Abstract
The capacity of immature B cells of the spleen and bone marrow to differentiate in vitro into cells representing mature end stage cells was investigated using BAFF and Notch pathway activators. Immature splenic and bone marrow B cells were found, in the presence of both of these activators, to mature into cells with follicular mature (FM) and marginal zone (MZ) cell phenotypes. Such cells were functionally responsive to B-cell-specific activation. The derivation in vitro of cells with a MZ phenotype was more robust from CD23− populations than CD23+ immature/transitional B cells suggesting a direct immature/T1 B cell to MZ cell differentiation pathway. Transcript analysis of the in vitro derived B cell populations demonstrated expression profiles similar to maturing B cells in vivo. FACS-purified populations of B220+CD19+CD21−CD23− cells from 2wk bone marrow gave rise to populations of CD21+CD23− cells with MZ cell phenotypes as well as CD21+CD23+ cells with FM cell phenotypes in percentages similar to those found in vivo. These data suggest that the commitment to a MZ and FM B cell phenotype is set prior to immature B cell release from the marrow.
Keywords: B cells, differentiation, bone marrow, spleen
Introduction
B cell development has been portrayed as proceeding in a linear fashion [1]; immature B cells migrate from the bone marrow to the spleen where they develop into transitional B cells (T1 and T2), which in turn give rise to marginal zone (MZ) and follicular mature (FM) B cells. Each of these developmental steps can be detected by its characteristic expression of cellular surface markers [2]. Meyer-Bahlburg and colleagues reported the identity of a T2 like cell (termed CD21int T2 B cell) that was responsive to the B cell activating cytokine BAFF (BlyS/TALL-1/zTNF4). This CD21int cell (which also expressed CD23) was shown to differentiate into CD23−MZ and CD23+FM end stage B cells [3]. Previously we observed that the transcriptional control sequences of the CD21 and CD23 genes were similar with common binding partners such that it was difficult to predict a pathway that would lead from a CD21+/CD23+ T2 cell into a CD21HI/CD23− MZ cell [4]. Instead we proposed that MZ cells were derived from CD21− and CD23−negative T1 cells, bypassing the T2 stage, allowing for the elevated expression of CD21 but repressing expression of CD23 [4]. Carey and colleagues recently demonstrated the potential of a direct lineage of N-deficient T1 B cells into a N-deficient MZ population [5]. Their data suggested mature MZ cells might be derived directly from T1 populations as well as via an intermediate T2 MZ precursor cell.
The BAFF and the Notch signaling pathways have emerged as critical signals for the development of mature splenic MZ cells. BAFF is a member of the TNF family (TNFSF13B) [6], which regulates B cell responses to BCR stimulation. BAFF functions by binding to three receptors: BR3 (BAFF receptor 3), BCMA (B cell maturation antigen), and TACI (transmembrane activator and CAML interactor) [7–10]. Animals lacking BAFF or the expression of BAFF-R demonstrate a deficiency of mature B cells in the spleen due to a maturation block between the early immature/T1 stages and the mature MZ and FM stages [11–14]. In the bone marrow, Hardy fractions A-D of marrow B cells do not express BAFF receptors but the Hardy fraction E cells express both BR3 and TACI [15]. The expression of BR3 and TACI is maintained on the surface of the T1, T2, T3, FO and MZ cells [15–17].
The Notch signaling pathway, specifically the Notch2 receptor, is essential for MZ B cell development [18]. Notch2-deficient animals lack MZ B cells but have normal numbers of FM cells [18]. A loss of MZ B cells is also seen in animals deficient for Delta-like-1 (DL1) (a Notch ligand) [19, 20], and RBPj, a DNA binding protein downstream of Notch signaling [21]. Animals lacking Notch2 or Dll1 show haplo-insufficiency for MZ B cell differentiation.
Making use of constitutive Notch signaling, Schmitt and Zúñiga-Pflücker developed an in vitro culture system capable of supporting T cell development from hematopoietic progenitor cells (HPC) [22]. This system utilized the OP9 bone marrow stromal line retrovirally transduced to express the Notch ligand, DL1. Using the DL1 cell line, Schmitt and Zúñiga-Pflücker were able to induce a normal progression of T cell development from HPCs. Derivation of T cells, however, occurred at the cost of B cell development; the culture system suppressed growth of HPCs into B cells. Alternatively, when pre-B cells from adult bone marrow were cultured on OP9 cells expressing DL1, they continued to develop into B cells. Thomas et al demonstrated the successful use of the OP9-DL1 expression system for delivering a Notch dependent signal to follicular B cells in culture. [23].
As described above, we and others have proposed that all, or some, of the MZ cells in the adult spleen fail to pass through a T2 CD21+/CD23+ intermediate cell stage but instead mature directly from immature bone marrow B cells (Hardy fraction E, CD23−) or splenic T1 CD21−/CD23− precursors into CD21+/CD23− MZ cells. To test this hypothesis we sought to develop an in vitro culture system where we could imitate in vivo B cell development. Using culture systems of immature splenic and marrow B cells in the presence of Notch ligand signaling and BAFF cytokine, we have developed such a culture system that demonstrates that the majority of cells that differentiate into a MZ phenotype do so from a CD23− intermediate cell, thus confirming the model of MZ cell derivation from immature bone marrow and splenic T1 intermediate cells. Interestingly immature B cells from the marrow cultured in the presence of Notch and BAFF signaling do not differentiate into the same cell type, but instead create a heterogeneous population of cells with phenotypes of both MZ and FM cells suggesting that the determination to create such lineages is pre-set prior to the migration of immature B cells into the peripheral lymphoid compartments.
