Abstract
All addictive drugs produce tolerance and addicts compensate by increasing drug exposure. Thus, the quantity of illicit drug ingested is related to the severity of addiction. Unfortunately, there are no objective methods to estimate intake for most addictive drugs. Using experimenter-administered doses of deuterium-labeled R-methamphetamine (R-[−]-MA-d3), we have developed a method to estimate the amount of abused methamphetamine intake in addicts enrolled in clinical trials. This study assessed the pharmacokinetics, pharmacodynamics, and tolerability of single oral doses of R-MA in healthy adults to select a dose of R-MA-d3 to be used as a biomarker for estimation the amount of methamphetamine abuse. This was a five-session randomized, double-blind, placebo-controlled, balanced crossover study in eight subjects. Oral R-(−)-MA was dosed at 0 mg, 1 mg, 2.5 mg, 5 mg, or 10 mg; bioavailability was estimated by slow intravenous dosing (30 minutes) of 2.5 mg R-(−)-MA-d3 given with the 2.5 mg R-(−)-MA oral dose condition. Pharmacokinetic and pharmacodynamic measures were obtained. No serious adverse events occurred during the study and all doses of R-MA were well tolerated. Linear pharmacokinetics was observed within our oral dose range of 1 to 10 mg. Complete bioavailability and pharmacologic inactivity were found for all oral doses. These characteristics indicate the advantage of using a small oral R-(−)-MA-d3 dose as a biomarker to estimate exposure to abused methamphetamine. Based on these results, 5 mg R-(−)-MA-d3 has been selected as the biomarker dose in future studies. Preliminary findings from our study indicate that experimenter-administered oral R-(−)-MA-d3 may allow estimation of abused methamphetamine intake and exposure. Knowledge of the quantity of methamphetamine intake may allow better estimation of disease severity and treatment efficacy. Experience gained from this study also can be applied to the management of other drug dependence problems such as cocaine, cannabinoid, and opiate addiction.
Keywords: methamphetamine, abuse, biomarker
INTRODUCTION
Methamphetamine (MA) addiction is a worldwide public health crisis. MA epidemics are occurring in the United States, Asia, northern Europe, and Australia.1 The MA epidemic continues to spread, producing increasing pressures on social, health, and criminal justice systems worldwide. There is a pressing need to develop treatments to manage this addictive disease with diminishing abused, illicit drug use as a primary goal.2 In pharmacotherapy trials, a decrease in the proportion of “positive” urinalysis tests is a key outcome measure that is used to infer therapeutic efficacy.3 However, this test is qualitative in nature, monitoring the presence or absence of illicit drug use in a binary fashion. The amount of illicit drug use is usually estimated by self-report, an often noisy measure that lacks objective validation. To address the need for a quantitative method to estimate the dose of illicit MA consumed by addicts, we have developed a method to provide a continuous and objective evaluation of the efficacy of the tested treatment.
MA has a chiral center with two stereoisomers, S-(+)-MA (commonly called d-MA) and R-(−)-MA (commonly called l-MA). Addicts generally prefer and abuse the S isomer.4 In the United States, almost all illicit MA is pure S-(+)-MA, although in southeast Asia, illicit racemic MA is found. R-(−)-MA is the active ingredient in the over-the-counter Vicks inhaler (called levmetamfetamine or l-desoxyephedrine by the manufacturer). Each inhaler contains 50 to 75 mg R-(−)-MA, a potentially psychoactive dose. Despite the easy availability of substantial amounts of R-(−)-MA in this product, reports of abuse are rare.
In previous studies of the pharmacology of R-(−)-MA, we found lesser subjective and cardiovascular effects than equivalent doses of S-(+)-MA despite similar pharmacokinetic (PK) profiles.4 These findings suggested that R-(−)-MA would not be psychoactive or abused, making R-(−)-MA oral dosing safe and ethical in addicts. The similar PK of S-(+)-MA and R-(−)-MA suggested that administering known doses of R-(−)-MA would allow estimation of the quantity of abused MA ingestion. Using R-(−)-MA as an “internal standard” for ingestion eliminates the need to correct for differences in absorption and excretion resulting from sex, race, age, weight, diet, and physiological parameters, including blood flow, urine flow, and especially body fluid pH. Thus, the aim of this study was to select a dose of R-(−)-MA to be used as a biomarker of MA exposure in clinical trials. This study defines the PK, pharmacodynamics, and tolerability of a range of oral doses of R-(−)-MA, a necessary first step in validating experimenter-administered R-(−)-MA as a method to quantify MA exposure. We defined the ideal dose for R-(−)-MA being a biomarker as that which produced low plasma levels that were reliably above the assay limit of quantification (5 ng/mL in plasma and 10 ng/mL in urine) and resulted in easily measured, relatively constant (across subjects) urinary levels from 24 to 48 hours after dosing.
