Abstract
Background
Obstructive sleep apnea (OSA) or habitual snoring and asthma are known comorbid conditions in men and non-pregnant women. This comorbidity has not been evaluated among pregnant women. We assessed the habitual snoring-asthma relationship among pregnant women.
Methods
A cohort of women (N=1,335) were interviewed during pregnancy, and we ascertained participants’ asthma status and collected information about habitual snoring, before and during pregnancy. Logistic regression procedures were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs).
Results
Compared with non-asthmatics, the adjusted OR among asthmatics for snoring before pregnancy was 2.13 (95%CI 1.10–4.12). The odds of snoring during early pregnancy was 1.79-fold (OR=1.79; 95%CI 1.07–3.01). Associations were more pronounced among overweight (≥25 kg/m2) asthmatics (OR=5.39; 95%CI 2.27–12.75).
Conclusions
We report a cross-sectional association of habitual snoring and asthma among pregnant women. If confirmed, pregnant asthmatics may benefit from more vigilant screening and management of OSA or habitual snoring during pregnancy.
Keywords: Asthma, Obstructive Sleep Apnea, Sleep Disordered Breathing, Habitual Snoring, Obesity, Pregnancy
INTRODUCTION
Obstructive sleep apnea (OSA), a common sleep-related breathing disorder with snoring as the cardinal symptom, is characterized by repetitive complete or partial occlusion of the upper airway during sleep despite continuing ventilatory effort (1, 2). Snoring, which is produced by vibrations of the soft tissues, is a good marker for OSA (3). Obesity, (4) pregnancy (5, 6) and postmenopausal status (7, 8) are well known risk factors of OSA. Asthma, an inflammatory disease of the lower respiratory tract, manifests as intermittent constriction of the bronchial airways with symptoms that are often exaggerated at night (9, 10). Investigators have attributed the nocturnal deterioration of asthma symptoms to a number of factors, including alterations in the autonomic tone, changes in hormonal secretion, circadian changes in inflammatory cells and cytokines, and the possible role of acid reflux (9–11).
Hudgel and Shucard (12) and Catterall et al (13) were the first to publish reports documenting the co-occurrence of OSA and asthma; and to describe more frequent and severe hypoxemic episodes particularly during rapid eye movement (REM) sleep. These seminal observations have been corroborated and expanded upon by a large number of studies conducted in men, non-pregnant women, children and adolescents (10, 14–17). Fitzpatrick et al, (14) in their community-based study of 1,478 British adults noted that asthmatics were more likely than their unaffected counterparts to report frequent snoring (i.e., ≥4 nights a week) and excessive daytime sleepiness. The clinical and public health implications of observed OSA-asthma comorbidity has been underscored by results from studies which indicate that treatment for OSA improves asthma symptoms (18–20), and disease-specific quality of life (21). Collectively, available data indicate that OSA (or habitual snoring) and asthma is likely to have a bidirectional relationship in which each condition has the potential to exacerbate the other. On the basis of available epidemiological and clinical literature, the National Asthma Education and Prevention Program Expert Panel currently recommend evaluating OSA or habitual snoring as a potential contributor to poor asthma control (22).
Prior studies, however, have excluded pregnant women; hence very little is known about the co-occurrence of OSA and asthma in pregnancy. This gap in the literature is particularly troublesome since available evidence suggest: (1) markedly increased prevalence of sleep disordered breathing or snoring during pregnancy (5, 23); and (2) increased risks of medical complications of pregnancy including gestational diabetes mellitus and preeclampsia among women who are habitual snorers during pregnancy (24–29). To the best of our knowledge no study has evaluated the co-occurrence of OSA and asthma among pregnant women. We assessed associations of maternal self-reported habitual snoring (a good marker for OSA(3)) before and during early pregnancy with maternal asthma diagnosis prior to pregnancy. We hypothesized that maternal habitual snoring before and during early pregnancy are positively associated with maternal history of asthma.
MATERIALS AND METHODS
Study population and setting
This analysis is based on data collected from a cohort of women attending prenatal care clinics (for routine prenatal care) affiliated with Swedish Medical Center in Seattle, Washington, USA. Eligible women started prenatal care before 20 weeks gestation, were 18 years of age or older, could speak and read English, and planned to carry the pregnancy to term and to deliver at the hospital. At between 8–19 weeks (mean and standard deviation: 16.3±2.6) weeks gestation, participants reported sociodemographic, behavioral, and health characteristics in a structured interview. After delivery, study personnel abstracted data from participants’ hospital labor and delivery medical records and clinic records. Between December 2003 and July 2006, 1,393 (83%) of 1,685 approached women consented to participate. We excluded 58 women who did not complete the interview. Thus, 1,335 women remained for analysis. All study procedures were approved by the Institutional Review Board of Swedish Medical Center. All participants provided written informed consent.