Results
Derivation of B cells with MZ- and FM-like phenotypes from immature splenic B cells requires BAFF and Notch signaling
The in vitro differentiation of immature thymic T cells into mature cell types has been aided by the development of culture conditions that supply the key cues for T cell survival and development [22]. We sought to develop a similar system for the in vitro differentiation of immature bone marrow and splenic B cells. We reasoned that an in vitro culture system utilizing a stromal cell monolayer supplemented with exogenous BAFF and Notch signaling would facilitate both the survival of B cells in culture and their differentiation into end stage phenotypes.
The first experiments using these culture conditions were with total splenocytes obtained from 8wk or 2wk old C57BL/6 mouse spleens. Cells were cultured for 72h under these various culture conditions, RNA isolated and analyzed for the expression of B cell marker genes. The expression levels of CD21 and CD23 were elevated with BAFF signaling (Supplemental Figure 1). Expression levels of CD19 remained constant for the 8wk splenocytes but showed increased level of expression in both of the BAFF cultures of the 2wk splenocytes. These transcript data indicated that cells in culture maintain, or expand, the gene expression profiles they possessed at the beginning of culture conditions but did not inform us as to whether such cells were differentiating into end stage mature populations. To test for this possibility, culture experiments were repeated and evaluated with FACS analysis using antibodies capable of differentiating B cell subsets.
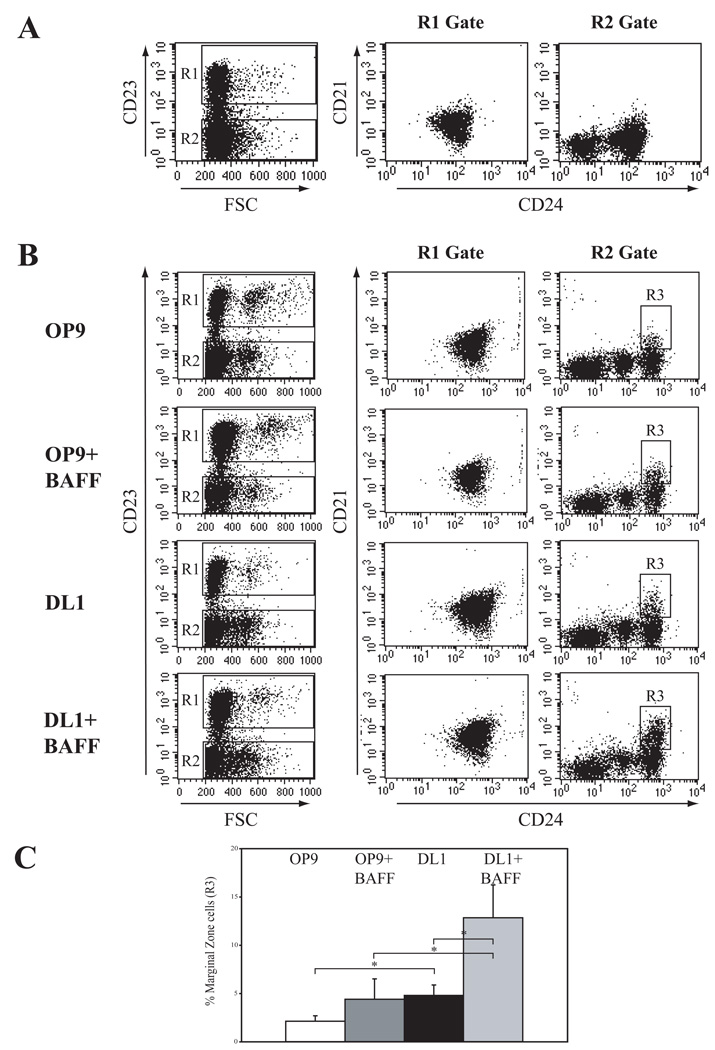
Total naïve splenocytes were obtained from immature 2wk old animals. Spleens from 2wk old animals are, when compared to mature animals, enriched for maturing T1 and T2 B cell subsets. As shown in Figure 1A, there are very few cells with a MZ phenotype (CD23−CD21HICD24+) present in the spleens of 2wk mice. Splenocytes from 2wk animals were then cultured for 72h in the presence or absence of BAFF and Notch (Figure 1B). CD23+ cells (R1 gate) and CD23− cells (R2 gate) were then analyzed for CD21 and CD24 expression to identify MZ B cell populations (R3 gate) and FM/T2 cells (R1 gate). Culturing cells with DL1+BAFF provided for a robust expansion of B cells with a MZ phenotype. The relative percentage of live, CD23− cells with the MZ phenotype (R3 gate) was quantified and found to be significantly increased (p≤ 0.05) in the DL1+BAFF cultures compared to the three other culture conditions (Figure 1C). On average, plating 5 × 106 total 2wk splenocytes within the DL1+BAFF culture resulted in 1×106 live cells after 72h, with B220+ cells comprising about 50% of this population. About 1% of the total cultured cells (1 × 104) possessed the MZ-like phenotype of CD23−CD21+CD24+. The robust response of the 2wk spleen sample to the DL1+BAFF culture allowed us to optimize the time required to maintain the viability of the B cells in culture as well as expand their differentiation (72h) (Supplemental Figure 2).
Figure 1. BAFF and Notch signaling combine to increase the presence of MZ-like B cells from spleens of immature animals.
(A) Freshly-isolated total cells from spleens of 2 wk-old mice were analyzed for CD23 expression, and CD23− cells (R1 gate) and CD23+ cells (R2 gate) were analyzed for CD21 and CD24 expression. (B) Flow cytometric analysis of splenocytes from 2 wk-old mice and cultured on OP9 and DL1 cell lines in the presence or absence of BAFF. To identify MZ-like B cells, live cells were gated on CD23 expression (FSC vs. CD23). CD23− cells (R2) were then examined for expression of CD21 and CD24. R3 delineates the area where MZ B cells would be found. Data shown are representative of four independent experiments. (C) Graph of the relative percentages of MZ-like B cells identified under different culture conditions from total cultured cells. Data mean + SD of four experiments. *p≤0.05, Student’s t-test.
