Abstract
Axonally specific microtubule-associated protein tau is an important component of neurofibrillary tangles found in AD (Alzheimer's disease) and other tauopathy diseases such as CTE (chronic traumatic encephalopathy). Such tau aggregate is found to be hyperphosphorylated and often proteolytically fragmented. Similarly, tau is degraded following TBI (traumatic brain injury). In the present study, we examined the dual vulnerability of tau to calpain and caspase-3 under neurotoxic and neurodegenerative conditions. We first identified three novel calpain cleavage sites in rat tau (four-repeat isoform) as Ser130↓Lys131, Gly157↓Ala158 and Arg380↓Glu381. Fragment-specific antibodies to target the major calpain-mediated TauBDP-35K (35 kDa tau-breakdown product) and the caspase-mediated TauBDP-45K respectively were developed. In rat cerebrocortical cultures treated with excitotoxin [NMDA (N-methyl-d-aspartate)], tau is significantly degraded into multiple fragments, including a dominant signal of calpain-mediated TauBDP-35K with minimal caspase-mediated TauBDP-45K. Following apoptosis-inducing EDTA treatment, tau was truncated only to TauBDP-48K/45K-exclusively by caspase. Cultures treated with another apoptosis inducer STS (staurosporine), dual fragmentation by calpain (TauBDP-35K) and caspase-3 (TauBDP-45K) was observed. Tau was also fragmented in injured rat cortex following TBI in vivo to BDPs of 45–42 kDa (minor), 35 kDa and 15 kDa, followed by TauBDP-25K. Calpain-mediated TauBDP-35K-specific antibody confirmed robust signals in the injured cortex, while caspase-mediated TauBDP-45K-specific antibody only detected faint signals. Furthermore, intravenous administration of a calpain-specific inhibitor SNJ-1945 strongly suppressed the TauBDP-35K formation. Taken together, these results suggest that tau protein is dually vulnerable to calpain and caspase-3 proteolysis under different neurotoxic and injury conditions.
Keywords: cell death, neurodegeneration, protease, tau protein, tauopathy, traumatic brain injury (TBI)
Abbreviations: AD, Alzheimer's disease; CCI, controlled cortical impact; CSF, colony-stimulating factor; CTE, chronic traumatic encephalopathy; DMEM, Dulbecco's modified Eagle's medium; DTT, dithiothreitol; NMDA, N-methyl-d-aspartate; STS, staurosporine; TAI, traumatic axonal injury; TauBDP-35K, 35 kDa tau-breakdown product; TBI, traumatic brain injury; TBST, TBS and 0.05% Tween-2
INTRODUCTION
Axonally specific microtubule-associated protein tau is an important factor in AD (Alzheimer's disease) as tau aggregate is found in a form of neurofibrillary tangle. Besides tau hyperphosphorylation, proteolytic processing of tau was suggested to facilitate tau deposit formation (Zemlan et al., 2003; Gabbita et al., 2005; Arnaud et al., 2009). Similarly, tau is also degraded following acute TBI (traumatic brain injury) and with neurotoxin exposure in vitro and in vivo (e.g. Methamphetamine and Ecstasy) (Warren et al., 2005, 2006, 2007; Arnaud et al., 2009). Siman et al. (2004) reported that tau BDP (breakdown product) can be detected in neuronal culture media following neurodegenerative challenge and in CSF (colony-stimulating factor) from human TBI patients. Several studies have also reported increased levels of tau protein in CSF from brain-injured patients (Zemlan et al., 2002; Franz et al., 2003) and from patients who experienced ischaemic stroke (Bitsch et al., 2002). A cleaved form of tau was specifically identified in the hippocampus, cortex after in vivo kainite administration and a rat model of TBI (Zemlan et al., 2003; Gabbita et al., 2005). However, the exact protease(s) involved in c-tau formation has not been elucidated.
There are two cellular cysteine proteases (calpain and caspase-3) that are capable of tau processing. Tau protein is a substrate for calpain in vitro (Johnson et al., 1989; Litersky et al., 1993; Yang and Ksiezak-Reding, 1995; Yen et al., 1999). Yang and Ksiezak-Reding (1995) and Yen et al. (1999) previously demonstrated that, under the in vitro digestion paradigm, calpain produces N-terminal truncation as well as a cleavage approx. 100 residues from the C-terminal of full-length four-repeat human tau (441 residues). Park and Ferreira (2005) reported that calpain could in fact produce a neurotoxic 17-kDa tau fragment. Zhang JY et al. (2009) also showed that autophagy inhibition in rat brain also cause tau proteolysis by calpain. Yet, specific calpain cleavage sites in tau protein have never been reported. Tau is also cleaved by caspase-3 in cultured neuronal cells under the apoptotic paradigms that mimic neurodegeneration (Canu et al., 1998; Chung et al., 2001; Rohn et al., 2002; Krishnamurthy and Sneige, 2002; Gamblin et al., 2003). It was further determined that tau was cleaved by caspase-3 in vitro at two major cleavage sites: between Asp25 and Gln26 and between Asp421 and Ser422 in human tau (Chung et al., 2001; Rohn et al., 2002). In rat, the first cleavage sequence is not conserved. Tau truncated at Asp421 is also found as a component of neurofibrillary tangle of Alzheimer's brain (Guillozet-Bongaarts et al., 2005).
In TBI and ischaemic brain injury, axons are highly vulnerable neuronal structures to mechanical and chemical insults (e.g. sodium and calcium homoeostasis disturbances) and excitotoxicity to the brain. Evidence of axonal damage following TBI has been documented extensively, and prolonged and sustained loss of white matter (Gale et al., 1995; Bramlett and Dietrich, 2002) and increased demyelination (Ng et al., 1994; Gale et al., 1995) have been detected, although the underlying biochemical mechanisms are not completely understood. Structurally, the damaged axon undergoes progressive changes including swelling, vacuolization and, occasionally, disconnection and fragmentation. Ultrastructural features, such as neurofilament compaction, misalignment and disassembly, microtubule loss, increased axolemmal permeability and mitochondrial swelling and disruption of cristae also occur (Christman et al., 1994; Pettus et al., 1994; Buki et al., 1999, 2000). TAI (traumatic axonal injury) is a consequence of a cascade of mechanical and biochemical events that have only recently begun to be elucidated. Increased permeability of the axolemma and subsequent Ca2+ influx initiate the activation of various proteases and mitochondrial dysfunction, leading to degradation of the axonal cytoskeleton and disturbances in axonal transport (Kampfl et al., 1997; Buki et al., 2000; Knoblach et al., 2002; Medana and Esiri, 2003). Wallerian degeneration has been documented following TBI in humans (Adams et al., 2000), but not in rodents. While TAI is recognized as an important pathological component of acute brain injury, the precise biochemical mechanisms of TAI are unknown. The microtubule-associated tau is preferentially localized in the axon (Binder et al., 1985; Kosik and Finch, 1987) and tau degradation is associated with axonal disruption (Higuchi et al., 2002).
In the present study, we sought to elucidate the major tau cleavage sites produced by calpain. We also test the hypothesis that tau protein might be differentially susceptible to proteolytic attack by calpain and caspase-3 respectively depending on the type of neurotoxic or neurodegenerative conditions.
MATERIALS AND METHODS
In vivo model of TBI
A CCI (controlled cortical impact) device was used to model TBI in rats (Dixon et al., 1991; Pike et al., 1998). It generated injured brain tissue including hippocampus and cortex. Adult male (280–300 g) Sprague–Dawley rats (Harlan, Indianapolis, IN, U.S.A.) were anaesthetized with 4% isoflurane in a carrier gas of 1:1 O2/N2O (4 min), followed by maintenance anaesthesia of 2.5% isoflurane in the same carrier gas. Core body temperature was monitored continuously by a rectal thermistor probe and maintained at 37±1°C by placing an adjustable temperature-controlled heating pad beneath the rats. Animals were mounted in a stereotactic frame in a prone position and secured by ear and incisor bars. A midline cranial incision was made, the soft tissues reflected and a unilateral (ipsilateral to site of impact) craniotomy (7 mm diameter) was performed adjacent to the central suture, midway between bregma and lambda. The dura matter was kept intact over the cortex. Brain trauma is produced by impacting the right cortex (ipsilateral cortex) with a 5 mm diameter aluminium impactor tip (housed in a pneumatic cylinder) at a velocity of 3.5 m/s with a 1.6 mm compression and 150 ms dwell time (compression duration). These injuries are associated with different magnitudes of local cortical contusion and more diffuse axonal damage. Velocity was controlled by adjusting the pressure (compressed N2) supplied to the pneumatic cylinder. Velocity and dwell time were measured by a linear velocity displacement transducer (Shaevitz™ model 500 HR; Lucas, Detroit, MI, U.S.A.) that produced an analogue signal that was recorded by a storage-trace oscilloscope (BK Precision, model 2522B; Placentia, CA, U.S.A.). Sham-injured control animals underwent identical surgical procedures but did not receive an impact injury. Appropriate pre- and post-injury management was maintained to ensure compliance with guidelines set forth by the University of Florida Institutional Animal Care and Use Committee and the National Institutes of Health guidelines detailed in the Guide for the Care and Use of Laboratory Animals. Different brain tissue regions were collected at a maximum of eight time points (2, 6, 24 h, and 2, 3, 5, 7, 14 days) after CCI, as described below.