METHODS
Subjects
Eight subjects participated in this study. The subjects were not dependent on any psychoactive drug (except nicotine or caffeine) according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Revised criteria. Inclusion criteria were age 18 to 50 years, no recent (less than 3 months) use of MA, and ability to give informed consent. Exclusion criteria included use of medications containing MA (eg, the Vicks inhaler) or medications that are metabolized to MA (eg, selegiline) within the last month and significant medical or psychiatric illnesses. The protocol was approved by the California Pacific Medical Center and University of California–San Francisco Institutional Review Boards and written informed consent was obtained for all subjects. The study was carried out in accordance with the Declaration of Helsinki.
Study Design
An inpatient, placebo-controlled five-session, partially Latin Square balanced, ascending-dose design study with four oral and one intravenous R-(−)-MA challenges was performed in eight healthy, non-MA-using subjects. All doses were given at least 1 hour after and at least 2 hours before food. Intravenous doses were slowly administered over 30 minutes by an infusion pump starting 1.5 hours after oral dosing. Intravenous doses were prepared by the research pharmacist with R-(−)-MA-d3 dissolved in an adequate volume of saline (usually 50 mL) to assure easy and accurate administration. The oral placebo was lactose; the intravenous placebo was 0.9% NaCl. The doses and conditions were: 1) 1 mg R-(−)-MA orally followed 1.5 hours later by a 30-minute placebo infusion; 2) 2.5 mg R-(−)-MA orally followed 1.5 hours later by a 30-minute infusion of 2.5 mg R-(−)-MA-d3; 3) 5 mg R-(−)-MA orally followed 1.5 hours later by a 30-minute placebo infusion; 4) 10 mg R-(−)-MA orally followed 1.5 hours later by a 30-minute placebo infusion; and 5) oral placebo followed 1.5 hours later by a 30-minute placebo infusion.
Because deuterium labeling does not alter the pharmacology of MA, we used unlabeled MA as the oral dose and labeled MA only for the intravenous dosing condition; in clinical trials, we will use deuterium-labeled MA.
Pharmacokinetic Measures
Sample Collection
Plasma samples were collected at 15 minutes before and 0.5, 1, 1.5, 2, 2.5, 3, 4, 8, 12, 24, and 48 hours after dosing. All urine was collected in 24-hour intervals with separate aliquots saved from each void.
Bioassay
Our published gas chromatography method was used to measure concentrations of R-(−)-MA and R-(−)-AMP in plasma and urine.5 MA and AMP levels in plasma or urine are measured simultaneously by gas chromatography mass spectrophotometry following conversion to the N-propyl derivatives. The p-methyl analogs of each analyte are used as internal standards. The method has sensitivity, precision, and accuracy suitable for measuring PK parameters in humans after modest doses of MA and has been applied to studies of the PKs of S-(+)-MA. The limit of quantitation in plasma is 2 ng/mL, and the limit of quantitation in urine is 10 ng/mL. Interday coefficients of variation for both analytes in plasma were less than 10% over the concentration range of 10 to 200 ng/mL with accuracy ranging from 92% to 100%. In urine, coefficients of variation were less than 15% over the range of 10 to 5000 ng/mL with accuracy ranging from 93% to 112%.
Pharmacokinetic Analysis
Pharmacokinetic parameters were calculated by the noncompartmental trapezoidal method using the software package WinNonlin Professional (Version 5.2; Pharsight, Mountain View, CA). The area under the plasma concentration–time curve (AUC) was calculated from time 0 hour to time of the last plasma sample and with extrapolation to infinity using the terminal rate constant k. Other parameters included terminal half-life, the maximal plasma concentration (Cmax), and the time to maximal concentration.