Splenocytes taken from 2wk old mice derived in the DL1+BAFF culture were also analyzed for the expression of B cell markers, CD1d and surface IgD. In Supplemental Figure 3A, the derivation of a B220+CD1dHICD21+ population was evident in the DL1+BAFF culture. The number of CD21+IgD+ and CD23+IgD+ cells is markedly enhanced in the DL1+BAFF cultures (Supplemental Figure 3B).
Although the 2wk cultured splenic B cells assume the cell surface receptor expression profiles similar to mature B cell subsets, it was important to demonstrate that these cells were still responsive to BCR activation. Calcium flux assays were carried out using the 2wk splenic cultures maintained on either the OP9 cell line alone or with the DL1+BAFF for 72 h. Figure 2 demonstrates that IgM crosslinking of the DL1+BAFF cells resulted in calcium release comparable to freshly isolated mature splenocytes, while those cells cultured on the OP9 monolayer demonstrated a marked reduction of total calcium flux upon activation.
Figure 2. Immature B cells differentiated in the DL1+BAFF culture conditions maintain responsiveness to BCR stimulation.
Calcium flux responses were compared in splenocytes from 2 wk-old mice cultured with the DL1+BAFF (thick black line) to or on OP9 cells (black dashed line). The response of cultured cells was compared to the response from freshly isolated splenocytes from mature animals (shaded area). Ionomycin (maximal calcium release) (thin black line) was included as a positive control for Fluo-4. Arrow denotes time of introduction of α-IgM. Data are representative of three independent experiments.
Culture of mature splenic B cells in the presence of BAFF and Notch signaling fails to preferentially expand any mature B cell subset
The same culture conditions described above were used for the analysis of B cells from spleens of mature mice (Figure 3A). Splenocytes from the culture were analyzed for the expression of CD23, and the CD23+ cells (R1 gate) and CD23− cells (R2 gate) cells were further analyzed for the relative expression of CD21 and CD24. As shown in Figure 3B, similar percentages of CD23+ and CD23− cells were maintained in the OP9 and DL1 cell conditions, but the overall percentages of CD23+ cells increased with the addition of BAFF. The number of cells with the MZ phenotype (R3 gate) did not significantly change in any of the cultures indicating that mature MZ cells fail to respond to DL1+BAFF culture conditions, similar to the loss of proliferation potential found in MZ cells in vivo [24, 25].
Figure 3. Constitutive BAFF and Notch signaling does not significantly increase the numbers of MZ-like B cells in splenocytes from mature animals.
(A) Total cells from spleens of 8 wk-old mice were analyzed for CD23 expression, and CD23− cells (R1 gate) and CD23+ cells (R2 gate) were analyzed for CD21 and CD24 expression. (B) Flow cytometric analysis of splenocytes from 8wk old mice and cultured on OP9 and DL1 cell lines +/− BAFF. CD23− cells, based on FSC vs. CD23 (R2), were then examined for expression of CD21 and CD24. R3 delineates the area where MZ B cells would be found. Data are representative of three independent experiments.
Immature marrow B cells cultured with BAFF and Notch signaling also yield cells with MZ and FM phenotypes
Mouse bone marrow contains a significant population of CD19+ immature B cells that express low levels of the BAFF receptors, BR3 and TACI [15]. We chose to analyze marrow cells from 2wk old mice since the marrow lacks re-circulating mature B cells found in the marrow of mature animals (Figure 4A). Total marrow from 2wk old animals was cultured for 72 h under the conditions described above and analyzed (Figure 4B). There was a dramatic expansion in cells possessing the MZ phenotype (R3 gate) when cultured with the DL1+BAFF. The relative percentage of live, CD23− cells with the MZ phenotype (R3 gate) was quantified and found to be significantly increased (p≤ 0.05) in DL1+BAFF cultures compared to the three other culture conditions (data not shown). These data indicate that 2wk marrow immature B cells are responsive to both Notch ligand and BAFF signaling and have the potential to differentiate into cells with FM- and MZ-like phenotypes.
Figure 4. Cells with a MZ-like B cell phenotype can develop from bone marrow cells stimulated with BAFF and Notch.
(A) Total cells from bone marrow of 2 wk-old mice were analyzed for CD23 expression, and CD23− cells (R1 gate) and CD23+ cells (R2 gate) were then analyzed for CD21 and CD24 expression. (B) Flow cytometric analysis of bone marrow cells from 2 wk-old mice cultured on OP9 and DL1 cell lines+/− BAFF. Cells were first gated on CD23 expression (FSC vs. CD23). MZ B cells (R3) were identified from CD23− cells (R2) also stained with CD21 and CD24. Data are representative of three independent experiments.
We then tested the ability of cultured marrow cells (taken from 10wk animals and cultured in DL1+BAFF for 72 h) to maintain their normal B cell functions by analyzing their response to simulation with α-IgM and LPS. As shown in Supplemental Figure 4 activation by α-IgM and LPS resulted in the blast activation of the majority of the B220+ cells.
Derivation in vitro of B cells with a MZ phenotype cells from immature splenic and marrow CD23− B cells
The derivation of B cells with a mature phenotype from samples that are enriched for immature subsets could either be a result of the expansion of a small subset of cells or the differentiation of immature cells. We chose to address this question by analyzing the level of cellular proliferation within these cultures with CFSE labeling, and by culturing FACS-sorted purified B cell subsets.
