Abstract
AIMS
To assess pharmacokinetics and pharmacodynamics of a 10 mg intravenous sildenafil bolus in pulmonary arterial hypertension (PAH) patients stabilized on 20 mg sildenafil orally three times daily.
METHODS
Pharmacokinetic parameters were calculated using noncompartmental analysis.
RESULTS
After an acute increase, plasma concentrations stabilized within the range reported previously for a 20 mg oral tablet. At 0.5 h, mean ± SD changes from baseline were −8.4 ± 11.7 mmHg (systolic pressure), −2.6 ± 7.3 mmHg (diastolic pressure) and −3.5 ± 10.4 beats min−1 (heart rate). There was no symptomatic hypotension.
CONCLUSIONS
Although further research is warranted, a 10 mg sildenafil intravenous bolus appears to provide similar exposure, tolerability and safety to the 20 mg tablet.
Keywords: intravenous, pharmacokinetics, phosphodiesterase type 5 inhibitor, sildenafil
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Pulmonary arterial hypertension (PAH) is a rare syndrome of dyspnoea and fatigue due to an increase in pulmonary vascular resistance. Although the disease remains incurable, targeted therapies have been shown to be beneficial to patients. Oral sildenafil (20 mg three times daily) is a phosphodiesterase type 5 inhibitor with proven efficacy on exercise capacity, symptoms and pulmonary haemodynamics in patients with PAH.
WHAT THIS STUDY ADDS
It is advisable that chronic therapies for PAH, including oral sildenafil, not be interrupted. However, patients may not be able to take their medication because of an intervening illness or a requirement to undergo general anaesthesia, preventing oral drug administration. This study demonstrates that an intravenous bolus of 10 mg sildenafil in stable patients with PAH is safe, well tolerated and maintains plasma levels to preserve drug exposure.
Introduction
Pulmonary arterial hypertension (PAH) is a rare syndrome of dyspnoea and fatigue due to an increase in pulmonary vascular resistance [1]. It may be idiopathic or associated with other factors (i.e. connective tissue disease, left-to-right cardiac shunts, portal hypertension, HIV infection or exposure to drugs and toxins). Pulmonary arterial hypertension is severely disabling, incurable and rapidly progressive to right ventricular dysfunction and death, but patients benefit from therapies targeting specific pathways [1]. Oral sildenafil citrate is a phosphodiesterase type 5 inhibitor (PDE5i) indicated for the treatment of PAH (World Health Organization Functional Class II and III).
As interruption of PAH therapy, including oral sildenafil, is potentially harmful, an alternative route of administration is needed when oral administration is not appropriate or feasible. The intravenous route appears most suitable.
Based on the calculated absolute bioavailability of oral sildenafil and the PDE5i activity and pharmacokinetics of the main metabolite, desmethylsildenafil [2, 3], a 10 mg intravenous bolus of sildenafil is expected to maintain equivalent PDE5i activity to a 20 mg oral dose. This study assesses the safety, tolerability and pharmacokinetics of a 10 mg intravenous sildenafil bolus in patients with PAH who were stabilized on 20 mg oral sildenafil three times daily.
Patients and methods
This single-centre open-label study was conducted in compliance with the revised Declaration of Helsinki and with independent ethics committee approval. Eligible were adults (≥18 years old) with PAH who, while taking 20 mg oral sildenafil three times daily for ≥1 month, with or without additional PAH therapies, were haemodynamically stable, i.e. blood pressure (BP) ≥ 90/50 mmHg (sitting or, for patients confined to bed, supine) and no symptomatic postural hypotension or drop in BP of ≥20 mmHg systolic (SBP) or ≥10 mmHg diastolic (DBP) on standing. A single 10 mg intravenous bolus dose of sildenafil (12.5 ml of 0.8 mg ml−1 solution) replaced the morning oral sildenafil dose. Patients remained in the clinic for ≥6 h, kept recumbent most of the time and before BP checks. Telephone follow-up was 7 days later. Adverse events were elicited. Heart rate (HR) and BP (duplicate sitting and standing measurements) were monitored at screening, at predose and at 0.5, 1, 2, 3 and 6 h postdose.