Data Collection
Interviewer-administered questionnaires were completed by participants in the analytical population at a mean gestational age of 16.3 weeks. Characteristics assessed using the questionnaire (i.e., self-administered) included maternal age, height, pre-pregnancy weight, reproductive and medical history including her history of asthma, and average nightly sleep duration (before and during early pregnancy). Maternal history of asthma diagnosis was determined by response to the questions “Has a doctor ever told you that you have asthma?” Sleep disordered breathing before and during pregnancy was assessed by asking women about the frequency of snoring during the index pregnancy. Specifically they were asked “Since becoming pregnant, when you are asleep, to the best of your knowledge, have you snored?” Responses were as follows: (i) all of the time, (ii) most of the time, (iii) some of the time, (iv) a little of the time, and (v) none of the time. From this information, we categorized participants as habitual snorers if they reported snoring most or all of the time; all other women were classified as non-snorers. The same question and categorization scheme was used to assess snoring during the year before pregnancy.
Statistical analytical methods
We compared the frequency distribution of sociodemographic, lifestyle, behavioral and medical history characteristics of participants according to whether or not they had received a physician diagnosis of asthma prior to the index pregnancy. All continuous variables are presented as mean ± standard deviation (30). We used unadjusted and multivariable-adjusted logistic regression models to calculate odds ratios (ORs) and 95% confidence intervals (CIs) of the association between snoring and asthma. Separate models were fitted for snoring before and during pregnancy, respectively. In multivariable models, we adjusted for maternal age (continuous), parity (nulliparous, multiparous), and pre-pregnancy body mass index (continuous). Additional adjustment for the other covariates listed in Table 1 (including maternal cigarette smoking status or history of chronic hypertension) did not substantially change the effect estimates. We evaluated the isolated and joint effect of asthma history and pre-pregnancy overweight status on the odds of snoring before and during pregnancy. We classified women by the joint distribution of prior history of asthma diagnosis (no vs. yes) and pre-pregnancy overweight status (< 25 vs. ≥25 kg/m2) resulting in the following categories: lean non-asthmatics (reference); lean asthmatics; overweight non-asthmatics; and overweight asthmatics. All analyses were performed using Stata 9.0 statistical software (Stata, College Station, TX). All reported confidence intervals were calculated at the 95% level. All reported p-values are two-tailed and set at 0.05 levels.
Table 1.
Characteristics of the study population according to asthma status, Seattle, Washington, USA, 2003–2006
| Physician Diagnosed Asthma |
|||
|---|---|---|---|
| Yes N=200 |
No N=1,135 |
||
| Characteristics | % | % | *P-value |
| Maternal Age (years) | 32.8 ± 4.7 | 33.4 ± 4.4 | 0.08 |
| <30 | 24.5 | 17.4 | 0.08 |
| 30–34 | 38.5 | 42.2 | |
| 35–39 | 31.5 | 32.4 | |
| ≥40 | 5.5 | 8.0 | |
| Non-Hispanic white race/ethnicity | 13.0 | 12.1 | 0.71 |