If the expansion of B cells within the 2wk splenocyte culture was the result of the proliferation of a small subset of starting cells, then CFSE staining should demonstrate that cells in DL1+BAFF cultures had undergone numerous divisions. Alternatively, if the culture conditions were to induce cellular differentiation of the immature cells, division cycles might be muted. As shown in Figure 5, when 2wk splenocytes are labeled with CFSE and placed in either the OP9 or DL1+BAFF cultures for 72 h, there is a single burst of proliferation within the first 24 h. The majority of the live DL1+BAFF cells (Figure 5A,B) then cease division in the culture. Alternatively, the OP9 cultured cells undergo additional divisions. These data indicate that the bulk of the B220+ cells have only undergone one to two cycles of replication after 72 h of culture in the DL1+BAFF conditions.
Figure 5. Immature B cells demonstrate limited proliferation when differentiated in the presence of Notch and BAFF signaling.
Total splenocytes from 2wk animals were labeled with CFSE and placed in OP9 or DL1+BAFF cultures for 72 h. Histograms show fluctuations in CFSE intensity at 0, 24, 48, and 72 h. A) Labeled splenocytes (thick line) and unlabeled splenocytes (dashed line). B) B220+ labeled (thick line) and unlabeled splenocytes (dashed line). Data are representative of three independent experiments.
We next utilized FACS sorting of immature B cells to purify cells prior to in vitro DL1+BAFF culture. Live cells from 2wk spleen samples were sorted into B220+CD23+ and B220+CD23− populations (Figure 6A), maintained for 72 h in the DL1+BAFF culture, and analyzed by FACS (Figure 6B). The originally sorted B220+CD23− cells gave rise to a significant population of CD23+ cells (R1 gate) that, upon additional analysis, were also CD21+CD24+ (FM cells). The sorted B220+CD23− cells also maintained a population of CD23− cells (gate R2) that were either of the MZ (CD21HICD24+)(gate R3) or T1 (CD21−CD24+) phenotypes. Interestingly the B220+CD23+ sorted cells, cultured with DL1+BAFF, generated a population of CD23− cells that were also CD24+CD21HI (thus possessing the MZ phenotype). The same results were obtained from splenic B cell population purified by magnetic bead separation (Supplemental Figure 5). These data indicate that the majority of the cells possessing a MZ-like phenotype obtained in these culture conditions are consistently CD23−, but that a small subset of CD23−CD21HICD24+ cells can be derived from CD23+ precursor cells.
Figure 6. Highly purified CD23− B cell subsets give rise to MZ-like cells during in vitro culture.
(A) Total splenocytes were obtained from 2 wk-old C57BL/6 mice and enriched for B cells by CD90 depletion. These cells were then stained with B220 and CD23 and sorted into B220+CD23− and B220+CD23+ populations. (B) The B220+CD23− and B220+CD23+ populations were cultured for 72 h in the presence of BAFF and Notch and analyzed by flow cytometry for development of MZ-like B cells (R3). Data are representative of three independent experiments.
The in vitro culture analysis was also carried out using FACS sorted and purified bone marrow B cells. Cells were first gated upon the lack of surface expression of CD21 and CD23 (Figure 7A, left panel), and then sorted based upon the expression of both B220 and CD19 (right panel). This pool of cells would include the BR3+ cells of the Hardy E subset [15] as well as those of less differentiated BR3− marrow subsets (Hardy subsets B–D) [15, 26]. The sorted cells were grown either on the OP9 cells alone, or in the DL1+BAFF culture, and analyzed for MZ and FM cell phenotypes (Figure 7B). Immature B cells incubated with the OP9 culture retained the same surface phenotype as the original sorted cells, however the DL1+BAFF culture demonstrated an expansion of both CD23+CD21+ (T2 and FM phenotype) cells as well as the CD21HICD23− phenotype of MZ cells from the original CD23−/CD21− cells. Interestingly the DL1+BAFF culture of these B220+CD19+CD21−CD23− cells gave rise to both CD23+CD21+ and CD23−CD21+ cells suggesting that the fate of such cells to become MZ or FM cells was determined prior to their incubation in the DL1+BAFF culture conditions.
Figure 7. Highly purified CD21−CD23− immature bone marrow B cells give rise to MZ-like cells during in vitro culture.
(A) Total bone marrow cells from 2 wk-old C57BL/6 animals were enriched for B cells by CD90 depletion. These cells were then stained with CD23 and CD21; the CD21−CD23− population was analyzed for B220+CD19+ cells, which were FACS-purified for culture. (B) The CD19+B220+CD21−CD23− sorted marrow cells were cultured on either OP9 monolayer alone, or on DL1 monolayer with the addition of BAFF for 72 h, and analyzed by flow cytometry for development of FM and MZ-like B cells. Data are representative of three independent experiments. Line in the lower four plots indicates demarcation between CD21− and CD21+ cells.
Transcriptional profiling of in vitro derived mature B cells
The DL1+BAFF culture conditions with immature B cells produced an increased number of cells with MZ-like phenotype. We sought to determine if such cells were bone fide MZ cells or simply an intermediate step towards a mature MZ phenotype. One method to accomplish this was to isolate MZ-like cells from the DL1+BAFF cultured splenocytes and compare their gene expression profiles with purified mature B cells. Accordingly, MZ-like cultured cells and freshly isolated mouse MZ and FM splenic B cells were highly purified by FACS sorting and interrogated by quantitative PCR. As shown in Figure 8, the expression level of CD21 by the in vitro derived cells more closely matched that of purified FM cells than MZ cells, while the expression of CD23 more closely matched that of the in vivo purified MZ cells. CD19 expression was also elevated in the MZ sample while the cultured cells and FM cells demonstrated equivalent levels of expression. We then examined the expression of a number of genes have been shown to be preferentially expressed in MZ cells, such as CD9, Dtx1, Marcks, Sema7a, Abcb1a, Dtx1 and CD81 [18, 27, 28] (Figure 8). Expression of these genes demonstrates variable expression in the cultured cell samples compared to the purified MZ and FM populations. Thus while the cultured MZ-like cells possess the cell surface protein phenotype associated with in vivo cells, they have not yet attained an expression profile identical to that found in cells in vivo.