Brain tissue collection and preparation
At the appropriate time-points (2, 6, 24 h, and 2, 3, 5, 7, 14 days) after CCI, animals were anaesthetized and immediately killed by decapitation. Brains were immediately removed, rinsed with ice-cold PBS and halved. The five different brain regions in right hemispheres (cerebrocortex, subcortical white matter, hippocampus and corpus callosum) were rapidly dissected, rinsed in ice cold PBS, snap-frozen in liquid nitrogen and frozen at −80°C until use. For the left hemisphere, the same tissue as the right side was collected. For Western-blot analysis, the brain samples were pulverized with a small mortar and pestle set over dry ice to a fine powder. The pulverized brain tissue powder was then lysed for 90 min at 4°C with 50 mM Tris/HCl (pH 7.4), 5 mM EDTA, 1% (v/v) Triton X-100, 1 mM DTT (dithiothreitol), 1×protease inhibitor cocktail (Roche Biochemicals). The brain lysates were then centrifuged at 15000 g for 5 min at 4°C to clear and remove insoluble debris, snap-frozen and stored at −85°C until used.
Rat primary cerebrocortical culture and neurotoxic challenges
Cerebrocortical cells harvested from 1-day old Sprague–Dawley rat brains were plated on poly-l-lysine coated on six-well culture plates (Erie Scientific, Portsmouth, NH, U.S.A.) according to a previously cited method (Nath et al., 1998) at a density of 4.36×105 cells/ml. Cultures were maintained in DMEM (Dulbecco's modified Eagle's medium) with 10% fetal bovine serum in a humidified incubator in an atmosphere of 10% CO2 at 37°C. After 5 days in culture, the medium was changed to DMEM with 5% horse serum. Subsequent media changes were performed thrice a week. Experiments were performed on days 10 to 11 in vitro when astroglia had formed a confluent monolayer beneath morphologically mature neurons.
Neurotoxic challenges and pharmacologic intervention
In addition to untreated controls, the following conditions were used: NMDA (N-methyl-d-aspartate; 300 μM; Sigma) for 3–24 h as an excitotoxic challenge (Nath et al., 2000); apoptotic inducers STS (staurosporine) (0.5 μM; Sigma, St. Louis, MO, U.S.A.) that activates calpain and caspase-3 for 24 h (Zhang Z et al., 2009); the Ca2+ chelator EDTA (5 mM; Sigma) for up to 24 h as a caspase-dominated challenge (Waterhouse et al., 1996; Chiesa et al., 1998; McGinnis et al., 1999; Zhang Z et al., 2009). For pharmacological intervention, cultures were pretreated 1 h before the STS, EDTA or NMDA challenge with the calpain inhibitor SNJ-1945 (Senju Pharmaceuticals, Kobe, Japan) (Shirasaki et al., 2005; Oka et al., 2006; Koumura et al., 2008), or the pan-caspase inhibitor Z-VAD (OMe)-FMK (R&D, Minneapolis, MN, U.S.A.). Cell lysates were collected and lysed with the same lysis buffer as described above.
In vitro protease digestion of tau in naïve brain lysate and pure recombinant rat tau
For these experiments, Brain tissue collection and preparation are the same as described above, except without the use of protease inhibitor cocktail (see above). In vitro protease digestion of naïve rat hippocampus lysate (30 μg) or purified recombinant human tau (Panvera Co., Madison, WI, U.S.A.) with purified proteases, human calpain-2 (BD Bioscience, Catalogue no. 208715, 1 μg/μl) and recombinant human caspase-3 (BD Bioscience, 1 unit/μl) was performed in a buffer containing 100 mM Tris/HCl (pH 7.4) and 20 mM DTT. For calpain-2, 2 mM CaCl2 was also added, and then incubated at room temperature (25°C) for 30 min. In addition, 2 mM EDTA was added for caspase-3 and the mixture was incubated at 37°C for 4 h. The protease reaction was stopped by the addition of PAGE-sample buffer.
Generation of calpain and caspase-3-specific TauBDP-antibodies
To ascertain whether tau fragments detected originated from calpain or caspase-3 proteolysis, we raised TauBDP-45K (caspase) and TauBDP-35K (calpain)-specific antibodies. A synthetic peptide (Cys-C6-SIDMVD-COOH) based on tau C-terminal of tauBDP-45K generated by caspase-3 (Chung et al., 2001) and another peptide (NH2-KDRTGN-C6-Cys) based on the new N-terminal of the calpain-mediated TauBDP-35K were custom made (California Peptide, Napa, CA, U.S.A.). A C6 linker and N-terminal cysteine were introduced for the subsequent coupling of the peptide to KLH (keyhole limpet haemocyanin) protein using a sulfo-link cross-linking reagent (Pierce). After coupling efficiency determinations, peptides were dialysed, concentrated, and 2 mg of conjugated protein was used for multiple antigen injections into two rabbits. After 3 months, collected serum samples from the rabbits were subjected to affinity purification using the same synthetic peptide coupled to sulfo-linked resins (Pierce). Affinity-purified antibody was dialysed against TBS (20 mM Tris/HCl, pH 7.4 and 150 mM NaCl), before it was concentrated and stored in 50% glycerol at −20°C.
Immunoblotting analysis
After SDS/PAGE in a Tris/glycine buffer system and electrotransfer, blotting membranes were blocked for 1 h at ambient temperature in 5% (w/v) non-fat dried skimmed milk powder in TBST (TBS and 0.05% Tween-2), then incubated in primary monoclonal tau antibody (Cedarlane, Tau-1, catalogue no. CLT9007) in TBST with 5% milk at 1:50 dilution as recommended by the manufacturer at 4°C overnight. This was followed by three washes with TBST and a 2 h incubation at ambient temperature with a secondary antibody linked to biotinylated secondary antibody (Amersham, catalogue no. RPN1177v1) followed by a 30 min incubation with streptavidin-conjugated alkaline phosphatase (colorimetric method). Then a colorimetric development was performed with a one-step BCIP (5-bromo-4-chloroindol-3-yl phosphate)/NBT (Nitro Blue Tetrazolium) reagent (KPL, catalogue no. 50-81-08). Molecular masses of intact tau protein and its potential breakdown products (TauBDPs) were assessed by running alongside rainbow-coloured molecular-mass standards (Amersham, catalogue no. RPN800V). When desired, Western blots were probed with polyclonal antibody specific to anti-activated calpain-1 new N-terminal (anti-NH2-LGRHENA) or pro-calpain-2 N-terminal (anti-SHERAIK). Results shown are representative of three separate experiments. Semi-quantitative evaluation of tau protein TauBDPs levels were performed via computer-assisted densitometric scanning (Epson XL3500 high-resolution flatbed scanner) and image analysis with Image J software [NIH (National Institutes of Health)]. Uneven loading of samples into different wells might occur despite careful protein concentration determination and careful sample handling and gel loading (20 mg per well). To overcome this source of variability, we performed Western blotting using the same sample against β-actin (monoclonal, Sigma, no. A5441) as a control.
Statistical analyses
Densitometric results were acquired in arbitrary densitometric units. Changes in any outcome parameter were compared with the appropriate control group. Thus, magnitude of change from control in one model system could be directly compared with magnitude of change from any other model system. In this study, six replicate results were evaluated by Student's t test and ANOVA and post-hoc Tukey tests. A value of P<0.05 was taken as significant.