Samples for plasma concentration measurement (sildenafil and desmethylsildenafil) were collected predose, 5 min postdose and up to 6 h postdose. Concentrations were measured using liquid chromatography with tandem mass spectrometry (Covance Bioanalytical Services, LLC, Indianapolis, IN, USA), with calibration range of 1.00–500 ng ml−1 and sildenafil vs. desmethylsildenafil precision (% coefficient of variance) of ≤4.6% vs. ≤4.7% and accuracy of 1.0–2.6% vs. −0.3–0.9% [4]. Concentrations were compared with those reported previously for PAH patients taking 20 mg oral sildenafil three times daily [4, 5]. Pharmacokinetic parameters were calculated using noncompartmental analysis and (because the intravenous dose replaced the morning oral dose) an assumed steady state and a dosing interval of 8 h based on three times daily oral dosing.
It was planned that 12 patients would complete the study. Adverse events suggestive of excessive vasodilatation (hypotension and syncope) in three or more of 12 patients were to be regarded as a warning signal providing strong evidence of an increased adverse event rate due to intervention (because there is <0.02 probability of observing this many adverse events when the rate is ≤5%).
Results
The study was truncated after 10 patients because safety data suggested that the last two patients were unlikely to significantly impact the overall conclusions. Five male and five female patients with PAH, either idiopathic (n= 6) or associated with appetite suppressant intake (1), portal hypertension (1), congenital heart disease (1) or HIV infection (1), participated. All were in Functional Class II–III and on stable medication, without any episodes of clinical worsening, for at least 4 weeks. Mean (range) age and body mass index were 59.5 (46–76) years and 29.2 (20.7–41.1) kg m−2, respectively. Sildenafil treatment was monotherapy (n= 2) or combination with oral bosentan (n= 7) or oral bosentan plus subcutaneous treprostinil (n= 1).
The baseline mean ± standard deviation (SD) sitting SBP, DBP and HR were 120.1 ± 17.1 mmHg, 69.6 ± 12.2 mmHg and 73.3 ± 8.5 beats min−1, respectively. At 0.5 h, mean ± SD changes from baseline in sitting SBP, DBP and HR were −8.4 ± 11.7 mmHg, −2.6 ± 7.3 mmHg and −3.5 ± 10.4 beats min−1, respectively. It is not known whether greater BP changes occurred within the 0.5 h after dosing, before the first BP assessment. However, no clinically relevant changes of BP, cases of symptomatic postural hypotension or symptoms of hypotension were observed, even in the three patients who had marked changes in BP and HR in the first 2 h postdose.
A moderate urinary tract infection and fatal ventricular fibrillation related to underlying PAH (7 days postdose) were deemed unrelated to treatment. Only the following mild adverse events in two patients were considered possibly treatment related: flushing (at dosing) and mild flatulence (10 min postdose); and mild hot flush (at dosing).
After an acute increase, sildenafil plasma concentrations appeared to stabilize within the range reported previously for patients with PAH who were taking 20 mg oral sildenafil three times daily (Figure 1). Pharmacokinetic analysis (Table 1) showed a higher than expected ratio of desmethylsildenafil to sildenafil (45%), probably resulting from high baseline values of metabolite carryover from oral dosing.
Figure 1.