Figure 8. Expression levels of MZ-specific genes in MZ-like B cells developed in culture compared to vivo derived MZ and FM B cells.
DL1+BAFF cultured MZ cells were obtained by culturing total splenocytes from 2 wk-old mice for 72 h in the presence of Notch and BAFF. Freshly isolated MZ and FM cells were obtained from 10–12 wk-old mice by FACS. RNA was isolated from each set of cells and used in RT-PCR analysis of MZ-specific genes. Gene expression levels shown are relative to 1000 copies of β-actin and show mean + SD from three independent experiments. *p≤0.05, Student’s t-test. (A) Expression levels of CD21, CD23, CD19, Dtx1, Marcks, Sema7a, and CD81 between cultured MZ-like cells and freshly isolated MZ and FM B cells. (B) Expression levels for Abcb1a and CD9 between each set of cells.
Previously we defined a series of genes using grid array analysis whose expression was preferentially induced or extinguished during in vivo B cell maturation [28]. We selected a number of these indicator genes to probe gene expression patterns in cells obtained from the DL1+BAFF cultures. Three genes that show reduced expression with the onset of B cell maturation are Bst1, E2F7, and Tcf19; the expression of these genes is also depressed in the marrow and spleen samples in the cultured cells compared to the controls (Supplemental Figure 6). Alternatively, Spic, Klf12, Icosl, Mef2c and Zfp318 demonstrate elevated expression during B cell maturation, and the expression of these genes is also elevated in the cells cultured in the DL1+BAFF media compared to controls.
Discussion
In this report we detail the phenotypes of maturing B cells cultured in the presence of constitutive Notch and BAFF activation. We have demonstrated that immature marrow and spleen B cell populations can be induced to assume FM- and MZ-like phenotypes in such cultures, and that gene expression profiles derived from such cells confirm their progressive maturation and differentiation.
A variety of culture systems have been used in the past for the development of murine and human B cells. Most of these have defined requirements for development of B cell precursors from bone marrow intermediates [29, 30]. Alternatively, a variety of conditions have been defined for maintaining mature B cells in culture and facilitating isotype switching following activation [31–34]. To the best of our knowledge, our report is the first that attempts to derive mature MZ and FM cells in vitro from immature and transitional splenic and marrow B cell precursors.
The B cell culture system we have developed consists of three critical components. First, the cells are maintained on a monolayer, the OP9 cell line [35, 36]. This cell line has been previously shown to facilitate the maturation of B cells from lymphoid precursors into B220+ cells that secrete IgM after induction with LPS and IL-7 [37]. Second, the culture system utilized continuous Notch signaling via the use of the OP9 line constitutively expressing the Delta-like-1 Notch ligand. Third, the BAFF cytokine was included within this culture to enhance cell survival and facilitate the differentiation of immature B cells.
The use of the DL1+BAFF culture system demonstrated that both Notch and BAFF signaling were required for optimal outgrowth of the populations of B cells with the FM- and MZ-like phenotypes. The MZ-like cells derived in the DL1+BAFF culture system described in this report possess virtually the same cell surface expression phenotype as MZ cells isolated freshly from the animal. To determine if the cultured cells shared the functional potential of native MZ cells, we compared the gene expression profiles of the MZ-like cells obtained from the DL1+BAFF with wild type MZ and FM cells obtained from naïve spleens. This comparison indicated the cultured cells expressed the expected genes but did not do so in total accordance with the cell populations (Figure 8) suggesting that the in vitro culture system appropriately induces the differentiation of the MZ cells but that a final maturation signal(s) present in vivo is missing. Such a signal could include tonic BCR activation [39], LPS and/or CD40 signaling [32, 33] or cytokine/chemokine activation [40].
The Guidos lab recently suggested that optimal Notch signaling is required for MZ cell development [38] thus our culture system (especially for the 2wk marrow population) could have been predicted to only generate MZ cells (CD23−CD21HI) and not FM cells (CD23+/CD21+/CD24lo). However, the generation of both CD23+CD21+ and CD23−CD21HI cells from FACS sorted immature marrow populations (B220+CD19+CD21−CD23−) (Figure 7) as well as immature splenocytes (Figure 6) suggests the DL1+BAFF culture conditions serve to aid in the differentiation of cells with predetermined fates. Thus the potential of an immature B cell leaving the marrow to assume a MZ or FM phenotype may be set prior to the entry of the cells into the spleen and not directly due to the micro-environment of the spleen that the immature cell finds itself.
Past models of MZ and FM cell development have predicted a linear model in which immature B cells enter the spleen after release from the marrow, taking on a T1 phenotype [1]. These cells then, via the action of BAFF, mature to a T2 (and T3) stage cell. The pathway then bifurcates with one branch to the MZ cell and the second to FM cells [41]. More recent models have taken into account that T3 cells may well be anergic B cells [42]. An alternative model first proposed based upon transcriptional control pathways, and later confirmed by analysis of N-diversity regions suggests that MZ cells may be totally, or in part, generated directly from the T1 cells without first cycling through a T2 CD23+ intermediate [4, 5]. This model can also be predicted from mathematical modeling of maturing B cell populations [43]. Our ability to generate MZ-like cells in vitro with the DL1+BAFF culture allowed us to directly test this question; do MZ cells require a T2 intermediate, or can they be directly derived from a CD23− T1 cell? The data presented in this report suggests that the bulk of cells with the MZ phenotype will be derived from CD23− cells and will not cycle through a CD23+ intermediate step. However, a small population of CD23+ B cells also possesses the potential to generate cells with a MZ phenotype. This pathway is indicative of the canonical T1 to T2 to MZ or FM pathway. Thus both pathways can generate cells with a MZ-like phenotype although the alternative T1 to MZ pathway is likely to be more robust in the presence of BAFF and Notch signaling.