RESULTS
Identification of major calpain cleavage sites in rat tau protein and validating tau-fragment-specific antibodies
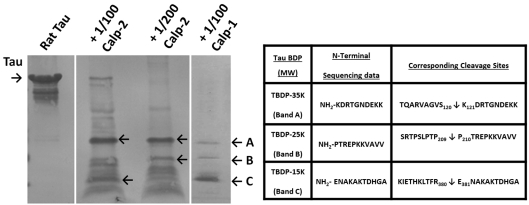
While the two major caspase-3 cleavage sites (Asp25↓Gln26 and between Asp421↓Ser422) in human tau (4R isoform) has been mapped out (Chung et al., 2001; Rohn et al., 2002), with the C-terminal cleavage site conserved in rat tau (4R isoform) (Asp412↓Ser413), the exact calpain cleavage sites have not been identified. We subjected recombinant rat tau (441-residue four-repeat isoform) with calpain-2 at two different protease/substrate ratios: 1:100 and 1:200 and calpain-1 at 1:100 (Figure 1). A number of fragments can be visualized by Coomassie Brilliant Blue staining. Both calpain-1 and -2 generated major tau fragments of the same size. We subjected these fragments to N-terminal microsequencing and were able to identify the internal N-terminal sequence of three peptide fragments: TauBDP-35K (band A), TauBDP-25K (band B) and TauBDP-15K (band C). They yielded newly exposed N-terminal of NH2-KDRTGNDEKK, NH2-PTREPKKVAVV and NH2-ENAKAKTDHGA, corresponding to cleavage sites between Ser130↓Lys131; Gly157↓Ala158 and Arg380↓Glu381 respectively (Figure 1). This multiple cleavage breakdown pattern is consistent with previous studies on calpain proteolysis of tau (Johnson et al., 1989; Litersky et al., 1993; Yang and Ksiezak-Reding, 1995; Yen et al., 1999).
Figure 1. Purified rat tau fragmentation by calpain.
Recombinant rat tau protein (50 μg) was digested in vitro by human calpain-1 (at protease/substrate ratio of 1:100) or rat calpain-2 (at protease/substrate ratios of 1:100 and 1:200). Intact protein and tau fragments were resolved by SDS/PAGE and transferred to PVDF membrane. Proteins were visualized by Coomassie Brilliant Blue staining. Both calpain-1 and -2 generated major tau fragments of the same size. Several major fragments (A, B and C) were subjected to Edman N-terminal microsequencing. The table shows the identified in vitro calpain cleavage sites of rat tau.
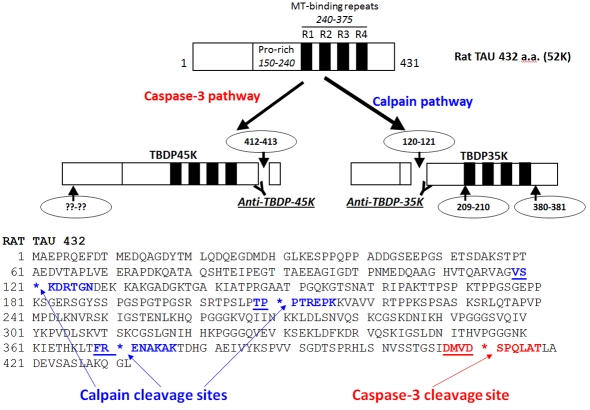
With this new information, in Figure 2, we mapped out the various cleavage sites and the corresponding Tau-BSPs that are produced. In addition, to readily distinguish calpain-mediated TauBDP versus caspase-mediated Tb-BDP formation, we developed fragment-specific antibodies to target the major calpain-mediated TauBDP-35K (35 kDa tau-breakdown product) (based on neo-N-terminal peptide NH2-KDRTGN), and the caspase-mediated TauBDP-45K (based on neo-C-terminal peptide GSIDMVD-COOH) respectively (Figure 2).
Figure 2. Schematic of tau proteolysis by the calpain and caspase-3 pathways.
In this model, rat tau-4 repeat (4R) isoform with 432 residues is shown. It has four microtubule (MT)-binding repeats. Caspase-3 cleaves near the C-terminal (at Asp412↓Ser413), and an unknown N-terminal site producing key caspase fragment doublet of 45–48 kDa (TauBDP-45K). On the other hand, calpain cleaves at least three internal sites (Ser120↓Lys121; Pro209↓Pro2108 and Arg380↓Glu381) producing a key calpain fragment of 35 kDa (TauBDP-35K)
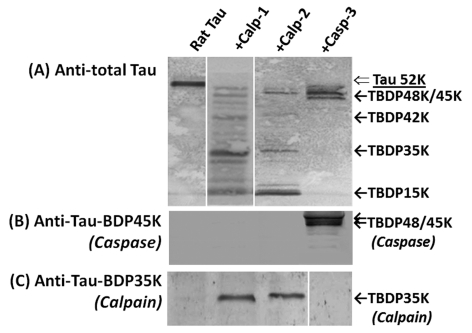
To validate the fidelity of these two fragment-specific antibody tools, we subjected rat tau protein in small quantities (100 ng) to either calpain-2 or caspase-3 digestion. We then probed untreated rat tau and digested tau samples by SDS/PAGE followed by immunoblotting with total tau, TauBDP-35K and TauBDP-45K antibodies. As expected, both calpain-1 and -2 digested tau into several immunoreactive fragments (42, 35 and 15 kDa), while caspase-3 digestion only produced limited fragment doublet of 48 kDa/45 kDa (Figure 3). Probing with TauBDP-35K (calpain) and TauBDP-45K (caspase) antibodies confirmed that these antibodies are fragment specific with no cross-reactivity with intact protein or other fragments (Figure 3).
Figure 3. Tau protein in rat brain lysate is sensitive to in vitro calpain and caspase-3 digestion.
The lysate of naïve rat hippocampus was in vitro cleaved by calpain-1, calpain-2 and caspase-3: Control; Calpain-1 (1:500 protease/substrate ratio); calpain-2; (1:200 protease/substrate ratio); or caspase-3 digestion (1:50 protease/substrate ratio). The pattern of the tau protein fragmentation was monitored with total tau antibody (A) or antibodies specific to caspase-mediated TauBDP-45K (B) and calpain-mediated TauBDP-35K (C) respectively.
Examining tau protein integrity in cultured cerebrocortical neurons subjected to neurotoxic and neurodegenerative conditions
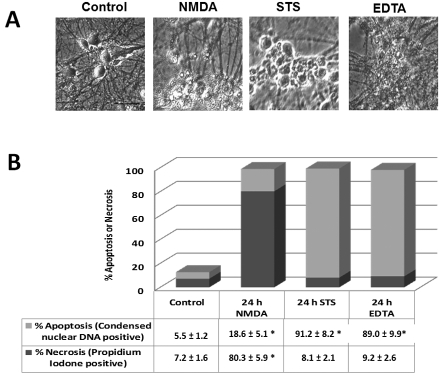
Next, a number of neurotoxic conditions were carefully selected to reflect excitotoxicity (mixed necrosis and apoptosis) and two apoptosis-inducing agents that evokes and calpain and/or caspase activation to mimic neurodegenerative conditions. In our design, rat cerebrocortical cultures were treated as an excitotoxic challenge NMDA (Nath et al., 1998); apoptotic inducer STS (0.5 μM) that activates calpain and caspase-3 for 24 h (Zhang Z et al., 2009); the Ca2+ chelator EDTA as a caspase-dominated challenge (Waterhouse et al., 1996; Chiesa et al., 1998; McGinnis et al., 1999; Zhang Z et al., 2009), percentage neuronal necrosis and percentage apoptosis were also assessed by using propidium iodide labelling of nuclei at 24 h for necrosis, while Hoechst 33342 stained condensed nuclear DNA as evidence for % apoptosis. Figure 4(A) shows NMDA, EDTA and STS treatments all caused extensive neurodegeneration in vitro. Figure 4(B) further shows that, while NMDA induced a mixed necrosis/apoptosis phenotype, while both STS and EDTA produced a robust apoptosis phenotype.
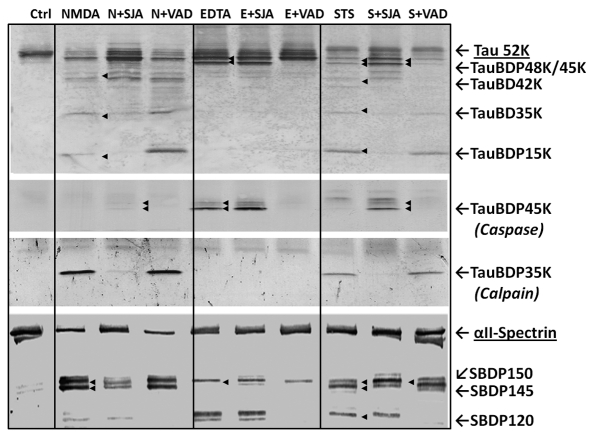
Figure 4. Tau fragmentation pattern in neurotoxin-challenged rat cerebrocortical neurons and the contribution of calpain and/or caspase.
Rat cerebrocortical cultures were either untreated (control), or treated with excitotoxin (NMDA, 200 μM), apoptosis-inducers calcium chelator EDTA (2 mM) or STS (0.5 μM) for 24 h. (A) All three neurotoxic conditions cause extensive neurodegeneration when observed by phase contrast microscopy. (B) Percentage neuronal necrosis and percentage apoptosis were also assessed by using propidium iodine labelling of nuclei at 24 h for necrosis, while Hoechst 33342 stained condensed nuclear DNA as evidence for percentage apoptosis, using Genescript Double Stain Apoptosis Detection Kit (Hoechst 33342/PI). Shown are means±S.D. (n = 5). *Indicates statistical significance (P = 0.05; Student's two-tailed t test).