| Annual household income (US$) | |||
| <30,000 | 4.0 | 1.6 | 0.10 |
| 30,000–69,999 | 12.0 | 14.2 | |
| ≥ 70,000 | 78.5 | 79.8 | |
| Missing | 5.5 | 4.4 | |
| Nulliparous | 69.0 | 57.9 | 0.003 |
| Parity | |||
| ….0 | 69.0 | 57.9 | 0.01 |
| ….1 | 24.5 | 33.8 | |
| ….2+ | 6.5 | 8.2 | |
| Education ≤ high school | 3.5 | 2.7 | 0.55 |
| Unmarried | 9.5 | 8.1 | 0.51 |
| Pre-gestational diabetes mellitus | 2.0 | 1.1 | 0.26 |
| Pre-gestational chronic hypertension | 4.0 | 4.1 | 0.97 |
| Migraine | 23.5 | 18.7 | 0.11 |
| Mood/anxiety disorders | 10.0 | 6.9 | 0.12 |
| Family history of diabetes mellitus | 14.0 | 14.6 | 0.82 |
| Family history of hypertension | 50.5 | 50.3 | 0.96 |
| Employed during pregnancy | 83.0 | 77.9 | 0.10 |
| Smoked during pregnancy | 3.0 | 5.5 | 0.14 |
| No prenatal vitamin | 3.0 | 2.5 | 0.66 |
| No exercise during pregnancy | 5.0 | 7.8 | 0.17 |
| Multifetal pregnancy | 2.5 | 3.4 | 0.49 |
| Pre-pregnancy body mass index (kg/m2)* | 24.6 ± 5.3 | 23.4 ± 4.5 | <0.001 |
| Normal (18.5–24.9) | 68.0 | 70.2 | 0.02 |
| Lean (<18.5) | 1.0 | 5.2 | |
| Overweight (25–29.9) | 20.5 | 17.5 | |
| Obese (≥30) | 10.5 | 7.1 | |
Mean ± standard deviation (30)
P-value from Student t test for continuous variable or from Chi-square test for categorical variables
RESULTS
Women with a prior history of physician diagnosed asthma were more likely to be younger, nulliparous, and to be overweight and obese when compared with women who did not have a history of asthma (Table 1). Other characteristics including marital status, annual household income, race/ethnicity, physical activity and multivitamin use during pregnancy were similar for women with and without a history of asthma. The frequency distribution of study subjects according to asthma and snoring status (before and during pregnancy) are summarized in Table 2. Women with a history of asthma were more likely to report snoring most or all of the time before (7.0% vs. 3.2%) and during pregnancy (11.0% vs. 6.4%), as compared with those with no asthma history. Women with a history of asthma were more likely to report habitual snoring before pregnancy (OR=2.32; 95% CI 1.23–4.40) than those without the history (Table 3). After adjusting for maternal age, parity, and pre-pregnancy body mass index, the association was slightly attenuated and remained statistically significant (adjusted OR=2.13; 95% CI 1.10–4.12). Asthmatics were also more likely to report habitual snoring during pregnancy (adjusted OR=1.79; 95% CI 1.07–3.01), as compared with their non-asthmatic counterparts.
Table 2.
Sleep disordered breathing reported by pregnant women with and without a medical history of asthma, Seattle, Washington, USA, 2003–2006
| Physician Diagnosed Asthma |
|||
|---|---|---|---|
| Yes N=200 |
No N=1,135 |
||
| Sleep Disordered Breathing | % | % | *P-value |
| Snoring before pregnancy | |||
| Never | 59.5 | 63.0 | 0.05* |
| A little of the time | 17.5 | 20.3 | |
| Some of the time | 14.0 | 12.5 | |
| Most or all the time | 7.0 | 3.2 | |
| Missing | 2.0 | 1.0 | |
| Snoring during pregnancy | |||
| Never | 54.5 | 57.8 | 0.01* |
| A little of the time | 17.5 | 18.3 | |
| Some of the time | 12.0 | 15.4 | |
| Most or all the time | 11.0 | 6.4 | |
| Missing | 5.0 | 2.0 | |
P-value from Chi-square test for categorical variables
Table 3.