Materials and Methods
Mice and Cell Isolation
C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained at the Comparative Medicine Facility (University of Utah), according to Protocol #07-7004. Total splenocytes and bone marrow cells were isolated as previously described [2]. For CD23+ cell purification with magnetic beads, total splenocytes were resuspended in labeling buffer, labeled with CD23 biotinylated antibody (B3B4, eBioscience, San Diego, CA), washed and incubated with streptavidin microbeads (Miltenyi Biotec Inc., Auburn, CA) and separated into CD23− and CD23+ populations. This method yielded a CD23+ cell population that was ≥95% pure while the CD23− cell population was ≥80% pure (data not shown). To select for only B cells, total splenocytes were sorted into B220+CD23− and B220+CD23+ populations using the FACSVantage (BD Biosciences)(B220, RA3-6B2, BD Biosciences, San Jose, CA)). Total splenocytes were depleted of T cells by magnetic separation with CD90 magnetic beads (Miltenyi Biotec Inc.). Remaining cells were then stained with B220 and CD23 and then sorted into each population. A similar B220 bead enrichment strategy was followed to sort B220+CD19+CD21−CD23− bone marrow cells for use in the OP9 and DL1+BAFF cultures (CD19,eBio1D3, eBioscience: CD21, eBio8D9, eBioscience). MZ and FM cells were sorted as described in the manuscript.
In vitro B cell culture system
OP9 and OP9-DL1 cell lines were maintained in high glucose DMEM (Invitrogen, Carlsbad, CA) containing 20% bovine growth serum (HyClone, Logan, UT) and Pen-strep (Invitrogen). On Day 0, 5×106 total splenocytes or sorted cells from 2-week and 8-week old mice were plated on 80–90% confluent stromal lines in RPMI (10% bovine growth serum, Pen-strep). A single dose of BAFF (0.5ug/ml, Peprotech, Rocky Hill, NJ) was added at day zero [44]. On Day 3, splenocytes were removed from culture and analyzed as described.
Flow Cytometry
Cultured cells were isolated and stained with antibodies to the following cell surface proteins: CD21 (7G6, BDBiosciences), CD23, CD24 (M1/69, BDBiosciences), CD1d (1B, eBioscience), B220 (RA3-6B2, eBioscience), and IgD (11–26, eBioscience) [2]. Mature B cells were identified using antibody cocktails with CD23/CD21/CD24, B220/CD21/Cd1d, CD21/IgD, and CD23/IgD. Samples were stained with TO-PRO 3 (Invitrogen) for viability. Cells were then analyzed with the FACSCalibur and data was plotted using CellQuest Pro (BD Biosciences). During analysis, contaminating stromal cells were eliminated with FSC v SSC gates. Plots are representative of 10,000 live events.
RNA isolation and RT-PCR
RNA was isolated from cultured cells using the Illustra RNAspin mini RNA isolation kit from GE Healthcare (Buckinghamshire, England). Total isolated RNA was reverse transcribed into cDNA, providing a template for quantitative PCR using the Roche LightCyler (Indianapolis, IN). Oligonucleotides used for quantitative PCR are listed in Supplemental.
In Vitro Cell Proliferation Assay with CFSE
Total splenocytes were isolated from 2 wk mice, RBCs lysed and remaining cells resuspended at 1×106 cells/ml final concentration in pre-warmed (37°C) 1X PBS + 0.1% BSA. CFSE was added (CellTrace CFSE cell proliferation kit, C34554, Invitrogen) at a 1 µM final concentration and the cells were incubated at 37°C in the dark for 10 min. CFSE labeling was quenched by adding 5 volumes of ice-cold RPMI to the cells and incubating them on ice for 5 min. After incubation, cells were spun down and washed 3X in RPMI and plated at 5×106 cells/well in a 6-well dish on either OP9 or DL1 cells in the presence of BAFF as previously described. Variations in the intensity of CFSE labeling were detected with the FACSCalibur at 0, 24, 48, and 72 h. Cells were collected at each time point and further stained with B220 to identify labeled B cell populations in the mixed splenocyte culture. CFSE-labeled 2PK3 cells and unlabeled, cultured splenocytes were included as controls.
Calcium Flux Assay
Responses of in vivo and in vitro-derived cell populations to BCR stimulation were determined by analyzing changes in the fluorescence levels of Fluo-4, AM (F-14217, Invitrogen) over time as previously described [45]. For analysis, changes in Fluo-4 fluorescence were detected using the FACSCanto (BD Biosciences) for continuous data acquisition. Cells were stimulated with 10 µg of goat F(ab’)2 anti-mouse IgM (1022-01, Southern Biotech, Birmingham, AL). Ionomycin (#407950, Calbiochem/EMD Biosciences, Gibbstown, NJ) was used as a control for Fluo-4 loading. The kinetics of the calcium response was plotted using FlowJo (Tree Star, Inc., Oregon). Data are shown as a percentage of responding cells with the response threshold set by treating 98% of cells in the baseline acquisition period as non-responders.