We then investigated the relative contributions of calpain and caspase-3 in the fragmentation of tau in cultured neurons under several neurodegenerative conditions. With NMDA treatment, tau is significantly degraded into multiple fragments (42K, 35K and 15K) including a dominant signal of calpain-mediated TauBDP-35K with minimal caspase-mediated TauBDP-45K (Figure 5, top three panels).
Figure 5. Tau fragmentation pattern in neurotoxin-challenged rat cerebrocortical neurons and the contribution of calpain and/or caspase.
Rat cerebrocortical cultures were either untreated (control), or treated with excitotoxin (NMDA, 200 μM), apoptosis-inducer calcium chelator EDTA (2 mM) or STS (0.5 μM) for 24 h. For tau fragmentation analysis, neurotoxin challenges were undertaken in the absence of presence of either calpain inhibitor SNJ-1945 (20 μM) or caspase inhibitor Z-VAD-FMK (20 μM). After 24 h, cell lysates were harvested for protein and immunoblotting analysis with total tau monoclonal antibody (top panel), antibody specific to caspase-mediated TauBDP-45K (second panel), antibody specific to calpain-mediated TauBDP-35K (third panel) or αII-spectrin monoclonal antibody (bottom panel). Results shown are representative of three separate experiments.
When caspase inhibitor (Z-VAD; 20 μM) was added to the NMDA condition, no significant changes of tau breakdown pattern were observed. In contrast, when inhibitor (SNJ-1945; 20 μM) was added to the NMDA condition, it significantly reduced the lower-molecular-mass fragment, including complete blockade of the calpain-mediated TauBDP-35K (Figure 5, top and third panels), and yet some high-molecular-mass fragments (425–48K) persisted. Interestingly, when a blot was probed with the anti-caspase-mediated TauBDP-45K antibody, TauBDP-45K/48K were detected in the calpain inhibitor-NMDA co-treatment lane (Figure 5, second panel). Consistent with the above, when established calpain/caspase dual-substrate αII-spectrin (Wang, 2000) was probed, it clearly showed that NMDA-yielded prominent calpain-mediated SBDP150/SBDP145, with minor bands of caspase-3-mediated SBDP120 (Figure 5, bottom panel). These fragments are strongly inhibited with their respective protease inhibitors (SNJ and Z-VAD). Taken together, these results suggest that in NMDA paradigm, calpain is the dominant pathway in tau fragmentation with a more minor contribution of caspase.
When the neuronal culture was treated with apoptosis-inducing EDTA, tau was truncated only to TauBDP-48K/45K, as confirmed by total tau blot and caspase-mediated anti-TauBDP-45K blot (Figure 5, top two panels). Both fragments, as expected are caspase inhibitor (Z-VAD) sensitive, but insensitive to calpain inhibitor (SNJ-1945) (Figure 5). Thus, EDTA challenge produced a straight caspase-dominant tau fragmentation condition. The αII-spectrin breakdown pattern again confirmed the presence of caspase-mediated SBDP120, but not calpain-generated fragment SBDP145 (Figure 5, bottom panel).
When the culture was treated with another apoptosis inducer, STS, a balance of higher-molecular-mass (45--48 kDa) and low-molecular-mass (35 and 15 kDa) TauBDPs were observed (Figure 5, top panel). The 48/45K fragments were caspase-mediated, as confirmed by the tau-45K fragment-specific antibody blot as well as its sensitivity to caspase inhibitor (Z-VAD) (Figure 5, second panel). Similarly, the involvement of calpain was confirmed by the TauBDP-35K-specific antibody and its sensitivity to calpain inhibitor SNJ-1945 (Figure 5, third panel). Importantly, the presence of calpain inhibitor strongly elevated the TauBDP-48K/45K by both total tau blot and anti-TauBDP-48/45K blot (Figure 5, second panel), suggesting the dual involvement of both calpain and caspase. This is also consistent with αII-spectrin breakdown pattern. Calpain inhibitor blocked mainly the SBDP145, but not the SBDP120, while the caspase inhibitor blocked the SBDP120, but not SBDP145. It is noted that both inhibitors failed to block the formation of SBDP150, as both calpain and caspase can produce an SBDP150 of almost identical molecular mass (Wang, 2000) (Figure 5B, bottom panel). Taken together, with STS treatment produces a neurodegenerative paradigm where there is a dual and balanced contribution of both calpain and caspase in tau fragmentation.
Immunoblot analysis of tau protein integrity in rat brain after experimental TBI
TBI is a risk factor for subsequent development of AD. Thus, we were interested in the tau fragmentation process under this acute neurodegenerative condition. We hypothesize that such tau fragmentation might have downstream consequences leading to tauopathy and possible development of AD. We subjected rats to CCI (controlled cortical impact), an experimental model of TBI, as previously established (Pike et al., 1998). We then harvested cortical and hippocampal tissues for immunoblotting examination with antibodies against total tau protein (Figure 6), specific to calpain-mediated TauBDP-35K and caspase-mediated TauBDP-45K respectively (Figure 7). We have previously detected significant αII-spectrin breakdown products in the traumatic injured rat brain (Pike et al., 1998).
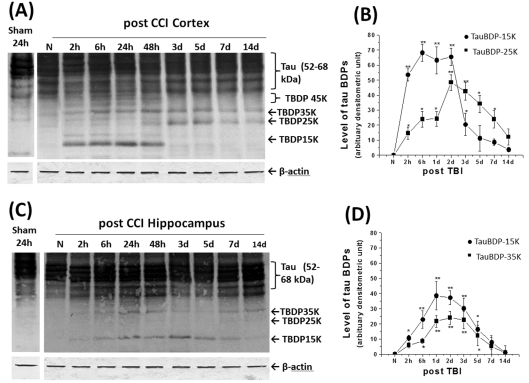
Figure 6. Time course of tau protein fragmentation in rat cortex and hippocampus following TBI.
Western blot results show TauBDP-35K, TauBDP-25K and TauBDP-15K accumulated in injured rat cortex following TBI at various time points (A). The densitometric results revealed that TauBDP-35 K/15K and TauBDP-25K accumulated in the different time points after TBI (B). In the injured rat hippocampus, immunoblots revealed tau TauBDP-35K and TauBDP-15K are the key fragments and they accumulated in a similar temporal profile in the TBI group (C), (*P<0.05; **P<0.001) when compared with naïve group (D). β-Actin blots were also performed routinely as protein-loading evenness controls, thus ruling out technical artefacts. Results are from n = 6 (B, D). Student's t test was used to compare TBI time points with naïve (*P<0.05; **P<0.001).
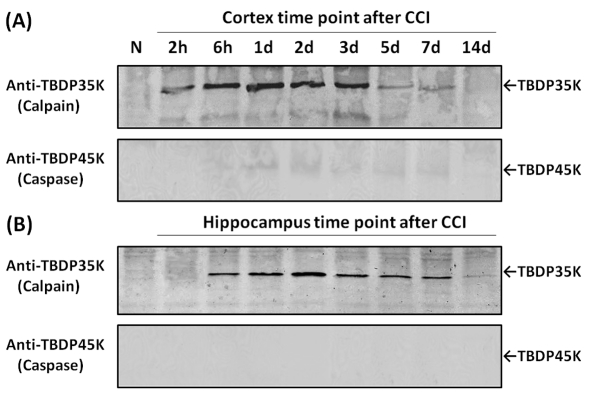
Figure 7. Examination of relative contribution of calpains versus caspase-3 in tau proteolysis in rat cortex and hippocampus following TBI with tau-fragment-specific antibodies.
Western blot of naïve and CCI cortex (A) and hippocampus (B) time course samples similar to those of Figure 5. However, these blots were probed with polyclonal antibody specific to caspase-mediated TauBDP-45K (lower panel), or polyclonal antibody specific to calpain-mediated TauBDP-35K (upper panel). Results shown are representative of three separate experiments.
In the naïve cortex, tau protein was detected by immunoblots as a cluster of bands approx. 52–68 kDa, most likely representing the various isoforms and phosphorylation states of tau protein (Figure 6). Sham (24 h after craniotomy) did not significantly generate TauBDPs. Next, we examined the temporal profile of TBI-induced tau proteolysis in the rat cortex. In the rat ipsilateral cortex of the TBI group, immunoblots revealed that TauBDP-35K and TauBDP-15K accumulated relatively rapidly, followed by the delayed appearance of TauBDP-25K (Figure 6A). When the density of two TauBDPs of the TBI group was compared with the naïve group, the results showed that the TauBDP-15K increased rapidly, as early as 2 h after TBI, and sustained over 48 h, followed by its resolution for 3–14 days (Figures 6A and 6B). On the other hand, TauBDP-25K, while significantly elevated in 2–6 h, did not reach its peak until 48 h and this level was sustained until at least 7 days (Figure 6B). Other fragments (such as TauBDP-45K) can be observed, but they are minor fragments (Figure 6A). Furthermore, we routinely performed β-actin blots as controls and found even sample loading (see Figure 6A). In addition, in the contralateral cortex, no tau proteolysis was observed in all three groups, even when tau immunoblots were intentionally overdeveloped (results not shown).