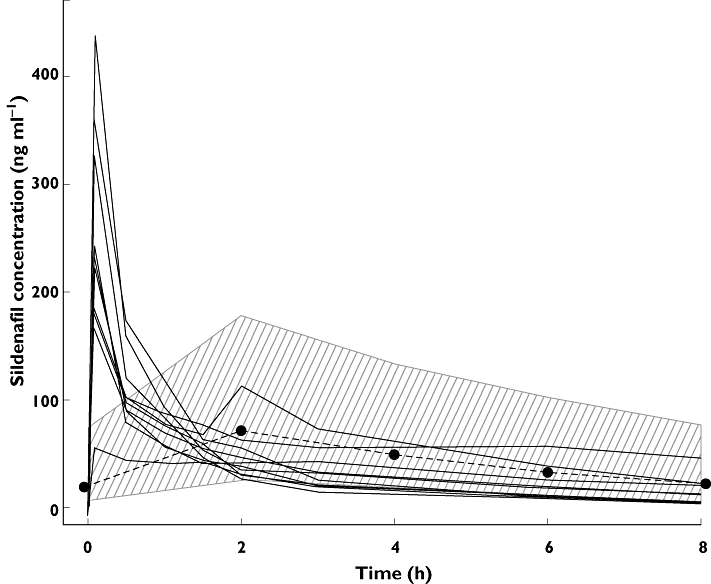
In 10 patients with pulmonary arterial hypertension (PAH) who were stabilized on 20 mg oral sildenafil three times daily and had their morning oral sildenafil dose replaced with a 10 mg bolus intravenous sildenafil dose, individual sildenafil plasma concentrations after the bolus dose are shown (continuous lines). These are superimposed on plasma concentrations associated with oral sildenafil 20 mg three times daily dosing in a population pharmacokinetic model established using the data from 206 patients with PAH in a multinational, randomized, double-blind, placebo-controlled trial to evaluate the safety and efficacy of oral sildenafil (20, 40 or 80 mg three times daily) over a 12 week period: 95% range of the individual predicted concentrations (hatched area) and the mean concentration time profile (bold dashed black line) [6, 7].
Table 1.
Summary of sildenafil and desmethylsildenafil pharmacokinetic parameters after intravenous administration of a single 10 mg bolus dose to patients with pulmonary arterial hypertension
| Parameter (units)* | Sildenafil(n= 10) | Desmethyl sildenafil(n= 10) |
|---|---|---|
| AUC0–6 (ng h ml−1) | 301.2 (31%) | 123.1 (79%) |
| AUC0–8 (ng h ml−1) | 329.7 (34%) | 147.3 (86%) |
| C0 (ng·ml−1) | 248.4 (48%) | – |
| Cmax (ng ml−1) | 213.3 (45%) | 30.8 (62%) |
| tmax (min) | 5 | 35 |
| t½ (h) | 3.2 ± 1.7 | – |
| CL (l h−1) | 32.2 ± 12.3 | – |
| Vz (l) | 137.3 ± 52.0 | – |
| MR | 0.45 (0.19–2.06) |
Maximum observed plasma drug concentration (Cmax) and time to maximum observed plasma drug concentration (tmax) are unadjusted data. Half-life (t½) was calculated as ln(2)/kel, where kel is the terminal phase rate constant calculated by linear regression of the log-linear concentration–time curve. The area under the curve from time 0 to 6 h (AUC0–6) was calculated using the linear/log trapezoidal method and extrapolated to 8 h (AUC0–8) based on kel. C0 was back-extrapolated using the slope (in semilog scale) of the first two observed concentrations. The AUC0–8 was used to calculate CL (dose/AUC0–8), Vz (dose/AUC0–8×kel) and the metabolite ratio (MR = AUC0–8 desmethylsildenafil/AUC0–8 sildenafil). Results are presented as geometric mean [% coefficient of variance (CV)] for AUC0–6, AUC0–8, C0, and Cmax; median for tmax; arithmetic mean ± SD for t½, CL and Vz, and geometric mean (range) for MR.
Discussion
This is the first study to administer a 10 mg intravenous sildenafil bolus to patients with PAH. The results are supported by previous studies that assessed intravenous sildenafil in adults with pulmonary hypertension, ischaemic heart disease or coronary heart disease [4, 6–9]; despite some initial bolus injections administered over 5 min (e.g. 5.25 mg [4] and 26.25 mg [9]), sildenafil was administered predominantly as an infusion, achieved mean peak plasma concentration (Cmax) values of up to 1822 ng ml−1 (associated with doses of up to 80 mg infused over 40 min) [6] and elicited no tolerability or safety concerns. This suggests that a 10 mg intravenous bolus dose, which achieved an estimated mean baseline concentration (C0) value of <250 ng ml−1 in the present study, has a wide safety margin.