Supplementary Material
Acknowledgements
The authors would like to thank members of both Weis labs for their insightful and stimulating critiques of this work. This work was supported by grants from the National Institute of Allergy and Infectious Diseases (AI-24158, JHW: AI-32223, JJW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Institute of Allergy and Infectious Diseases or the National Institutes of Health. A.C.J. was supported by the Training Program in Microbial Pathogenesis, 5T32-AI-055434. We would like to thank J.C. Zuniga-Pflucker for making the OP9 and DL1 lines available for our use.
Footnotes
Conflict of interest.
There are no conflicts of interest for any of the authors.
References
- 1.Allman D, Pillai S. Peripheral B cell subsets. Curr Opin Immunol. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roundy KM, Spangrude G, Weis JJ, Weis JH. Partial rescue of B cells in microphthalmic osteopetrotic marrow by loss of response to type I IFNs. Int Immunol. 2005;17:1495–1503. doi: 10.1093/intimm/dxh327. [DOI] [PubMed] [Google Scholar]
- 3.Meyer-Bahlburg A, Andrews SF, Yu KO, Porcelli SA, Rawlings DJ. Characterization of a late transitional B cell population highly sensitive to BAFF-mediated homeostatic proliferation. J Exp Med. 2008;205:155–168. doi: 10.1084/jem.20071088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debnath I, Roundy KM, Weis JJ, Weis JH. Defining in vivo transcription factor complexes of the murine CD21 and CD23 genes. J Immunol. 2007;178:7139–7150. doi: 10.4049/jimmunol.178.11.7139. [DOI] [PubMed] [Google Scholar]
- 5.Carey JB, Moffatt-Blue CS, Watson LC, Gavin AL, Feeney AJ. Repertoire-based selection into the marginal zone compartment during B cell development. J Exp Med. 2008;205:2043–2052. doi: 10.1084/jem.20080559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancro MP. The BLyS family of ligands and receptors: an archetype for niche-specific homeostatic regulation. Immunol Rev. 2004;202:237–249. doi: 10.1111/j.0105-2896.2004.00212.x. [DOI] [PubMed] [Google Scholar]
- 7.Kalled SL. Impact of the BAFF/BR3 axis on B cell survival, germinal center maintenance and antibody production. Semin Immunol. 2006;18:290–296. doi: 10.1016/j.smim.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, Hession C, Schneider P, Sizing ID, Mullen C, Strauch K, Zafari M, Benjamin CD, Tschopp J, Browning JL, Ambrose C. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 9.Thompson JS, Schneider P, Kalled SL, Wang L, Lefevre EA, Cachero TG, MacKay F, Bixler SA, Zafari M, Liu ZY, Woodcock SA, Qian F, Batten M, Madry C, Richard Y, Benjamin CD, Browning JL, Tsapis A, Tschopp J, Ambrose C. BAFF binds to the tumor necrosis factor receptor-like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J Exp Med. 2000;192:129–135. doi: 10.1084/jem.192.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y, Bressette D, Carrell JA, Kaufman T, Feng P, Taylor K, Gan Y, Cho YH, Garcia AD, Gollatz E, Dimke D, LaFleur D, Migone TS, Nardelli B, Wei P, Ruben SM, Ullrich SJ, Olsen HS, Kanakaraj P, Moore PA, Baker KP. Tumor necrosis factor (TNF) receptor superfamily member TACI is a high affinity receptor for TNF family members APRIL and BLyS. J Biol Chem. 2000;275:35478–35485. doi: 10.1074/jbc.M005224200. [DOI] [PubMed] [Google Scholar]
- 11.Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, Rixon M, Schou O, Foley KP, Haugen H, McMillen S, Waggie K, Schreckhise RW, Shoemaker K, Vu T, Moore M, Grossman A, Clegg CH. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 12.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J Immunol. 2004;173:2245–2252. doi: 10.4049/jimmunol.173.4.2245. [DOI] [PubMed] [Google Scholar]
- 14.Amanna IJ, Dingwall JP, Hayes CE. Enforced bcl-xL gene expression restored splenic B lymphocyte development in BAFF-R mutant mice. J Immunol. 2003;170:4593–4600. doi: 10.4049/jimmunol.170.9.4593. [DOI] [PubMed] [Google Scholar]
- 15.Stadanlick JE, Kaileh M, Karnell FG, Scholz JL, Miller JP, Quinn WJ, 3rd, Brezski RJ, Treml LS, Jordan KA, Monroe JG, Sen R, Cancro MP. Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nat Immunol. 2008;9:1379–1387. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Park CS, Yoon SO, Li L, Hsu YM, Ambrose C, Choi YS. BAFF supports human B cell differentiation in the lymphoid follicles through distinct receptors. Int Immunol. 2005;17:779–788. doi: 10.1093/intimm/dxh259. [DOI] [PubMed] [Google Scholar]
- 17.O'Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin LL, Mantchev GT, Bram RJ, Noelle RJ. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saito T, Chiba S, Ichikawa M, Kunisato A, Asai T, Shimizu K, Yamaguchi T, Yamamoto G, Seo S, Kumano K, Nakagami-Yamaguchi E, Hamada Y, Aizawa S, Hirai H. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity. 2003;18:675–685. doi: 10.1016/s1074-7613(03)00111-0. [DOI] [PubMed] [Google Scholar]
- 19.Hozumi K, Negishi N, Suzuki D, Abe N, Sotomaru Y, Tamaoki N, Mailhos C, Ish-Horowicz D, Habu S, Owen MJ. Delta-like 1 is necessary for the generation of marginal zone B cells but not T cells in vivo. Nat Immunol. 2004;5:638–644. doi: 10.1038/ni1075. [DOI] [PubMed] [Google Scholar]