The rat hippocampus is another highly vulnerable region following experimental TBI. In the naïve and sham hippocampus, tau is expressed again as a cluster of isoforms of 52–68 kDa (Figure 6C). In the rat ipsilateral hippocampus of the TBI group, tau immunoblots revealed the accumulation of two fragments (TauBDP-35K and TauBDP-15K) from 2 h to 5 days after TBI (Figure 6C). When the density of TauBDP-35K and TauBDP-15K of TBI group was compared with the naïve group, the results showed that both TauBDPs rose as early as 2–6 h after TBI, peaked at 1–2 days and were sustained over 5 days (Figure 6D).
To further distinguish if any of these fragments were derived from calpain and/or caspase proteolysis, we employed the two tau-fragment-specific antibodies. In the injured cortex, we found robust signals of calpain-mediated TauBDP-35K from 2 h to 5 days, receding in 5 and 7 days (Figure 7A). In contrast, we only observed a faint caspase-mediated TauBDP-45K signal (from 1 to 7 days) on overexposure of the blot development (Figure 7A). In the injured hippocampus, the TauBDP-35K signal was again very robust from 6 h to 7 days, while TauBDP-45K was not detectable (Figure 7B). These results suggest that CCI-induced neurodegeneration is accompanied by the formation and accumulation of calpain-mediated TauBDPs with only minor contributions to caspase-generated tau fragments in the injured cortex.
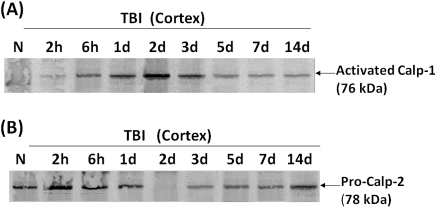
In addition, we also sought to compare tauBDP-35K formation with calpain-1 and calpain-2 temporal activation profiles. To do this, we employed anti-activated calpain-1 new-N-terminal (anti-NH2-LGRHENA) antibody (A), or pro-calpain-2 N-terminal (anti-SHERAIK) antibody. Figure 8 shows that, in animals following TBI, both calpain-1 and calpain-2 are autolytically activated in injured cortex. Calpain-1 has an early peak on day 2 and 3 and subsided afterward, while calpain-2 also peaked on day 2 but was sustained to 3 and 5 days (Figure 8). The calpain-1 activation matches very well with the appearance of TauBDP-35K (calpain-specific) that also peaked at day 2 and 3 in injured cortex (Figure 7). Taken together, these results argue that calpain-1 might be the major isoform cleaving tau.
Figure 8. Examination of autolytic activation of calpain-1 and calpain-2 in rat cortex following TBI.
Western blot of naïve and cortex CCI time course samples similar to those in Figure 6. However, these blots were probed with polyclonal antibody specific to anti-activated calpain-1 new N-terminal (anti-NH2-LGRHENA) (A), or pro-calpain-2 N-terminal (anti-SHERAIK) (B). Results shown are representative of three separate experiments.
Suppression of TauBDP-35K by calpain inhibitor administration in vivo
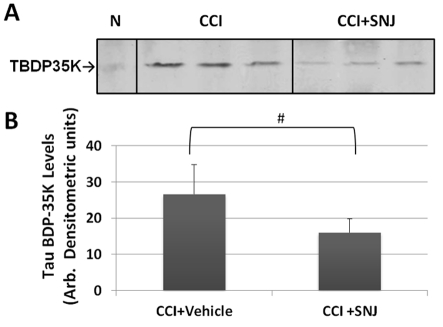
Finally, to ascertain that the prominent TauBDP-35K generated after CCI was indeed calpain-mediated, we administered a calpain-specific inhibitor agent (SNJ-1945) (Shirasaki et al., 2005; Oka et al., 2006; Koumura et al., 2008) (100 mg/kg, i.v. bolus) immediately after CCI. Cortical tissue was probed with a TauBDP-35K-specific antibody (Figure 9). Densitometric analysis shows that SNJ-1945 was effective in suppressing TBI-induced TauBDP-35K by 60% (P<0.035, Student unpaired t test).
Figure 9. Attenuation of calpain-generated TauBDP-35K after CCI by administration of calpain inhibitor in vivo.
Ipsilateral cortical tissue lysate were probed with anti-TauBDP-35K antibody 6 h after controlled cortical impact [1.6 mm with a calpain inhibitor SNJ-1945 (100 mg/kg bolus, i.v.) given immediately after CCI]. Top panel: representative immunoblots [naïve (N) control was also shown]. (B) Quantification of TauBDP-35K levels (n = 5) shown are means±S.D. #Indicates statistical significance (P = 0.035; Student's two-tailed t test).
DISCUSSION
Tau protein is a major neuronal microtubule-associated protein primarily localized in the axonal compartment of neurons (Kosik and Finch, 1987). Owing to the presence of several alternative splicing sites and multiple phosphorylation sites, tau protein usually appears on immunoblots as multiple bands of approx. 52–68 kDa. Under normal conditions, tau promotes tubulin assembly and stability and is involved in axon elongation and transport (Garcia and Cleveland, 2001; Avila et al., 2004). Tau interacts with microtubules at the tubulin-binding domain to induce tubulin assembly, stabilizes the polymer and minimizes microtubule disassembly (reviewed by Garcia and Cleveland, 2001; Drubin and Kirschner, 1986). Tau abnormality is linked to several neurodegenerative disorders referred to as ‘tau pathologies’, a heterogeneous group of age-dependent cognitive disorders, most notably AD and CTE (chronic traumatic encephalopathy; reviewed by Garcia and Cleveland, 2001). Axonally specific microtubule-associated protein tau is an important factor in AD as tau aggregate is found in a form of neurofibrillary tangle. Besides hyperphosphorylation, proteolytic processing of tau was suggested to facilitate this tau deposit formation (Zemlan et al., 2003; Gabbita et al., 2005; Arnaud et al., 2009). Similarly, tau is degraded following acute TBI and with neurotoxin exposure (e.g. Methamphetamine) (Warren et al., 2005, 2007; Arnaud et al., 2009). Both cellular cysteine proteases (calpain and caspase-3) are capable of tau processing. In addition, there is now mounting evidence that repeated concussion (or mild TBI) experienced by impact sport athletes (e.g. football and hockey players and boxers) can lead to a brain disorder called CTE – a tauopathy disease not dissimilar to the tau pathological component of AD (McKee et al., 2009). In addition, there are epidemiological data linking prior TBI (especially among males) to increased risk of developing AD later in life (Van Den Heuvel et al., 2007; Kiraly and Kiraly, 2007). Thus, it is possible that tau fragmentation in acute brain injury will somehow facilitate or enable tau aggregate deposit formation (Yoshiyama et al., 2005; Uryu et al., 2007).
Calpain and caspase-3 cysteine proteases are important mediators of cell death and dysfunction in numerous CNS (central nervous system) diseases and injuries, including TBI (McIntosh et al., 1996; Bartus, 1997; McCracken et al., 1999; Wang, 2000; Pike et al., 2004). There is also evidence of calpain and caspase-3 activation in injured axons following experimental TBI (Saatman et al., 1996a; Newcomb et al., 1997; Pike et al., 1998; Buki et al., 1999, 2000; Beer et al., 2000). In addition, inhibitors of both calpains and caspase-3 can confer neuroprotection after TBI in animal models (Nath et al., 1996; Saatman et al., 1996b, 2000; Posmantur et al., 1997; Clark et al., 2000). Tau is vulnerable to both of these proteases in vitro (Yang and Ksiezak-Reding, 1995; Yen et al., 1999; Chung et al., 2001; Rohn et al., 2002; Gamblin et al., 2003; Park and Ferreira, 2005; Guillozet-Bongaarts et al., 2005; Delobel et al., 2005; Arnaud et al., 2009). The major cleavage site for caspase-3 on rat tau has been mapped out at the C-terminal Asp412↓ Ser413 (Rohn et al., 2002; Gamblin et al., 2003), while calpain produces multiple fragments including a neurotoxic fragment of 17 kDa (Park and Ferreira, 2005), but exact calpain cleavage sites remained elusive.