Pharmacodynamic Measures
Physiological Measures
Blood pressure, heart rate, skin and tympanic temperature, respiratory rate, and pulse oxygen saturation were monitored noninvasively at 15 minutes before and 0.25, 0.5, 0.75, 1.5, 1.75, 2, 2.5 3, 4, 6, 8, 12, 18, 24, 30, 36, and 48 hours after dosing.
Subjective Measures
Verbal ratings of global intoxication (on a scale ranging from 0 to 100) and visual analog ratings of various acute R-(−)-MA effects were obtained at the same time as plasma samples. Scales were anchored on the left with the 0 representing “not at all” and on the right with the 100 representing “extremely.” Visual analog items included: “any drug” effect, “good drug” effect, “drug liking,” “high,” “stimulated,” “clear-headed,” and “want more drug.” These items were selected based on significant effects in previous S-(+)-MA studies.4,6
Statistical Analysis
To assess dose-proportionality in R-(−)-MA PK, conventional bioequivalence criteria were used to determine differences between dose conditions. Analysis of variance will be done on log-transformed PK parameters to test for differences between dose levels. Dose proportionality will be stated if the 90% confidence interval of the ratio for dose-corrected AUC or Cmax is within the interval of 0.8 to 1.25.7 Statistical analyses were performed with GraphPad Prism Version 5 (GraphPad Software, San Diego, CA).
RESULTS
Tolerability of Methamphetamine
All R-(−)-MA doses were well tolerated and no serious adverse events occurred. Heart rate, systolic blood pressure, and diastolic blood pressure in each dose condition did not differ from placebo. No clinical significant changes in core temperature, respiratory rate, oxygen saturation, electrocardiography, physical examination, biochemical tests, or hematologic parameters were evident.
Pharmacokinetic Results
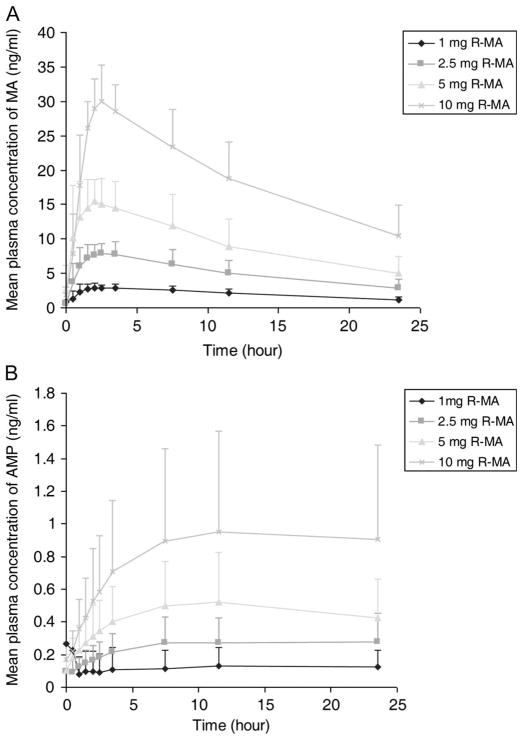
Plasma concentration of R-(−)-MA and the metabolite R-(−)-AMP increased proportionately with R-(−)-MA dosage from 1 mg to 10 mg; plasma concentration–time profiles are shown in Figure 1 and major PK parameters of R-(−)-MA are presented in Table 1. R-(−)-MA is rapidly absorbed with a time to maximal concentration of 1.95 ± 0.94 hour. Major PK parameters such as AUC and Cmax were normalized by dose correction under 2.5-mg, 5-mg, and 10-mg sessions. No significant difference in corrected AUC and Cmax were seen compared with the 1-mg session, suggesting linear PKs within our experimental dose range. The apparent clearance of R-(−)-MA was constant over the dose range; the average percent of oral R-(−)-MA excreted as parent drug and AMP is approximately 44% and 3%, respectively, which are similar to results found in our previous studies.8
FIGURE 1.
Mean plasma concentration–time curves for R-(−)-MA (A) and R-(−)-AMP (B) under different oral doses.
TABLE 1.