- 20.Kuroda K, Han H, Tani S, Tanigaki K, Tun T, Furukawa T, Taniguchi Y, Kurooka H, Hamada Y, Toyokuni S, Honjo T. Regulation of marginal zone B cell development by MINT, a suppressor of Notch/RBP-J signaling pathway. Immunity. 2003;18:301–312. doi: 10.1016/s1074-7613(03)00029-3. [DOI] [PubMed] [Google Scholar]
- 21.Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, Suzuki A, Nakano T, Honjo T. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol. 2002;3:443–450. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 23.Thomas M, Calamito M, Srivastava B, Maillard I, Pear WS, Allman D. Notch activity synergizes with B-cell-receptor and CD40 signaling to enhance B-cell activation. Blood. 2007;109:3342–3350. doi: 10.1182/blood-2006-09-046698. [DOI] [PubMed] [Google Scholar]
- 24.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 25.Forster I, Muller W, Schittek B, Rajewsky K. Generation of long-lived B cells in germ-free mice. Eur J Immunol. 1991;21:1779–1782. doi: 10.1002/eji.1830210732. [DOI] [PubMed] [Google Scholar]
- 26.Rumfelt LL, Zhou Y, Rowley BM, Shinton SA, Hardy RR. Lineage specification and plasticity in CD19− early B cell precursors. J Exp Med. 2006;203:675–687. doi: 10.1084/jem.20052444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kin NW, Crawford DM, Liu J, Behrens TW, Kearney JF. DNA microarray gene expression profile of marginal zone versus follicular B cells and idiotype positive marginal zone B cells before and after immunization with Streptococcus pneumoniae. J Immunol. 2008;180:6663–6674. doi: 10.4049/jimmunol.180.10.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Debnath I, Roundy KM, Dunn DM, Weiss RB, Weis JJ, Weis JH. Defining a transcriptional fingerprint of murine splenic B-cell development. Genes Immun. 2008 doi: 10.1038/gene.2008.70. [DOI] [PubMed] [Google Scholar]
- 29.Whitlock CA, Witte ON. Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc Natl Acad Sci U S A. 1982;79:3608–3612. doi: 10.1073/pnas.79.11.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grawunder U, Melchers F, Rolink A. Interferon-gamma arrests proliferation and causes apoptosis in stromal cell/interleukin-7-dependent normal murine pre-B cell lines and clones in vitro, but does not induce differentiation to surface immunoglobulin-positive B cells. Eur J Immunol. 1993;23:544–551. doi: 10.1002/eji.1830230237. [DOI] [PubMed] [Google Scholar]
- 31.Hodgkin PD, Castle BE, Kehry MR. B cell differentiation induced by helper T cell membranes: evidence for sequential isotype switching and a requirement for lymphokines during proliferation. Eur J Immunol. 1994;24:239–246. doi: 10.1002/eji.1830240138. [DOI] [PubMed] [Google Scholar]
- 32.Maliszewski CR, Grabstein K, Fanslow WC, Armitage R, Spriggs MK, Sato TA. Recombinant CD40 ligand stimulation of murine B cell growth and differentiation: cooperative effects of cytokines. Eur J Immunol. 1993;23:1044–1049. doi: 10.1002/eji.1830230510. [DOI] [PubMed] [Google Scholar]
- 33.Armitage RJ, Macduff BM, Spriggs MK, Fanslow WC. Human B cell proliferation and Ig secretion induced by recombinant CD40 ligand are modulated by soluble cytokines. J Immunol. 1993;150:3671–3680. [PubMed] [Google Scholar]
- 34.Punnonen J, de Vries JE. IL-13 induces proliferation, Ig isotype switching, and Ig synthesis by immature human fetal B cells. J Immunol. 1994;152:1094–1102. [PubMed] [Google Scholar]
- 35.Nakano T. Lymphohematopoietic development from embryonic stem cells in vitro. Semin Immunol. 1995;7:197–203. doi: 10.1016/1044-5323(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 36.Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 37.Carlyle JR, Michie AM, Furlonger C, Nakano T, Lenardo MJ, Paige CJ, Zuniga-Pflucker JC. Identification of a novel developmental stage marking lineage commitment of progenitor thymocytes. J Exp Med. 1997;186:173–182. doi: 10.1084/jem.186.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan JB, Xu K, Cretegny K, Visan I, Yuan JS, Egan SE, Guidos CJ. Lunatic and manic fringe cooperatively enhance marginal zone B cell precursor competition for delta-like 1 in splenic endothelial niches. Immunity. 2009;30:254–263. doi: 10.1016/j.immuni.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 40.Cyster JG. Lymphoid organ development and cell migration. Immunol Rev. 2003;195:5–14. doi: 10.1034/j.1600-065x.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 41.Srivastava B, Quinn WJ, 3rd, Hazard K, Erikson J, Allman D. Characterization of marginal zone B cell precursors. J Exp Med. 2005;202:1225–1234. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, Cambier JC. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 43.Shahaf G, Allman D, Cancro MP, Mehr R. Screening of alternative models for transitional B cell maturation. Int Immunol. 2004;16:1081–1090. doi: 10.1093/intimm/dxh109. [DOI] [PubMed] [Google Scholar]
- 44.Woodland RT, Fox CJ, Schmidt MR, Hammerman PS, Opferman JT, Korsmeyer SJ, Hilbert DM, Thompson CB. Multiple signaling pathways promote B lymphocyte stimulator dependent B-cell growth and survival. Blood. 2008;111:750–760. doi: 10.1182/blood-2007-03-077222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacobson AC, Weis JJ, Weis JH. CD21 signaling via C3 regulates Purkinje cell protein 4 expression. Mol Immunol. 2009;46:1488–1493. doi: 10.1016/j.molimm.2008.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.