In patients stabilized on oral sildenafil for the treatment of PAH, intravenous sildenafil would allow the continuation of therapy when oral medication cannot be taken or absorbed, such as perioperatively. Anaesthetic management and underlying illness may predispose PAH patients to significant perioperative risk for major complications, including pulmonary hypertensive crisis and death [10]. Intravenous sildenafil was used successfully for the prevention of pulmonary hypertensive crises during acute intestinal malabsorption in an infant with PAH [11]. Further research is warranted in patients in whom oral administration is not appropriate or feasible, and to address the safety and tolerability of repeated administration in a less stable population or in emergency situations.
The data from this study, combined with data in healthy subjects and other patients, were used to build an integrated intravenous population pharmacokinetic model, for use in developing a model for haemodynamic parameters. A separate publication is planned.
In conclusion, this study suggests that a 10 mg intravenous sildenafil bolus will provide similar PDE5i exposure, tolerability and safety to those provided by a 20 mg tablet and offers a treatment option in PAH patients when oral administration may not be appropriate or feasible.
Acknowledgments
This study was sponsored by Pfizer Inc. and conducted by investigators contracted by and under the direction of the sponsor. Assistance was provided by Michele Schroeder and the Pfizer Inc. Phase 1 Unit (Brussels, Belgium). Editorial support was provided by Deborah Campoli-Richards of Complete Healthcare Communications, Inc., and was funded by Pfizer Inc.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219–63. doi: 10.1183/09031936.00139009. [DOI] [PubMed] [Google Scholar]
- 2.Revatio® (Sildenafil Citrate) Prescribing Information. New York, NY: Pfizer Inc.; 2009. [Google Scholar]
- 3.Muirhead GJ, Rance DJ, Walker DK, Wastall P. Comparative human pharmacokinetics and metabolism of single-dose oral and intravenous sildenafil. Br J Clin Pharmacol. 2002;53(Suppl 1):13S–20S. doi: 10.1046/j.0306-5251.2001.00028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Data on File. New York, NY: Pfizer Inc.; 2009. [Google Scholar]
- 5.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–57. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 6.Jackson G, Benjamin N, Jackson N, Allen MJ. Effects of sildenafil citrate on human hemodynamics. Am J Cardiol. 1999;83(Suppl 5A):C13–C20. doi: 10.1016/s0002-9149(99)00043-0. [DOI] [PubMed] [Google Scholar]
- 7.Mikhail GW, Prasad SK, Li W, Rogers P, Chester AH, Bayne S, Stephens D, Khan M, Gibbs JS, Evans TW, Mitchell A, Yacoub MH, Gatzoulis MA. Clinical and haemodynamic effects of sildenafil in pulmonary hypertension: acute and mid-term effects. Eur Heart J. 2004;25:431–6. doi: 10.1016/j.ehj.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Suntharalingam J, Hughes RJ, Goldsmith K, Doughty N, George P, Toshner M, Sheares KK, Pepke-Zaba J. Acute haemodynamic responses to inhaled nitric oxide and intravenous sildenafil in distal chronic thromboembolic pulmonary hypertension (CTEPH) Vascul Pharmacol. 2007;46:449–55. doi: 10.1016/j.vph.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Robinson SD, Ludlam CA, Boon NA, Newby DE. Phosphodiesterase type 5 inhibition does not reverse endothelial dysfunction in patients with coronary heart disease. Heart. 2006;92:170–6. doi: 10.1136/hrt.2004.059683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaise G, Langleben D, Hubert B. Pulmonary arterial hypertension: pathophysiology and anesthetic approach. Anesthesiology. 2003;99:1415–32. doi: 10.1097/00000542-200312000-00027. [DOI] [PubMed] [Google Scholar]
- 11.Lammers AE, Haworth SG, Pierce CM. Intravenous sildenafil as an effective treatment of pulmonary hypertensive crises during acute intestinal malabsorption. Cardiol Young. 2006;16:84–6. doi: 10.1017/S1047951105002155. [DOI] [PubMed] [Google Scholar]