Pharmacokinetic Parameters for R-(−)-MA Under Different Oral Doses
| Parameters | 1 mg R-MA | 2.5 mg R-MA | 5 mg R-MA | 10 mg R-MA |
|---|---|---|---|---|
| AUC0–24 (ng·h/mL) | 47.6 ± 12.7 | 123.7 ± 39.5 | 223.4 ± 76.8 | 441.6 ± 99 |
| 1 | 0.97 (0.87–1.08) | 1.10 (0.97–1.24) | 1.06 (0.95–1.20) | |
| AUC0–infinity (ng·h/mL) | 73.0 ± 29.2 | 188.6 ± 89.0 | 325.4 ± 155.3 | 694.7 ± 325.2 |
| 1 | 0.98 (0.84–1.14) | 1.16 (0.96–1.40) | 1.05 (0.83–1.34) | |
| Cmax (ng/mL) | 3.3 ± 0.6 | 8.8 ± 1.4 | 17.8 ± 2.6 | 31.4 ± 5.2 |
| 1 | 0.94 (0.85–1.03) | 0.93 (0.83–1.03) | 1.05 (0.98–1.13) | |
| T1/2 (hours) | 14.1 ± 3.7 | 14.2 ± 4.4 | 12.2 ± 4.8 | 14.7 ± 6.3 |
| Tmax (hours) | 1.9 ± 1.1 | 2.1 ± 1.1 | 1.4 ± 0.8 | 2.4 ± 0.6 |
| CL (L/h) | 15.8 ± 6.5 | 15.5 ± 5.3 | 19.1 ± 11.1 | 16.3 ± 4.8 |
| Vd (L) | 297.2 ± 65.2 | 291.0 ± 70.0 | 288.5 ± 74.3 | 315.5 ± 72.9 |
| MA percent recovered in the urine (%) | 44.3 ± 18.4 | 40.8 ± 13.9 | 49.0 ± 17.8 | 42.6 ± 20.2 |
| AMP percent recovered in the urine (%) | 3.3 ± 3.3 | 2.8 ± 1.5 | 2.8 ± 1.3 | 2.5 ± 1.3 |
AUC, area under the concentration–time curve; Cmax, maximal plasma concentration; T1/2, half-life; Tmax, time to maximal concentration; CL, clearance; Vd, volume of distribution; MA, methamphetamine.
To estimate absolute bioavailability, we gave 2.5 mg R-(−)-MA-d3 intravenously with 2.5 mg R-(−)-MA orally. Major PK parameters for each subject are summarized in Table 2. The half-life is similar between the intravenous and oral routes. However, a larger AUCPO than AUCIV was seen. Thus, absolute bioavailability calculated based on AUCPO/AUCIV is close to 1 in this study, indicating complete absorption of the oral dose.
TABLE 2.
Pharmacokinetic (PK) Parameters for 2.5 Mg R-(−)-MA After Oral (PO) and Intravenous (IV) Administration
| PK Parameters | IV | PO |
|---|---|---|
| Cmax (ng/mL) | 11.03 ± 3.66 | 8.6 ± 1.1 |
| T1/2 (h) | 13.6 ± 4.4 | 13.6 ± 4.3 |
| AUC0–t (ng·h/mL) | 108.5 ± 28.2 | 123.3 ± 43.0 |
Cmax, maximal plasma concentration; T1/2, time to maximal concentration; AUC, area under the concentration–time curve.
Pharmacodynamic Results
Heart rate, systolic blood pressure, and diastolic blood pressure in each dose condition were not affected by R-(−)-MA compared with placebo. Results of subjective measures were similar with no differences from placebo on visual analog scales or clinical parameters (Fig. 2). The lack of pharmacologic activity in this dose range indicates the advantage of using R-(−)-MA as a marker dose.
FIGURE 2.
Mean of maximal subjective response under different dose sessions (n = 8). MA, methamphetamine; po, orally; iv, intravenously.
DISCUSSION
In this study, we show that small oral doses of 1 to 10 mg R-(−)-MA are well tolerated, have no significant pharmacologic activity, and demonstrate linear PK and complete bioavailability. Based on these data, we have selected 5 mg as the preferred biomarker dose because it is the lowest dose that met all the requirements.