In the present study, the primary objective was to examine the relative vulnerability of tau to calpain and caspase-3 attack under neurotoxic and neurodegenerative conditions. We first identified three major calpain cleavage sites in rat tau (four-repeat 442-residue isoform) as Ser130↓Lys131, Gly157↓Ala158 and Arg380↓Glu381 (Figures 1 and 2). We then developed fragment-specific antibodies to target the major calpain-mediated TauBDP-35K as well as the major caspase-mediated TauBDP-45K respectively (Figure 3).
In rat cerebrocortical culture treated with excitotoxin (NMDA), tau is significantly degraded into multiple fragments, including a dominant signal of calpain-mediated TauBDP-35K with minimal caspase-mediated TauBDP-45K (Figure 4). When cultures were treated with apoptosis-inducing EDTA, tau was truncated only to TauBDP-48K/45K, which are both caspase inhibitor sensitive. Cultures treated with another apoptosis inducing STS, dual fragmentation by calpain (TauBDP-35K) and caspase-3 (TauBDP-45K) was observed (Figure 5).
Our results revealed that 52–68 kDa tau protein in lysate from naïve rat hippocampus was degraded in vitro into main smaller fragments (TauBDP-35K, -25K and -15K) by calpain-2 and large fragments (TauBDP-45/48K) by caspase-3. Thus, the calpain-mediated tau protein fragmentation pattern in vitro (TauBDP-35K, -25K and -15K) (Figure 3) matched very well with in vivo tau proteolysis after TBI (Figure 6). We also noted a TauBDP-15K in vitro, in cell culture and in vivo (Figures 3, 5 and 6) might correspond to the 17 kDa calpain fragment reported previously (Park and Ferreira, 2005). In addition, the antibody specific to the calpain-mediated TauBDP-35K readily detected this fragment in both injured cortex and hippocampus for sustained post-TBI intervals (Figure 7). Since the controlled cortical impact device targets the cortex, there was more focal injury in the ipsilateral cortex tissue than in the hippocampal tissue, which was impacted indirectly by contusive force. As a result, there is more extensive tau proteolysis in the cortex than in the hippocampus (Figure 7). Equally importantly, TauBDP-35K generated after CCI was suppressed by systemic administration of calpain-specific inhibitor agent (SNJ-1945) (Figure 9). These results are consistent with those reported by Sinjoanu et al. (2008) that calpain inhibitor A-705253 inhibits β-amyloid-induced tau cleavage in hippocampal neurons.
Regarding the contribution of caspase to tau fragmentation in vivo, we know that rat tau is cleaved by caspase-3 between Asp412 and Ser413 (Chung et al., 2001; Rohn et al., 2002). Since this cleavage produced large TauBDPs of 45K/48K (Figure 3), almost indistinguishable from the intact tau clusters, it was difficult to ascertain whether these fragments were detected in TBI using total tau antibody (Figure 5). Therefore, we developed an antibody (anti-TauBDP-45K) that only detected the caspase-3 generated new tau C-terminal (SSTGSIDMVD-COOH), but does not recognize the intact tau or calpain-produced TauBDPs (Figures 2 and 3). Using this fragment-specific antibody, we observed that the caspase-3-generated TauBDP-45K/48K was readily detected during in vitro caspase-3 digestion (Figure 3), but was only minimally detected in injured cortex in vivo after TBI (upon overdevelopment of the blot) (Figure 6). Taken together, these results strongly suggest that tau protein is primarily attacked in vivo by calpain with only minor contributions by caspase-3 following TBI in rats. This is in contrast to chronic neurodegenerative paradigms where tau is cleaved more prominently by caspase-3 (Canu et al., 1998; Chung et al., 2001; Rohn et al., 2002; Krishnamurthy and Sneige, 2002). In addition, more minor caspase-3 activity in our TBI model could in fact be due to some inhibitory pathway for caspase. Also, caspase-3 expressed at high levels in brain from fetal rats and rat pups, but is down-regulated in adult rats (Shimohama et al., 1999), possibly explaining the low TauBDP-45K levels in vivo after brain injury (Figure 6), while in rat in vitro cultures (14 div), there was more caspase-3 participation (Figure 5).
In summary, following acute brain injury, tau protein is predominantly vulnerable to calpain attack following acute TBI with minor contributions by caspase. Yet, in cultured neurons, different acute neurotoxic challenges result in various degrees of calpain and caspase contributions in tau fragmentation. Thus, caution should be exercised when studying tau fragmentation. Employing calpain- and caspase-generated tau fragment-specific tools will add to the fidelity of such tau fragmentation analysis. In addition, if preserving tau integrity is a therapeutic goal, depending on the pathological condition, one might consider the use of calpain and/or caspase inhibitory agents. Lastly, to study the possible linkage between acute head trauma and subsequent development of tauopathy (CTE and AD), it will be very important to examine if the calpain-generated TauBDP-35K, in addition to caspase-generated TauBDP-45K, can be detected in aggregated tau or neurofibrillary tangles. These efforts are now ongoing in our laboratories.
ACKNOWLEDGEMENTS AND DISCLAIMER
K.K.W. and R.L.H. hold equity in Banyan Biomarkers Inc. a company commercializing technology of detecting brain injury biomarkers. We acknowledge Dr J. Inoue and Dr M. Azuma (Research Laboratories, Senju Pharmaceutical, Kobe, Japan) for generously providing the supply of SNJ-1945 for this work.
Footnotes
This work was supported by NINDS-NIH [1R21NS052322] (to M.C.L.), [R01 NS049175] (to K.K.W.); Department of Defense (DoD) [Grant No. DAMD17-03-1-0066] (to R.L.H.).
REFERENCES
- Adams JH, Graham DI, Jennett B. The neuropathology of the vegetative state after an acute brain insult. Brain. 2000;123:1327–1338. doi: 10.1093/brain/123.7.1327. [DOI] [PubMed] [Google Scholar]
- Arnaud LT, Myeku N, Figueiredo-Pereira ME. Proteasome-caspase-cathepsin sequence leading to tau pathology induced by prostaglandin J2 in neuronal cells. J Neurochem. 2009;110:328–342. doi: 10.1111/j.1471-4159.2009.06142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J, Perez M, Lucas JJ, Gomez-Ramos A, Maria IS, Moreno F, Smith M, Perry G, Hernandez F. Assembly in vitro of tau protein and its implications in Alzheimer's disease. Curr Alzheimer Res. 2004;1:97–101. doi: 10.2174/1567205043332207. [DOI] [PubMed] [Google Scholar]
- Bartus RT. The calpain hypothesis of neurodegeneration: evidence for a common cytotoxic pathway. Neuroscientist. 1997;3:314–327. [Google Scholar]
- Beer R, Franz G, Srinivasan A, Hayes RL, Pike BR, Newcomb JK, Zhao X, Schmutzhard E, Poewe W, Kampfl A. Temporal profile and cell subtype distribution of activated caspase-3 following experimental traumatic brain injury. Neurochemistry. 2000;75:1264–1273. doi: 10.1046/j.1471-4159.2000.0751264.x. [DOI] [PubMed] [Google Scholar]
- Binder LI, Frankfurter A, Rebhun LI. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsch A, Horn C, Kemmling Y, Seipelt M, Hellenbrnd U, Stiefel M, Ciesielczyk B, Cepek L, Bahn E, Ratzka P, Prange H, Otto M. Serum tau protein level as a marker of axonal damage in acute ischemic stroke. Eur Neurol. 2002;47:45–51. doi: 10.1159/000047946. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol. 2002;103:607–614. doi: 10.1007/s00401-001-0510-8. [DOI] [PubMed] [Google Scholar]
- Buki A, Okonkwo DO, Wang KKW, Povlishock JT. Cytochrome c release and caspase activation in traumatic axonal injury. J Neurosci. 2000;20:2825–2834. doi: 10.1523/JNEUROSCI.20-08-02825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buki A, Siman R, Trojanowski JQ, Povlishock JT. The role of calpain-mediated spectrin proteolysis in traumatically induced axonal injury. J Neuropathol Exp Neurol. 1999;58:365–375. doi: 10.1097/00005072-199904000-00007. [DOI] [PubMed] [Google Scholar]