Odds ratios (OR) and 95% confidence intervals (CI) of habitual snoring before and during pregnancy according to maternal history of asthma, Seattle, Washington, USA, 2003–2006
| Asthma Diagnosis |
||||
|---|---|---|---|---|
| Yes N=200 |
No N=1,135 |
Unadjusted OR (95% CI) |
Adjusted* OR (95% CI) |
|
| Maternal Snoring | % | % | ||
| Habitual Snoring Before Pregnancy | ||||
| No | 91.0 | 95.9 | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 7.0 | 3.2 | 2.32 (1.23–4.40) | 2.13 (1.10–4.12) |
| Habitual Snoring During Pregnancy | ||||
| No | 84.0 | 91.5 | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 11.0 | 6.4 | 1.86 (1.13–3.08) | 1.79 (1.07–3.01) |
Adjusted for maternal age (continuous), parity (categorical), and pre-pregnancy body mass index (continuous) Column percentages do not add up to 100% due to missing values
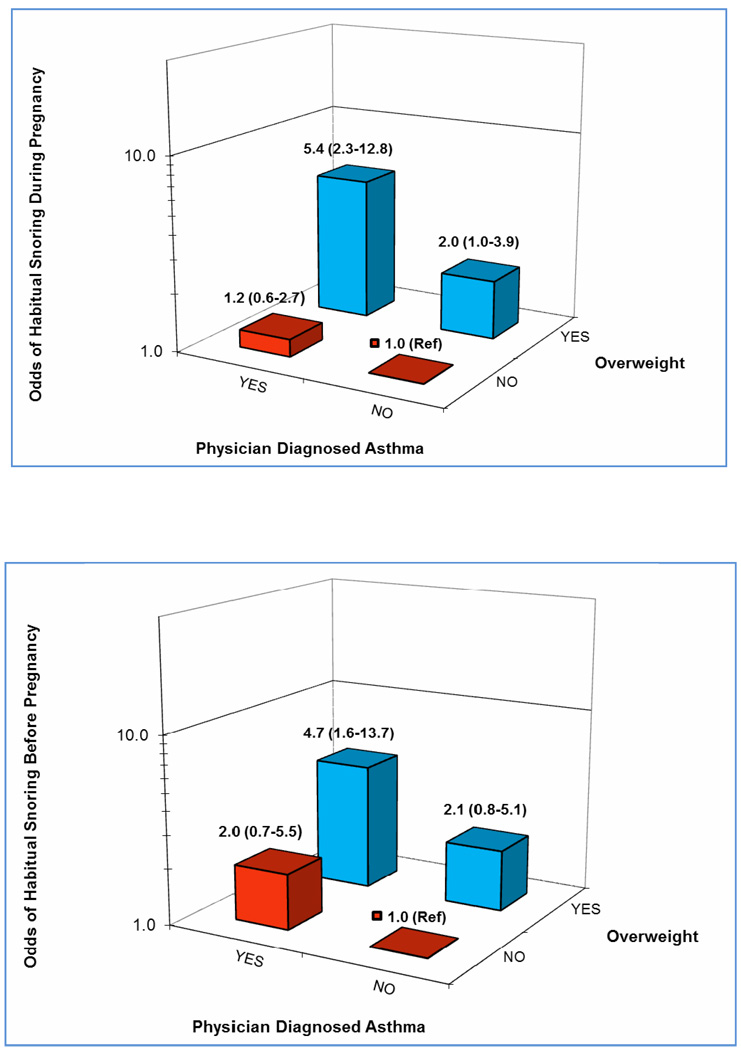
We evaluated the joint effect of asthma history and pre-pregnancy overweight status (Figure 1) and noted that overweight asthmatic had the highest odds of habitual snoring before and during pregnancy, respectively. Compared with lean non-asthmatics, the multivariable-adjusted OR among overweight asthmatics for snoring before pregnancy was 4.71 (adjusted OR=4.71; 95% CI 1.62–13.71). Similarly, overweight asthmatics had a 5.39-fold increased odds of snoring during pregnancy (adjusted OR=5.39; 95% CI 2.27–12.75), as compared with lean non-asthmatics.
Figure 1.
Adjusted odds ratio (OR) of habitual snoring before pregnancy (a) and during pregnancy (b) according to maternal history of physician diagnosed asthma (yes vs. no) and maternal pre-pregnancy overweight status (<25kg/m2 vs. ≥25kg/m2). Reported ORs and (95% confidence intervals (CIs) were adjusted for maternal age, race/ethnicity, and parity
DISCUSSION
Approximately 15% of the cohort reported having a medical diagnosis of asthma prior to the study pregnancy. Overall, asthmatics were more likely than non-asthmatics to report habitual snoring before and during pregnancy. The odds of habitual snoring were particularly elevated among overweight asthmatics. Overweight asthmatics had over a 4-fold increased odds of habitual snoring before (adjusted OR=4.71) and during (adjusted OR=5.39) as compared with lean non-asthmatics.
