Gentamicin dosing in critically ill patients. Acta Anaesthesiol Scand. 2001;45:734–40. doi: 10.1034/j.1399-6576.2001.045006734.x. [DOI] [PubMed] [Google Scholar]
- 29.Shlipak MG, Stehman-Breen C, Fried LF, Song X, Siscovick D, Fried LP, Psaty BM, Newman AB. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43:861–7. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 30.Wasen E, Isoaho R, Vahlberg T, Kivela SL, Irjala K. Association between markers of renal function and C-reactive protein level in the elderly: confounding by functional status. Scand J Clin Lab Invest. 2008;68:484–91. doi: 10.1080/00365510701854983. [DOI] [PubMed] [Google Scholar]
- 31.Singh D, Whooley MA, Ix JH, Ali S, Shlipak MG. Association of cystatin C and estimated GFR with inflammatory biomarkers: the Heart and Soul Study. Nephrol Dial Transplant. 2007;22:1087–92. doi: 10.1093/ndt/gfl744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duffull SB, Dooley MJ, Green B, Poole SG, Kirkpatrick CM. A standard weight descriptor for dose adjustment in the obese patient. Clin Pharmacokinet. 2004;43:1167–78. doi: 10.2165/00003088-200443150-00007. [DOI] [PubMed] [Google Scholar]
- 33.Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–41. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 34.Rossi S. Australian Medicines Handbook. 11th edn. Adelaide, SA: Australian Medicines Handbook Pty Ltd; 2010. [Google Scholar]