- Canu N, Dus L, Barbato C, Ciotti MT, Brancolini C, Rinaldi AM, Novak M, Cattaneo A, Bradbury A, Calissano P. Tau cleavage and dephosphorylation in cerebellar granule neurons undergoing apoptosis. J Neurosci. 1998;8:7061–7074. doi: 10.1523/JNEUROSCI.18-18-07061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa R, Angeretti N, Del Bo R, Lucca E, Munna E, Forloni G. Extracellular calcium deprivation in astrocytes: regulation of mRNA expression and apoptosis. J Neurochem. 1998;70:1474–1483. doi: 10.1046/j.1471-4159.1998.70041474.x. [DOI] [PubMed] [Google Scholar]
- Christman CW, Grady MS, Walker SA, Holloway KL, Povlishock JT. Ultrastructural studies of diffuse axonal injury in humans. J Neurotrauma. 1994;11:173–186. doi: 10.1089/neu.1994.11.173. [DOI] [PubMed] [Google Scholar]
- Chung CW, Song YH, Kim IK, Yoon WJ, Rye BR, Jo DG, Woo HN, Kwon YK, Kim HH, Gwag BJ, Mook-Jung IH, Jung YK. Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol Dis. 2001;8:162–172. doi: 10.1006/nbdi.2000.0335. [DOI] [PubMed] [Google Scholar]
- Clark RS, Kochanek PM, Watkins SC, Chen M, Dixon CE, Seidberg NA, Melick J, Loeffert JE, Nathaniel PD, Jin KL, Graham SH. Caspase-3 mediated neuronal death after traumatic brain injury in rats. J Neurochem. 2000;74:740–753. doi: 10.1046/j.1471-4159.2000.740740.x. [DOI] [PubMed] [Google Scholar]
- Delobel P, Leroy O, Hamdane M, Sambo AV, Delacourte A, Buée L. Proteasome inhibition and Tau proteolysis: an unexpected regulation. FEBS Lett. 2005;579:1–5. doi: 10.1016/S0014-5793(05)01501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Kirschner MW. Tau protein function in living cells. J Cell Biol. 1986;103:2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz G, Beer R, Kampfl A, Engelhardt K, Schmutzhard E, Ulmer H, Deisenhammer F. Amyloid beta 1-42 and tau in cerebrospinal fluid after severe traumatic brain injury. Neurology. 2003;60:1457–1461. doi: 10.1212/01.wnl.0000063313.57292.00. [DOI] [PubMed] [Google Scholar]
- Gabbita SP, Scheff SW, Menard RM, Roberts K, Fugaccia I, Zemlan FP. Cleaved-tau: a biomarker of neuronal damage after traumatic brain injury. J Neurotrauma. 2005;22:83–94. doi: 10.1089/neu.2005.22.83. [DOI] [PubMed] [Google Scholar]
- Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, Lapointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau, linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci USA. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SD, Johnson SC, Bigler ED, Blatter DD. Nonspecific white matter degeneration following traumatic brain injury. J Int Neuropsychol Soc. 1995;1:17–28. doi: 10.1017/s1355617700000060. [DOI] [PubMed] [Google Scholar]
- Garcia ML, Cleveland DW. Going new places using an old MAP: tau, microtubules and human neurodegenerative diseases. Curr Opin Cell Biol. 2001;13:41–48. doi: 10.1016/s0955-0674(00)00172-1. [DOI] [PubMed] [Google Scholar]
- Guillozet-Bongaarts AL, Garcia-Sierra F, Reynolds MR, Horowitz PM, Fu Y, Wang T, Cahill ME, Bigio EH, Berry RW, Binder LI. Tau truncation during neurofibrillary tangle evolution in Alzheimer's disease. Neurobiol Aging. 2005;26:1015–1022. doi: 10.1016/j.neurobiolaging.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Lee VM, Trojanowski JQ. Tau and axonopathy in neurodegenerative disorders. Neuromol Med. 2002;2:131–150. doi: 10.1385/NMM:2:2:131. [DOI] [PubMed] [Google Scholar]
- Johnson GV, Jope RS, Binder LI. Proteolysis of tau by calpain. Biochem Biophys Res Commun. 1989;163:1505–1511. doi: 10.1016/0006-291x(89)91150-9. [DOI] [PubMed] [Google Scholar]
- Kampfl A, Posmantur RM, Zhao X, Schmutzhard E, Clifton GL, Hayes RL. Mechanisms of calpain proteolysis following traumatic brain injury: implications for pathology and therapy: a review and update. J Neurotrauma. 1997;14:121–134. doi: 10.1089/neu.1997.14.121. [DOI] [PubMed] [Google Scholar]
- Kiraly M, Kiraly SJ. Traumatic brain injury and delayed sequelae: a review--traumatic brain injury and mild traumatic brain injury (concussion) are precursors to later-onset brain disorders, including early-onset dementia. ScientificWorldJournal. 2007;7:1768–1776. doi: 10.1100/tsw.2007.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblach SM, Nikolaeva M, Huang X, Fan L, Krajewski S, Reed JC, Faden AI. Multiple caspases are activated after traumatic brain injury: evidence for involvement in functional outcome. J Neurotrauma. 2002;19:1155–1170. doi: 10.1089/08977150260337967. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Finch EA. MAP2 and tau segregate into dendritic and axonal domains after the elaboration of morphologically distinct neurites: an immunocytochemical study of cultured rat cerebrum. J Neurosci. 1987;7:3142–3153. doi: 10.1523/JNEUROSCI.07-10-03142.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumura A, Nonaka Y, Hyakkoku K, Oka T, Shimazawa M, Hozumi I, Inuzuka T, Hara H. A novel calpain inhibitor, ((1S)-1((((1S-1-benzyl-3-cyclopropylamino-2,3-dioxoproptyl)amino)carbonyl)-3-methylbutyl carbamic acid 5-methoxy-3-oxapentyl ester, protects neuronal cells from cerebral ischemia-induced damage in mice. Neuroscience. 2008;157:309–318. doi: 10.1016/j.neuroscience.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy S, Sneige N. Molecular and biologic markers of premalignant lesions of human breast. Adv Anat Pathol. 2002;9:185–197. doi: 10.1097/00125480-200205000-00002. [DOI] [PubMed] [Google Scholar]
- Litersky JM, Scott CW, Johnson GV. Phosphorylation, calpain proteolysis and tubulin binding of recombinant human tau isoforms. Brain Res. 1993;604:32–40. doi: 10.1016/0006-8993(93)90349-r. [DOI] [PubMed] [Google Scholar]
- McCracken E, Hunter AJ, Patel S, Graham DI, Dewar D. Calpain activation and cytoskeletal protein breakdown in the corpus callosum of head-injured patients. J Neurotrauma. 1999;16:749–761. doi: 10.1089/neu.1999.16.749. [DOI] [PubMed] [Google Scholar]
- McGinnis KM, Gnegy ME, Park YH, Mukerjee N, Wang KK. Procaspase-3 and poly (ADP) ribose polymerase (PARP) are calpain substrates. Biochem Biophys Res Commun. 1999;263:94–99. doi: 10.1006/bbrc.1999.1315. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Smith DH, Meaney DF, Kotapka MJ, Gennarelli TA, Graham DI. Neuropathological sequelae of traumatic brain injury: relationship to neurochemical and biomechanical mechanisms. Lab Invest. 1996;74:315–341. [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in human CNS diseases. Brain. 2003;126:515–530. doi: 10.1093/brain/awg061. [DOI] [PubMed] [Google Scholar]
- Nath R, McGinnis KJ, Nadimpalli R, Stafford D, Wang KKW. Effects of ICE-like proteases and calpain inhibitors on neuronal apoptosis. NeuroReport. 1996;8:249–255. doi: 10.1097/00001756-199612200-00050. [DOI] [PubMed] [Google Scholar]
- Nath R, Probert Jr A, McGinnis KM, Wang KKW. Evidence for activation of caspase-3-like protease in excitotoxins- and hypoxia/hypoglycemia-injured neurons. J Neurochem. 1998;71:186–195. doi: 10.1046/j.1471-4159.1998.71010186.x. [DOI] [PubMed] [Google Scholar]
- Nath R, Scott M, Nadimpalli R, Gupta R, Wang KKW. Activation of apoptosis-linked caspase(s) in NMDA-injured brains in neonatal rats. Neurochem Int. 2000;36:119–126. doi: 10.1016/s0197-0186(99)00112-6. [DOI] [PubMed] [Google Scholar]
- Newcomb JK, Kampfl A, Posmantur RM, Zhao X, Pike BR, Liu SJ, Clifton GL, Hayes RL. Immunohistochemical study of calpain-mediated breakdown products to alpha-spectrin following controlled cortical impact injury in the rat. J Neurotrauma. 1997;14:369–383. doi: 10.1089/neu.1997.14.369. [DOI] [PubMed] [Google Scholar]