One puzzling outcome was the unexpected bioavailability of more than one for most subjects. This unexpected finding may be partly the result of the following reasons. First, some portion of the intravenous dose may have been lost during administration. A dead space in the intravenous tubing of approximately 2 mL was inherent in the design of the infusion system. Because the drug was dissolved in 50 mL saline, this might account for a 4% decrease in delivered dose. Second, we cannot rule out possible carryover effects from previous oral dose sessions or self-administered illicit MA use during the trial. Predose MA levels were detected in several subjects and with a level greater than 5% of the Cmax. Although AUC0–t was adjusted to them by subtracting the area contributed by the predose level to get closer to the actual value, data manipulation in such a manner will still introduce some variation.9 Finally, variance in assay results could account for up to a 10% systemic error. Overall, we estimate the percentage of error from all sources does not exceed 15% of the total dose. Thus, we still obtain a bioavailability of approximately 85% for the oral doses. Our previous study has also found a relatively high bioavailability of MA from intranasal or smoked administration with 79% and 67%, respectively.6 This relatively high oral bioavailability supports the use of R-(−)-MA as an orally administered marker dose.
In conclusion, this is the first study supporting development of a semiquantitative method for estimating abused drug exposure. R-(−)-MA is essentially completely absorbed and produces dose proportional increases in urine levels. We plan to apply the method in clinical trials, allowing continuous estimates of MA abuse during the trial. Using a known dose of a reference compound will help us to better control a variety of factors affecting the disposition of MA. This is the first step for a potentially important change in the evaluation of MA treatments efficacy. Knowledge of the quantity of MA intake may allow better estimation of disease severity and treatment efficacy. Knowledge gained from this study also can be applied to the management and assessment of disease severity in other addictions; cocaine, opiate, and cannabinoid addictions are natural targets.
References
- 1.Schifano F, Corkery JM, Cuffolo G. Smokable (‘ice,’ ‘crystal meth’) and non smokable amphetamine-type stimulants: clinical pharmacological and epidemiological issues, with special reference to the UK. Ann Ist Super Sanita. 2007;43:110–115. [PubMed] [Google Scholar]
- 2.Rose ME, Grant JE. Pharmacotherapy for methamphetamine dependence: a review of the pathophysiology of methamphetamine addiction and the theoretical basis and efficacy of pharmacotherapeutic interventions. Ann Clin Psychiatry. 2008;20:145–155. doi: 10.1080/10401230802177656. [DOI] [PubMed] [Google Scholar]
- 3.CPDD. Meeting. Washington, DC: Consensus statement on evaluation of outcome of pharmacotherapy for substance abuse/dependence; 1999. [Accessed March 30, 2010.]. Available at: www.cpdd.vcu.edu/Media/FactSheets/nida_cpdd_report.pdf. [Google Scholar]
- 4.Mendelson J, Uemura N, Harris D, et al. Human pharmacology of the methamphetamine stereoisomers. Clin Pharmacol Ther. 2006;80:403–420. doi: 10.1016/j.clpt.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Jacob P, III, Tisdale EC, Panganibanki K, et al. Gas chromatographic determination of methamphetamine and its metabolite amphetamine in human plasma and urine following conversion to N-propyl derivatives. J Chromatogr. 1995;664:449–457. doi: 10.1016/0378-4347(94)00479-o. [DOI] [PubMed] [Google Scholar]
- 6.Harris DS, Boxenbaum H, Everhart ET, et al. The bioavailability of intranasal and smoked methamphetamine. Clin Pharmacol Ther. 2003;74:475–486. doi: 10.1016/j.clpt.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Hummel J, McKendrick S, Brindley C, et al. Exploratory assessment of dose proportionality: review of current approaches and proposal for a practical criterion. Pharm Stat. 2009;8:38–49. doi: 10.1002/pst.326. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Everhart T, Fernandez E, et al. Formation of the para-hydroxy methamphetamine metabolite is not enantiomer specific. Reno, NV. The College on Problems of Drug Dependence 71st Annual Meeting; June 20–25, 2009. [Google Scholar]
- 9.Patterson S, Jones B. Dealing With Unexpected Challenges. Section 5.5 Carry-over. Chapman & Hall/CRC; 2006. Bioequivalence and statistics in clinical pharmacology; pp. 147–154. [Google Scholar]