To the best of our knowledge, no epidemiological studies to date have examined the comorbidity of OSA and asthma among pregnant women. Nevertheless, several studies, generally conducted in men and non-pregnant women (10, 14–17) and children (17) have examined the co-occurrence of OSA and asthma. Our results corroborate previous reports of increased prevalence of habitual snoring among asthmatics; and extend this literature to include observations of such associations among pregnant women. In a population-based, cross sectional study of 5,424 Swedish adults aged 20–69 years, Larsson et al (31) noted that 10.7% of participants reported snoring as a problem. After adjusting for age, gender and cigarette smoking the authors noted that asthmatics has a 1.62 increased odds of reporting snoring as a problem (OR=1.62; 95% CO 1.16–2.27). In a clinic based study (32) asthmatics were more likely to report frequent snoring (18.5% vs. 8.0%, p<0.001) than primary care general internal medicine patients. However, there was no clear evidence of a higher prevalence of snoring or other symptoms of OSA among asthmatics versus the primary care patient comparison group. In another clinic-based study, Teodorescu and et al (10), reported that asthmatic women had a 2-fold greater odds of prevalent OSA (95% CI 1.1–4.1) than men. The authors also noted that the odds of OSA increased with the dose of inhaled corticosteroids (ICSs), a likely surrogate marker of asthma severity. The odds of self-reported habitual snoring were increased 1.11-fold with low-dose ICSs (OR= 1.11; 95% CI 0.31–4.03), 2.59-fold with medium-dose use (OR=2.59; 95% CI 0.96–6.97), and 3.67-fold (OR=3.67; 95% CI 1.34–10.01) with high-dose use (p-for trend=0.004). Results from the Busselton Health Survey (33), a longitudinal study designed to assess risk factors for the incident habitual snoring in Australian adults aged 25–74 years. The authors noted that asthmatics were 2.8-times more likely (OR=2.8; 95% CI 1.4–5.6), than non-asthmatics to begin snoring during the 14-year follow-up period. Finally, results from small clinical studies using polysomnography to objectively assess OSA symptoms revealed higher frequencies of OSA in asthmatics, particularly those patients with difficult-to-control asthma (34). For example, Yigla et al (34), using the respiratory disturbance index (RDI) of ≥5 to define OSA, the RDI values were significantly higher in the continuous oral corticosteroid therapy subgroup (21.4±3.4 vs. 11.1±1.6, p <0.05). On balance, evidence from diverse study populations, and research designs document cross-sectional associations between snoring and asthma; and evidence from a longitudinal study suggest that asthma may be an independent risk factor for the development of snoring and suggests that the associations may be causal (33).
The biological mechanisms underlying observed OSA-asthma associations are yet to be fully elucidated. However, investigators have proposed several plausible and compelling hypotheses by which asthma could lead to the development or worsening of OSA (10, 34–36). Asthma could deleteriously impact the patency of the upper airway. Frequent nocturnal asthma attacks may result in sleep deprivation and fragmentation of sleep that could lead to increased upper airway collapsibility during sleep (36, 37). Some investigators (38, 39) have noted that reduction in lung volume during sleep, particularly during REM sleep; and systemic inflammation-related weakening of respiratory muscles (40, 41) may account for observed associations. Nasal congestion or the presence of nasal polyps, common in asthmatics, is also thought to lead to increased nasal resistance or obstruction resulting in respiratory disturbances in sleep (40). Recent data reporting a very high prevalence of OSA in a small group of severe asthmatic patients on chronic or intermittent systemic steroids suggests that the use of systemic steroids may increase upper airway collapsibility (34). The observed dose-dependent relationships of ICSs and OSA symptoms (10) may reflect a relationship of asthma severity with OSA, with ICS doses as a mere surrogate of asthma severity.
Several limitations of our study merit discussion and consideration. First, maternal asthma status was based on self-reports made during interviews and on medical records review. Although the questions we used to ascertain maternal asthma status have been widely used in National Health and Nutrition Examination Surveys (NHANES) and other large epidemiological studies (42), and investigators have documented good agreement between asthma classification based on self-reports with information derived from medical records review (43, 44), we cannot exclude the possibility of that asthma status was underreported or over reported in our study. Second, maternal habitual snoring before and during pregnancy was obtained from self-report, and thus is likely susceptible to misclassification. However, we note that the use of self-reported snoring as a tool to detect OSA and its symptoms is well established. Investigators have shown that self-reported snoring correlates well with objective findings from nocturnal polysomnography, especially in frequent snorers. Snoring that is infrequent or non-habitual has not been shown to be a useful screen for sleep disordered breathing in large epidemiologic studies (30, 45). It was therefore necessary, as we have done in our analysis, to distinguish frequent snorers from infrequent snorers in our study. Lastly, the generalizability of our study may also be limited as our cohort was primarily comprised of Non-Hispanic White and well-educated women. The concordance of our results with those from other studies that have included racially, ethnically and geographically diverse populations, however, serve to attenuate these concerns.