- Ng HK, Mahaliyana RD, Poon WS. The pathological spectrum of diffuse axonal injury in blunt head trauma: assessment with axon and myelin strains. Clin Neurol Neurosurg. 1994;96:24–31. doi: 10.1016/0303-8467(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Oka T, Walkup RD, Tamada Y, Nakajima E, Tochigi A, Shearer TR, Azuma M. Amelioration of retinal degeneration and proteolysis in acute ocular hypertensive rats by calpain inhibitor ((1S)-((((1S)-1-benzyl-3-cyclopylamino-2,3-dioxopropyl)amino)carbonyl)-3-methylbutyl carbamic acid 5-methoxy-3-oxapentyl ester. Neuroscience. 2006;141:2139–2145. doi: 10.1016/j.neuroscience.2006.05.060. [DOI] [PubMed] [Google Scholar]
- Park SY, Ferreira A. The generation of a 17 kDa neurotoxic fragment: an alternative mechanism by which tau mediates beta-amyloid-induced neurodegeneration. J Neurosci. 2005;25:5365–5375. doi: 10.1523/JNEUROSCI.1125-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettus EH, Christman CW, Giebel ML, Povlishock JT. Traumatically induced altered membrane permeability: its relationship to traumatically induced reactive axonal change. J Neurotrauma. 1994;11:507–522. doi: 10.1089/neu.1994.11.507. [DOI] [PubMed] [Google Scholar]
- Pike BR, Zhao X, Newcomb JK, Posmantur RM, Wang KKW, Hayes RL. Regional calpain and caspase-3 proteolysis of alpha-spectrin after traumatic brain injury. NeuroReport. 1998;9:2437–2442. doi: 10.1097/00001756-199808030-00002. [DOI] [PubMed] [Google Scholar]
- Pike BR, Flint J, Dave JR, Lu XC, Wang KK, Tortella FC, Hayes RL. Accumulation of calpain and caspase-3 proteolytic fragments of brain-derived alphaII-spectrin in cerebral spinal fluid after middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2004;24:98–106. doi: 10.1097/01.WCB.0000098520.11962.37. [DOI] [PubMed] [Google Scholar]
- Posmantur R, Kampfl A, Siman R, Liu J, Zhao X, Clifton GL, Hayes RL. A calpain inhibitor attenuates cortical cytoskeletal protein loss after experimental traumatic brain injury in the rat. Neuroscience. 1997;77:875–888. doi: 10.1016/s0306-4522(96)00483-6. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Rissman RA, Head E, Cotman CW. Caspase activation in the Alzheimer's disease brain: tortuous and torturous. Drug News Perspect. 2002;15:549–557. doi: 10.1358/dnp.2002.15.9.740233. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Bozyczko-Coyne D, Marcy V, Siman R, McIntosh TK. Prolonged calpain-mediated spectrin breakdown occurs regionally following experimental brain injury in the rat. J Neuropathol Exp Neurol. 1996a;55:850–860. doi: 10.1097/00005072-199607000-00010. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Murai H, Bartus RT, Smith DH, Hayward NJ, Perri BR, McIntosh TK. Calpain inhibitor AK295 attenuates motor and cognitive deficits following experimental brain injury in the rat. Proc Natl Acad Sci USA. 1996b;93:3428–3433. doi: 10.1073/pnas.93.8.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatman KE, Zhang C, Bartus RT, McIntosh TK. Behavioral efficacy of posttraumatic calpain inhibition is not accompanied by reduced spectrin proteolysis, cortical lesion, or apoptosis. J Cereb Blood Flow Metab. 2000;20:66–73. doi: 10.1097/00004647-200001000-00010. [DOI] [PubMed] [Google Scholar]
- Shimohama S, Tanino H, Fujimoto S. Changes in caspase expression in Alzheimer's disease: comparison with development and aging. Biochem Biophys Res Commun. 1999;256:381–384. doi: 10.1006/bbrc.1999.0344. [DOI] [PubMed] [Google Scholar]
- Shirasaki Y, Miyashita H, Yamaguchi M, Inoue J, Nakamura M. Exploration of orally available calpain inhibitors: peptidyl alpha-ketoamides containing an amphiphile at P3 site. Bioorg Med Chem. 2005;13:4473–4484. doi: 10.1016/j.bmc.2005.04.059. [DOI] [PubMed] [Google Scholar]
- Siman R, McIntosh TK, Soltesz KM, Chen Z, Neumar RW, Roberts VL. Proteins released from degenerating neurons are surrogate markers for acute brain damage. Neurobiol Dis. 2004;16:311–320. doi: 10.1016/j.nbd.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Sinjoanu RC, Kleinschmidt S, Bitner RS, Brioni JD, Moeller A, Ferreira A. The novel calpain inhibitor A-705253 potently inhibits oligomeric beta-amyloid-induced dynamin 1 and tau cleavage in hippocampal neurons. Neurochem Int. 2008;53:79–88. doi: 10.1016/j.neuint.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryu K, Chen XH, Martinez D, Browne KD, Johnson VE, Graham DI, Lee VM, Trojanowski JQ, Smith DH. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol. 2007;208:185–192. doi: 10.1016/j.expneurol.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuvel C, Thornton E, Vink R. Traumatic brain injury and Alzheimer's disease: a review. Prog Brain Res. 2007;161:303–316. doi: 10.1016/S0079-6123(06)61021-2. [DOI] [PubMed] [Google Scholar]
- Wang KKW. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- Warren MW, Kobeissy FH, Hayes RL, Gold MS, Wang KKW. Concurrent calpain and caspase-3 mediated proteolysis of αII-spectrin and tau in rat brain after methamphetamine exposure: A similar profile to traumatic brain injury. Life Sci. 2005;78:301–309. doi: 10.1016/j.lfs.2005.04.058. [DOI] [PubMed] [Google Scholar]
- Warren MW, Kobeissy FH, Liu MC, Hayes RL, Gold MS, Wang KK. Ecstasy toxicity: a comparison to methamphetamine and traumatic brain injury. J Addict Dis. 2006;25:115–123. doi: 10.1300/J069v25n04_11. [DOI] [PubMed] [Google Scholar]
- Warren MW, Zheng WR, Kobeissy F, Liu MC, Hayes RL, Gold MS, Liu MC, Wang KKW. Calpain and caspase mediated proteolysis of αII-spectrin and tau in rat cerebrocortical neuron cultures after ecstasy (MDMA) or methamphetamine exposure Int. J Neuropsypharm. 2007;9:1–11. doi: 10.1017/S1461145706007061. [DOI] [PubMed] [Google Scholar]
- Waterhouse N, Kumar S, Song Q, Strike P, Sparrow L, Dreyfuss G, Alnemri ES, Litwack G, Lavin M, Watters D. Heteronuclear ribonucleoproteins C1 and C2, components of the spliceosome, are specific targets of interleukin 1beta-converting enzyme-like proteases in apoptosis. J Biol Chem. 1996;271:29335–29341. doi: 10.1074/jbc.271.46.29335. [DOI] [PubMed] [Google Scholar]
- Yang LS, Ksiezak-Reding H. Calpain-induced proteolysis of normal human tau and tau associated with paired helical filaments. J Biochem. 1995;233:9–17. doi: 10.1111/j.1432-1033.1995.009_1.x. [DOI] [PubMed] [Google Scholar]
- Yen S, Easson C, Nacharaju P, Hutton M, Yen SH. FTDP-17 tau mutations decrease the susceptibility of tau to calpain I digestion. FEBS Lett. 1999;461:91–95. doi: 10.1016/s0014-5793(99)01427-1. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Uryu K, Higuchi M, Longhi L, Hoover R, Fujimoto S, McIntosh T, Lee VM, Trojanowski JQ. Enhanced neurofibrillary tangle formation, cerebral atrophy, and cognitive deficits induced by repetitive mild brain injury in a transgenic tauopathy mouse model. J Neurotrauma. 2005;22:134–1141. doi: 10.1089/neu.2005.22.1134. [DOI] [PubMed] [Google Scholar]
- Zemlan FP, Jauch EC, Mulchahey JJ, Gabbita SP, Rosenberg WS, Speciale SG, Zuccarello M. C-tau biomarker of neuronal damage in severe brain injured patients: association with elevated intracranial pressure and clinical outcome. Brain Res. 2002;23:131–139. doi: 10.1016/s0006-8993(02)02920-7. [DOI] [PubMed] [Google Scholar]
- Zemlan FP, Mulchahey JJ, Gudelsky GA. Quantification and localization of kainic acid-induced neurotoxicity employing a new biomarker of cell death: cleaved microtubule-associated protein-tau (C-tau). Neuroscience. 2003;121:399–409. doi: 10.1016/s0306-4522(03)00459-7. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Peng C, Shi H, Wang S, Wang Q, Wang JZ. Inhibition of autophagy causes tau proteolysis by activating calpain in rat brain. J Alzheimers Dis. 2009;16:39–47. doi: 10.3233/JAD-2009-0908. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Larner S, Liu MC, Zheng W, Hayes RL, Wang KKW. Multiple αII-spectrin breakdown products distinguish calpain and caspase dominated necrotic and apoptotic cell death pathways. Apoptosis. 2009;14:1289–1298. doi: 10.1007/s10495-009-0405-z. [DOI] [PubMed] [Google Scholar]