In summary, we found increased odds of habitual snoring before and during pregnancy among pregnant asthmatics as compared with their non-asthmatic counterparts. Observed associations were particularly strong among overweight asthmatics. Despite noted study limitations, our results among pregnant women are consistent with a larger body of work documenting associations between OSA and asthma among men, non-pregnant women and children. Large well designed prospective cohort studies that allow for the comprehensive examination of OSA-asthma comorbidity among pregnant women are warranted. Such studies should include objective assessments of the full spectrum of OSA symptoms and should include comprehensive assessments of environmental, behavioral, and genetic risk factors of asthma and OSA. Enhanced understanding of the epidemiology and shared pathophysiological mechanisms of OSA and asthma are expected to provide important information needed for enhancing the diagnosis and treatment of these disorders in pregnant women.
ACKNOWLEDGEMENTS
This research was supported by awards from the National Institutes of Health (R01HD-055566 and R01HD-32562). The authors are indebted to the staff of the Center for Perinatal Studies for their expert technical assistance.
Footnotes
COMPETING INTERESTS
The authors declare that they have no competing interests.
Contributor Information
Michelle A. Williams, Email: mwilliam@uw.edu.
Bizu Gelaye, Email: mirt@uw.edu.
Chunfang Qiu, Email: Chun-fag.Qiu@swedish.org.
Neway Fida, Email: newayg@yahoo.com.
Swee May Cripe, Email: smtang@uw.edu.
REFERENCES
- 1.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360(9328):237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Netzer NC, Hoegel JJ, Loube D, Netzer CM, Hay B, Alvarez-Sala R, Strohl KP. Prevalence of symptoms and risk of sleep apnea in primary care. Chest. 2003;124(4):1406–1414. doi: 10.1378/chest.124.4.1406. [DOI] [PubMed] [Google Scholar]
- 4.Kalra M, Inge T, Garcia V, Daniels S, Lawson L, Curti R, Cohen A, Amin R. Obstructive sleep apnea in extremely overweight adolescents undergoing bariatric surgery. Obes Res. 2005;13(7):1175–1179. doi: 10.1038/oby.2005.139. [DOI] [PubMed] [Google Scholar]
- 5.Colten HR, Altevogt BM Institute of Medicine. Washington, DC: Institute of Medicine : National Academies Press; Committee on Sleep M, Research: Sleep disorders and sleep deprivation : an unmet public health problem. 2006 [PubMed]
- 6.Loube DI, Poceta JS, Morales MC, Peacock MD, Mitler MM. Self-reported snoring in pregnancy. Association with fetal outcome. Chest. 1996;109(4):885–889. doi: 10.1378/chest.109.4.885. [DOI] [PubMed] [Google Scholar]
- 7.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 8.Schoenfeld A, Ovadia Y, Neri A, Freedman S. Obstructive sleep apnea (OSA)-implications in maternal-fetal medicine. A hypothesis. Med Hypotheses. 1989;30(1):51–54. doi: 10.1016/0306-9877(89)90125-4. [DOI] [PubMed] [Google Scholar]
- 9.Kakkar RK, Berry RB. Asthma and obstructive sleep apnea: at different ends of the same airway? Chest. 2009;135(5):1115–1116. doi: 10.1378/chest.08-2778. [DOI] [PubMed] [Google Scholar]
- 10.Teodorescu M, Consens FB, Bria WF, Coffey MJ, McMorris MS, Weatherwax KJ, Palmisano J, Senger CM, Ye Y, Kalbfleisch JD, et al. Predictors of habitual snoring and obstructive sleep apnea risk in patients with asthma. Chest. 2009;135(5):1125–1132. doi: 10.1378/chest.08-1273. [DOI] [PubMed] [Google Scholar]
- 11.Payne RJ, Kost KM, Frenkiel S, Zeitouni AG, Sejean G, Sweet RC, Naor N, Hernandez L, Kimoff RJ. Laryngeal inflammation assessed using the reflux finding score in obstructive sleep apnea. Otolaryngol Head Neck Surg. 2006;134(5):836–842. doi: 10.1016/j.otohns.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Hudgel DW, Shucard DW. Coexistence of sleep apnea and asthma resulting in severe sleep hypoxemia. JAMA. 1979;242(25):2789–2790. [PubMed] [Google Scholar]
- 13.Catterall JR, Douglas NJ, Calverley PM, Brash HM, Brezinova V, Shapiro CM, Flenley DC. Irregular breathing and hypoxaemia during sleep in chronic stable asthma. Lancet. 1982;1(8267):301–304. doi: 10.1016/s0140-6736(82)91567-7. [DOI] [PubMed] [Google Scholar]
- 14.Fitzpatrick MF, Martin K, Fossey E, Shapiro CM, Elton RA, Douglas NJ. Snoring, asthma and sleep disturbance in Britain: a community-based survey. Eur Respir J. 1993;6(4):531–535. [PubMed] [Google Scholar]
- 15.Janson C, De Backer W, Gislason T, Plaschke P, Bjornsson E, Hetta J, Kristbjarnarson H, Vermeire P, Boman G. Increased prevalence of sleep disturbances and daytime sleepiness in subjects with bronchial asthma: a population study of young adults in three European countries. Eur Respir J. 1996;9(10):2132–2138. doi: 10.1183/09031936.96.09102132. [DOI] [PubMed] [Google Scholar]
- 16.Mansfield LE, Diaz G, Posey CR, Flores-Neder J. Sleep disordered breathing and daytime quality of life in children with allergic rhinitis during treatment with intranasal budesonide. Ann Allergy Asthma Immunol. 2004;92(2):240–244. doi: 10.1016/S1081-1206(10)61554-2. [DOI] [PubMed] [Google Scholar]
- 17.Ross KR, Hart MA, Storfer-Isser A, Kibler AM, Johnson NL, Rosen CL, Kercsmar CM, Redline S. Obesity and obesity related co-morbidities in a referral population of children with asthma. Pediatr Pulmonol. 2009;44(9):877–884. doi: 10.1002/ppul.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan CS, Woolcock AJ, Sullivan CE. Nocturnal asthma: role of snoring and obstructive sleep apnea. Am Rev Respir Dis. 1988;137(6):1502–1504. doi: 10.1164/ajrccm/137.6.1502. [DOI] [PubMed] [Google Scholar]
- 19.Ciftci TU, Ciftci B, Guven SF, Kokturk O, Turktas H. Effect of nasal continuous positive airway pressure in uncontrolled nocturnal asthmatic patients with obstructive sleep apnea syndrome. Respir Med. 2005;99(5):529–534. doi: 10.1016/j.rmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Guilleminault C, Quera-Salva MA, Powell N, Riley R, Romaker A, Partinen M, Baldwin R, Nino-Murcia G. Nocturnal asthma: snoring, small pharynx and nasal CPAP. Eur Respir J. 1988;1(10):902–907. [PubMed] [Google Scholar]
- 21.Lafond C, Series F, Lemiere C. Impact of CPAP on asthmatic patients with obstructive sleep apnoea. EUROPEAN RESPIRATORY JOURNAL. 2007;29(2):307–311. doi: 10.1183/09031936.00059706. [DOI] [PubMed] [Google Scholar]
- 22.National Heart, Lung Blood Institute. National Asthma Education Program. Expert Panel on the Management of, Asthma Expert Panel report 3 guidelines for the diagnosis and management of asthma. [ http://purl.access.gpo.gov/GPO/LPS93946]
- 23.Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;27(7):1405–1417. doi: 10.1093/sleep/27.7.1405. [DOI] [PubMed] [Google Scholar]
- 24.Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 36(4):849–855. doi: 10.1183/09031936.00021810. [DOI] [PubMed] [Google Scholar]
- 25.Facco FL, Grobman WA, Kramer J, Ho KH, Zee PC. Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. Am J Obstet Gynecol. 203(2):e141–e145. doi: 10.1016/j.ajog.2010.03.041. 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louis JM, Auckley D, Sokol RJ, Mercer BM. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol. 202(3):e261–e265. doi: 10.1016/j.ajog.2009.10.867. 261. [DOI] [PubMed] [Google Scholar]
- 27.Qiu C, Enquobahrie D, Frederick IO, Abetew D, Williams MA. Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: a pilot study. BMC Womens Health. 10:17. doi: 10.1186/1472-6874-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Champagne K, Schwartzman K, Opatrny L, Barriga P, Morin L, Mallozzi A, Benjamin A, Kimoff RJ. Obstructive sleep apnoea and its association with gestational hypertension. Eur Respir J. 2009;33(3):559–565. doi: 10.1183/09031936.00122607. [DOI] [PubMed] [Google Scholar]
- 29.Venkata C, Venkateshiah SB. Sleep-disordered breathing during pregnancy. J Am Board Fam Med. 2009;22(2):158–168. doi: 10.3122/jabfm.2009.02.080057. [DOI] [PubMed] [Google Scholar]
- 30.Gislason T, Benediktsdottir B, Bjornsson JK, Kjartansson G, Kjeld M, Kristbjarnarson H. Snoring, hypertension, and the sleep apnea syndrome. An epidemiologic survey of middle-aged women. Chest. 1993;103(4):1147–1151. doi: 10.1378/chest.103.4.1147. [DOI] [PubMed] [Google Scholar]
- 31.Larsson LG, Lindberg A, Franklin KA, Lundback B. Symptoms related to obstructive sleep apnoea are common in subjects with asthma, chronic bronchitis and rhinitis in a general population. Respir Med. 2001;95(5):423–429. doi: 10.1053/rmed.2001.1054. [DOI] [PubMed] [Google Scholar]
- 32.Auckley D, Moallem M, Shaman Z, Mustafa M. Findings of a Berlin Questionnaire survey: comparison between patients seen in an asthma clinic versus internal medicine clinic. Sleep Med. 2008;9(5):494–499. doi: 10.1016/j.sleep.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Knuiman M, James A, Divitini M, Bartholomew H. Longitudinal study of risk factors for habitual snoring in a general adult population: the Busselton Health Study. Chest. 2006;130(6):1779–1783. doi: 10.1378/chest.130.6.1779. [DOI] [PubMed] [Google Scholar]
- 34.Yigla M, Tov N, Solomonov A, Rubin AH, Harlev D. Difficult-to-control asthma and obstructive sleep apnea. J Asthma. 2003;40(8):865–871. doi: 10.1081/jas-120023577. [DOI] [PubMed] [Google Scholar]
- 35.Bohadana AB, Hannhart B, Teculescu DB. Nocturnal worsening of asthma and sleep-disordered breathing. J Asthma. 2002;39(2):85–100. doi: 10.1081/jas-120002190. [DOI] [PubMed] [Google Scholar]
- 36.Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481–485. doi: 10.1164/ajrccm.150.2.8049833. [DOI] [PubMed] [Google Scholar]
- 37.Leiter JC, Knuth SL, Bartlett D., Jr. The effect of sleep deprivation on activity of the genioglossus muscle. Am Rev Respir Dis. 1985;132(6):1242–1245. doi: 10.1164/arrd.1985.132.6.1242. [DOI] [PubMed] [Google Scholar]
- 38.Ballard RD, Irvin CG, Martin RJ, Pak J, Pandey R, White DP. Influence of sleep on lung volume in asthmatic patients and normal subjects. J Appl Physiol. 1990;68(5):2034–2041. doi: 10.1152/jappl.1990.68.5.2034. [DOI] [PubMed] [Google Scholar]
- 39.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol. 1988;65(5):2124–2131. doi: 10.1152/jappl.1988.65.5.2124. [DOI] [PubMed] [Google Scholar]
- 40.Reid MB, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: involvement of muscle myofilaments. Am J Respir Crit Care Med. 2002;166(4):479–484. doi: 10.1164/rccm.2202005. [DOI] [PubMed] [Google Scholar]
- 41.Silvestri M, Bontempelli M, Giacomelli M, Malerba M, Rossi GA, Di Stefano A, Rossi A, Ricciardolo FL. High serum levels of tumour necrosis factor-alpha and interleukin-8 in severe asthma: markers of systemic inflammation? Clin Exp Allergy. 2006;36(11):1373–1381. doi: 10.1111/j.1365-2222.2006.02502.x. [DOI] [PubMed] [Google Scholar]
- 42.Lateef TM, Merikangas KR, He J, Kalaydjian A, Khoromi S, Knight E, Nelson KB. Headache in a national sample of American children: prevalence and comorbidity. J Child Neurol. 2009;24(5):536–543. doi: 10.1177/0883073808327831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evaluation of National Health Interview Survey diagnostic reporting. Vital and health statistics Series 2, Data evaluation and methods research. 1994;(120):1–116. [PubMed] [Google Scholar]
- 44.Edwards WS, Winn DM, Collins JG. Evaluation of 2-week doctor visit reporting in the national health interview survey. Vital Health Stat 2. 1996;(122):1–46. [PubMed] [Google Scholar]
- 45.Jennum P, Hein HO, Suadicani P, Gyntelberg F. Risk of ischemic heart disease in self-reported snorers. A prospective study of 2,937 men aged 54 to 74 years: the Copenhagen Male Study. Chest. 1995;108(1):138–142. doi: 10.1378/chest.108.1.138. [DOI] [PubMed] [Google Scholar]